Abstract
Objective:
The goal of this study was to evaluate differences in levator ani hematoma formation within 3 days of delivery between adult women after their first vaginal delivery and adult women who have had multiple vaginal deliveries.
Methods:
This was a cross-sectional study at a single institution from 2013 to 2015 using a high-resolution endovaginal ultrasound transducer to identify postvaginal delivery hematoma formation. Logistic regression was used to examine the association between hematoma formation and vaginal parity while considering potential confounders including induction, vaginal operative delivery, vaginal birth after cesarean, fetal weight, fetal head circumference, race and ethnicity, body mass index, age at delivery, gestational age, and length of second-stage labor.
Results:
Ninety women (46 vaginal-primiparous; 44 vaginal-multiparous) were included in this study. After adjusting for oxytocin use, length of second-stage labor, and body mass index, the odds of pelvic floor hematoma of 1000 mm3 or greater were 2.93 (95% confidence interval, 0.78–10.91) times greater in women after their first vaginal delivery compared with women with a history of multiple vaginal deliveries. The adjusted odds of pelvic floor hematoma of 1500 mm3 or greater were 6.02 (95% confidence interval, 1.09–33.24) times greater in vaginal-primiparous compared with vaginal-multiparous women.
Conclusions:
Although the prevalence of pelvic floor hematoma was higher in vaginal-primiparous women than vaginal-multiparous women after vaginal delivery, hematomas were present in both groups. Future prospective studies are needed to evaluate the additive effect of multiple vaginal deliveries on the pelvic floor.
Keywords: pelvic floor hematoma, postpartum levator ani hematoma, 3D pelvic floor ultrasound
During vaginal delivery, the levator ani muscles are stretched1 and passage of the neonate can cause avulsion of these muscles from the insertion site at the inferior pubic ramus and the pelvic side walls. Avulsion of the levator ani muscle from the pubic bone has been reported in 13% to 36% of women after first vaginal delivery.2-5 Levator ani avulsion is highly associated with pelvic organ prolapse later in life.6-8 It has been shown that documented levator ani hematomas near the pubic bone after delivery result in persistent avulsion at 3 months postpartum.9
Previous studies using 3-dimensional (3D) transperineal or 3D endovaginal ultrasonography (EVUS) have shown that the prevalence of asymptomatic pelvic floor hematoma within 72 hours after delivery ranged between 24% and 71%.9-11 However, these studies only included women after their first vaginal delivery. In addition, the presence of hematoma was ultrasonically assessed as “present” or “absent” without any objective quantitative assessment.
The goal of our study was to evaluate whether levator ani muscle hematoma size differed between vaginal-primiparous and vaginal-multiparous women in the immediate postpartum period using a high-resolution endovaginal ultrasound transducer to identify hematoma formation.
METHODS
Vaginal Primiparous Versus Vaginal Multiparous
Traditionally, women are considered primiparous if they have had one delivery and multiparous if they have had more than one delivery, regardless of the mode of delivery. For this study, patients with one vaginal delivery were classified as vaginal primiparous and those with multiple vaginal deliveries were classified as vaginal multiparous. For example, a woman with 2 births who had a cesarean delivery with the first child and then recently delivered vaginally was categorized as vaginal primiparous rather than multiparous for analysis in the present study. Deliveries of gestations of less than 20 weeks did not contribute to the number of deliveries tallied for the participants.
Study Design
This was a cross-sectional study of women who gave birth at a tertiary health center in the Oklahoma between November 21, 2013, and August 21, 2015. Women were recruited postpartum while recovering in the mother/baby unit. Inclusion criteria included the following: (1) within 3 days of vaginal delivery; (2) first, second, or third delivery; (3) singleton gestation; (4) 18 years or older; (5) 36 weeks of gestation or more at delivery; and (6) ability to read and understand English. After determination of inclusion of first, second, or third delivery, women were then categorized as vaginal primiparous or vaginal multiparous as defined previously. Women were excluded if the current delivery was their fourth delivery or greater.
Quantification of Hematoma
The outcome of interest was ultrasonographically visualized and quantified hematoma. All participants were scanned by the same registered sonographer (LED) with the same high-frequency 3D EVUS (8838 probe, 6-12 MHZ 360° side-fire rotational scanner; B-K Medical, Peabody, Mass) within 3 days of vaginal delivery. The 3D EVUS is a high-resolution ultrasound technique, which provides detailed information about the pelvic floor muscle structures.12,13 Cadaveric correlation has shown the 3D EVUS can accurately image the levator ani muscles.12 A 3D EVUS transducer inserted approximately 6 cm into the vaginal canal allows for use of higher frequencies to optimize imaging of the adjacent structures. The use of the 3D EVUS allows for visualization of the pubic bone, urethra, anal canal, and levator ani muscles in any plane including the traditional sagittal, axial, and coronal planes. In this study, the volumes were obtained following a previously described imaging approach.12,14
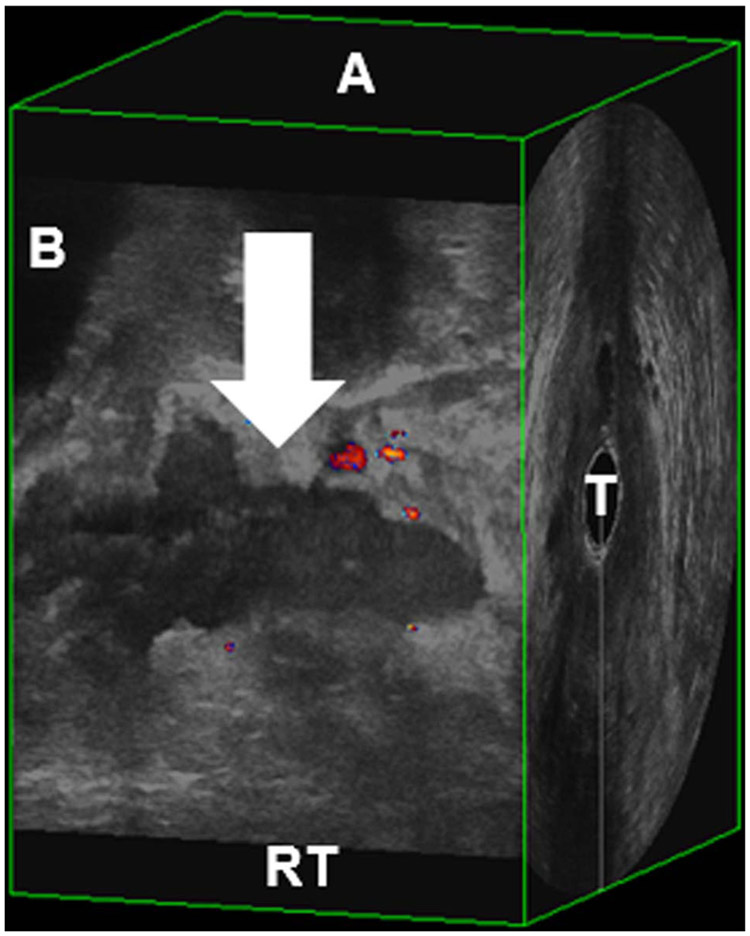
The 3D volumes were examined caudad to cephalad and then from right to left. The levator ani muscles were thoroughly examined for signs of hematoma, which sonographically appears as an inhomogeneous, hypoechoic, or anechoic area of disruption of the striated echotexture of the muscle (Fig. 1). To quantitatively assess the hematomas, the hematomas were measured in 3 planes. The ellipsoid volume formula, length × height × width × 0.523, was used.
FIGURE 1.
Sagittal view of hematoma. Arrow, hematoma; A, anterior; B, bladder; RT, right pelvic floor; T, transducer.
We wanted to limit the outcome to hematomas that appeared large enough to create a defect at the junction of the levator ani and the pubic bone (avulsion). Based on clinical expertise, a cut point of 1000 mm3 or greater was used to define hematomas large enough to produce a clinically significant defect. Therefore, “microhematomas” were defined as hematomas with a volume of less than 1000 mm3 and “macrohematomas” were defined as hematomas of 1000 mm3 or greater.
Statistical Analysis
Sample size estimates were calculated using PS: Power and Sample Size (Dupont WD; Plummer WD). A study by Van Delft et al9 found hematomas in 24% of primiparous women after delivery. Assuming a difference of 24%, a hematoma formation of 5% in the vaginal-multiparous women and 29% in vaginal-primiparous women, and using an α value of 0.05, we would need at least 36 women in the vaginal-primiparous group and 36 women in the vaginal-multiparous group to have 80% power to detect a statistically significant difference. We targeted 45 women in each group to ensure that at least 36 in each group would have quality ultrasound scans and complete data for analysis.
Logistic regression was used to calculate a crude odds ratio (OR) and 95% confidence intervals (CIs) for the association between vaginal parity and hematoma. Potential confounders were chosen based on clinical knowledge and previous literature. Potential confounders considered were as follows: (1) augmentation/induction (use of pharmaceuticals or mechanical measures), (2) vaginal operative delivery (forceps/vacuum), (3) vaginal birth after caesarean, (4) fetal weight (>4000 g vs ≤4000 g), (5) fetal head circumference (≥35.5 cm vs <35.35 cm), (6) maternal race (white, African-American, or multiracial), (7) maternal ethnicity (Hispanic vs non-Hispanic), (8) maternal body mass index (BMI) immediately before delivery (<30 kg/m2 vs ≥ 30 kg/m2), (9) maternal age at delivery (>35 years vs ≤ 35 years), (10) gestational age at delivery (categorized as 36 weeks 0 days to 38 weeks 0 day, 38 weeks 1 day to 40 weeks 0 day, and ≥40 weeks 1 day), and (11) length of second-stage labor (>110 minutes vs ≤110 minutes). An interaction term combining each covariate and vaginal parity was entered into the model to evaluate interaction (P < 0.05). Each covariate was then individually entered into the model with vaginal parity to evaluate potential confounding. All covariates resulting in a 20% or more change in point estimate were included in the final model. To assess possible interaction between confounders in the final model, all pair-wise interactions for confounders in the model were evaluated.
Sensitivity Analysis
A sensitivity analysis was conducted to evaluate the impact of changing the cut point for the outcome (hematoma formation). We included cut offs of 750 mm3 or greater, 1500 mm3 or greater, and 2000 mm3 or greater and compared the results to the 1000-mm3 or greater cut point. The OR estimates and 95% CI were compared across the 4 groups based on the final adjusted model obtained using the 1000-mm3 or greater cut point.
SAS 9.3 (SAS Institute, Cary, NC) was used for statistical analysis. Statistical significance was determined using an α value of 0.05.
Ethics Approval
This study was approved by the institutional review board (IRB) at the University of Oklahoma Health Sciences Center (IRB# 3324). Patients gave informed consent in accordance with the Declaration of Helsinki before involvement in any study activities.
RESULTS
Ninety (34%) of 268 eligible women consented to participate in this study. Sixty-two percent (56/90) delivered between 38 weeks 1 day and 40 weeks 0 day. Seventy-nine percent (71/90) of the women identified themselves as white. Forty-six (51%) of 90 women were categorized as vaginal-primiparous and 44 (49%) were categorized as vaginal multiparous (Table 1).
TABLE 1.
Demographics of All Participants and Demographics by Vaginal-Primiparous Compared With Vaginal-Multiparous Status
| Variable (N = 90) | All Participants (N = 90) | Vaginal-Primiparous (n = 46) | Vaginal-Multiparous (n = 44) | P |
|---|---|---|---|---|
| Race, n (%) | ||||
| White | 71 (79) | 35 (76) | 36 (82) | 0.55 |
| African American | 18 (20) | 10 (22) | 8 (18) | |
| Multiracial | 1 (1) | 1 (2) | 0 (0) | |
| Ethnicity, n (%) | ||||
| Hispanic | 15 (17) | 8 (17) | 7 (16) | 0.98 |
| Non-Hispanic | 73 (81) | 37 (81) | 36 (82) | |
| Unknown | 2 (2) | 1 (2) | 1 (2) | |
| Maternal age at delivery, y | ||||
| Range | 18–45 | 18–45 | 18–38 | 0.07 |
| Median | 27 | 25.5 | 28 | |
| BMI, kg/m2 | ||||
| Range | 21.61–44.23 | 23.23–44.23 | 21.61–43.94 | 0.11 |
| Mean (SD) | 30.82 (5.68) | 31.76 (6.00) | 29.85 (5.20) | |
| Vaginal operative delivery,* n (%) | ||||
| No | 87 (97) | 44 (96) | 43 (98) | 0.58 |
| Yes | 3 (3) | 2 (4) | 1 (2) | |
| VBAC, n (%) | ||||
| No | 83 (92) | 39 (85) | 44 (100) | 0.0070 |
| Yes | 7 (8) | 7 (15) | 0 (0) | |
| Oxytocin use, n (%) | ||||
| No | 55 (61) | 22 (48) | 33 (75) | 0.0082 |
| Yes | 35 (39) | 24 (52) | 11 (25) | |
| Transcervical balloon catheter, n (%) | ||||
| No | 80 (89) | 38 (83) | 42 (95) | 0.05 |
| Yes | 10 (11) | 8 (17) | 2 (5) | |
| Prostaglandin E2, n (%) | ||||
| No | 88 (98) | 44 (96) | 44 (100) | 0.16 |
| Yes | 2 (2) | 2 (4) | 0 (100) | |
| Misoprostol, n (%) | ||||
| No | 84 (93) | 42 (91) | 42 (55) | 0.43 |
| Yes | 6 (7) | 4 (8) | 2 (5) | |
| Second-stage labor, † min | ||||
| Range | 1.00–280.00 | 3.00–280.00 | 1.00–157.00 | 0.0004 |
| Mean (SD) | 47.12 (54.57) | 67.69 (63.97) | 26.55 (32.69) | |
| Head circumference, cm | ||||
| Range | 31.00–37.00 | 31.00–36.00 | 32.00–37.00 | 0.0024 |
| Mean (SD) | 34.20 (1.32) | 33.79 (1.23) | 34.63 (1.29) | |
| Infant weight at delivery, n (%) | ||||
| ≤4000 g | 85 (94) | 45 (98) | 40 (91) | 0.15 |
| >4000 g | 5 (6) | 1 (2) | 4 (9) | |
| Gestational age group, n (%) | ||||
| 36w0d–38w0d | 12 (13) | 5 (11) | 7 (16) | 0.76 |
| 38w1d–40w0d | 56 (62) | 29 (63) | 27 (61) | |
| ≥40w1d | 22 (25) | 12 (26) | 10 (23) | |
| Hematoma, n (%) | ||||
| Present | 23 (26) | 18 (39) | 5 (11) | 0.02 |
| Absent | 67 (74) | 28 (61) | 39 (89) | |
No participants in this study had forcep-assisted delivery, only vacuum assistance.
Second-stage labor information missing for 6 participants. N = 84.
VBAC, vaginal birth after caesarean.
Although hematomas were present in both groups, larger hematomas were more prevalent in vaginal-primiparous women (Table 2). Macrohematomas occurred in 39% (18/46) of the vaginal-primiparous women compared with 11% (5/44) vaginal-multiparous women. The unadjusted odds of macrohematoma were 5.01 (95% CI, 1.66–15.11) times greater for vaginal-primiparous women compared with vaginal-multiparous women.
TABLE 2.
Frequency of Macro-Hematoma in Vaginal-Primiparous and Vaginal-Multiparous Women by Hematoma Size
| Hematoma Size | Vaginal-Primiparous (n = 46) |
Vaginal-Multiparous (n = 44) |
|---|---|---|
| 0–750 mm3 | 26 (57%) | 35 (80%) |
| 750–999 mm3 | 2 (4%) | 4 (9%) |
| 1000–1499 mm3 | 6 (13%) | 3 (7%) |
| 1500–1999 mm3 | 3 (7%) | 1 (2%) |
| ≥2000 mm3 | 9 (20%) | 1 (2%) |
None of the women in this study had a forcep-assisted delivery and only 3 women (2 vaginal-primiparous; 1 vaginal-multiparous) had vacuum-assisted deliveries. Of the women who had vacuum-assisted deliveries, only one had a macrohematoma.
No interaction was present when each covariate was individually entered into the model as an interaction term with vaginal parity. Of the potential confounders considered, oxytocin use, duration of second-stage labor, and BMI changed the crude point estimate by 20% or more and were included in the final model (Table 3). Six women (4 vaginal-primiparous; 2 vaginal-multiparous) were missing information on duration of second-stage labor; therefore, 84 women were included in the multivariable analysis. Of those with missing second-stage labor duration, 3 had macrohematomas. After adjusting for oxytocin use, length of second-stage labor, and BMI, the odds of pelvic floor hematoma were 2.93 (95% CI 0.78–10.91) times greater in women after their first vaginal delivery compared with women with history of multiple vaginal deliveries.
TABLE 3.
Logistic Regression Model (n = 84)
| Variable |
*Adjusted OR |
95% Wald Confidence Limits |
|---|---|---|
| Vaginal parity (primiparous vs multiparous) | 2.93 | 0.78–10.91 |
| Oxytocin (yes vs no) | 8.12 | 2.25–29.76 |
| † Duration of second-stage labor (>110 min vs ≤ 110 min) | 3.37 | 0.67–17.04 |
| BMI (obese vs not obese) | 0.25 | 0.07–0.89 |
Odds ratios are adjusted for all other variables in the table.
Second-stage labor information missing for 6 participants (n = 84).
Because the cut point of 1000 mm3 or greater for macrohematoma was chosen based on clinical expertise, a sensitivity analysis to evaluate the impact of using various cut points was conducted (Table 4). When adjusting for the same confounders but using a larger cut point of 1500 mm3 or greater, there was a significant association between vaginal parity and pelvic floor hematoma (adjusted OR, 6.02 [95% CI, 1.09–33.24]).
TABLE 4.
Sensitivity Analysis of Results of Final Model by Varying the Size Criterion for Defining Macrohematoma
| Macrohematoma Present |
Macrohematoma Present |
Macrohematoma Present |
OR (95% CI) Adjusted for Oxytocin Use, Duration of Second-Stage Labor, and BMI |
|
|---|---|---|---|---|
| Outcome | Total (N = 90) | Vaginal-Primiparous (n = 46) |
Vaginal-Multiparous (n = 44) |
*n = 84 |
| Macrohematoma, ≥750 mm3 | 29 (32%) | 20 (43%) | 9 (20%) | 1.83 (0.61–5.47) |
| Macrohematoma, ≥1000 mm3 | 23 (26%) | 18 (39%) | 5 (11%) | 2.93 (0.78–10.91) |
| Macrohematoma, ≥1500 mm3 | 14 (16%) | 12 (26%) | 2 (5%) | 6.02 (1.09–33.24) |
| Macrohematoma, ≥2000 mm3 | 10 (11%) | 9 (20%) | 1 (2%) | 6.94 (0.75–64.63) |
Second-stage labor information missing for 6 participants (n = 84).
DISCUSSION
Our study found a prevalence of pelvic floor hematoma in vaginal-primiparous women of 39% and 11% in vaginal-multiparous women. The current study demonstrated that the odds of pelvic floor hematoma at the 1000 mm3 or greater cut point, after adjusting for oxytocin use, length of second-stage labor, and BMI, were higher in vaginal-primiparous compared with vaginal-multiparous women; however, this was not a statistically significant association. The adjusted odds of hematomas 1500 mm3 or greater were 6 times higher in vaginal-primiparous women compared with vaginal-multiparous women and was statistically significant. It should be noted that there were only 14 women with macrohematomas 1500 mm3 or greater. Of these, 12 were in the vaginal-primiparous group and 2 in the vaginal-multiparous group. Although hematomas were present in both groups at all cut points, larger hematomas were more prevalent in vaginal-primiparous women, corresponding to most levator ani avulsions resulting from first vaginal delivery.15 It is likely that the association between vaginal parity and hematomas 2000 mm3 or greater was not statistically significant because of low prevalence of hematomas of that size.
At the time of this article, only 3 previous studies have included hematoma formation as part of their outcome assessment.9-11 However, none of the previous studies have compared vaginal-primiparous women with vaginal-multiparous women. Only one previous study examined hematoma formation as the primary outcome and used 3D high-resolution EVUS, as in our study. In this study by Van Delft et al,9 hematoma formation occurred in 24% of primiparous women after vaginal delivery. A potential explanation for our higher prevalence of 39% compared with the 24% reported by Van Delft et al9 was the subjective assessment of hematomas. Van Delft et al9 visually assessed for the presence of a hematoma subjectively as “present” or “absent” and may have missed capturing hematomas approximately 1000 mm3 without objective quantitative assessment.
A major strength of this study was the quantitative assessment of the hematomas. Another strength of this study was that only one sonographer scanned all the participants and reviewed all ultrasound volumes, which allowed for consistency in the quantitative assessment of pelvic floor hematoma. However, a limitation of only one sonographer reviewing the volumes is the inability to assess interrater and intrarater variability. There is also the possibility of selection bias. Women with uncomplicated deliveries may have been discharged from the hospital before the opportunity to approach them for enrolment, and others may have declined to participate in the study. It is possible that refusal to participate was due to factors associated with the presence of a hematoma, such as pain due to trauma during a difficult vaginal delivery. If a painful delivery was associated with hematoma formation and women were declining to participate because of pain, our sample underestimated the prevalence of hematoma formation in this population. The IRB approval for this study did not include data collection on participants who refused; therefore, reason for refusal was not collected. This was a cross-sectional study; therefore, temporality was not assessed. Previous studies have demonstrated that hematoma formation does not occur before delivery.16,17 Although we cannot definitively say that the hematomas observed in this study occurred during delivery, it is unlikely that women would develop spontaneous hematomas in their skeletal muscles. Finally, it is unknown how long hematoma formation in the levator ani muscles would take to reach maximum volume, but it is suspected that this process could take up to 24 hours after vaginal delivery. Therefore, for women scanned within 24 hours of delivery, their hematoma volume may have been underestimated. Unfortunately, we did not record timing of procedure but most women were scanned the following day after delivery. For a better understanding of the formation and resolution of pelvic floor hematomas, a prospective study design where timing of procedure relative to delivery is considered and multiple serial ultrasounds after delivery is necessary.
The current study is novel in using the 3D EVUS to image and compare vaginal-primiparous and vaginal-multiparous women immediately after vaginal delivery. It is also the first to quantitatively measure pelvic floor hematomas postpartum. This study demonstrated that future studies evaluating pelvic floor hematoma formation and subsequent avulsions should include both vaginal-primiparous and vaginal-multiparous women and quantitative assessment.
CONCLUSIONS
This cross-sectional study demonstrated that pelvic floor hematomas were present in both vaginal-primiparous and vaginal-multiparous women after vaginal delivery. When adjusting for oxytocin use, length of second-stage labor, and BMI, the odds of a larger pelvic floor hematoma (≥1500 mm3) were greater in women after their first vaginal delivery compared with women with a history of multiple vaginal deliveries. Although the prevalence of pelvic floor hematoma was higher in vaginal-primiparous women than vaginal-multiparous women after vaginal delivery, hematomas were present in both groups. This suggests value in pursuing a prospective study design evaluating the additive effect of multiple vaginal deliveries on the pelvic floor.
Acknowledgments
D.R.T. was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number 1K01HL135466.
Footnotes
The authors have declared they have no conflicts of interest.
REFERENCES
- 1.Lien KC, Mooney B, Delancey JO, et al. Levator ani muscle stretch induced by simulated vaginal birth. Obstet Gynecol 2004;103(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dietz HP, Lanzarone V. Levator trauma after vaginal delivery. Obstet Gynecol 2005;106(4):707–712. [DOI] [PubMed] [Google Scholar]
- 3.Shek KL, Dietz HP. Intrapartum risk factors for levator trauma. BJOG 2010;117(12):1485–1492. [DOI] [PubMed] [Google Scholar]
- 4.DeLancey JO, Kearney R, Chou Q, et al. The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet Gynecol 2003;101(1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shek KL, Dietz HP. Can levator avulsion be predicted antenatally. Am J Obstet Gynecol 2010;202(6):586.e1–586.e6. [DOI] [PubMed] [Google Scholar]
- 6.Dietz HP, Moegni F, Shek KL. Diagnosis of levator avulsion injury: a comparison of three methods. Ultrasound Obstet Gynecol 2012;40(6):693–698. [DOI] [PubMed] [Google Scholar]
- 7.Caudwell-Hall J, Kamisan Atan I, Martin A, et al. Intrapartum predictors of maternal levator ani injury. Acta Obstet Gynecol Scand 2017;96(4):426–431. [DOI] [PubMed] [Google Scholar]
- 8.Durnea CM, Khashan AS, Kenny LC, et al. Prevalence, etiology and risk factors of pelvic organ prolapse in premenopausal primiparous women. Int Urogynecol J 2014;25(11):1463–1470. [DOI] [PubMed] [Google Scholar]
- 9.van Delft K, Thakar R, Shobeiri SA, et al. Levator hematoma at the attachment zone as an early marker for levator ani muscle avulsion. Ultrasound Obstet Gynecol 2014;43(2):210–217. [DOI] [PubMed] [Google Scholar]
- 10.Albrich SB, Laterza RM, Skala C, et al. Impact of mode of delivery on levator morphology: a prospective observational study with three-dimensional ultrasound early in the postpartum period. BJOG 2012;119(1):51–60. [DOI] [PubMed] [Google Scholar]
- 11.Aydin S, Tuncel MA, Aydin CA, et al. Do we protect the pelvic floor with non-elective cesarean? A study of 3-D/4-D pelvic floor ultrasound immediately after delivery. J Obstet Gynaecol Res 2014;40(4):1037–1045. [DOI] [PubMed] [Google Scholar]
- 12.Shobeiri SA, Leclaire E, Nihira MA, et al. Appearance of the levator ani muscle subdivisions in endovaginal three-dimensional ultrasonography. Obstet Gynecol 2009;114(1):66–72. [DOI] [PubMed] [Google Scholar]
- 13.Shobeiri SA, Rostaminia G, White D, et al. The determinants of minimal levator hiatus and their relationship to the puborectalis muscle and the levator plate. BJOG 2013;120(2):205–211. [DOI] [PubMed] [Google Scholar]
- 14.Santoro GA, Wieczorek AP, Dietz HP, et al. State of the art: an integrated approach to pelvic floor ultrasonography. Ultrasound Obstet Gynecol 2011;37:381–396. [DOI] [PubMed] [Google Scholar]
- 15.Kamisan Atan I, Lin S, Dietz HP, et al. It is the first birth that does the damage: a cross-sectional study 20 years after delivery. Int Urogynecol J 2018;29(11):1637–1643. [DOI] [PubMed] [Google Scholar]
- 16.van Delft K, Sultan AH, Thakar R, et al. The relationship between postpartum levator ani muscle avulsion and signs and symptoms of pelvic floor dysfunction. BJOG 2014;121(9):1164–1171. [DOI] [PubMed] [Google Scholar]
- 17.Blasi I, Fuchs I, D’Amico R, et al. Intrapartum translabial three-dimensional ultrasound visualization of levator trauma. Ultrasound Obstet Gynecol 2011;37(1):88–92. [DOI] [PubMed] [Google Scholar]