Abstract
Background
This study investigated the effect of the reduced dose of systemic chemotherapy (SYS) on recurrence patterns in patients receiving adjuvant hepatic artery infusion (HAI) chemotherapy after complete colorectal liver metastases (CRLM) resection.
Methods
Patients undergoing complete CRLM resection between 2000 and 2007 were selected from a prospectively-maintained database and categorized as receiving SYS or HAI+SYS. Those with pre and/or intra-operative extrahepatic disease, documented death or recurrence within 30 days of CRLM resection were excluded. Competing risk, Fine and Gray’s tests were used to compare SYS vs HAI+SYS for time-to-organ recurrence.
Results
Of 361 study patients, 153 (42.4%) received SYS and 208 (57.6%) received HAI+SYS. The median follow up for survivors was 100 months (range=12–185) and 156 months (range=18–217) for SYS and HAI+SYS, respectively. The 5-year cumulative incidence (CI) of any liver recurrence was greater for those receiving SYS (SYS= 41.9% vs. HAI+SYS= 28.6%, p=0.005). The 5-year CI of developing any lung or extrahepatic recurrence for SYS patients was 36.2% and 47.9% compared to 44.5% (p=0.242) and 51.7% (p=0.551), respectively, in patients receiving HAI+SYS.
Conclusion
Despite the reduced dose of systemic chemotherapy, adjuvant HAI+SYS after CRLM resection is not associated with a significantly increased risk of extrahepatic recurrence.
Keywords: Colorectal liver metastases, adjuvant hepatic arterial infusion therapy, extrahepatic recurrence
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in the United States. The estimated number of new colorectal cancer cases for 2019 was approximately 145,000.1 Although the incidence and mortality of CRC have been declining, it is still considered the second leading cause of cancer deaths worldwide.2–5 By 2030, the incidence rates of colon and rectal cancer in patients aged 20 to 34 is projected to increase by 90% and 124%, and patients aged 35 to 49 will increase by 28% and 46%, respectively.6 At the time of CRC diagnosis, 15–20% of patients will have synchronous colorectal liver metastasis (CRLM), and of those patients with synchronous CRLM, the disease is confined to the liver in 77% of the cases.4,7,8 Ultimately, around 10% of resected CRC patients will develop metachronous liver metastases making the liver the most common site of recurrence.9,10
The prognosis of patients with untreated CRLM is poor with a median survival time of 6 months, whereas, patients with unresected CRLM have a 16–24 months median survival and a 5-year overall survival of less than 5%.11–15 Even though resection of CRLM is the only treatment that offers the best chance of long-term survival and the only chance of cure, recurrence will occur in up to 75% of patients.16–19 Treatment failure is most commonly due to liver or lung recurrences which are the two most common sites of initial recurrence.12,20–22 Therefore, preventing liver and lung recurrence is crucial for improving overall survival (OS). Adjuvant systemic chemotherapy (SYS) is associated with a marginally improved short term disease-free survival with no overall survival benefit, but is still the standard of care in the Unites States.20,23–25 Adding floxuridine, a 5-fluorouracil analog, delivered by hepatic arterial infusion to systemic chemotherapy (HAI+SYS) in the adjuvant setting has been shown to improve intrahepatic recurrence-free survival, overall survival, and liver-related mortality.26–30 The rationale for using HAI is that CRLM derive nutritive blood supply from the hepatic artery and floxuridine is nearly completely extracted by the liver. Therefore, the extrahepatic environment is not exposed to HAI chemotherapy. The purpose of adding systemic chemotherapy to the HAI treatment is to both enhance the effects of HAI therapy in the liver and decrease the risk for extrahepatic recurrence. However, to decrease liver related toxicity, the dose of systemic chemotherapy is reduced while the patient is receiving HAI chemotherapy. The effect this therapy may have on recurrence patterns outside the liver has not been thoroughly studied. The aim of this study is to investigate the rates and patterns of extrahepatic recurrence in patients receiving HAI+SYS compared to SYS after complete resection of CRLM.
Methods
Patient Selection
From a prospectively-maintained liver resection database, all patients undergoing complete resection of CRLM from January 2000 to December 2007 were identified. Exclusion criteria included patient who received preoperative HAI, incomplete resection, ablation only, extrahepatic disease known before or discovered at the time of surgery, as well as loss of follow up, recurrence, or death within 30 days of hepatectomy. Patients are typically offered adjuvant HAI, however, due to a number of factors including patient preference, physician preference and logistical issues not all patients receive adjuvant HAI therapy. Patients who received at least one infusion of adjuvant HAI chemotherapy in addition to systemic chemotherapy were included in the HAI+SYS treatment group. If patients had an HAI pump placed but never received an infusion of floxuridine, they were included in the SYS group. This cohort is a subset of a group previously studied for associations between perioperative therapy and cause of death.29 This study was approved by our Institutional Review Board.
Patient characteristics definitions
When the primary colorectal tumor was present in the cecum, ascending or proximal two-thirds of the transverse colon, it was categorized as occurring on the right, whereas if it was present in the distal one-third of the transverse colon, splenic flexure, descending, or sigmoid colon, it was categorized as occurring on the left. If the colorectal tumor was present beyond the rectosigmoid junction, then it was categorized as occurring in the rectum. The time between the primary colorectal tumor resection and the date of CRLM diagnosis was defined as the disease-free interval. If patients had their last chemotherapy treatment within 6 months of CRLM resection, they were considered to have received preoperative chemotherapy. The clinical risk score was calculated based on adding a point for the those with disease-free interval less than 12 months, node-positive primary colorectal tumor, largest CRLM diameter greater than 5 cm, more than one CRLM, and preoperative CEA greater than 200 ng/ml.16
Recurrence Definitions
All patients were followed to the date of death or last follow up. All recurrences for every patient throughout the follow up period were recorded. Recurrence-free survival (RFS) was defined as the time from CRLM resection to the first recurrence, date of last follow up, or death. Time to any liver recurrence was defined as the time from CRLM resection to the diagnosis date of first liver recurrence regardless of any prior extrahepatic recurrences, and death without liver recurrence was treated as a competing risk. Time to any lung recurrence was defined as the time from CRLM resection to the diagnosis date of first lung recurrence regardless of any prior extrapulmonic recurrences, and death without lung recurrence was treated as a competing risk. Time to any extrahepatic recurrence was defined as the time from CRLM resection to the diagnosis date of the first extrahepatic recurrence (including lung recurrence) regardless of any prior hepatic recurrences, and death without extrahepatic recurrence was treated as a competing risk.
Systemic Chemotherapy Dose
In our institution, patients receiving HAI+SYS receive a lower dose of systemic chemotherapy compared to patients receiving only systemic chemotherapy. FOLFOX is the standard of care adjuvant systemic chemotherapy regimen after complete CRLM resection. The dose for FOLFOX, when receiving HAI therapy, maintains the normal dose for Oxaliplatin but reduces the continuous infusion dose of 5-Flourouracil (5-FU) to 1000 mg/m2 from 1200 mg/m2 without giving any boluses. FOLFIRI is an alternative option for systemic chemotherapy which has a dose reduction when receiving HAI therapy including reduced irinotecan to 125 to 150 mg/m2 (from 180 mg/m2) and reduced continuous infusion dose of 5-flourouracil (5-FU) to 1000 mg/m2 (from 1200 mg/m2) without giving any boluses.
Statistical Analysis
Fisher’s exact test was used to compare differences between categorical variables and the Wilcoxon rank sum test was used for continuous variables. Kaplan-Meier and log-rank tests were used to compare RFS between the SYS and HAI+SYS groups. Competing risk methods, Fine and Gray’s test were used to compare SYS and HAI+SYS groups for time to specific organ recurrence. Our study looked at time to any recurrence (not first recurrence), and that precluded the use of multivariable analysis.
Results
Clinical characteristics of resected patients in the SYS and HAI+SYS groups
This study included 361 patients of whom 153 (42.4%) received SYS and 208 (57.6%) received HAI+SYS (Table 1). Patients receiving HAI+SYS were younger (59 vs. 62 years, p=0.028) and had more CRLM (2 vs. 1, p=0.002) at hepatectomy than those receiving SYS. The clinical risk score between the two groups was similar with the majority categorized as low risk (SYS=63% vs. HAI+SYS= 58%, p=0.329). Most patients in both groups had left-sided primary tumors and underwent major hepatectomies. Approximately half in both groups received preoperative systemic chemotherapy (SYS=52% and HAI+SYS=52%, p=>0.95).
Table 1. Perioperative characteristics of patients receiving SYS compared to HAI+SYS treatments after complete CRLM resection.
SYS= Systemic chemotherapy only, HAI+SYS= Hepatic artery infusion therapy + Systemic chemotherapy, CRLM= Colorectal liver metastases.
| SYS (n=153) | HAI+SYS (n=208) | P value | |
|---|---|---|---|
| Age (years) | 62 (31–84) | 59 (26–96) | 0.028 |
| Gender | 0.911 | ||
| Female | 53 (35) | 70 (34) | |
| Male | 100 (65) | 138 (66) | |
| BMI≥25 | 102 (67) | 152 (73) | 0.201 |
| Colon Tumor Location | 0.258 | ||
| Right | 43 (28) | 66 (32) | |
| Left | 58 (38) | 84 (40) | |
| Rectum | 52 (34) | 55 (26) | |
| Bilateral | 0 (0) | 3 (1) | |
| Primary T Status | 0.165 | ||
| 0 | 0 (0) | 3 (1) | |
| 1 | 9 (6) | 7 (3) | |
| 2 | 11 (7) | 23 (11) | |
| 3 | 102 (69) | 148 (72) | |
| 4 | 27 (18) | 26 (13) | |
| Node-positive primary | 95 (63) | 120 (58) | 0.329 |
| Preoperative CEA (ng/ml) | 10 (0.6–12560) | 8 (0.6–12325) | 0.487 |
| DFI (months) | 0.6 (0–106) | 1 (0–75) | 0.786 |
| Number of liver metastases | 1 (1–11) | 2 (1–9) | 0.002 |
| Size of larges metastases (cm) | 3.1 (0.5–22) | 3 (0.3–20) | 0.562 |
| Clinical Risk Score | 0.329 | ||
| High | 56 (37) | 88 (42) | |
| Low | 95 (63) | 120 (58) | |
| Preoperative Chemotherapy | 80 (52) | 109 (52) | >0.95 |
| Major Hepatectomy | 81 (53) | 125 (60) | 0.197 |
| Ablation | 9 (6) | 16 (8) | 0.537 |
Data are presented as median (range) for continuous variables and n (%) for categorical. BMI, body mass index, CEA, carcinoembryonic antigen, DFI, disease-free interval.
Recurrence Patterns
The median follow up for survivors in the SYS group was 100 months (range=12–185) and 156 months (range=18–217) in the HAI+SYS group. The ten-year overall RFS was 27.5% in the SYS group compared to 36.4% in the HAI+SYS group (p=0.262, Figure 1). The ten-year OS was 31% in patients receiving SYS compared to 51% in patients receiving HAI+SYS (p=0.004). The 5-year cumulative incidence (CI) of developing any intrahepatic recurrence was significantly higher in the patients receiving SYS compared to patients on HAI+SYS treatment (41.9% vs. 28.6%, p=0.005, Figure 2). The 5-year CI of developing any lung recurrence in patients receiving SYS was 36.2% compared to 44.5% in patients receiving HAI+SYS (p=0.230, Figure 3). The 5-year CI of developing any extrahepatic recurrence was 47.9% in patients receiving SYS compared to 51.7% in patients receiving HAI+SYS (p=0.538, Figure 4).
Fig 1:
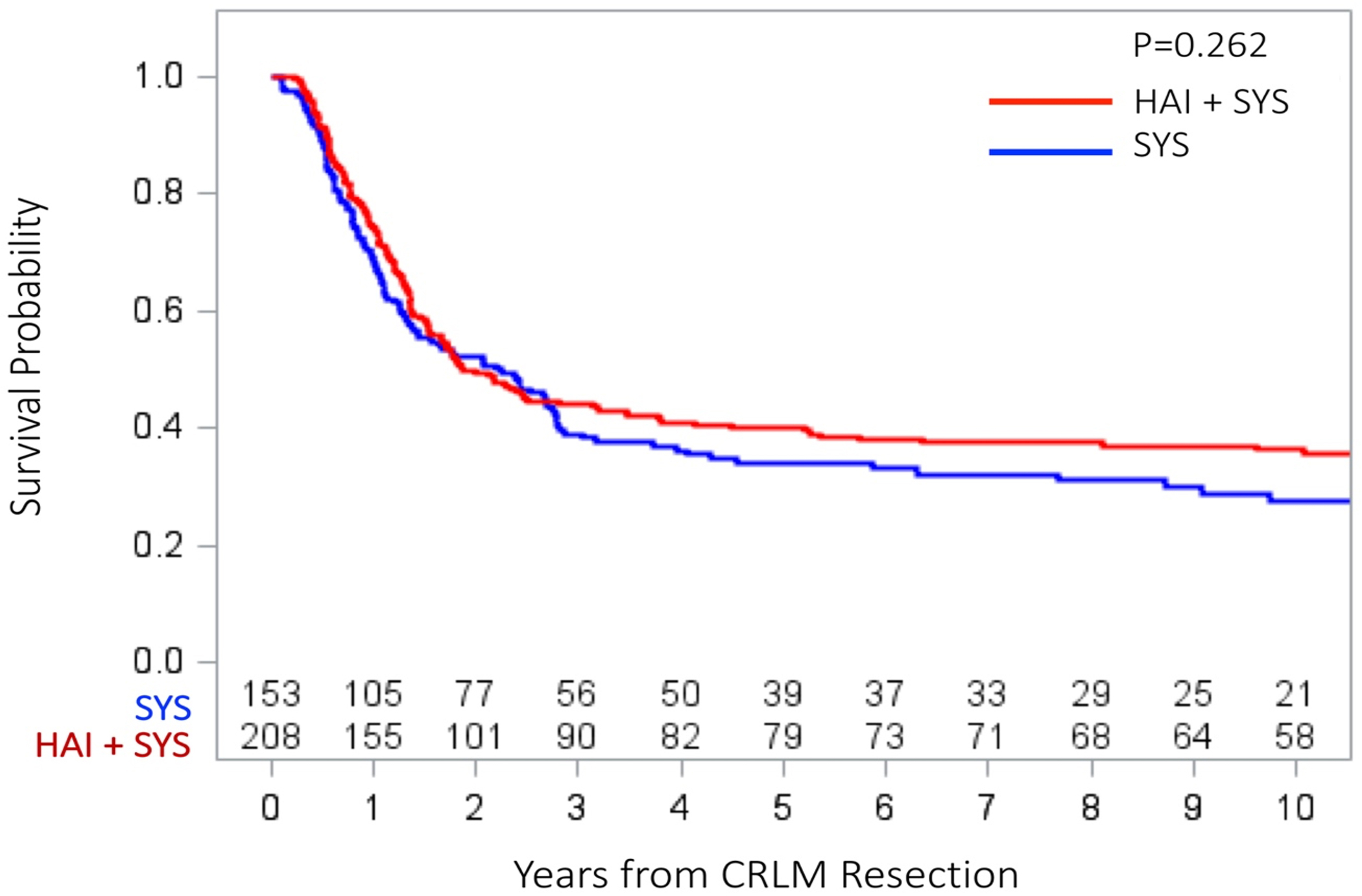
Overall recurrence‐free survival in patients receiving SYS compared with HAI + SYS treatments after complete CRLM resection. CRLM, colorectal liver metastases; HAI + SYS, hepatic artery infusion therapy + systemic chemotherapy; SYS, systemic chemotherapy only
Fig 2:
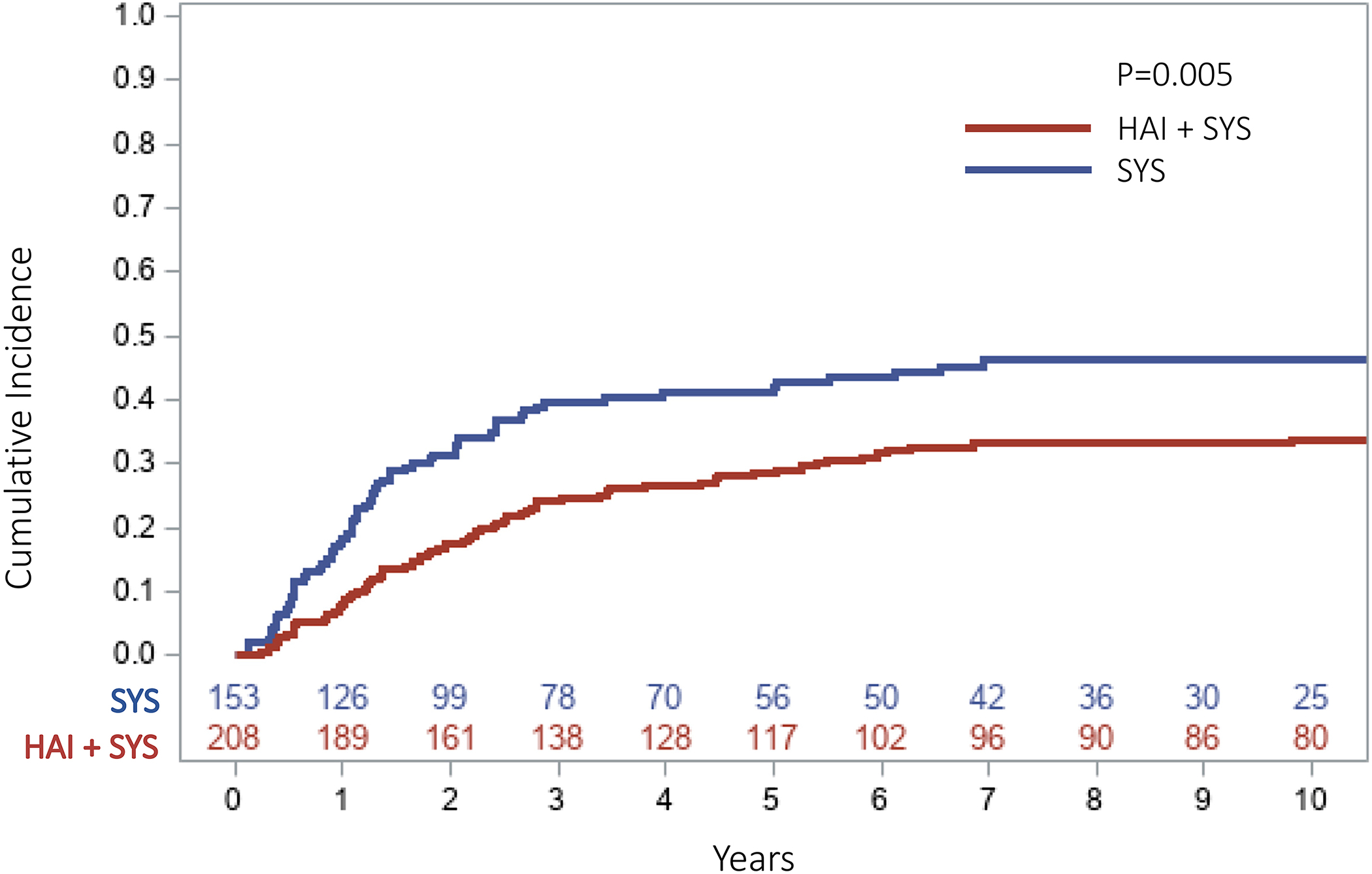
Cumulative incidence curve of any intrahepatic recurrence in patients receiving SYS compared with HAI + SYS treatments after complete CRLM resection (5‐year CI: SYS = 41.9% vs. HAI + SYS = 28.6%, p = .005). CI, cumulative incidence; CRLM, colorectal liver metastases; HAI + SYS, hepatic artery infusion therapy + systemic chemotherapy; SYS, systemic chemotherapy only
Fig 3:
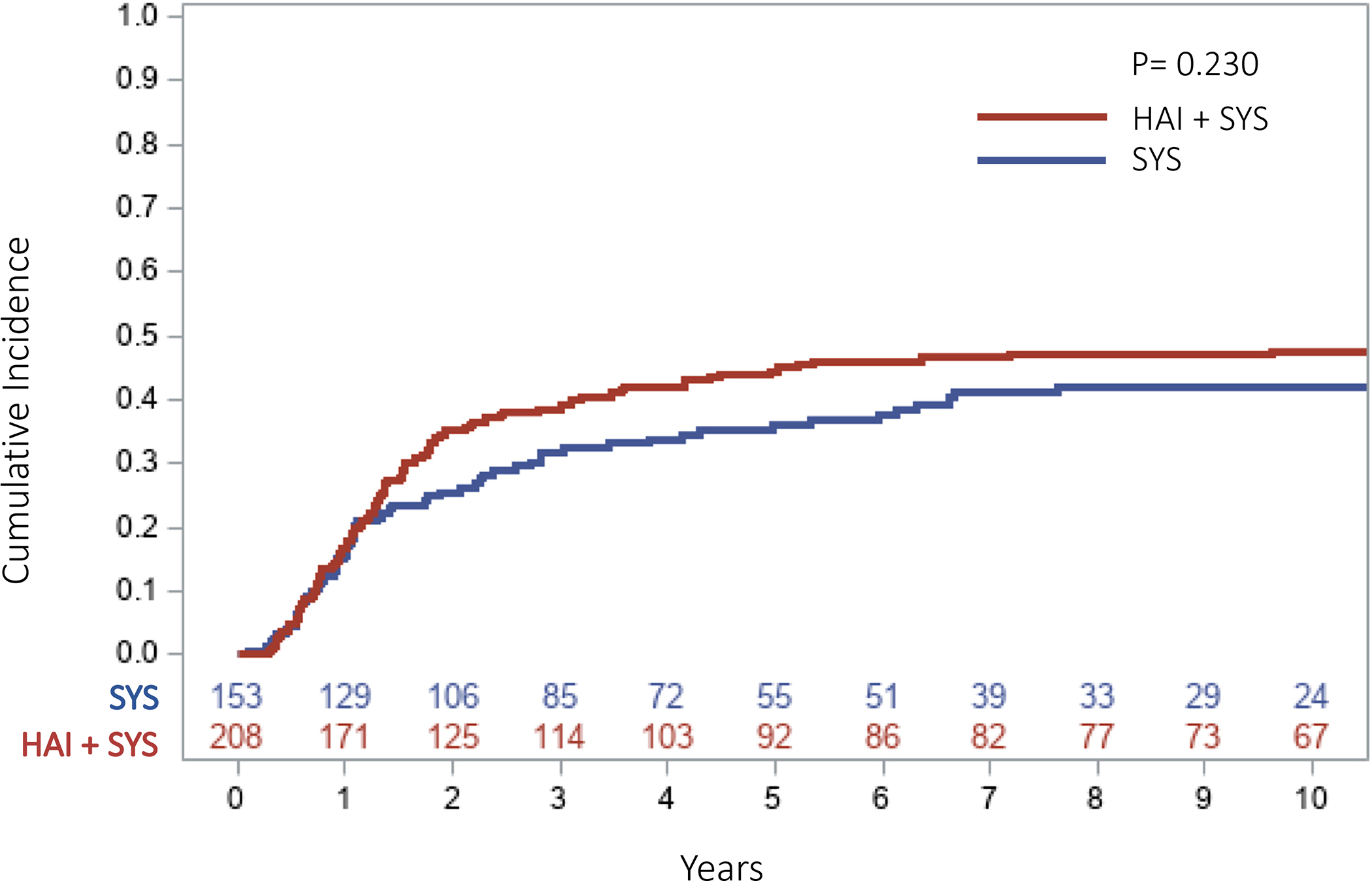
Cumulative incidence curve of any lung recurrence in patients receiving SYS compared with HAI + SYS treatments after complete CRLM resection (5‐year CI: SYS = 36.2% vs. HAI + SYS = 44.5%, p = .242). CI, cumulative incidence; CRLM, colorectal liver metastases; HAI + SYS, hepatic artery infusion therapy + systemic chemotherapy; SYS, systemic chemotherapy only
Fig 4:
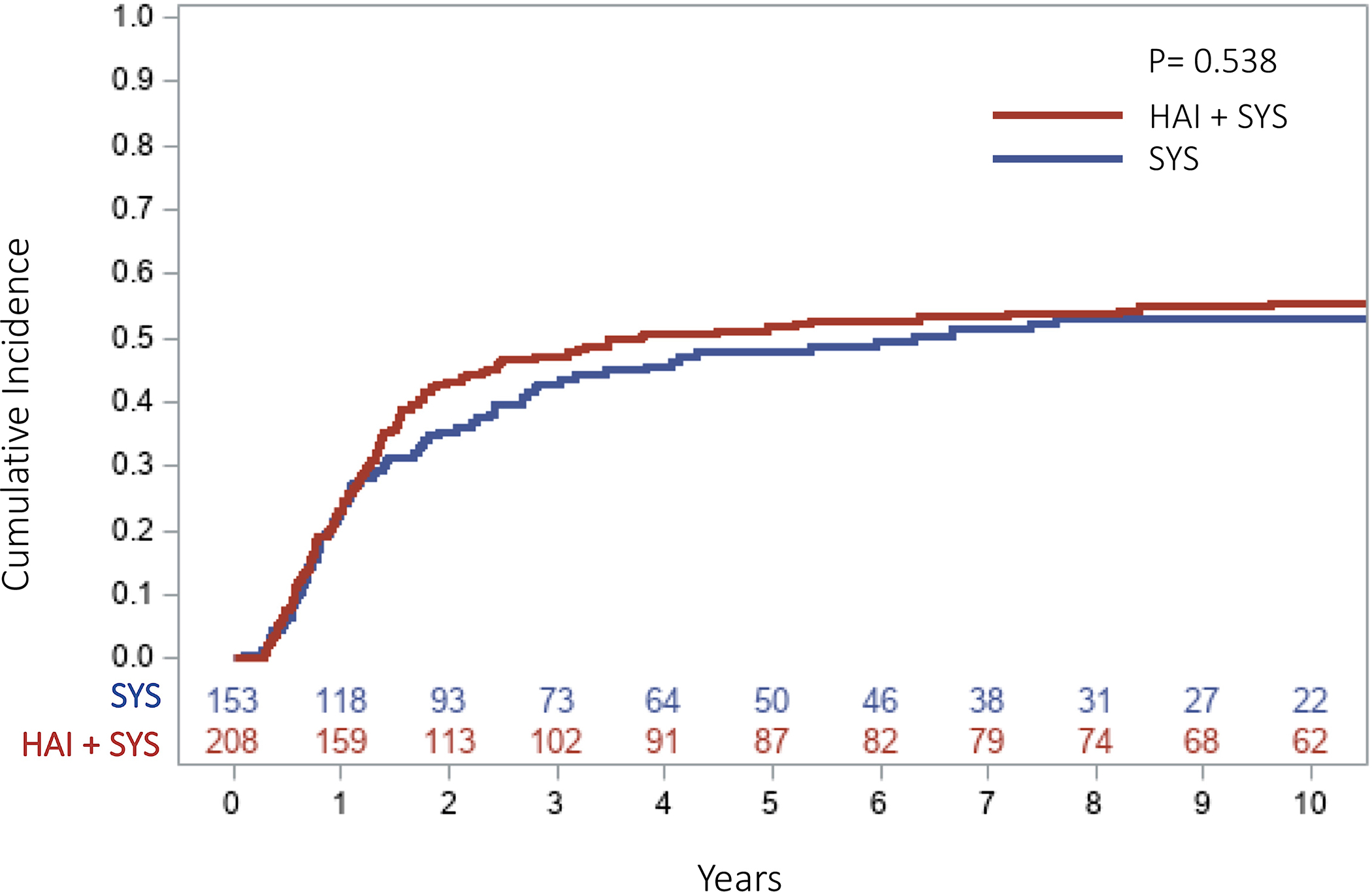
Cumulative incidence curve of any extrahepatic recurrence (including lung) in patients receiving SYS compared with HAI + SYS treatments after complete CRLM resection (5‐year CI: SYS = 47.9% vs. HAI + SYS = 51.7%, p = .551). CI, cumulative incidence; CRLM, colorectal liver metastases; HAI + SYS, hepatic artery infusion therapy + systemic chemotherapy; SYS, systemic chemotherapy only
Discussion
Recent studies have demonstrated that the 5-year overall survival of patients who underwent complete CRLM resection is approximately 50% compared to 27–44% in previous studies.17,25,31–35 Even though these studies show improvement in the overall survival, colorectal cancer is still a major cause of cancer death in the United States, and most patients will experience recurrence of disease after resection of CRLM.3 Multiple studies have investigated patterns of recurrence in patients who have undergone resection of CRLM. Many of these are outdated or underpowered and thus contradicting.21,36 More recent studies have shown that the initial recurrence after resection of CRLM involved the liver in 26–31% of the patients, and the lung in 28%.19,37 However, these studies only analyzed the initial recurrences and some included patients who received HAI therapy without addressing its effect on recurrence patterns. The strength of our study is that all recurrences over time were documented and patterns of extrahepatic recurrences in patient receiving HAI+SYS were compared to SYS.
Our study demonstrated that patients receiving adjuvant HAI+SYS are at significantly lower risk of developing any intrahepatic recurrence after CRLM resection compared to patients who received SYS. These results are in congruence with multiple previous studies establishing that adjuvant HAI chemotherapy is associated with better hepatic recurrence-free survival.26–28 The systemic chemotherapy that is administered concurrently to patients receiving HAI chemotherapy, is given at a reduced dose.38 Therefore, our study investigated whether this reduction of systemic chemotherapy necessary with the addition of HAI therapy subjects patients to an increased risk of developing extrahepatic recurrence. The results suggest that the 5-year CI of any lung recurrence in patients receiving HAI+SYS was not significantly different from patients on SYS treatment. The same results were observed when comparing the 5-year CI of any extrahepatic recurrence (inclusive of lung recurrence) between the two treatment groups. However, it is important to mention that there is an early diversion of the curves for lung recurrence after one year of resection in patients receiving HAI+SYS and SYS (Figure 3) which is also observed when analyzing any extrahepatic recurrences (Figure 4). The lowered dose of systemic chemotherapy that patients are receiving during HAI treatment may have put HAI+SYS patients at an early increased risk for extrahepatic disease which may explain the minor diversion seen in the curves. However, ultimately there was no significant difference in extrahepatic recurrence rates.
To understand the effect that HAI chemotherapy might have on the pathophysiology of metastasis, it is important to address the two models that describe the metastatic cascade. There are two ways by which cancer cells are thought to disseminate from the primary site. The first is via established solid metastases while the second is via hematogenous routes from the primary tumor to distant sites.39,40 According to the first model, it should be expected that by preventing intrahepatic recurrence, the incidence of extrahepatic recurrence will also decrease. If we apply the second model to our study, it would be expected that preventing intrahepatic recurrence will have no effect on the incidence of extrahepatic recurrence. This study demonstrates that HAI+SYS therapy is associated with decreased intrahepatic recurrence with no significant change in the incidence of extrahepatic recurrence. These patterns can be better explained by the second model of metastatic cascade. Currently, there is an ongoing trial in the Netherlands that is testing whether patients receiving adjuvant HAI therapy without systemic chemotherapy, after complete CRLM resection, compared to surgery only produces comparable outcomes.41 Since patients on this trial will not be receiving adjuvant systemic chemotherapy, the extrahepatic recurrence patterns of these patients will be essential to better understand the effect of HAI chemotherapy alone (without systemic chemotherapy) on disease recurrence outside the liver.
This study has some limitations that are important to discuss. First, the selection criteria for HAI therapy can be highly dependent on patient preference, physician preference, and logistical issues making it challenging to control for. Second, patients included in this study were treated between 2000 and 2007, and the chemotherapy regimens have been modified since that time with the introduction of FOLFOX and FOLFIRI in 2004 and 2005.42,43 However, it is unlikely that this change affected our findings since adjuvant systemic chemotherapy after CRLM resection does not decrease long-term incidence of recurrence.20,24,25,44 Third, the fact that we looked at all recurrences instead of initial recurrence only precluded the use of multivariable analysis. The reason for this is that we cannot account for the additional factors that occurred between recurrences (i.e., if for example a patient recurred in the liver first then the lungs, we cannot assess the effect of the liver recurrence, or any other new factors related to that, on the time to lung recurrence). However, the perioperative characteristics of both groups, including the Clinical Risk Score, were not significantly different (except for age and number of liver metastases).
Conclusion
Although patients on HAI+SYS therapy receive a reduced dose of systemic chemotherapy while on HAI treatment, the risk of developing lung or extrahepatic recurrence is not significantly increased. Patients receiving adjuvant HAI+SYS therapy after complete resection of CRLM have a significantly lower risk of developing intrahepatic recurrence compared to those receiving SYS.
Synopsis:
Adjuvant hepatic arterial infusion chemotherapy is associated with improved intrahepatic recurrence-free survival after resection of colorectal liver metastases. This study suggests no significant increased risk for developing extrahepatic recurrence with the addition of adjuvant hepatic arterial infusion chemotherapy, despite reduced dose of systemic chemotherapy.
Acknowledgments
FUNDING SOURCES: This work was supported in part by the NIH/NCI P30 CA008748 Cancer Center Support Grant.
Footnotes
Disclosures: The authors have no financial relationships related to this study.
Data Availability Statement:
Data available on request from the authors
References:
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: a cancer journal for clinicians. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2.Aran V, Victorino AP, Thuler LC, Ferreira CG. Colorectal cancer: epidemiology, disease mechanisms and interventions to reduce onset and mortality. Clinical colorectal cancer. 2016;15(3):195–203. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA: a cancer journal for clinicians. 2017;67(3):177–193. [DOI] [PubMed] [Google Scholar]
- 4.McMillan DC, McArdle CS. Epidemiology of colorectal liver metastases. Surgical oncology. 2007;16(1):3–5. [DOI] [PubMed] [Google Scholar]
- 5.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 6.Bailey CE, Hu C-Y, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA surgery. 2015;150(1):17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier A-M. Epidemiology and management of liver metastases from colorectal cancer. Annals of surgery. 2006;244(2):254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdalla EK, Vauthey J-N, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Annals of surgery. 2004;239(6):818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer-Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC cancer. 2014;14(1):810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valderrama-Treviño AI, Barrera-Mera B, Ceballos-Villalva JC, Montalvo-Javé EE. Hepatic metastasis from colorectal cancer. Euroasian journal of hepato-gastroenterology. 2017;7(2):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. ONCOLOGIST-MIAMISBURG-. 2008;13(1):51. [DOI] [PubMed] [Google Scholar]
- 12.De Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Annals of surgery. 2009;250(3):440–448. [DOI] [PubMed] [Google Scholar]
- 13.Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. The Lancet. 1994;343(8910):1405–1410. [DOI] [PubMed] [Google Scholar]
- 14.Bengtsson G, Carlsson G, Hafström L, Jönsson P-E. Natural history of patients with untreated liver metastases from colorectal cancer. The American Journal of Surgery. 1981;141(5):586–589. [DOI] [PubMed] [Google Scholar]
- 15.Masi G, Loupakis F, Pollina L, et al. Long-term outcome of initially unresectable metastatic colorectal cancer patients treated with 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) followed by radical surgery of metastases. Annals of surgery. 2009;249(3):420–425. [DOI] [PubMed] [Google Scholar]
- 16.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Annals of surgery. 1999;230(3):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World journal of surgery. 1995;19(1):59–71. [DOI] [PubMed] [Google Scholar]
- 18.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver: a prognostic scoring system to improve case selection, based on 1568 patients. Cancer: Interdisciplinary International Journal of the American Cancer Society. 1996;77(7):1254–1262. [PubMed] [Google Scholar]
- 19.D’Angelica M, Kornprat P, Gonen M, et al. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Annals of surgical oncology. 2011;18(4):1096–1103. [DOI] [PubMed] [Google Scholar]
- 20.Portier G, Elias D, Bouche O, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. Journal of Clinical Oncology. 2006;24(31):4976–4982. [DOI] [PubMed] [Google Scholar]
- 21.Ekberg H, Tranberg K-G, Andersson R, et al. Pattern of recurrence in liver resection for colorectal secondaries. World journal of surgery. 1987;11(4):541–546. [DOI] [PubMed] [Google Scholar]
- 22.Topal B, Kaufman L, Aerts R, Penninckx F. Patterns of failure following curative resection of colorectal liver metastases. European Journal of Surgical Oncology (EJSO). 2003;29(3):248–253. [DOI] [PubMed] [Google Scholar]
- 23.Power DG, Kemeny NE. Role of adjuvant therapy after resection of colorectal cancer liver metastases. J Clin Oncol. 2010;28(13):2300–2309. [DOI] [PubMed] [Google Scholar]
- 24.Mitry E, Fields AL, Bleiberg H, et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. Journal of clinical oncology. 2008;26(30):4906–4911. [DOI] [PubMed] [Google Scholar]
- 25.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. The lancet oncology. 2013;14(12):1208–1215. [DOI] [PubMed] [Google Scholar]
- 26.Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. New England Journal of Medicine. 1999;341(27):2039–2048. [DOI] [PubMed] [Google Scholar]
- 27.Kemeny NE, Gonen M. Hepatic arterial infusion after liver resection. New England Journal of Medicine. 2005;352(7):734–735. [DOI] [PubMed] [Google Scholar]
- 28.Kemeny MM, Adak S, Gray B, et al. Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy—an intergroup study. Journal of Clinical Oncology. 2002;20(6):1499–1505. [DOI] [PubMed] [Google Scholar]
- 29.Srouji R, Narayan R, Boerner T, et al. Addition of adjuvant hepatic artery infusion to systemic chemotherapy following resection of colorectal liver metastases is associated with reduced liver‐related mortality. Journal of Surgical Oncology. 2020;121(8):1314–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koerkamp BG, Sadot E, Kemeny NE, et al. Perioperative hepatic arterial infusion pump chemotherapy is associated with longer survival after resection of colorectal liver metastases: a propensity score analysis. Journal of Clinical Oncology. 2017;35(17):1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viganò L, Capussotti L, Lapointe R, et al. Early recurrence after liver resection for colorectal metastases: risk factors, prognosis, and treatment. A LiverMetSurvey-based study of 6,025 patients. Annals of surgical oncology. 2014;21(4):1276–1286. [DOI] [PubMed] [Google Scholar]
- 32.Abbas S, Lam V, Hollands M. Ten-year survival after liver resection for colorectal metastases: systematic review and meta-analysis. ISRN oncology. 2011;2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giuliante F, Ardito F, Vellone M, et al. Role of the surgeon as a variable in long‐term survival after liver resection for colorectal metastases. Journal of surgical oncology. 2009;100(7):538–545. [DOI] [PubMed] [Google Scholar]
- 34.Minagawa M, Makuuchi M, Torzilli G, et al. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Annals of surgery. 2000;231(4):487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jamison RL, Donohue JH, Nagorney DM, Rosen CB, Harmsen WS, Ilstrup DM. Hepatic resection for metastatic colorectal cancer results in cure for some patients. Archives of Surgery. 1997;132(5):505–511. [DOI] [PubMed] [Google Scholar]
- 36.Fortner JG. Recurrence of colorectal cancer after hepatic resection. The American journal of surgery. 1988;155(3):378–382. [DOI] [PubMed] [Google Scholar]
- 37.Butte JM, Gönen M, Allen PJ, et al. Recurrence after partial hepatectomy for metastatic colorectal cancer: potentially curative role of salvage repeat resection. Annals of surgical oncology. 2015;22(8):2761–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cercek A, D’Angelica M, Power D, et al. Floxuridine hepatic arterial infusion associated biliary toxicity is increased by concurrent administration of systemic bevacizumab. Annals of surgical oncology. 2014;21(2):479–486. [DOI] [PubMed] [Google Scholar]
- 39.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nature reviews cancer. 2004;4(6):448–456. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt-Kittler O, Ragg T, Daskalakis A, et al. From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proceedings of the National Academy of Sciences. 2003;100(13):7737–7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buisman F, Homs M, Grünhagen D, et al. Adjuvant hepatic arterial infusion pump chemotherapy and resection versus resection alone in patients with low-risk resectable colorectal liver metastases–the multicenter randomized controlled PUMP trial. BMC cancer. 2019;19(1):327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. Journal of Clinical Oncology. 2004;22(1):23–30. [DOI] [PubMed] [Google Scholar]
- 43.Colucci G, Gebbia V, Paoletti G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. Journal of Clinical Oncology. 2005;23(22):4866–4875. [DOI] [PubMed] [Google Scholar]
- 44.Primrose J, Falk S, Finch-Jones M, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. The lancet oncology. 2014;15(6):601–611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors


