Abstract
Rapid development of vaccines for COVID-19 has relied on the application of existing vaccine technologies. This work examines the maturity of ten technologies employed in candidate vaccines (as of July 2020) and NIH funding for published research on these technologies from 2000–2019. These technologies vary from established platforms, which have been used successfully in approved products, to emerging technologies with no prior clinical validation. A robust body of published research on vaccine technologies was supported by 16,358 fiscal years of NIH funding totaling $17.2 billion from 2000–2019. During this period, NIH funding for published vaccine research against specific pandemic threats such as coronavirus, Zika, Ebola, and dengue was not sustained. NIH funding contributed substantially to the advance of technologies available for rapid development of COVID-19 vaccines, suggesting the importance of sustained public sector funding for foundational technologies in the rapid response to emerging public health threats.
Keywords: NIH Funding, Vaccine, COVID-19, SARS-CoV-2
Abbreviations: CEPI, Coalition for Epidemic Preparedness Innovations; MeSH, Medical Subject Headings; WHO, World Health Organization; PMID, PubMed Identifiers; TLR9, toll-like receptor 9; TIME, Technology Innovation Maturation Evaluation (model); RePORT, (NIH) Research Portfolio Online Reporting Tools
1. Introduction
The COVID-19 pandemic has triggered a rapid mobilization of global vaccine development. With infection fatality rates approaching 1% [1], the prospect of long-term sequelae in those who recover [2], [3], and a high level of population immunity required to halt transmission [4], initiatives have proceeded at “warp speed” [5], [6]. The urgency of vaccine development necessitated the application of existing vaccine technologies.
Within six months of the first description of the SARS-CoV-2 virus [7], candidate vaccines employing many diverse methodologies entered development [8]. Some candidates incorporated technologies that had already been validated in successful products. Others incorporated technologies that had been previously shown to generate immune responses against human coronaviruses including Middle East Respiratory Syndrome virus (MERS-CoV) [9] and SARS-CoV-1 [10]. Some candidates applied technologies from registered veterinary vaccines against coronaviruses in domesticated species, including (chicken) Infectious Bronchitis Virus (IBV) [11] and bovine Betacoronavirus-1 [12]. Nascent technologies, such as genetically modified viral vectors or mRNA also played an important role, even though these technologies were not previously validated in clinical trials or registered products.
Rapid success of these initiatives was not guaranteed. Despite successful development of many vaccines, the challenge remained daunting. As late as 2013, evidence showed that the failure rate for vaccines entering development was as high as 94%, and that the average time from preclinical studies to approval was 10.7 years [13].
Research on technological innovation has demonstrated that the maturation level, or readiness, of many different technologies has been a critical determinant in their ability to generate products that satisfy market needs [14], [15], [16], [17], [18], [19]. Using a bibliometric model for the advance of basic biomedical research, we have found that few targeted therapeutics are successfully developed before research on both the drug target and therapeutic modality pass an analytically described established point, and that timelines of clinical development are significantly shorter when clinical trials commence after this point [20], [21]. Similarly, the maturation of vaccine technologies is recognized to be a factor in the success of vaccine development. For example, a model of vaccine development prepared for the Coalition for Epidemic Preparedness Innovations (CEPI) posits that technologies with “no licensure track-record,” have a substantially higher risk of failure than “well-established” technologies [22]. This is consistent with the suggestion that the high failure rate of vaccines in development prior to 2013 was due, in part, to the large number of candidate vaccines using unproven, nucleotide (DNA or RNA) technologies [13].
Research on the basic science and technologies underlying new drugs or vaccines, and the maturation of these technologies to the point that they can support efficient development, is funded primarily by the public sector, principally governments. We have previously examined the scale of NIH funding for the basic research and technologies underlying new drug approvals by identifying NIH funding cited in published research papers [21], [23]. These studies show that the NIH contributed more than $170 billion for research related to the 356 drugs approved from 2010–2019 or their drug targets, with more than 85% of this funding involving research on the drug targets rather than the drugs themselves. We have also observed that the majority of this research is funded through investigator-initiated research projects [21].
The present work examines the maturation of research on the technologies being used in candidate COVID-19 vaccines as well as the NIH funding supporting this research over the past twenty years. Specifically, we examine published research on vaccines incorporating attenuated or inactivated viruses, synthetic (recombinant) proteins, DNA or mRNA, or recombinant viral vectors as well as research on formulations including conventional adjuvants, virus-like particles, nanoparticles and toll-like receptor 9 (TLR9) agonists. We also examine research and NIH funding for vaccines aimed at coronaviruses and three unrelated viral pathogens that have been associated with epidemic transmission: Zika, Ebola, and dengue. We consider the impact of this prior research on accelerated efforts to develop a vaccine for COVID-19, and the importance of sustained public-sector funding in establishing a foundation for responding to pandemic outbreaks.
2. Materials and methods
Technologies utilized in candidate vaccines against COVID-19 were identified from the World Health Organization (WHO) “DRAFT Landscape of Candidate COVID-19 vaccines” [24]. Viruses with epidemic potential were identified from Plotkin [25] or the WHO Blueprint [26].
Searches were performed using PubMed (www.pubmed.gov; accessed June 3, 2020), with the updated Automatic Term Mapping (May, 2020) optimized with Medical Subject Headings (MeSH) terms or Boolean modifiers to increase specificity (Supplemental Table 1). PubMed Identifiers (PMIDs) with their respective publication year were recorded.
PMIDs acknowledging NIH funding were identified in NIH RePORTER data tables (https://exporter.nih.gov/ExPORTER_Catalog.aspx; accessed June 3, 2020) as described previously [23]. Each PMID was associated with a project year corresponding to the project number and year of publication using the “Link Tables for Project to Publication Associations.” Costs (since 2000) were derived from the “Project Data Table.” Publications occurring before the first year of the grant award or more than four years after the last year of the grant were excluded. Publications 1–4 years after the last year of the grant were associated with the project costs of the last year. All values are described after eliminating duplicate PMIDs, NIH-funded PMIDs, project years, and project costs arising from the identification of PMIDs in multiple searches, multiple sources of funding for some PMIDs, and multiple PMIDs related to many projects. “Unique” values across technologies are described after eliminating duplicates across technologies. Activity codes and the funding institute were determined from the project codes. Grant categories were derived from “NIH Types of Grant Programs 2020” (https://grants.nih.gov/grants/funding/funding_program.htm; accessed May, 2020). Costs are given in constant dollars inflation-adjusted to 2018 using the U.S. Bureau of Labor Statistics All Urban Consumer Prices (Current Series) (https://www.bls.gov/data/; accessed May, 2020). All analyses were performed in PostgreSQL and Excel.
The bibliographic Technology Innovation Maturation Evaluation (TIME) model assesses the maturation of a body of published research by modeling the rate of research accumulation, as described previously [20]. The model quantifies the “S-curve” of technology maturation described in other sectors [14], [15], [18], [19]. The TIME model fits an exponentiated logistic function to the accumulation of PubMed identified publications over time. The equation has the form:
where N is the cumulative number of publications, L is the upper limit of publications, r is the growth rate, t is time, and t 0 is the midpoint of exponential growth. This asymmetric, sigmoidal function exhibits the common logistic sigmoid function (“S-curve”) over log scales. The established (Te) point is defined as the point of minimum acceleration or logN’’(t)min. Results are visualized as annual publications, cumulative publications, or log cumulative publications to assess the suitability of the calculated curve fit.
3. Results
3.1. Research on vaccine technologies
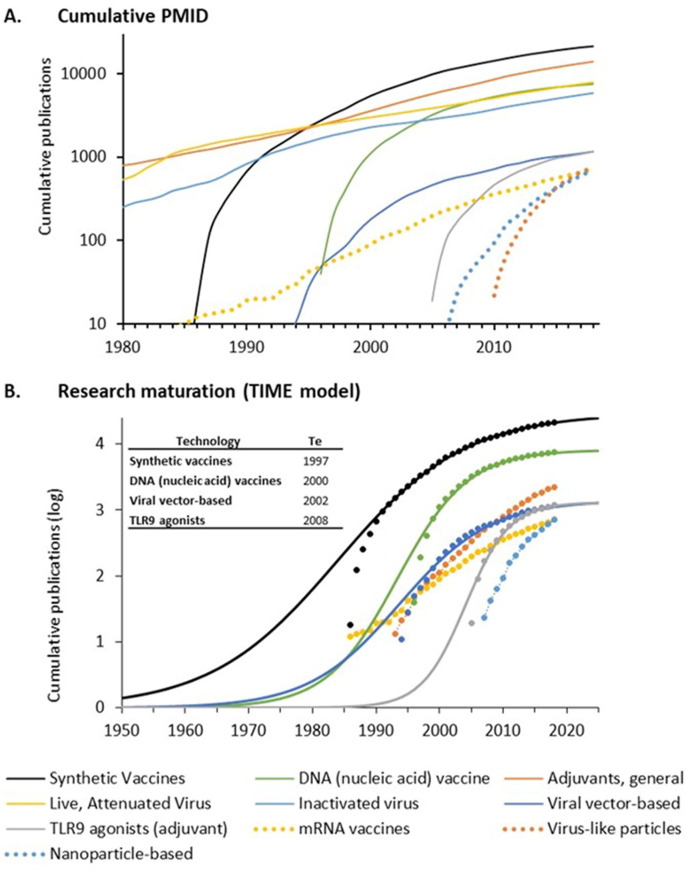
As of July 31, 2020, the WHO listed 165 candidate vaccines against COVID-19 [24]. PubMed searches were performed for ten of the technologies utilized in this portfolio of products. The ten technologies and the number of publications through 2019 are shown in Table 1 . The time course of publications on the ten technologies together is shown in Fig. 1 A. The time course of publication on individual technologies is shown in the interactive graphic at https://tabsoft.co/31EkYeK.
Table 1.
Publications in PubMed (PMID) and NIH funding associated with research on technologies used in candidate COVID-19 vaccines as well as vaccine development for selected epidemic threats.
| PMID (1960–2019) | NIH-funded PMID (1980–2019)1 | Project Years (1980–2019) | Project Costs (2000–2019)2 | |
|---|---|---|---|---|
| Selected vaccine technologies (unique)3 | 51530 | 8420 (16%) | 16358 | $17,171 |
| Synthetic Vaccines4 | 21742 | 3935 (18%) | 9755 | $9653 |
| Adjuvants, general | 14347 | 2132 (15%) | 5369 | $5642 |
| DNA (nucleic acid) vaccines5 | 7621 | 1464 (19%) | 3742 | $4585 |
| Live, Attenuated Virus | 8147 | 1399 (17%) | 3382 | $4053 |
| Viral vector-based | 1191 | 379 (32%) | 1010 | $1651 |
| Inactivated virus | 5929 | 515 (8.7%) | 1199 | $1469 |
| TLR9 agonists (adjuvant) | 1227 | 353 (29%) | 1082 | $1096 |
| mRNA vaccines | 767 | 174 (23%) | 534 | $943 |
| Virus-like particles | 801 | 161 (20%) | 418 | $583 |
| Nanoparticle-based | 761 | 121 (16%) | 334 | $519 |
| Vaccines against selected epidemic threats | ||||
| HIV | 5806 | 2024 (35%) | 6684 | $9184 |
| Coronavirus | 2435 | 388 (16%) | 625 | $767 |
| Ebola | 450 | 115 (26%) | 294 | $639 |
| Zika | 375 | 135 (36%) | 390 | $555 |
| Dengue | 725 | 110 (15%) | 231 | $331 |
Number of NIH-funded PMID and % of all PMID.
Costs given in millions of dollars inflation adjusted to 2018.
The “unique” number of PMID, NIH-funded PMID, Project Years, and Project Costs is greater than the sum of values for the individual technologies examined due to PMID addressing multiple technologies, being identified in multiple searches or having multiple sources of funding as well as Projects that produce multiple PMID. Duplication between technologies is eliminated in calculating “unique” values.
“Synthetic vaccines” is a MeSH term describing vaccines incorporating recombinant protein antigens.
Search performed for DNA vaccines included many reports describing mRNA technologies.
Fig. 1.
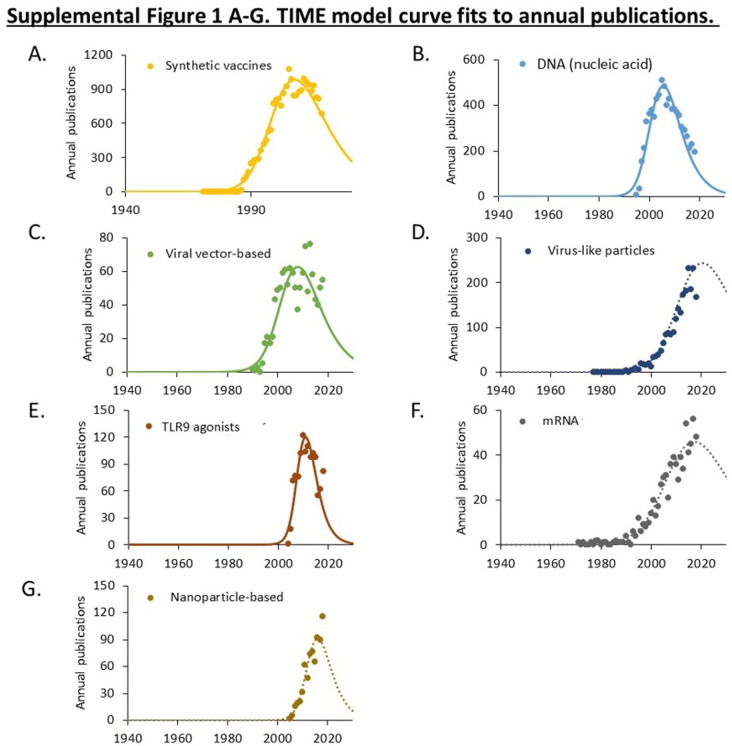
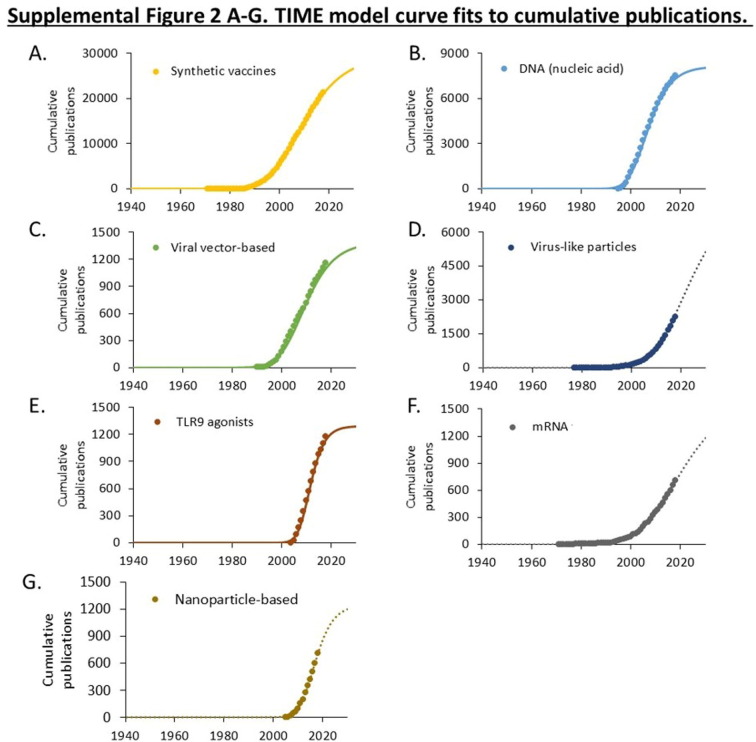
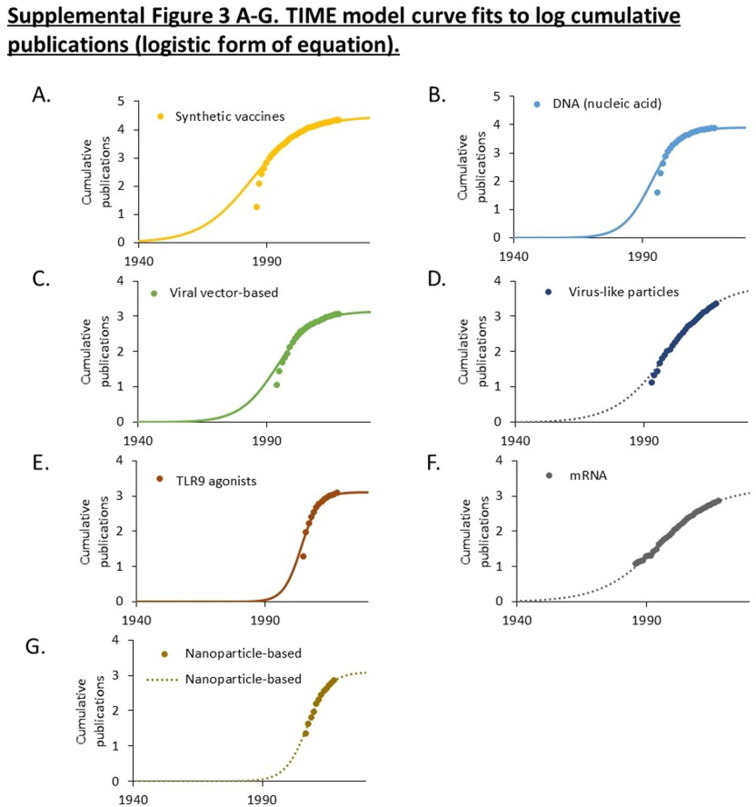
Publications related to ten vaccine technologies used in candidate COVID-19 vaccines. A. Cumulative PMIDs from a PubMed search over time. Data is shown on a logarithmic scale. B. Maturation of research using the TIME model. Cumulative publications are shown as symbols. Curves fitting the (exponentiated logistic) TIME model are shown as solid lines. The calculated established points for these technologies are shown in the box. Curves exhibiting exponential advance for technologies that have not yet reached an established point (Te) are shown as dotted lines. Supplemental Figs. 1–3 provide additional curve fits.
Technologies involving whole virus preparations, including live attenuated and inactivated viral vaccines, have been widely used in vaccines for polio, influenza, MMR, and other products since the 1950s. Research on these technologies has continued to accumulate since 1980 without evident acceleration or deceleration (Fig. 1A). These technologies were used in four of the first ten candidate vaccines to enter clinical trials against COVID-19, but only 12 (7.3%) candidate vaccines through July 31, 2020.
Synthetic vaccines incorporating recombinant proteins emerged with biotechnology in the 1980s. Exponential growth of research on synthetic vaccines was evident after 1985, but growth has slowed since the late 1990s (Fig. 1A). While only one of the first ten candidate COVID-19 vaccines to enter clinical trials employed synthetic vaccine technologies, they are used in 66 (40%) candidate vaccines through July 31, 2020.
Vaccines employing recombinant viral vectors emerged in the mid-1990s as an outgrowth of research on gene therapy. A period of exponential growth was evident in the 1990s, followed by slowing from the early 2000s to the present (Fig. 1A). Two of the first ten candidate COVID-19 vaccines in clinical trials used recombinant adenoviral vectors. Overall, recombinant viral vectors are employed in 31 (19%) candidate vaccines through July 2020.
DNA-based vaccine technologies also emerged in the mid-1990s as an outgrowth of research on gene therapy and exhibited a similar pattern of exponential advance and slowing. A related technology involving administration of mRNA derives from methods for translating purified mRNA in Xenopus oocytes and evidence that injection of purified viral mRNA into cultured cells could produce infectious virus [27]. These technologies have advanced significantly in recent years with the application of synthetic nucleotides, self-replicating mRNA, and improved formulations. One DNA vaccine and two mRNA vaccines were among the earliest candidate vaccines to enter human trials. At the end of July 2020, there were 36 (22%) nucleic acid-based vaccines in development, including 14 DNA vaccines and 22 mRNA vaccines.
Several other vaccine technologies are shown in Fig. 1A including the use of lipid nanoparticles or virus-like particles designed to enhance delivery and antigenicity of recombinant proteins and the incorporation of TLR9 agonists to stimulate specific immune pathways. These technologies were employed in 24 (15%) of the 165 candidate vaccines under development through July 2020.
Three technologies that originated in the 1950s, including live attenuated virus, inactivated virus, and adjuvants, which have been widely used in approved vaccines, have advanced continually since 1980 without evident acceleration or deceleration (Fig. 1A). The maturation of seven technologies emerging since 1980 was examined using the TIME model (Fig. 1B). This model fits the cumulative number of publications to an exponentiated logistic function and estimates the established (Te) point of this research as the point of maximum deceleration (Fig. 1B) [20], [21]. Curve fits for each technology are shown in Supplemental Figs. 1, 2, and 3.
Four technologies (synthetic, DNA, viral vector, and TLR9 agonists) exhibited a logistic pattern of growth and had estimated established points prior to 2010. Three technologies (virus-like particles, mRNA, and nanoparticles) exhibited an exponential, rather than logistic, pattern of growth (Fig. 1B, Supplemental Figs. 1, 2, and 3). This is indicative of technologies that have not yet reached the established point. Closer examination of mRNA technologies indicates that the rate of advance has slowed, though the point of maximum slowing (the established point) cannot be calculated with confidence.
3.2. NIH funding for published research on vaccine technologies
The total numbers of PMIDs, number and percent of NIH-funded PMIDs, project years, and project costs for ten vaccine technologies are shown in Table 1. We identified 51,530 unique publications related to these ten vaccine technologies, including 8,420 (16%) acknowledging NIH funding. This funding comprised 16,358 unique project years and $17.2 billion in project costs. The largest amount of funding was for synthetic (recombinant) vaccines ($9.65 billion) followed by adjuvants ($5.6 billion), DNA vaccines ($4.6 billion), and live attenuated virus ($4 billion). More than $1 billion in NIH funding was associated with published research on inactivated viral vector-based vaccines as well as TLR9 agonists as adjuvants. More than $500 million was invested in mRNA vaccines, virus-like particles, and nanoparticle-based vaccines.
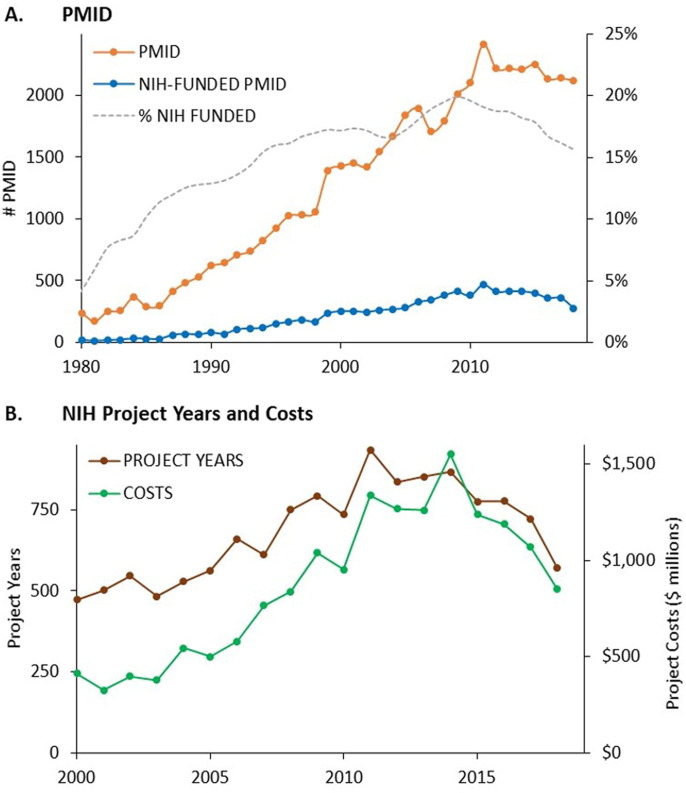
The time course of research publications, NIH-funded research publications, project years, and project costs for these ten vaccine technologies together are shown in Fig. 2 A–B. Data for each of the technologies individually is shown in the interactive graphic at https://tabsoft.co/31EkYeK.
Fig. 2.
NIH support for published research on ten vaccine technologies used in candidate COVID-19 vaccines. A. Annual PMIDs, NIH-funded PMIDs, and the fraction of PMIDs receiving NIH support for vaccine technologies. B. Annual project years and project costs associated with NIH-funded PMIDs 2000–2019.
Publication activity increased steadily from 1980 to 2010, with the percent of publications acknowledging NIH funding rising from 4% in 1980 to 20% in 2010. After 2010, annual publications and the percent of NIH-funded publications both decreased.
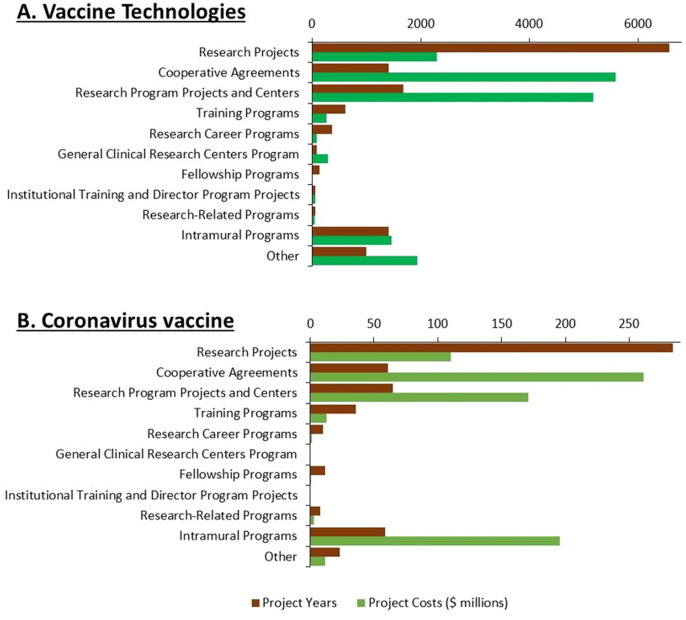
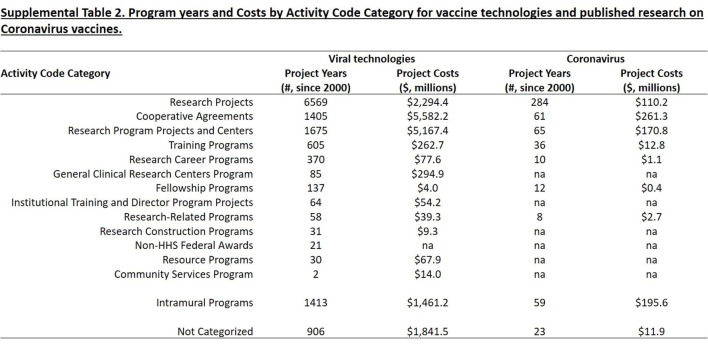
The type of research funding is shown in Fig. 3 A and Supplemental Table 2. While 40% of project years associated with research on these technologies represented investigator-initiated research projects, these accounted for only 8.9% of project costs. A greater fraction of funding involved cooperative agreements (44%), intramural programs (9.4%), and research program projects and centers (22.7%). This pattern resembles that observed for the foundational research underlying remdesivir [28], but differs sharply from that for other drugs approved from 2010–2019, where the majority of project years and costs involved investigator-initiated research projects [21].
Fig. 3.
NIH funding for published research on ten vaccine technologies and coronavirus vaccines by activity category. A. Number of project years and project costs for research on ten vaccine technologies by activity category 2000–2019. B. Number of project years and project costs for research on coronavirus vaccines 2000–2019.
3.3. Research and NIH funding for diseases with pandemic potential
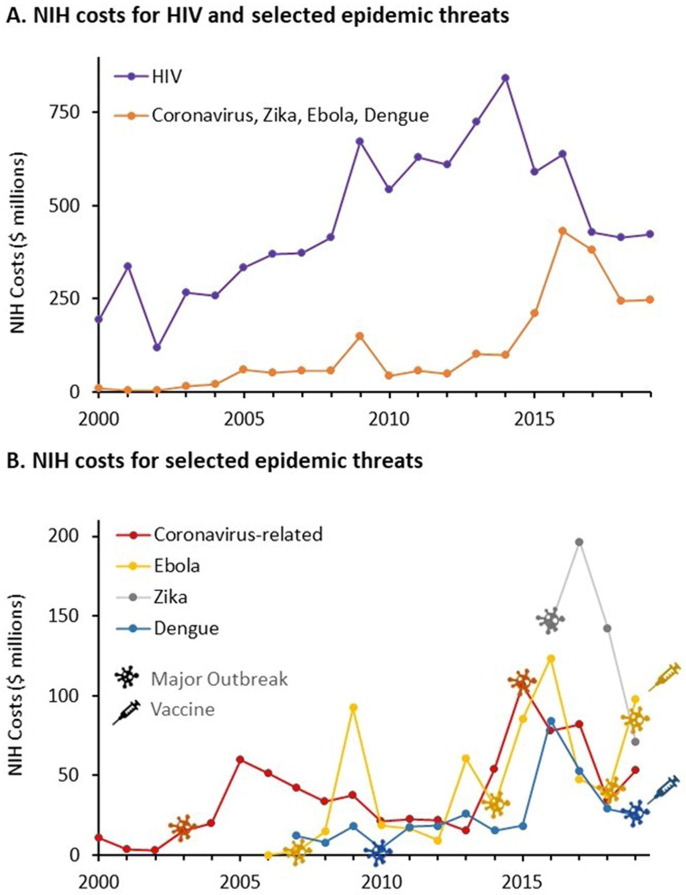
Table 1 shows the number of PMIDs, NIH-funded PMIDs, project years, and project costs associated with vaccine development for each of these pathogens as well as for HIV, which has been the subject of intensive vaccine research since the 1980s. Fig. 4 A shows the annual NIH project costs for Ebola, Zika, dengue, and coronavirus vaccines since 2000 compared to the project costs for research on HIV vaccines. Fig. 4B shows the annual NIH project costs for vaccine research on Ebola, Zika, dengue, and coronaviruses individually.
Fig. 4.
NIH costs for published search on vaccines for HIV and selected zoonotic pandemic threats (coronavirus, Ebola, Zika, dengue) 2000–2019. A. Annual NIH costs associated with published research on HIV vaccines compared to the cumulative total of research on coronavirus, Zika, Ebola, and dengue. B. Annual costs associated with published research on coronavirus, Zika, Ebola, and dengue. Symbols indicate years of major outbreaks as well as the years of vaccine approval.
NIH funding for research on HIV vaccines increased more than fourfold from 2000–2014, and has subsequently declined by half. There was little NIH funding for research on Ebola, Zika, dengue, or coronaviruses prior to 2014. NIH funding associated with publications on vaccines for Ebola virus exhibited a sharp peak in 2009, a larger peak from 2013–2015, and another resurgence in 2019. These peaks corresponded to a relatively small outbreak of Ebola in 2007, the major outbreak in 2014, which had limited spread to Europe and the US, and a recent event that began in 2018 (https://www.cdc.gov/vhf/ebola/history/chronology.html). A single peak of NIH funding associated with publications related to vaccines for Zika corresponds to the 2015–2017 outbreak in South America and the Caribbean that also affected several US states. A rise in funding associated with publications related to dengue was apparent in 2016, but dropped rapidly commensurate with the decline in reported cases in 2017 and 2018 (https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue).
The pattern of NIH funding for published research on coronavirus vaccines shows a similar correspondence with outbreaks of disease. NIH project costs associated with published research on coronaviruses peaked in 2005, then dropped steadily to 2013. A second peak of NIH funding associated with published research on coronavirus vaccines was evident in association with the MERS outbreak in 2012, peaking in 2015. Funding related explicitly to coronavirus vaccines declined rapidly after 2015 as this threat waned and other, more mediate threats, such as Zika, emerged.
Fig. 3B and Supplemental Table 2 show the activity code categories for the NIH projects associated with published research on coronavirus vaccines. While the majority of project years were associated with investigator-initiated research projects, only 14% of project costs were associated with research projects, while 34% went to cooperative agreements, 26% to intramural programs, and 22% to research program projects and centers.
4. Discussion
This analysis was undertaken in the context of evidence that there is a 94% failure rate for vaccines entering development, average clinical development time of more than 10 years [13], the exigency for rapid development of a COVID-19 vaccine [5], [6], and evidence that maturity of the underlying technologies is a significant factor in the efficiency of drug development [20], [21]. Using quantitative methods, we examined published research on ten technologies being employed in candidate COVID-19 vaccines, the maturity level, or readiness, of these technologies for clinical development, and the NIH funding for this research through the end of 2019. We also described NIH funding for vaccine development directed towards specific zoonotic threats including coronavirus, Zika, Ebola, and dengue.
Some of the technologies incorporated in candidate COVID-19 vaccines (live attenuated or inactivated virus, non-specific adjuvants) date from the mid-20th century and have been used to create many successful vaccine products including vaccines for polio, influenza, and childhood diseases. While we found that the research literature on these technologies continues to grow, there is no evidence of exponential acceleration since 1980, suggesting that these technologies had become well-established before that time.
The advance of research on synthetic (recombinant) vaccines, DNA vaccines, viral vectors, and TLR9 agonist-based adjuvants exhibited the characteristic “S-curve” pattern of technology advances with estimated established points prior to 2010. In contrast, research on mRNA vaccines, virus-like particles, and nanoparticles exhibited an exponential pattern of growth with only early evidence of slowing, and the established point cannot be estimated with confidence.
It should be emphasized that the TIME model does not identify individual publications or milestones in technology development. Rather, the model posits that the complete body of published research—including original insights or inventions, replication or refinement of previous observations, and/or refutation of erroneous results—contributes to the foundational knowledge required for efficient product development.
Studies of drug development demonstrate that few new drugs were approved before research on the biological target or class of drugs passed the established point [20], [21], [29]. Similarly, few products utilizing monoclonal antibodies [19], nucleotide therapeutics [30], or gene therapies [31] were approved before research on these modalities passed the established point. Evidence also shows that the timelines of clinical development from Phase 1 to approval is significantly shorter when clinical trials commence after the underlying technologies have passed this point [20], [21]. While the association between the point of maximum slowing of publication activity (the established point) and increased efficiency of product development is entirely empirical, it is likely that the slowing of publication activity reflects a stage in the advance of research when experiments are less likely to produce novel, publishable findings or introduce unanticipated new areas of investigation. So too, clinical investigations undertaken once the research has advanced to this stage may be more likely to achieve the predicted outcomes.
It should be cautioned that, while the TIME model has been extensively validated in studies of drug development, it has not been previously applied to vaccines. Published literature on vaccine development, however, suggests that the maturation of technology is an important factor in vaccine development as well. The success of the Salk vaccine was preceded by unsuccessful efforts to develop products using analogous technologies, and the rollout of the Salk vaccine itself was complicated by an outbreak of polio caused by inadequate inactivation of early batches of the product [32]. Similarly, early versions of vaccines for RSV and measles led to enhanced disease on re-exposure [33]. More recently, the dramatic decline in the success rate for vaccine development from 2003–2013 has been ascribed, in part, to numerous failures of then nascent DNA technologies [34]. In contrast, the remarkable safety of vaccines currently approved by the FDA [35], as well as the routine development of annual vaccines for evolving strains of influenza virus, are consistent with the efficiency of established vaccine technologies.
A relationship between technological maturity and the efficiency of vaccine development is incorporated in an economic model of vaccine development prepared for CEPI. In this model, vaccine technologies with no “licensure track-record” are assumed to have a higher risk of vaccine failure and higher development costs than “well-established” technologies [22]. While there is no “licensure track-record” for mRNA vaccines, this analysis suggests that research on mRNA vaccine technologies is just now achieving a level of technology maturation associated with product success.
The dramatic success of mRNA COVID-19 vaccines is also consistent with research on innovation suggesting that technologies which are not yet capable of meeting the performance standards of established markets, often generate important products that have a lower performance threshold [14], [15], [19]. In this context, the fact that the level and duration of gene expression required to stimulate an immune response may be lower than that required to achieve sustained, therapeutic levels of a protein, as well as the fact that clinical outcomes can be achieved without repetitive, long-term administration of the product, may have contributed to the early success of vaccines based on these technologies. It is also noteworthy that mRNA vaccines were able to enter preclinical and clinical development months earlier than vaccines using conventional technologies because nucleotide products can be synthesized, produced, and formulated using established, standard methods. In contrast, candidate vaccines using live attenuated virus, inactivated virus, or purified proteins required production and purification of virus or antigens with novel properties, requiring custom processes for production and quality control.
Viral vector vaccines employing adenoviral vectors also progressed rapidly through production and preclinical testing using standardized procedures. This progress was facilitated by the fact that two MERS vaccines utilizing these technologies had been previously produced and tested in Phase 1 clinical trials, before the waning of MERS precluded further testing [36], [37], and these technologies have advanced past the analytically-defined established point estimated by the model. The cost of vaccine discovery and development typically relies on both public and private sector investments. A 1997 report by the National Vaccine Advisory Committee concluded that “two thirds of all new vaccines provided worldwide have been produced by a US network of independent industrial, governmental, and academic partners engaged in vaccine research and development” [38]. A 2001 report by the World Bank, working with Gavi, the Vaccine Alliance, described the role of public-sector institutions as being primarily focused on basic science “measured by the number and value of the research manuscripts they publish in the scientific literature” [39], [40]. In contrast, industry focused on development, manufacture, and marketing of vaccine products [39], and more than 90% of all vaccine doses procured in the past five years have been manufactured by for-profit organizations [41].
A lack of sustainable investment is recognized as a limiting factor in developing vaccines for pandemic threats, particularly those initially impacting low-income countries [42]. Three of the four specific diseases examined in this report (coronavirus, Zika, and Ebola) were identified by the WHO as pandemic risks in the 2016 “R&D Blueprint for Action to prevent Epidemics” [26] and by CEPI as priority epidemic infectious diseases [22]. Our analysis describes a sporadic pattern of NIH funding related to these pathogens. In each case, we show that outbreaks of disease trigger an increase in grant-funded publication activity, which then wanes rapidly. The dramatic decrease in grant-funded publications related to coronavirus vaccines after 2015, despite recognition of coronavirus as a pandemic threat in the 2016 WHO report, is particularly striking. While this decrease may, in part, be due to the inability to conduct clinical trials of candidate products in the absence of active human infections, and uncertainty regarding the strains that may emerge in the future, mechanisms should be explored to sustain research aimed at preventing recognized pandemic threats, rather than simply responding to outbreaks of disease.
Our work also demonstrates that while the largest fractions of PMIDs and project years represented investigator-initiated research projects, a majority of the funding was associated with cooperative agreements or intramural research, two mechanisms for funding government-initiated research programs, or for research capacity through research program projects and centers. This pattern has been observed previously in examining the foundational research underlying development of remdesivir [28], but is distinctly different from NIH funding related to drugs in other therapeutic areas, where the large majority of research funding involves investigator-initiated research projects or research program projects and centers [21]. The lack of investigator-initiated, vaccine-focused research projects funded by the NIH is consistent with the observation that traditional grant mechanisms may not represent a robust mechanism for funding vaccine technologies or development [42], and demonstrates the importance of continued strategic initiatives by governments or non-governmental organizations.
Finally, this analysis emphasizes that NIH funding does not guarantee successful vaccine development. Despite decades of research and billions of dollars in NIH funding, there are no approved vaccines for HIV. In contrast, the NIH contributed little funding to the published research describing vaccines for Ebola or dengue viruses prior to approval of these products.
4.1. Limitations of this research
There are several important limitations to this study. First, this analysis is restricted to published research included in PubMed, and may not capture reports, patents, regulatory filings, unpublished clinical studies, or trade secrets. Also, this method is dependent on the efficiency of PubMed searches, and may not identify research without abstracts in PubMed or research that predates emergence of standard vocabularies and might be missed by search algorithms.
Second, it is not possible to associate costs with every NIH-funded publication due to incomplete RePORTER data and inconsistencies between publication dates and project years. Moreover, given an estimated lag of up to three years between research funding and publication [43], funding for research performed since 2018 may be underrepresented in this analysis.
Third, this analysis does not account for research funded by other government agencies including the Department of Defense or National Science Foundation, the $1.8 billion that the U.S. contributed to Gavi since 2009 [44], or research funded by other governments, philanthropies, academic institutions, or industry. It should also be noted that this analysis does not include research publications or funding in 2020 in response to the COVID-19 pandemic.
5. Conclusion
The Operation Warp Speed and Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) initiatives leveraged a variety of existing technologies in the race for a COVID-19 vaccine. This work illustrates the importance of a broad foundation of basic research over the past two decades, which advanced research on vaccine technologies that could be rapidly deployed in the response to the COVID-19 pandemic. To the extent that technological maturity contributes to the efficiency of new product development, NIH funding for this research in the years prior to the pandemic played an important role in the speed and success of COVID-19 vaccine development. This investment in basic research, along with investments related to developing and procuring the vaccine in response to the pandemic, should be considered in fully assessing the public sector’s contribution to these products, their price, and the distribution of profits arising from their sale.
These findings also demonstrate the importance of sustained public sector funding for foundational technologies in the ability to respond rapidly to emerging public health threats. Significantly, the majority of the NIH funding identified in this study came in the form of government-initiated cooperative agreements or intramural research, rather than investigator-initiated research projects. Even so, we observed a lack of sustained research or funding related to recognized, epidemic threats, including coronavirus, over the past two decades. Further consideration needs to be given to creating robust mechanisms for sustained funding of research on vaccines for recognized pandemic threats to ensure a rapid response to future outbreaks of disease.
6. Authorship
All authors attest they meet the ICMJE criteria for authorship.
7. Data sharing
Data used in this article are available from scholars.bentley.edu (Digital Commons).
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Ledley is Principle Investigator of a grant from the National Biomedical Research Foundation (a 501(c)(3) non-profit) to Bentley University.
Acknowledgments
Acknowledgements
This work was supported by a grant from the National Biomedical Research Foundation, Waltham, MA, to Bentley University. The funding source had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
The authors thank Prateet Shah for his contributions to this manuscript as well as Drs. Michael Boss and Nancy Hsiung for their constructive suggestions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.03.022.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
Supplementary figure 2.
Supplementary figure 3.
Supplementary figure 4.
Supplementary figure 5.
References
- 1.Basu A. Estimating The infection fatality rate among symptomatic COVID-19 cases In the United States: study estimates the COVID-19 infection fatality rate at the US county level. Health Aff. 2020 doi: 10.1377/hlthaff.2020.00455. [DOI] [PubMed] [Google Scholar]
- 2.Fiani B., Covarrubias C., Desai A., Sekhon M., Jarrah R. A contemporary review of neurological sequelae of COVID-19. Front Neurol. 2020;11:640. doi: 10.3389/fneur.2020.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demertzis Z.D., et al. Cardiac sequelae of novel coronavirus disease 2019 (COVID-19): a clinical case series. Eur Heart J-Case Rep. 2020 doi: 10.1093/ehjcr/ytaa179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flaxman S et al. Report 13: Estimating the number of infections and the impact of non-pharmaceutical interventions on COVID-19 in 11 European countries; 2020. www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-19/report-13-europe-npi-impact/.
- 5.O’Callaghan K.P., Blatz A.M., Offit P.A. Developing a SARS-CoV-2 vaccine at warp speed. JAMA. 2020;324(5):437–438. doi: 10.1001/jama.2020.12190. [DOI] [PubMed] [Google Scholar]
- 6.Corey L., Mascola J.R., Fauci A.S., Collins F.S. A strategic approach to COVID-19 vaccine R&D. Science. 2020;368(6494):948–950. doi: 10.1126/science.abc5312. [DOI] [PubMed] [Google Scholar]
- 7.Zhu N., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. DRAFT landscape of COVID-19 candidate vaccines. World Health Organization; 2020. www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines [accessed July 31, 2020].
- 9.Modjarrad K. MERS-CoV vaccine candidates in development: the current landscape. MethodsMol Biol. 2016;1403:269–284. doi: 10.1016/j.vaccine.2016.03.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McPherson C., et al. Development of a SARS coronavirus vaccine from recombinant spike protein plus delta inulin adjuvant. MethodsMol Biol. 2016;1403:269–284. doi: 10.1007/978-1-4939-3387-7_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J., et al. A self-adjuvanted nanoparticle based vaccine against infectious bronchitis virus. PloS ONE. 2018;13(9) doi: 10.1371/journal.pone.0203771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Decaro N., et al. A candidate modified-live bovine coronavirus vaccine: safety and immunogenicity evaluation. The New Microbiologica. 2009;32(1):109–113. [PubMed] [Google Scholar]
- 13.Pronker E.S., Weenen T.C., Commandeur H., Claassen E.H., Osterhaus A.D. Risk in vaccine research and development quantified. PloS ONE. 2013;8(3):e57755. doi: 10.1371/journal.pone.0057755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen C.M. Exploring the limits of the technology S-curve. Part I: Component technologies. Prod Operat Manage. 1992;1(4):334–357. [Google Scholar]
- 15.Christensen C.M. Harvard Business School Press; Boston, MA: 1997. The innovator's dilemma: when new technologies cause great firms to fail. [Google Scholar]
- 16.GAO. BEST PRACTICES: Better management of technology development can improve weapon system outcomes. General Accounting Office; 1999. www.gao.gov/products/NSIAD-99-162.
- 17.Clausing D., Holmes M. Technology readiness. Res-Technol Manage. 2010;53(4):52–59. [Google Scholar]
- 18.Foster R.N. Effective R&D operations in the ‘80s: boosting the payoff from R&D. Res Manage. 1982;25(1):22–27. [Google Scholar]
- 19.McNamee L.M., Ledley F.D. Patterns of technological innovation in biotech. Nat Biotechnol. 2012;30(10):937–943. doi: 10.1038/nbt.2389. [DOI] [PubMed] [Google Scholar]
- 20.McNamee L.M., Walsh M.J., Ledley F.D. Timelines of translational science: from technology initiation to FDA approval. PLoS ONE. 2017;12(5):e0177371. doi: 10.1371/journal.pone.0177371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cleary EG, Jackson, MJ, Ledley, FD. Government as the first investor in biopharmaceutical innovation; Evidence from new drug approvals 2010–2019 (Working Paper). INETEconomics; September 2020; www.ineteconomics.org/research/research-papers/government-as-the-first-investor-in-biopharmaceutical-innovation-evidence-from-new-drug-approvals-2010-2019.
- 22.Gouglas D., et al. Estimating the cost of vaccine development against epidemic infectious diseases: a cost minimisation study. Lancet Global Health. 2018;6(12):e1386–e1396. doi: 10.1016/S2214-109X(18)30346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cleary E.G., Beierlein J.M., Khanuja N.S., McNamee L.M., Ledley F.D. Contribution of NIH funding to new drug approvals 2010–2016. Proc Natl Acad Sci. 2018;115(10):2329–2334. doi: 10.1073/pnas.1715368115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO. DRAFT Landscape of candidate COVID-19 vaccines; 2020. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- 25.Plotkin S.A. Vaccines for epidemic infections and the role of CEPI. Hum Vac Immunother. 2017;13(12):2755–2762. doi: 10.1080/21645515.2017.1306615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO. World Health Organization, An R&D blueprint for action to prevent epidemics; 2016. https://www.who.int/blueprint/about/r_d_blueprint_plan_of_action.pdf [accessed May 1, 2018].
- 27.Schlake T., Thess A., Fotin-Mleczek M., Kallen K.-J. Developing mRNA-vaccine technologies. RNA Biol. 2012;9(11):1319–1330. doi: 10.4161/rna.22269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cleary EG, Jackson MJ, Folchman-Wagner Z, Ledley FD. Foundational research and NIH funding enabling Emergency Use Authorization of remdesivir for COVID-19. medRxiv; 2020.
- 29.Beierlein J.M., et al. Landscape of innovation for cardiovascular pharmaceuticals: From basic science to new molecular entities. Clin Ther. 2017;39(7):1409–1425. doi: 10.1016/j.clinthera.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Beierlein J.M., McNamee L.M., Ledley F.D. As technologies for nucleotide therapeutics mature, products emerge. Mole Therapy-Nucl Acids. 2017;9:379–386. doi: 10.1016/j.omtn.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ledley F., McNamee L., Uzdil V., Morgan I. Why commercialization of gene therapy stalled; Examining the life cycles of gene therapy technologies. Gene Ther. 2014;21(2):188–194. doi: 10.1038/gt.2013.72. [DOI] [PubMed] [Google Scholar]
- 32.Blume S.S. Lock in, the state and vaccine development: lessons from the history of the polio vaccines. Res Policy. 2005;34(2):159–173. [Google Scholar]
- 33.Lambert P.-H., et al. Consensus summary report for CEPI/BC March 12-13, 2020 meeting: assessment of risk of disease enhancement with COVID-19 vaccines. Vaccine. 2020;2020 doi: 10.1016/j.vaccine.2020.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stephens P. Vaccine R&D: past performance is no guide to the future. Vaccine. 2014;32(19):2139–2142. doi: 10.1016/j.vaccine.2014.02.047. [DOI] [PubMed] [Google Scholar]
- 35.Tau N., Yahav D., Shepshelovich D. Postmarketing safety of vaccines approved by the US Food and Drug Administration: a cohort study. Ann Intern Med. 2020 doi: 10.7326/M20-2726. [DOI] [PubMed] [Google Scholar]
- 36.Modjarrad K., et al. Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect Dis. 2019;19(9):1013–1022. doi: 10.1016/S1473-3099(19)30266-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Folegatti P.M., et al. Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral-vectored vaccine: a dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Vaccine Advisory Committee United States vaccine research: a delicate fabric of public and private collaboration. Pediatrics. 1997;100(6):1015–1020. doi: 10.1542/peds.100.6.1015. [DOI] [PubMed] [Google Scholar]
- 39.Batson A., Bekier M.M. Vaccines where they're needed. The McKinsey Quarterly Autumn. 2001:103. [Google Scholar]
- 40.Francis D.P. Successes and failures: worldwide vaccine development and application. Biologicals. 2010;38(5):523–528. doi: 10.1016/j.biologicals.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 41.WHO. Global Vaccine Market Report. World Health Organization; 2019. https://apps.who.int/iris/handle/10665/311278.
- 42.Rappuoli R., Black S., Bloom D.E. Vaccines and global health: in search of a sustainable model for vaccine development and delivery. Sci Transl Med. 2019;11(497):eaaw2888. doi: 10.1126/scitranslmed.aaw2888. [DOI] [PubMed] [Google Scholar]
- 43.Boyack K.W., Jordan P. Metrics associated with NIH funding: a high-level view. J Am Med Inform Assoc. 2011;18(4):423–431. doi: 10.1136/amiajnl-2011-000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.KFF. The U.S. and Gavi, the vaccine alliance. Kaiser Family Foundation; 2020; www.kff.org/global-health-policy/fact-sheet/the-u-s-and-gavi-the-vaccine-alliance/.