Abstract
Objective
To estimate the overall quality-adjusted life-years (QALYs) gained by averting 1 coronavirus disease 2019 (COVID-19) infection over the duration of the pandemic.
Methods
A cohort-based probabilistic simulation model, informed by the latest epidemiological estimates on COVID-19 in the United States provided by the Centers for Disease Control and Prevention and literature review. Heterogeneity of parameter values across age group was accounted for. The main outcome studied was QALYs for the infected patient, patient’s family members, and the contagion effect of the infected patient over the duration of the pandemic.
Results
Averting a COVID-19 infection in a representative US resident will generate an additional 0.061 (0.016-0.129) QALYs (for the patient: 0.055, 95% confidence interval [CI] 0.014-0.115; for the patient’s family members: 0.006, 95% CI 0.002-0.015). Accounting for the contagion effect of this infection, and assuming that an effective vaccine will be available in 3 months, the total QALYs gains from averting 1 single infection is 1.51 (95% CI 0.28-4.37) accrued to patients and their family members affected by the index infection and its sequelae. These results were robust to most parameter values and were most influenced by effective reproduction number, probability of death outside the hospital, the time-varying hazard rates of hospitalization, and death in critical care.
Conclusion
Our findings suggest that the health benefits of averting 1 COVID-19 infection in the United States are substantial. Efforts to curb infections must weigh the costs against these benefits.
Keywords: contagion effect, COVID-19, family, prevention, QALYs, spillover effect
Introduction
As the coronavirus disease 2019 (COVID-19) pandemic rampages throughout the world, difficult decisions about how we can curb its spread come to the forefront. In the absence of a vaccine and a dearth of effective medications against the infections, public health measures such as wearing masks, sheltering, and maintaining physical distances becomes essential. Undoubtedly, these measures are inconvenient and sometimes harmful to economic growth and the mental health of many people in the community. Yet the consequences of not following these measures could be devastating as well. In this article, we shed light on one aspect of the potential devastation that the spread of COVID-19 can cause. Specifically, we estimate the magnitude of health loss generated by an additional infection in the community over the pandemic’s longevity. We express this health loss in terms of quality-adjusted life-years (QALYs), which combine the impact from both the length of life and the quality-of-life losses.
Calculation of QALYs is standard in economic evaluation studies that compare the costs and benefits of medical and public health interventions.1 Most recently, the Institute for Clinical and Economic Review attempted to model the costs and QALYS for remdesivir as a treatment for COVID-19.2 However, these and many other such economic evaluations tend to focus on the primary patients in measuring the health benefits. A recent review identifies 14 cost-effectiveness studies of antiviral treatments for pandemics and outbreaks of respiratory illnesses, including COVID-19. None of those studies included the quality of life of family members affected during such ordeal.3 The approaches typically used produce a partial picture of society’s realized total health benefits by averting or treating an infection. The Second Panel on Cost-Effectiveness and Health recommended including broader “spillover” effects of patients’ health to family members and others in the society but called for more research work to show how to account for these spillovers.4 , 5 The recent ISPOR Task force on valuing healthcare technologies has also highlighted the need to account for these spillover effects.6 In the case of COVID-19, there are 2 specific spillovers to consider. One is the direct spillover to family members of the infected person; the other is the spillover to other society members through the contagion effect. We account for both these spillovers in this work.
Our work illustrates a modeling approach that considers competing risks of outcomes daily for a patient with COVID-19, calibrates the model outputs to US-based estimates, and accounts for patients’ and family members’ QALYs throughout a pandemic affected by a single infection. We use our results to highlight and discuss issues that one should consider in economic evaluations of COVID-19 vaccines.
Methods
Approach
Infectious disease models typically use a structural dynamic Susceptible - Exposed - Infectious - Recovered - Susceptible type (SIR/SIRS/SEIR/SEIRS) approach to assess spread in the community. In contrast, in this article, we present an alternative “reduced form” modeling approach (which has not been used previously) to focus on a single patient and the sequelae of infections arising out of the single patient. Our estimates of the experience of this index patient calibrate with the existing knowledge about the disease. Although our model does not directly model the complicated and heterogeneous transmission processes, it does capture the transmission parameters in a “reduced form” approach through the effective reproduction ratio (R(t)). R(t) is a direct function of an SEIR model’s structural parameters. Uncertainty in those structural parameter estimates are captured through the uncertainty in Rt.7 , 8 Moreover, our model also captures the reduced-form temporal trend in Rt after a vaccine is out.9 Thus, our model presents an opportunity to assess the impact of a vaccine or treatment for COVID-19 validly and more straightforwardly by relying on reduced-form epidemiological parameters derived from a full-scale validated SEIR model.
Our patient-level model relies on a traditional Markov-type model structure, with time-varying transitions across competing risks, to capture the experience of a single patient with a COVID-19 infection. Such an approach also helps us follow the concomitant experiences of the family members in detail. Finally, the contagion effect of that single infection is captured by current and future estimates of the effective reproduction numbers.
Simulation Model
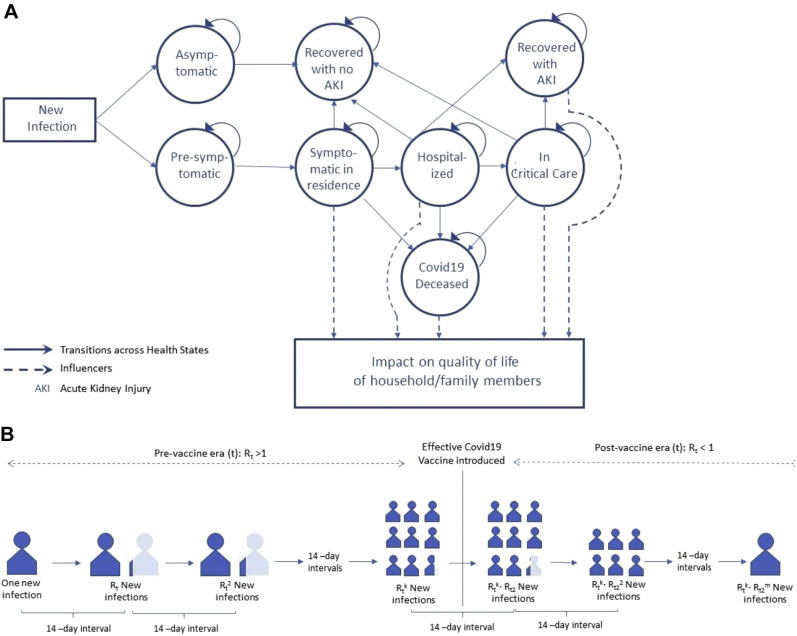
The experience of a representative patient with COVID-19 infection is illustrated in Figure 1 A. At the onset, we recognize a nontrivial probability of a COVID-19 infection to remain asymptomatic, in which case no quality of life is lost for that patient and their family members. Emerging evidence suggests that there may be some temporary lung damage in patients who are asymptomatic,10 but how that affects quality of life remains unclear. However, patients who are asymptomatic will continue to have a contagion effect, similar to a symptomatic patient. If the infection is symptomatic, the patient starts in a presymptomatic phase and daily follows a hazard to become symptomatic. Once symptomatic, the patient has a chance to be hospitalized, or they can die or recover without being hospitalized. Once hospitalized, the patients could get critical care (defined as admission to intensive care unit [ICU] or be on a ventilator) or die or recover without getting critical care. Finally, if the patient receives critical care, they can die or recover. If the patient recovers after getting critical care, there is a chance that such recovery will happen with long-term complications. Although many such long-term complications are reported in the literature,11 we only consider acute kidney injury as the most common long-term complication reported in US data.12
Figure 1.
COVID-19 quality-adjusted life-years model. (A) Patient and family member experience. (B) Contagion model.
The timeline of our model for the index patients starts with the index infection and continues till the patient recovers from the infection, unless they recover with acute kidney injury, in which case the timeline extends to the patient’s lifetime.
Quality-Adjusted Life-Years
Quality-adjusted life-years are calculated by accounting for the loss in quality-of-life weight for the specific health states that a patient experiences over its duration. We account for both the losses in QALYs experienced by the patient throughout a COVID-19 infection and the losses experienced by family members concomitant with the patient experiences (Fig. 1A). Based on the US national estimate of the average family size of 3.14 (and family size did not vary much across age),13 we account for the spillover effect on 2 family members.
Disutilities for various health states were obtained from literature estimates of quality of life losses (Table 2). These quality of life losses were divided by 365 to calculate QALYs lost per day that a patient remains in a particular health state. For example, the quality of life loss for symptomatic nonhospitalized COVID-19 health state was estimated to be –0.43 based on H1N1 patients’ experiences.14 Therefore, the disutility per day would be –0.0012 QALYs.
Table 2.
Quality of life utilities for patients with COVID-19 and their family members.
| Health state | Patients QALY loss∗ | Notes | Family member QALY loss∗ | Notes |
|---|---|---|---|---|
| Symptomatic infection | Mean(ΔQ1) = –0.43 ΔQ0~Uniform(–0.46, –0.40) |
Hollman et al (2013)35 Spain; EQ-5D; H1N1 outpatients, change from baseline |
ΔQ4~Uniform (–0.10, –0.015) | Wittenberg et al (2019)36 |
| Patient admitted to hospital | Mean(ΔQ2) = –0.50 ΔQ2~ Uniform(–0.60, –0.40) |
Barbut et al (2019)37 ICER Remdesivir Report38 |
Mean(ΔQ5) = -0.125 ΔQ5~Uniform (–0.15, –0.10) |
Interpolated between symptomatic patient and patient in ICU |
| Patient receiving critical care | Mean(ΔQ3) = –.60 ΔQ3~Uniform(–0.80, –0.40) |
Barbut et al (2019)37 ICER Remdesivir Report38 Hollman et al (2013)35 Spain; EQ-5D; H1N1 inpatient, change from baseline |
Mean(ΔQ6) = –.18 ΔQ6~Uniform(–0.21, –0.15) |
76% of family members of ICU patients showed signs of anxiety or depression (HAD scale, Pochard et al, 2005)39 Whynes et al (2009)40 (Eq-5D Disutility for either possible, other probable HADS-identified anxiety or depression ~ 0.24) |
| End-state | Lifetime QALE loss | Notes | Lifetime QALE loss | Notes |
|---|---|---|---|---|
| Patient with Acute Kidney Injury | ΔQ7 = x% of ΔQ9 <18: x = 10% (5% - 15%) 18-49: x = 12% (4% - 20%) 50-64: x = 19% (11% - 29%) 65-74: x = 22% (13%, 25%) 75+: x = 29% (17%, 43%) Beta distribution with a+b = 4000 |
Coca et al (2012)41 HR for overall mortality after AKI vs no AKI: 1.6 (95% CI 1.3–2.1) (N = 4000) |
ΔQ8: 10% (0, 20%) of ΔQ7 | Assumption. |
| Patient’s death | ΔQ9: QALE Loss <18 years: 23.97 18-49 years: 18.99 50-64 years: 12.83 65-74 years: 8.84 75+ years: 4.51 |
Fryback et al. (2007)42 US EQ-5D norm by age. 3% discounting. Vanness (2020)43 |
Mean(ΔQ8) = –0.35 for 1 year ΔQ10 ~ uniform(–0.10, –0.60) |
Comans et al (2013)44 EQ-5D disutility for bereavement of a family member and 1 year follow-up. |
QALY indicates quality-adjusted life-year.
All QALY losses converted to quality-adjusted days loss by dividing by 365.
Contagion Effect
The contagion effect of a single infection is illustrated in Figure 1B. We rely on evidence coming from population-level epidemiologic models to estimate the effective reproduction ratio (R(t)) and the uncertainty surrounding this estimate. Currently, R(t) < R0 (basic reproduction ratio) for COVID-19 due to a variety of individual-level protective behaviors (eg, wearing masks, practicing physical distancing) and policy levers (eg, shelter-in-place, business closures). While R0 for COVID-19 is estimated to be about 2.5,15 the effective R(t) currently is closer to but greater than 1.16 We account for uncertainty in this estimate using a uniform distribution (1, 1.2) and assume that this level will continue until an effective vaccine becomes available. To obtain a conservative estimate for the contagion effect,15 we assume the “generation time,” that is, time between an individual’s infection and the transmission of that infection to another individual, to be 14 days. We also assume that an effective vaccine will be available in 90 days.
Vaccine efficacy can vary. We do not make any specific assumption on vaccine efficacy level except that a prophylactic vaccine approved by the U.S. Food and Drug Administration is likely to have efficacy against acquisition of ≥70%. We allowed for a year for a non-perfect but suitable vaccine to help us reach herd immunity by the end of the year, which can be achieved by just 60% uptake. These estimates are in line with current modeling efforts with large SEIR models.17 Therefore, after a vaccine becomes available, we allow R(t) to decrease linearly over 1 year to reach levels of herd immunity or R(t)~0.8 In total, we account for the growth in infections from the index infection date over the next 15 months, with each new infection experiencing the probabilistically similar QALY loss for the patient and their family members as our index case.
Statistical Analysis
We carried out a probabilistic cohort-based simulation analysis of the model illustrated in Figure 1A. Of specific importance is to track different cohorts of infected individuals daily over the next set of competing risks that they face. For example, the cohort who would not remain asymptomatic will have a daily hazard of moving from presymptomatic to symptomatic status: those who become symptomatic will have competing risks of either dying outside hospital, recovering outside the hospital, or getting hospitalized; those who get hospitalized will face a daily competing hazard of dying in the hospital without critical care, recovering without critical care, or getting critical care; and, finally, those who get critical care will face a daily hazard of death versus recovery. We used an event-specific probability and distribution approach to simulating competing risks, where we first simulated the proportion of cohort belonging to each competing risk and then applied the subhazard of the event to model the time of incidence for the event.18 , 19 Where evidence exists, we stratified the probabilities and hazard estimates by age groups (Table 1 ). The infected person was followed until death or recovery. The resolution of final status occurred within 50 days in most of our simulations, but we followed through 180 days to capture any tails. Second-order (parameter) uncertainty for each parameter was propagated through the model using 10 000 deviates.
Table 1.
Estimates for probability and hazard parameters for Figure 1.
| Probability parameters | Distribution used | Notes |
|---|---|---|
| Asymptomatic infection | Beta(2,3) | Mean = .40, 5th percentile = .10; 95th percentile: .70. (CDC)20 |
| Age distribution of symptomatic cases | Dirichlet(69703, 616457, 325277, 142129, 166921) | <18 years: 5.2%; 18-49 years: 46.7%; 50-64 years: 24.6%; 65-74 years: 10.8%; 75+ years: 12.7%; (CDC21) |
| Hospitalized | Symptoms | <18 years: logit–1(ln(p0/(1- p0))+Normal(1.60, .30)) 18-49 years: p0 = Beta(1.5, 499) 50-64 years: logit–1 (ln(p0/(1- p0))+Normal(1.30, .10)) 65-74 years: logit–1 (ln(p0/(1- p0))+Normal(2.16, .13)) 75+ years: logit–1 (ln(p0/(1- p0))+Normal(3.63, .20)) |
18-49 years (Reference): .3% Odds ratio: <18 years: 4.96 (2.75-8.98); 50-64 years: 3.67 (3.01-4.48); 65-74 years: 8.7 (6.77-11.22); 75+ years: 37.87 (26.1-56.03); (CDC,22 Petrilli et al23) |
| Death outside hospital | Symptoms | <18 years: 0 18-49 years: p0 = Beta(2.2, 1000) 50-64 years: Beta(2.2, 1000) 65-74 years: Beta(9.5 1000) 75+ years: Beta(9.5, 1000) |
<18 years: 0%; 18-49 years: 0.22%; 50-64 years: 0.22% (assumption); 65-74 years: 0.95%; 75+ years: 0.95%; Based on % of total deaths outside hospital = 36.6%, IFR ~ 0.6% and CDC planning estimates (CDC12,15) |
| Critical Care | Hospitalized | <18 years: Beta(113, 1000) 18-49 years: Beta(113, 1000) 50-64 years: Beta(113, 1000) 65-74 years: Beta(183, 1000) 75+ years: Beta(183, 1000) |
<18 years: 11.3%; 18-49 years: 11.3%; 50-64 years: 11.3%; 65-74 years: 18.3%; 75+ years: 18.3%; Richardson et al (2020)34 |
| Death without Critical Care | Hospitalized | <18 years: 0 18-49 years: Beta(19, 1000) 50-64 years: Beta(19, 1000) 65-74 years: Beta(265, 1000) 75+ years: Beta(265, 1000) |
<18 years: 0; 18-49 years: 1.9%; 50-64 years: 1.9%; 65-74 years: 26.5%; 75+ years: 26.5%; Richardson et al (2020)12 |
| Death | Critical Care | <18 years: 0 18-49 years: Beta(637 1000) 50-64 years: Beta(637, 1000) 65-74 years: Beta(910, 1000) 75+ years: Beta(910, 1000) |
<18 years: 0; 18-49 years: 63.7%; 50-64 years: 63.7%; 65-74 years: 91%; 75+ years: 91%; Richardson et al (2020)12 |
| Acute Kidney Injury | Discharge from Critical Care | <18 years: Beta(111, 1000) 18-49 years: Beta(75, 1000) 50-64 years: Beta(75, 1000) 65-74 years: Beta(131, 1000) 75+ years: Beta(131, 1000) |
<18 years: 11.1%; 18-49 years: 7.5%; 50-64 years: 7.5%; 65-74 years: 13.1%; 75+ years: 13.1%; Richardson et al (2020)12 |
| Hazard parameters | Distribution used: Lognormal(mu, sigma) | Notes |
|---|---|---|
| Losing pre-symptomatic status | Mu~Uniform(1.51, 1.75) Sigma ~Uniform(0.45, 0.55) |
Mean days from exposure to symptom onset ~ 6 days (McAloon et al24) |
| Hospitalization | Symptoms | <18 years: mu~ Uniform(1, 2.5); sigma~(.15, .50) 18-49 years: mu~ Uniform(1,2.5); sigma~(.15, .50) 50-64 years: mu~ Uniform(1,2.5); sigma~(.15, .75) 65 - 74 years: mu~ Uniform(–.5,2); sigma~(.25, 1) 75+ years: mu~ Uniform(–.5,2); sigma~(.25, 1) |
18-49 years: 6 (3, 9) days 50-64 years: 6 (2, 9) days ≥65 years: 3 (0, 7) days (CDC15) |
| Death outside hospital | symptoms | <18 years: NULL 18-49 years: mu~ Uniform(2,3); sigma~(.30, .70) 50-64 years: mu~ Uniform(2,3); sigma~(.30, .80) 65-74 years: mu~ Uniform(1.5,3); sigma~(.30, .75) 75+ years: mu~ mu~ Uniform(1.5,3); sigma~(.30, .75) |
<18 years: NULL 18-49 years: 15 (9, 23) days 50-64 years: 15 (9, 25) days ≥65 years: 12 (7, 19) days (CDC23) |
| Recovery outside hospital |symptoms | First 7 days = 0; 7 days: uniform(0.3, .35) | Time to recovery ~ 10 days (CDC25) |
| Get critical care |Hospitalized | <18 years: mu~ Uniform(–0.5,1.2); sigma~(.95, 1.5) 18-49 years: mu~ Uniform(–0.5,1.2); sigma~(.95, 1.5) 50-64 years: mu~ Uniform(–0.5,2.3); sigma~(.5, 1.5) 65-74 years: mu~ Uniform(–1.8,1.8); sigma~(.75, 1.9) 75+ years: mu~ Uniform(–1.8,1.8); sigma~(.75, 1.9) |
<18 years: 3 (2, 5) days 18-49 years: 3 (2, 5) days 50-64 years: 6 (2, 11) days ≥65 years: 3 (1, 7) days (CDC14,15) |
| Death without critical care |Hospitalized | Same as Death outside hospital |symptoms | |
| Discharge without critical care |Hospitalized | <18 years: mu~ Uniform(–0.5,1.7); sigma~(.75, 1.5) 18-49 years: mu~ Uniform(–0.5,1.2.); sigma~(.95, 1.5) 50-64 years: mu~ Uniform(–0.5,1.7); sigma~(.75, 1.5) 65-74 years: mu~ Uniform(.3,2.2); sigma~(.5, 1.3) 75+ years: mu~ Uniform(.3,2.2); sigma~(.5, 1.3) |
<18 years: 4 (2, 7) days 18-49 years: 3 (2, 5) days 50-64 years: 4 (2, 7) days ≥65 years: 6 (3, 10) days (CDC15) |
| Death | Critical Care | <18 years: mu~ NULL 18-49 years: mu~ Uniform(1.5,2.7); sigma~(.2, .6) 50-64 years: mu~ Uniform(1.9,2.5); sigma~(.1, .2) 65-74 years: mu~ Uniform(1.75,2.6); sigma~(.1, .3) 75+ years: mu~ Uniform(1.75,2.6); sigma~(.1, .3) |
<18 years: NULL 18-49 years: 8 (5, 15) days 50-64 years: 9 (7, 12) days ≥65 years: 10 (6, 13) days (CDC15 + 1 day (Richardson et al12) |
| Discharge | Critical Care | <18 years: mu~ mu~ Uniform(1.2,2.6); sigma~(.2, .6) 18-49 years: mu~ Uniform(1.2,2.6); sigma~(.2, .6) 50-64 years: mu~ Uniform(1.8,2.4); sigma~(.1, .2) 65-74 years: mu~ Uniform(1.55,2.5); sigma~(.15, .35) 75+ years: mu~ Uniform(1.55,2.5); sigma~(.15, .35) |
<18 years: 7 (4, 14) days 18-49 years: 7 (4, 14) days 50-64 years: 8 (6, 11) days ≥65 years: 9 (5, 12) days (CDC15) |
Univariate sensitivity analysis was carried out for this probabilistic model. We studied how our main results changed when, for each parameter, we used a deterministic value equal to the 2.5 or the 97.5 percentile of the parameter distribution while keeping all other parameters probabilistic.
Results
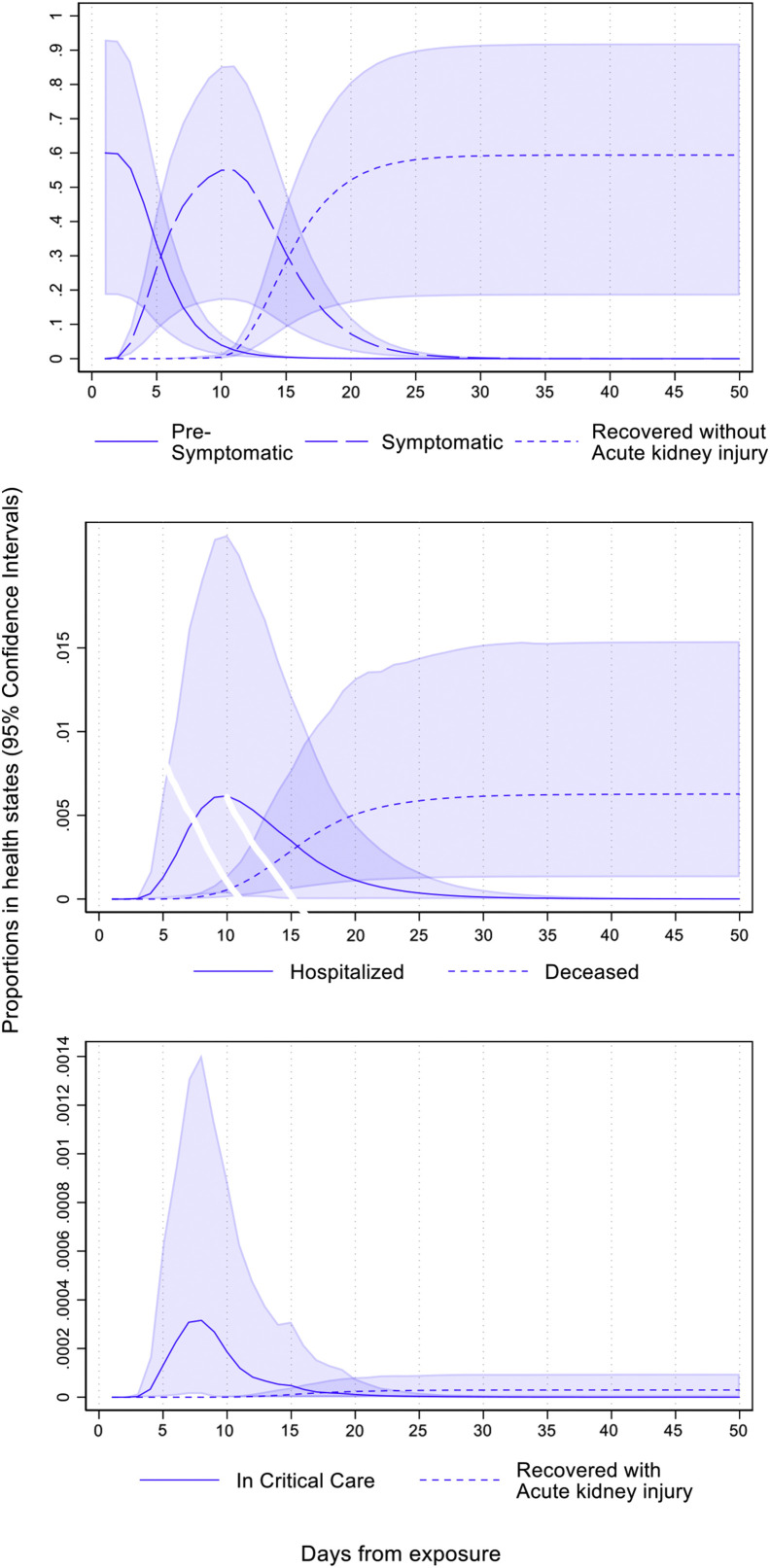
The probabilistic experiences of a single representative infection are shown in Figure 2 . There is about a 60% chance that an infected person will not remain asymptomatic. Among these, symptoms will appear for almost everyone within the first 15 days from the index day of infection (day 0) and most by the first 10 days. Close to 99% of those who become symptomatic (or 59.4% overall) recover without acute kidney disease. Almost all of these patients recover by day 25. The propensities to be in the hospital and critical care peak around the 10th day and the 7th day, respectively. This indicates that those who need critical care get hospitalized early. The probability of dying (corresponding to the infection fatality ratio) is 0.6%. Among those who become symptomatic, 0.005% recover with acute kidney injury. These estimates calibrate closely to existing evidence on COVID-19 in the United States.12 , 15 , 20, 21, 22, 23, 24, 25
Figure 2.
Proportion of initial cohort in different health states over days from exposure.
The main QALY results are shown in Table 3 . The QALY loss to the infected patient and their family members accumulates to –0.061 QALYs (95% confidence interval [CI] –0.129 to –0.016) for a single infection. Of this, 90% of the loss comes directly from patient experiences, and 10% from family members. Table 3 also shows the distribution of this loss between the patient and their family members by health states. When we applied this estimate of QALY loss for the index patient and their family members to account for the contagion effect, we found that over a 15-month period (with 3% discounting for the second year), where the vaccine arrives 3 months from now, the total QALY loss amounts to –1.51 QALYs (95% CI –4.37 to –0.28).
Table 3.
QALYs lost due to 1 representative COVID-19 infection and its distribution by health states.
| Health states | QALY loss to patients (% of total loss) [95% CI] | QALY loss to family members (% of total loss) [95% CI] | Total QALY loss (% of total loss) [95% CI] |
|---|---|---|---|
| Symptomatic nonhospitalized | –0.007 (11.5%) [–0.011 to –0.002] | –0.002 (3.3%) [–0.004 to –0.001] | –0.009 (14.8%) [–0.014 to –0.003] |
| Hospitalized, not in critical care | –0.00009 (0.15%) [–0.00028 to –0.00001] | –0.00004 (0.07%) [-0.00015 to 0] | –0.00013 (0.21%) [–0.00043 to –0.00001] |
| In critical care | –0.000003 (0.005%) [–0.00001 to –0.0000002] | –0.000002 (0.003%) [–0.000006 to –0.0000001] | –0.000005 (0.008%) [–0.000016 to –0.0000003] |
| Recovered with acute kidney diseases | –0.00005 (0.08%) [–0.00018 to –0.000003] | –0.000011 (0.02%) [–0.00004 to –0.000001] | –0.000065 (0.11%) [–0.00021 to –0.000004] |
| Death | –0.048 (78.7%) [–0.106 to –0.011] | –0.004 (6.6%) [–0.012 to –0.001] | –0.052 (85.2%) [–0.115 to –0.011] |
| TOTAL | –0.055 (90.2%)[–0.115 to –0.014] | –0.006 (9.8%)[–0.015 to –0.002] | –0.061 (100%) [–0.129 to –0.016] |
QALY indicates quality-adjusted life-year.
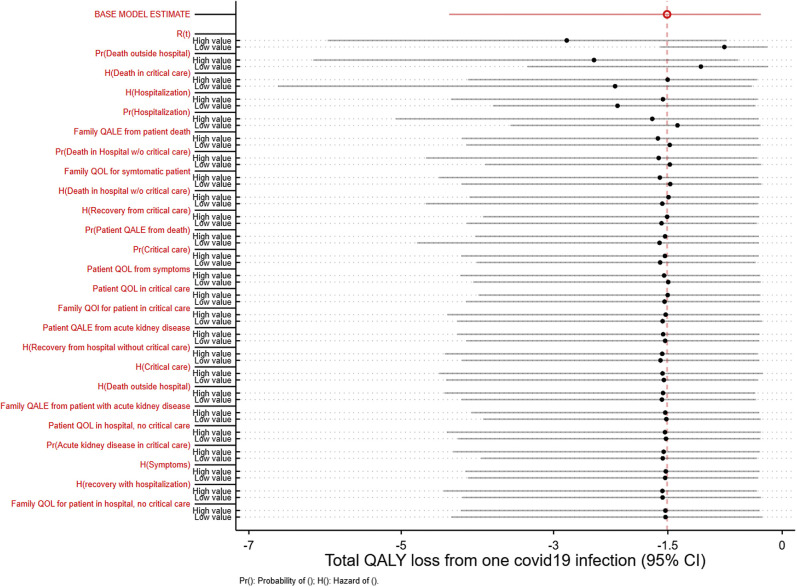
Figure 3 illustrates the sensitivity analysis of our main results based on different parameters in our model. Our base case results remain robust against most parameters. The most influential parameters are identified by the largest difference in the mean QALY loss estimate from holding the parameter value to its 2.5 and 97.5 percentile (Fig. 3). These parameters were the effective reproduction number, probability of death outside the hospital, the time-varying hazard rates of death in critical care, and the time-varying hazard rates of hospitalization.
Figure 3.
One-way sensitivity analysis for a probabilistic COVID-19 quality-adjusted life-years model.
Discussion
We developed a simulation model to comprehensively capture a COVID-19 infected patient’s experiences and their family members in the United States. We quantified these experiences using the loss in QALYs incurred. We then applied these estimates to account for the contagion effect of the index infection during the pandemic. We model the contagion effect under a conservative scenario where an effective vaccine becomes available in 3 months, following which it takes a year to achieve herd immunity.26 To our knowledge, this is the first instance where both patients’ and family members’ QALYs were modeled throughout a pandemic.
Our results show the QALY loss from 1 COVID-19 infection is substantial. To put this loss in perspective, we use the Tufts CEVR CEA registry27 to identify the incremental QALYs reported for other US interventions since 2008 across all possible scenarios (eg, perspective, time duration). Of the 1900 US-based incremental cost-effectiveness ratio estimates we identified, the potential gain in QALYs that could be achieved by preventing 1 COVID-19 infection was found to be greater than 90th percentile of the distribution of these estimates. There are many reasons for this. In general, the contagion effect of infection in a pandemic would account for a substantial portion of the value of prevention. Moreover, most, if not all, of the incremental cost-effectiveness ratios documented in the Tufts CEVR CEA registry do not account for the spillover effects of patients’ health on family members.
Our results also have implications for the evaluation of the upcoming COVID-19 vaccines. For the first vaccine that enters the market, its cost-effectiveness evaluation must compare the reduction and time profile of R(t) achieved by that vaccine versus the likelihood that R(t) initially stays high but starts declining due to its entry competitor vaccine in the future. This control arm for the evaluation of the first vaccine is illustrated in our present work, although in the case of an actual evaluation, one would model the entry of the competitor vaccine probabilistically. Finally, our model also suggests that there is a distinct first-mover advantage for a vaccine manufacturer. The first vaccine that comes to the market can show a larger effect from preventing infection than subsequent vaccines due to changing R(t)’s baseline levels (t).
Evaluation of vaccine should also account for any existing treatments of patients with COVID-19. The use of dexamethasone is already commonplace in the clinical care of patients who are hospitalized28; therefore, all our current estimates incorporate the effects of such uses. Recent trials have shown modest effects of remdesivir on the hazard of recovery of a patient who is hospitalized.29 Remdesivir did not significantly affect overall mortality,30 and most of its impact on time to recovery was concentrated among patients who did not need critical care at baseline. Consequently, the effect of remdesivir could be incorporated into our model by altering the hazard of recovery for hospitalized patients without critical care.29 However, as Figure 3 shows, our results are not sensitive to this parameter. Therefore, even though remdesivir may benefit some patients, it likely will have little influence on the overall value of a vaccine. In general, the value of prevention always hinges on the effectiveness of treatments. If effective treatments that can substantially reduce mortality become available in the future, the value of prevention can be revisited.
Existing evidence suggests the disproportionate toll of COVID-19 among minority communities. Our model would readily be extended to study the burden of an infection within separate communities, given detailed data on the distribution of infections by age group, alternate progression parameters, and effective reproduction ratios are established with these communities. However, substantial evidence gaps remain on these issues.
There are several limitations to our analysis. We believe that all of these drawbacks indicate that our estimate of the effect of preventing an additional COVID-19 infection is conservative. First, we account for acute kidney injury as the long-term complication of getting critical care with COVID-19. Emerging evidence suggests that there may be additional long-term complications associated with severe infections.11 Of specific concern is the evidence on the involvement of the heart (eg, myocarditis) in several recovered patients.31 However, the long-term consequences of these manifestations are yet to be established.32 Second, we assume that patients return to normal health immediately after recovery from infection. Some evidence suggests that return to normalcy may take many more days even after a patient becomes infection-free.33 Third, we assume the probability of death in patients below 18 years to be zero. There have been reports of about 90 COVID-19 deaths in the United States among children.15 For each of these cases, as more evidence accumulates, incorporating the effects would likely increase the impact of preventing a COVID-19 infection. Finally, our estimates of quality-of life weights do not directly come from patients with COVID-19, but rather patients experiencing severe influenzas or H1N1. Further works to assess the quality of life among patients with COVID-19 will be helpful.
Our model provides an alternate way to model the full impact of COVID-19 infection on QALYs of patients, family members, and the contagion effect. Efforts to curb COVID-19 infections must weigh the costs against these benefits.
Acknowledgments
Author Contributions: Concept and design: Basu, Gandhay
Acquisition of data: Basu, Gandhay
Analysis and interpretation of data: Basu, Gandhay
Drafting of the manuscript: Basu, Gandhay
Critical revision of paper for important intellectual content: Basu, Gandhay
Statistical analysis: Basu
Conflict of Interest Disclosures: Dr Basu reported receiving consulting fees from Salutis Consulting LLC, outside of the submitted work. He is an editor for Value in Health and had no role in the peer review process of this article. No other disclosures were reported.
Funding/Support: The authors received no financial support for this research.
Acknowledgment: We thank 3 anonymous reviewers for their helpful comments. We would like to thank Peter Neumann and the Center for the Evaluation of Value and Risk in Health at the Institute for Clinical Research and Health Policy Studies at Tufts Medical Center for providing us with the CEA registry data.
References
- 1.Sanders G.D., Maciejewski M.L., Basu A. Overview of cost-effectiveness analysis. JAMA. 2019;321(14):1400–1401. doi: 10.1001/jama.2019.1265. [DOI] [PubMed] [Google Scholar]
- 2.Whittington M.D., Campbell J.D. Alternative pricing models for remdesivir and other potential treatments for COVID-19. https://icer-review.org/wp-content/uploads/2020/06/ICER-COVID_Revised_Report_20200624.pdf
- 3.Dawoud D.M., Soliman K.Y. Cost-effectiveness of antiviral treatments for pandemics and outbreaks of respiratory illnesses, including COVID-19: a systematic review of published economic evaluations [e-pub ahead of print] Value Health. 2020 doi: 10.1016/j.jval.2020.07.002. Accessed August 14, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanders G.D., Neumann P.J., Basu A. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 5.Neumann P.J., Kim D.D., Trikalinos T.A. Future directions for cost-effectiveness analyses in health and medicine. Med Decis Making. 2018;38(7):767–777. doi: 10.1177/0272989X18798833. [DOI] [PubMed] [Google Scholar]
- 6.Lakdawalla D.N., Doshi J.A., Garrison L.P., Phelps C.E., Basu A., Danzon P.M. Defining elements of value in health care: a health economics approach: an ISPOR Special Task Force Report [3] Value Health. 2018;21(2):131–139. doi: 10.1016/j.jval.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 7.van den Driessche P. Reproduction numbers of infectious disease models. Infect Dis Model. 2017;2(3):288–303. doi: 10.1016/j.idm.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong H.G., Li Y. Estimation of time-varying reproduction numbers underlying epidemiological processes: a new statistical tool for the COVID-19 pandemic. PLoS One. 2020;15(7) doi: 10.1371/journal.pone.0236464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishiura H., Chowell G. The effective reproduction number as a prelude to statistical estimation of time-dependent epidemic trends. Math Stat Est Approach Epidemiol. 2009:103–121. [Google Scholar]
- 10.Long Q., Tang X., Shi Q. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 11.Potere N., Valeriani E., Candeloro M. Acute complications and mortality in hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Crit Care. 2020;24:389. doi: 10.1186/s13054-020-03022-1. recovered patients: is it mandatory? Sci Total Environ. 2020;729:139021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Provisional COVID-19 death counts by place of death and state Atlanta, GA: Centers for Disease Control and Prevention (US), National Center for Health Statistics (MD) https://www.cdc.gov/nchs/nvss/vsrr/covid19/index.htm
- 13.United States Census Bureau America’s families and living arrangements: 2019. https://www.census.gov/data/tables/2019/demo/families/cps-2019.html
- 14.Centers for Disease Control and Prevention Characteristics and clinical outcomes of adult patients hospitalized with COVID-19 - Georgia, March 2020. https://www.cdc.gov/mmwr/volumes/69/wr/mm6918e1.htm [DOI] [PMC free article] [PubMed]
- 15.American Academy of Pediatrics Children and COVID-19: state-level data report. https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/
- 16.Abbott S., Hellewell J., Thompson R.N. Estimating the time-varying reproduction number of SARS-CoV-2 using national and subnational case counts [version 1; peer review: awaiting peer review] https://wellcomeopenresearch.org/articles/5-112
- 17.Makhoul M., Ayoub H.H., Chemaitelly H. Epidemiological impact of SARS-CoV-2 vaccination: mathematical modeling analyses. medRxiv. 2020 doi: 10.1101/2020.04.19.20070805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barton P., Jobanputra P., Wilson J., Bryan S., Burls A. The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis. Health Technol Assess. 2004;8(11):104. doi: 10.3310/hta8110. [DOI] [PubMed] [Google Scholar]
- 19.Degeling K., Koffijberg H., Franken M.D., Koopman M., IJzerman M.J. Comparing strategies for modeling competing risks in discrete-event simulations: a simulation study and illustration in colorectal cancer. Med Decis Making. 2019;39(1):57–73. doi: 10.1177/0272989X18814770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention COVID-19 pandemic planning scenarios. https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html
- 21.Centers for Disease Control and Prevention Coronavirus disease 2019 case surveillance: United States, January 22–May 30, 2020. https://www.cdc.gov/mmwr/volumes/69/wr/mm6924e2.htm?s_cid=mm6924e2_w#T2_down [DOI] [PMC free article] [PubMed]
- 22.Centers for Disease Control and Prevention Estimated influenza illnesses, medical visits, hospitalizations, and deaths in the United States: 2018–2019 influenza season. https://www.cdc.gov/flu/about/burden/2018-2019.html
- 23.Petrilli C.M., Jones S.A., Yang J. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McAloon C.G., Collins A., Hunt K. The incubation period of COVID-19: a rapid systematic review and meta-analysis of observational research. BMJ Open. 2020;10:e039652. doi: 10.1136/bmjopen-2020-039652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention Duration of isolation and precautions for adults with COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html
- 26.Jena A.B., Worsham C.M. If we want to beat COVID-19, we need to get a lot better at vaccinating people. https://www.washingtonpost.com/opinions/2020/07/07/if-we-want-beat-covid-19-we-need-get-lot-better-vaccinating-people/
- 27.CEVR Tuft Medical Center CEA registry. https://cevr.tuftsmedicalcenter.org/databases/cea-registry
- 28.Stauffer W.M., Alpern J.D., Walker P.F. COVID-19 and dexamethasone: a potential strategy to avoid steroid-related Strongyloides hyperinfection. JAMA. 2020;324(7):623–624. doi: 10.1001/jama.2020.13170. [DOI] [PubMed] [Google Scholar]
- 29.Beigel J.H., Tomashek K.M., Dodd L.E. Remdesivir for the treatment of COVID-19: preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 30.Pan H., Peto R., Karim Q.A., WHO Solidarity trial consortium Repurposed antiviral drugs for COVID-19 –interim WHO SOLIDARITY trial results [e-pub ahead of print] medRxiv. 2020 doi: 10.1056/NEJMoa2023184. Accessed August 14, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li L.Q., Huang T., Wang Y.Q. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitrani R.D., Dabas N., Goldberger J.J. COVID-19 cardiac injury: implications for long-term surveillance and outcomes in survivors. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.06.026. S1547-5271(20)30625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balachandar V, Mahalaxmi I, Subramaniam M, et al. Follow-up studies in COVID-19 [DOI] [PMC free article] [PubMed]
- 34.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hollmann M., Garin O., Galante M., Ferrer M., Dominguez A., Alonso J. Impact of influenza on health-related quality of life among confirmed (H1N1) 2009 patients. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0060477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wittenberg E., James L.P., Prosser L.A. Spillover effects on caregivers’ and family members’ utility: a systematic review of the literature. PharmacoEconomics. 2019;37:475–499. doi: 10.1007/s40273-019-00768-7. [DOI] [PubMed] [Google Scholar]
- 37.Barbut F., Galperine T., Vanhems P. Quality of life and utility decrement associated with Clostridium difficile infection in a French hospital setting. Health Qual Life Outcomes. 2019;17:6. doi: 10.1186/s12955-019-1081-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whittington M.D., Campbell J.D. Alternative pricing models for remdesivir and other potential treatments for COVID-19. Institute for Clinical and Economic Review Report. May 1, 2020. https://icer-review.org/wp-content/uploads/2020/06/ICER-COVID_Initial_Abstract_05012020.pdf
- 39.Pochard F., Darmon M., Fassier T. Symptoms of anxiety and depression in family members of intensive care unit patients before discharge or death: a prospective multicenter study. J Crit Care. 2005;20(1):90–96. doi: 10.1016/j.jcrc.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Whynes D.K., TOMBOLA Group Responsiveness of the EQ-5D to HADS-identified anxiety and depression. J Eval Clin Pract. 2009;15(5):820–825. doi: 10.1111/j.1365-2753.2008.01102.x. [DOI] [PubMed] [Google Scholar]
- 41.Coca S.G., Singanamala S., Parikh C.R. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fryback D.G., Dunham N.C., Palta M. US norms for six generic health-related quality-of-life indexes from the National Health Measurement study. Med Care. 2007;45(12):1162–1170. doi: 10.1097/MLR.0b013e31814848f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanness D. Quality-adjusted life expectancies in the US. Private Communications.
- 44.Comans T., Visser V., Scuffham P. Cost-effectiveness of a community-based crisis intervention program for people bereaved by suicide. Crisis. 2013;34(6):390–397. doi: 10.1027/0227-5910/a000210. [DOI] [PubMed] [Google Scholar]