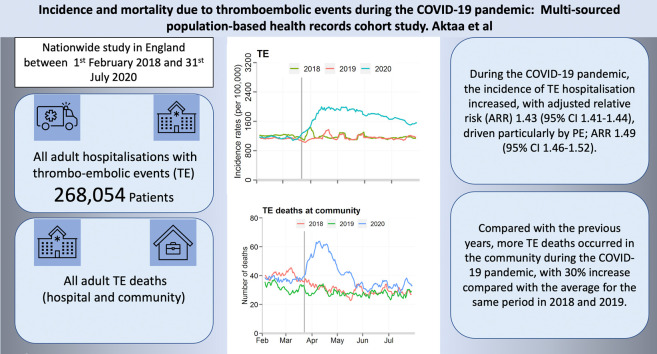
Abstract
Background
Evidence supports an excess of deaths during the COVID-19 pandemic. We report the incidence and mortality of thrombo-embolic events (TE) during the COVID-19 pandemic.
Methods
Multi-sourced nationwide cohort study of adults (age ≥18 years) admitted to hospital with TE and deaths from TE in England (hospital and community) between 1st February 2018 and 31st July 2020. Relative risks, adjusted for age, sex, atrial fibrillation, co-morbidities and time trend comparing before and during the COVID-19 pandemic were estimated using Poisson regression.
Findings
Of 272,423 patients admitted with TE to 195 hospitals, 86,577 (31.8%) were admitted after 2nd March 2020 (first COVID-19 death in the UK). The incidence of TE hospitalised increased during the COVID-19 pandemic from 1090 to 1590 per 100,000 (absolute risk change 45.9% [95% CI 45.1–46.6%], adjusted relative risk [ARR] 1.43 [95% CI 1.41–1.44]) driven particularly by pulmonary embolism; 1.49, 95% CI 1.46–1.52. TE were more frequent among those with COVID-19; 1.9% vs. 1.6%, absolute risk change 21.7%, 95% CI 21.0–22.4%, ARR 1.20, 95% CI 1.18–1.22. There was an increase in the overall mortality from TE during the pandemic (617, 6.7% proportional increase compared with the historical baseline), with more TE deaths occurring in the community compared with the historical rate (44% vs. 33%).
Interpretation
The COVID-19 pandemic has resulted in an increase in the incidence of hospitalised TE. There were more deaths from TE in the community highlighting a number of mechanisms including the hypercoagulable state associated with COVID-19 infection and potential impact of delays in seeking help.
Research in context
Evidence before this study
We searched PubMed on 16 November 2020 for articles that documented the incidence and mortality of thrombo-embolic events (TE) during the COVID-19 pandemic using the search terms “COVID-19” OR “Coronavirus*” OR “2019-nCOV” OR “SARS-CoV” AND (“Thromboembolism” OR “Venous Thromboembolism” OR “thromboembol*”) with no language or time restrictions. The majority of data on TE in COVID-19 pertains to hospitalised patients from retrospective cohort studies. One study found that TE in hospitalised patients was associated with an increased mortality rate (adjusted hazard ratio 1.82; 95% CI 1.54–2.15). A systematic review and meta-analysis of 35 studies in 9249 hospitalised patients calculated an overall pooled incidence of TE of 17.8% (95% CI: 9.9–27.4%), rising to 22.9% (95% CI: 14.5–32.4%) in patients admitted to intensive care (ICU). The most contemporary data are from a cohort of 1114 patients (715 outpatient, 399 hospitalised, 170 admitted to ICU). With robust COVID-19-specific therapies and widespread thromboprophylaxis the prevalence of venous TE in ICU patients was reported as 7% (n = 12) when catheter-/device-related events were excluded, and among the outpatients there was no TE reported. No published studies have used nationwide data to investigate TE during the pandemic or the effect of the pandemic on outcomes of patients with TE but without Covid-19.
Added value of this study
This retrospective multi-sourced nationwide unlinked cohort study compares the overall incidence and mortality of TE prior to and during the COVID-19 pandemic. We found an increased incidence of TE despite only a small proportion having a diagnosis of COVID-19. This may highlight the lack of testing, particularly in the community during the initial phase of the pandemic, and the possibility of other factors contributing to TE risk, such as decreased daily activity mandated by home quarantine and alterations in medication concordance. Mortality from TE was higher in the community during the pandemic and this highlights that adverse societal effects of the pandemic, such as aversion to seeking medical assessment, may precipitate worse outcomes related to TE.
Implications of all the available evidence
Evidence suggests that COVID-19 produces a hypercoagulable state and thromboprophylaxis is recommended in hospitalised patients to prevent excess mortality from TE. Whether to anticoagulate non-hospitalised ambulatory patients with COVID-19 will be answered by ongoing trials. Clinicians should consider the risks posed by decreased daily activity and fear of medical contact, and provide appropriate advice to patients.
Keywords: COVID-19, Thrombo-embolic events, Mortality, Pulmonary embolism
Graphical abstract
1. Introduction
Thrombo-embolism has been described as one of the major cardiovascular (CV) complications of coronavirus disease 19 (COVID-19) contributing to worse outcomes [[1], [2], [3], [4], [5], [6]]. Pathophysiological mechanisms linked to SARS-CoV-2, which causes COVID-19, could predispose infected people to arterial and venous thrombo-embolic events (hereafter collectively referred to as TE), including the inflammatory response to viraemia [[7], [8], [9], [10]], endothelial function disorder in the lung as elsewhere [11], and the hypercoagulable state described in COVID-19 patients [4,12]. The pandemic may also have had unintended consequences associated with changes in health seeking behaviour, which could affect the potential to prevent and treat TE in people not infected with-COVID-19 [12]. The response of the public and the health system to the pandemic may, therefore, be associated with excess deaths secondary to TE in the community, which has been reported for a range of other CV conditions [[13], [14], [15], [16]].
The United Kingdom is unique in that it has a suite of continuous capture, full populace, nationwide datasets such as the Civil Registration Deaths Data and Hospital Episode Statistics (HES). During the pandemic, these datasets have demonstrated critical value in showing how the pandemic has affected the health of people and with a potential to inform mitigation strategies now that a second wave has occurred.
This study aimed to investigate, using nationwide data from HES and the Civil Registration Deaths Data in England, the patterns of change in admissions with different phenotypes of TE, as well as the causes and place of TE-related deaths antecedent, compared with during the COVID-19 pandemic. We hypothesised that patients' characteristics may differ during the pandemic as a result of a new pathology - highlighting the hypercoagulable state associated with the COVID-19 contagion. Furthermore, we anticipated an increase in TE-related deaths occurring in the community because of the changes in health-seeking behaviour during the pandemic.
2. Methods
2.1. Data collection
HES consists of International Statistical Classification of Disease-10th Revision (ICD-10) codes regarding demographical, clinical, administrative and patient information of all patients admitted to any hospital in England. We identified TE on the basis of the ICD-10 codes (Supplement Table 1) recorded at the principle or primary position for patients hospitalised between 1st February 2018 and 31st July 2020 and included only the index hospitalisations for TE during the study period in the analysis; to avoid analysing replicate events for the same patient, re-hospitalisations due to TE during the study period were excluded. Admissions with TE were classified as arterial (including stroke and arterial thromboembolic events), and venous (including pulmonary embolism [PE] and deep venous thrombosis [DVT]). Patients with acute coronary syndrome (ACS) were excluded from the analysis, because data on acute CV events, including ACS have been reported elsewhere [15].
2.2. Death data
We obtained all certified and registered deaths in England for deceased ≥18 years of age, between 1st February 2018 and 31st July 2020 as recorded in the Civil Registration Deaths Data of the Office for National Statistics (ONS) [17]. We used the ICD-10 codes corresponding to the immediate cause of death and contributing causes as registered on the Medical Certificate of Cause of Death (MCCD) regardless of the location of death. The MCCD is completed by the doctor who attended the deceased during their last illness within 5 days unless there is to be a coroner's post-mortem or an inquest. TE directly leading to death were categorised as venous (PE and DVT), and arterial, and then deaths were classified according to the COVID-19 status. ICD-10 codes ‘U071’ (confirmed) and ‘U072’ (suspected) were used to identify whether a death was related to COVID-19 on any part of the MCCD. The place of death as recorded on the MCCD was classified as community (home, care home and hospice) or hospital.
2.3. Statistical analyses
Baseline characteristics were described using numbers and percentages for categorical data. Data were stratified by COVID-19 status (infected or not infected), age band (<50, 50–59, 60–69, 70–79, 80+ years), sex and Charlson co-morbidity index (CCI) [18]. Since AF is associated with TE, such as ischaemic stroke, the incidence of different TE phenotypes were each adjusted for AF incidence. Given that there was a decline in admission during the pandemic [14,19,20], we estimated the proportion of TE admissions (adjusted for presence of AF) from all admissions in the corresponding day for the previous two years and compared this with the proportion of TE admissions from all admissions in the corresponding day from 2nd March 2020. This date was chosen for the time series comparison because it corresponded to the first COVID-19 death in the UK.
Incidence rates for admission with TE were standardised per 100,000 admissions. The number of daily deaths was presented using a 7-day simple moving average (the mean number of daily deaths for that day and the preceding 6 days) from 1st February up to 31st July, adjusted for seasonality. A Poisson regression model was fitted to estimate the relative risk, adjusted for age, sex, AF, CCI, and time trend (before and after the COVID-19 pandemic).
For the categories of TE death, the ICD-10 code on the MCCD was counted only once per deceased. Thus, the overall rate of TE death represents the number of people with a direct TE-related death. In light of the fact that people may have had more than one of the predefined TE events leading to death, analyses for each of the predefined TE categories represent the number of events (not people) per category. For the purposes of this investigation, TE that contributed, but did not directly lead to death were excluded from the analyses. The TE-related excess death rate was derived by subtracting total TE deaths during the COVID-19 pandemic up to the end of the period of analysis and the average total TE deaths in the same time period of 2018 and 2019.
All tests were two sided and statistical significance considered as p < 0.05. Statistical analyses were performed in R V.4.0.0.
3. Results
3.1. Admissions
Data were available for 272,423 admissions relating to pre-specified TE codes from 195 National Health Service (NHS) hospitals in England over the 3-year study period. Of those, 86,577 (31.8%) patients were admitted during the COVID-19 pandemic and 132,357 (48.6%) were women. The age, co-morbidities and TE phenotypes of patients admitted with TE prior to the pandemic were comparable to those for patients admitted during it (Table 1 ).
Table 1.
Patient characteristics for admissions in England with arterial and venous thrombo-embolic events between 1st Feb 2018 and 31st July 2020, by study period (pre-COVID-19 and during COVID-19), and COVID-19 status.
| Study period |
COVID-19 status |
|||
|---|---|---|---|---|
| Before COVID-19 period | COVID-19 period | COVID-19 +ve | COVID-19 -ve | |
| N | 185,846 | 86,577 | 84,728 | 1849 |
| Women (%) | 90,904 (48.9) | 41,453 (47.9) | 40,594 (47.9) | 859 (46.5) |
| Age (%) | ||||
| 0–49 | 22,943 (12.4) | 10,944 (12.8) | 10,760 (12.8) | 184 (10.2) |
| 50–59 | 21,837 (11.8) | 10,850 (12.6) | 10,651 (12.7) | 199 (11.0) |
| 60–69 | 31,920 (17.3) | 15,146 (17.6) | 14,846 (17.7) | 300 (16.6) |
| 70–79 | 47,039 (25.5) | 21,829 (25.4) | 21,380 (25.4) | 449 (24.9) |
| 80+ | 60,603 (32.9) | 27,059 (31.5) | 26,389 (31.4) | 670 (37.2) |
| Charlson comorbidity index | ||||
| 0 | 31,611 (17.0) | 14,711 (17.0) | 14,455 (17.1) | 256 (13.8) |
| 1 | 43,147 (23.2) | 18,572 (21.5) | 18,287 (21.6) | 285 (15.4) |
| 2 | 36,159 (19.5) | 16,050 (18.5) | 15,771 (18.6) | 279 (15.1) |
| 3 | 26,739 (14.4) | 12,611 (14.6) | 12,307 (14.5) | 304 (16.4) |
| 4+ | 48,190 (25.9) | 24,633 (28.5) | 23,908 (28.2) | 725 (39.2) |
| Atrial fibrillation (%) | 36,413 (19.6) | 16,708 (19.3) | 16,223 (19.1) | 485 (26.2) |
| Arterial TE (%) | 119,373 (64.2) | 55,166 (63.7) | 53,975 (63.7) | 1191 (64.4) |
| Venous TE (%) | 66,473 (35.8) | 31,411 (36.3) | 30,753 (36.3) | 658 (35.6) |
| DVT (%) | 30,586 (16.5) | 13,852 (16.0) | 13,712 (16.2) | 140 (7.6) |
| PE (%) | 35,575 (19.1) | 17,767 (20.5) | 17,162 (20.3) | 605 (32.7) |
Note: frequency (percentage) was reported for each category. TE, thrombo-embolic events; DVT, deep vein thrombosis; PE, pulmonary embolism.
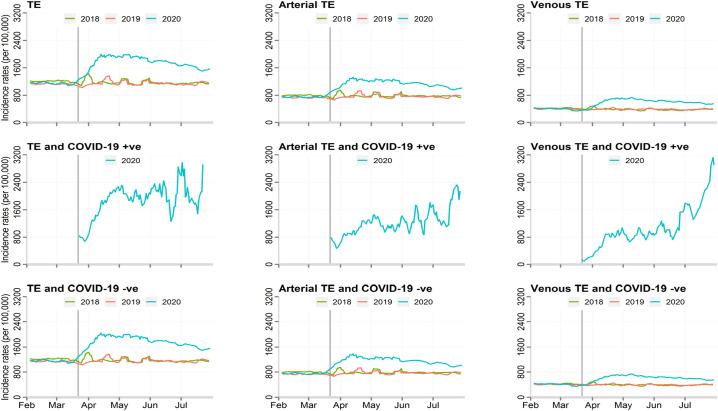
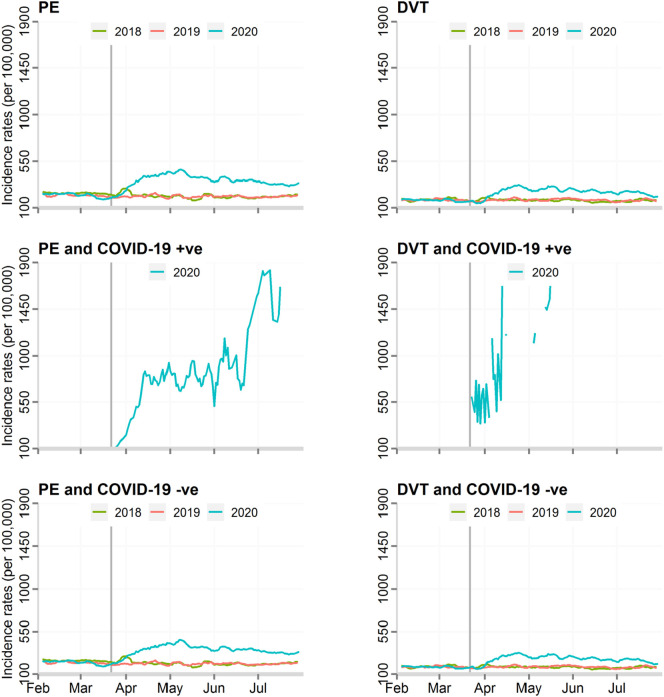
There was an increase in the standardised incidence rate of admissions with TE during the COVID-19 pandemic. When compared with the background number for all admissions during these two periods, TE accounted for 1.4% of all hospital admissions before the pandemic and 2.0% during the pandemic, equating to 500 more people being hospitalised with TE per 100,000 admissions (absolute risk increase 45.9% [95% CI 45.1–46.6%], adjusted relative risk 1.43 [95% CI 1.41–1.44]) (Table 2 ). While the most frequent manifestation of TE related to arterial pathologies, there was an increase in all types of TE. The largest increase was seen in venous TE (adjusted relative risk 1.44, 95% CI 1.42–1.47) and in particular PE (1.49, 95% CI 1.46–1.52) during, compared with before, the pandemic (Fig. 1, Fig. 2 ). Moreover, adjustment for demographics and co-morbidities, including AF, made little difference to the direction or magnitude of the relative increase in TE admissions (Table 2, Supplement Fig. 1).
Table 2.
Incidence rates, absolute risk change and adjusted relative risk between pre-COVID-19 and COVID-19 periods.
| Incidence rate (per 100,000) |
Absolute risk change |
Unadjusted relative riska |
Adjusted relative riskb |
||
|---|---|---|---|---|---|
| Conditions | Pre-COVID-19 | COVID-19 period | Percentage (95% CI) | RR (95% CI) | RR (95% CI) |
| All patients | |||||
| TE | 1090 | 1590 | 45.9% (45.1–46.6%) | 1.42 (1.41–1.43) | 1.43 (1.41–1.44) |
| Arterial TE | 720 | 1036 | 43.8% (42.9–44.7%) | 1.40 (1.39–1.41) | 1.42 (1.40–1.44) |
| Venous TE | 369 | 554 | 50.0% (48.6–51.3%) | 1.46 (1.45–1.48) | 1.44 (1.42–1.47) |
| DVT | 164 | 233 | 42.2% (40.3–44.1%) | 1.48 (1.46–1.50) | 1.46 (1.42–1.49) |
| PE | 205 | 329 | 60.8% (59.0–62.7%) | 1.54 (1.52–1.56) | 1.49 (1.46–1.52) |
A Poisson regression model was used to calculate the relative risk, accounted for time trend.
A Poisson regression model was used to calculate the relative risk, adjusted for age, sex, COVID-19 status, Charlson Comorbidity Index and time trend. TE, thromboembolic events; DVT, deep vein thrombosis; PE, pulmonary embolism.
Fig. 1.
Incidence rates of thromboembolic events, by the type of thromboembolic event and COVID-19 status.
TE; thrombo-embolic events.
Fig. 2.
Incidence rates of DVT and PE events, by COVID-19 status.
DVT, deep vein thrombosis; PE, pulmonary embolism.
TE were more frequent among those diagnosed with COVID-19 infection, 1.9% vs. 1.6%, absolute risk change 21.7% 95% CI 21.0–22.4% (adjusted relative risk 1.20, 95% CI 1.18–1.22) (Fig. 1, Fig. 2). The greatest increase in TE risk with COVID-19 infections was observed in venous TE (1.87, 95% CI 1.85–1.89), with PE demonstrating the greatest risk increase (2.96, 95% CI 2.91–3.00) (Supplement Table 2).
3.2. Deaths
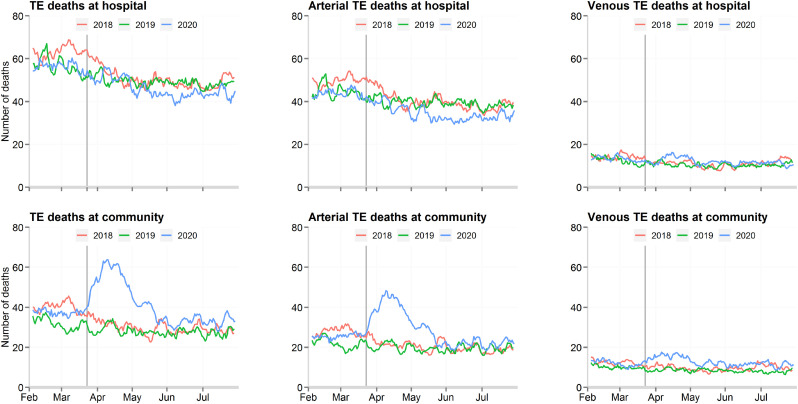
During the COVID-19 pandemic study period, there were 4374 and 5476 deaths relating to TE in the community and hospital settings, respectively (Fig. 3 ). In the community, this represented a 1289 (30%) increase in the deaths compared with the average for the same period in 2018 and 2019. In hospital, there was a 672 (11%) decrease in TE-related deaths in the same time period. Both arterial and venous TE accounted for the increase in TE-deaths in the community during the pandemic, as shown in Fig. 3. However, arterial TE contributed to the greatest excess in death. Deaths from TE were more frequent among patients not diagnosed with COVID-19, compared with patients who had the infection (5.6% vs. 1.5%).
Fig. 3.
Daily number of thromboembolic related deaths, by the type of thromboembolic event and place of death.
TE; thrombo-embolic events.
4. Discussion
This nationwide study describes, using full populace data, the incidence and mortality attributed to TE during the COVID-19 pandemic compared with previous years. We have illustrated that the pandemic has resulted in an increase in the incidence of all phenotypes of TE, and was associated with an abrupt rise in TE-related deaths. Nearly half of these deaths occurred in the community, with estimated rates being substantially higher than those of previous years. During the COVID-19 pandemic, the most frequent phenotype of TE in England was arterial, including stroke, but the highest risk increase was observed in venous TE, particularly PE, with only a small proportion being confirmed COVID-19 cases. Although infection with SARS-COV-2 was associated with an increase in TE, deaths from TE were more frequent among those not diagnosed with COVID-19, possibly signifying a lack of testing for COVID-19 during the first stage of the pandemic, particularly in the community setting.
This research provides insights into potential mechanisms behind the excess in deaths during the COVID-19 pandemic. We showed that the baseline characteristics of those admitted with TE were similar during compared with before the pandemic, suggesting that TE most affect patients classically at risk. In addition, our study illustrated that TE were more frequent among patients diagnosed with COVID-19, and that adjusting for co-morbidities made little difference to the direction or magnitude of this association. As such, our findings support the notion that COVID-19 predisposes to TE both within and outside the pulmonary vasculature. While this predisposition may be partially explained by the historical risk-factors for TE, other mechanisms may exist, such as the distortion of the endothelial thrombotic/fibrinolytic balance, inflammatory storm, and alveolar injury [11,21]. These processes can contribute to immunothrombosis in situ [22,23], and may explain the substantial increase in PE incidence observed in our study, as well as that previously reported in venous TE in the context of COVID-19 despite the use of thromboprophylaxis [6,24].
Previous studies have described the incidence and outcomes of TE in patients hospitalised with confirmed COVID-19 infection [6,[25], [26], [27], [28], [29]]. Our study extends this knowledge by comparing the overall incidence and mortality of TE prior to and during the COVID-19 pandemic, as well as by COVID-19 status. Infection with the SARS-COV-2 virus was associated with an increase in TE - supporting the notion of COVID-19 precipitating a prothrombotic state [12,30]. Yet, we also found an increase in the incidence of TE despite only 2.2% having a diagnosis of COVID-19. This is important because testing for COVID-19 was insufficient in the early stages of the pandemic both in the hospital setting [31,32] and in the community [33]. In addition, some patients may have had false negative results for COVID-19 [34]. Thus, and given that the greatest magnitude of increased TE risk during the pandemic was seen in venous TE, our study suggests that other factors such as decreased daily activity mandated by home quarantine - so called ‘seated immobility syndrome’- [35] and alterations in medication concordance [12] may have contributed to the increased incidence of TE during the COVID-19 pandemic.
The current study shows that while the mortality of TE declined in hospital, it increased substantially in the community during the pandemic. This supports the findings of an earlier report which suggested that the COVID-19 outbreak was associated with a sharp rise in the number of out-of-hospital deaths related to TE [36]. This rise in TE-deaths – so called mortality harvesting – which occurred in the community highlights how adverse societal effects of the pandemic, such as aversion to seeking medical assessment, may precipitate worse outcomes in TE, and raises the possibility that a second mechanism– delay by the public in seeking help for fear of catching COVID-19 in hospital [15].
TE associated deaths in patients with confirmed COVID-19 infection are likely to be underestimated in our study given the lack of testing during the early phases of the pandemic [31,32], and the reliance on ONS data [37]. However, the observed increase in TE mortality in non-COVID patients highlights the potential indirect repercussions of the pandemic on TE management and outcomes. While these findings may be explained by insufficient detection and diagnosis of COVID-19 infection, other factors may have contributed. That is, sub-optimal treatment of non-COVID patients at risk of TE because of the pressures of the pandemic on healthcare services, and the late presentation to hospital of patients with TE will have adversely affected prognosis [15].
Although our study has many strengths, it nonetheless has some limitations. The exclusion of ICD codes for ACS may have resulted in an underestimation for the overall impact of the COVID-19 on the incidence of TE. However, given that these data have been previously described [15], we opted to present here the rates of venous TE, as well as other non-coronary arterial TE during the pandemic. Another limitation is that non-fatal TE that did not lead to hospitalisation were not captured in our study. The MCCD were completed by any doctor (not just the attending doctor) during the COVID-19 pandemic and the duration of time over which the deceased was not seen before referral to the coroner was extended from 14 to 28 days. Moreover the documentation of causes of death could be ‘to the best of their knowledge and belief’ without diagnostic proof, if appropriate and to avoid delay [38]. This may have resulted in misclassification bias, with under-reporting of the deaths directly due to TE disease in preference to COVID-19 infection (which is a notifiable disease under the Health Protection (Notification) Regulations 2010) or respiratory disease. Our analysis will have excluded a small proportion of deaths under review by the coroner, though typically these will have been unnatural in aetiology. Equally, coding of TE in HES may be inaccurate, and our study may have under-estimated the incidence of TE. That is because we only included TE at the principle or primary position of hospital admission diagnoses, and, thus, did not capture non-fatal TE that occurred during the hospital stay for patients admitted with a non-TE illness during the pandemic.
5. Conclusion
This nationwide analysis of hospitalisations and deaths from TE during the COVID-19 pandemic found an increase in the incidence of all phenotypes of TE, particularly PE and a rise of TE-related deaths in the community. The increased incidence of TE during the pandemic and with COVID-19 infection, which appeared not to be associated with people having different co-morbidities, suggests a hypercoagulable state associated with the infection. The rise in death in the community during the pandemic, compared with previous years, highlights possible fears around or delays in seeking help.
Data sharing statement
We used routinely collected data from electronic health records using HES data to obtain information about TE hospitalisation, and death register to obtain mortality data. The ICD codes used are provided in the supplementary material. Data used for this study will be available upon approval by NHS Digital UK.
Funding
None.
Ethical approval
Not applicable.
CRediT authorship contribution statement
SA and CPG were responsible for the study design and concept. JW and MR performed the data cleaning and data analysis. RN conducted the literature search. SA and CPG wrote the first draft of the manuscript and all authors participated in the writing of the paper.
Declaration of competing interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.thromres.2021.03.006.
Appendix A. Supplementary data
Supplementary material
References
- 1.Spyropoulos A.C., Weitz J.I. Hospitalized COVID-19 patients and venous thromboembolism. Circulation. 2020;142(2):129–132. doi: 10.1161/CIRCULATIONAHA.120.048020. [DOI] [PubMed] [Google Scholar]
- 2.Gupta A., Madhavan M.V., Sehgal K., et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 3.Driggin E., Madhavan M.V., Bikdeli B., et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J. Am. Coll. Cardiol. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao D., Zhou F., Luo L., et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. The Lancet Haematology. 2020;7(9) doi: 10.1016/S2352-3026(20)30217-9. e671-e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piazza G., Campia U., Hurwitz S., et al. Registry of arterial and venous thromboembolic complications in patients with COVID-19. J. Am. Coll. Cardiol. 2020;76(18):2060–2072. doi: 10.1016/j.jacc.2020.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merrill J.T., Erkan D., Winakur J., James J.A. Emerging evidence of a COVID-19 thrombotic syndrome has treatment implications. Nat. Rev. Rheumatol. 2020;16(10):581–589. doi: 10.1038/s41584-020-0474-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danzi G.B., Loffi M., Galeazzi G., Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? European Heart Journal. 2020;41(19):1858. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H., Liu L., Zhang D., et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395(10235):1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Libby P., Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020;41(32):3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. <span class=“subtitle”><em>JACC</em> State-of-the-Art Review</span>. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kansagra A.P., Goyal M.S., Hamilton S., Albers G.W. Collateral effect of Covid-19 on stroke evaluation in the United States. N. Engl. J. Med. 2020;383(4):400–401. doi: 10.1056/NEJMc2014816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mafham M.M., Spata E., Goldacre R., et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396(10248):381–389. doi: 10.1016/S0140-6736(20)31356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J., Mamas M.A., Mohamed M.O., et al. Place and causes of acute cardiovascular mortality during the COVID-19 pandemic. Heart. 2021;107:113–119. doi: 10.1136/heartjnl-2020-317912. heartjnl-2020-317912. [DOI] [PubMed] [Google Scholar]
- 16.Wu J., Mamas M.A., de Belder M.A., Deanfield J.E., Gale C.P. Second decline in admissions with heart failure and myocardial infarction during the COVID-19 pandemic. J. Am. Coll. Cardiol. 2021;77(8):1141–1143. doi: 10.1016/j.jacc.2020.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.User guide to mortality statistics. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/methodologies/userguidetomortalitystatisticsjuly2017 Available.
- 18.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Wu J., Mamas M., Rashid M., et al. Patient response, treatments, and mortality for acute myocardial infarction during the COVID-19 pandemic. European Heart Journal - Quality of Care and Clinical Outcomes. 2020:1–9. doi: 10.1093/ehjqcco/qcaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solomon M.D., McNulty E.J., Rana J.S., et al. The Covid-19 pandemic and the incidence of acute myocardial infarction. N. Engl. J. Med. 2020;383(7):691–693. doi: 10.1056/NEJMc2015630. [DOI] [PubMed] [Google Scholar]
- 21.Gu S.X., Tyagi T., Jain K., et al. Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat. Rev. Cardiol. 2020:1–16. doi: 10.1038/s41569-020-00469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicolai L, Leunig A, Brambs S, et al. Vascular neutrophilic inflammation and immunothrombosis distinguish severe COVID-19 from influenza pneumonia. Journal of Thrombosis and Haemostasis; n/a(n/a). [DOI] [PMC free article] [PubMed]
- 23.van Dam L.F., Kroft L.J.M., van der Wal L.I., et al. Clinical and computed tomography characteristics of COVID-19 associated acute pulmonary embolism: a different phenotype of thrombotic disease? Thromb. Res. 2020;193:86–89. doi: 10.1016/j.thromres.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poissy J., Goutay J., Caplan M., et al. Pulmonary embolism in patients with COVID-19. Circulation. 2020;142(2):184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 25.Thomas W., Varley J., Johnston A., et al. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb. Res. 2020;191:76–77. doi: 10.1016/j.thromres.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunutsor S.K., Laukkanen J.A. Incidence of venous and arterial thromboembolic complications in COVID-19: a systematic review and meta-analysis. Thromb. Res. 2020;196:27–30. doi: 10.1016/j.thromres.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oxley T.J., Mocco J., Majidi S., et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N. Engl. J. Med. 2020;382(20) doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bilaloglu S., Aphinyanaphongs Y., Jones S., Iturrate E., Hochman J., Berger J.S. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324(8):799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helms J., Tacquard C., Severac F., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliver D. David Oliver: Let’s be open and honest about covid-19 deaths in care homes. BMJ. 2020;369:m2334. doi: 10.1136/bmj.m2334. [DOI] [PubMed] [Google Scholar]
- 32.Griffin S. Covid-19: “staggering number” of extra deaths in community is not explained by covid-19. BMJ. 2020;369:m1931. doi: 10.1136/bmj.m1931. [DOI] [PubMed] [Google Scholar]
- 33.Iacobucci G. Covid-19: lack of capacity led to halting of community testing in march, admits deputy chief medical officer. BMJ. 2020;369:m1845. doi: 10.1136/bmj.m1845. [DOI] [PubMed] [Google Scholar]
- 34.Variation in false-negative rate of reverse transcriptase polymerase chain reaction–based SARS-CoV-2 tests by time since exposure. Annals of Internal Medicine. 2020;173(4):262–267. doi: 10.7326/M20-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hitos K., Cannon M., Cannon S., Garth S., Fletcher J.P. Effect of leg exercises on popliteal venous blood flow during prolonged immobility of seated subjects: implications for prevention of travel-related deep vein thrombosis. J. Thromb. Haemost. 2007;5(9):1890–1895. doi: 10.1111/j.1538-7836.2007.02664.x. [DOI] [PubMed] [Google Scholar]
- 36.Benzakoun J., Hmeydia G., Delabarde T., et al. Excess out-of-hospital deaths during the COVID-19 outbreak: evidence of pulmonary embolism as a main determinant. Eur. J. Heart Fail. 2020;22(6):1046–1047. doi: 10.1002/ejhf.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raleigh V.S. Tackling UK’s mortality problem: covid-19 and other causes. BMJ. 2020;369:m2295. doi: 10.1136/bmj.m2295. [DOI] [PubMed] [Google Scholar]
- 38.Guidance for doctors completing medical certificates of cause of death in England and Wales. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/877302/guidance-for-doctors-completingmedical-certificates-of-cause-of-death-covid-19.pdf Available.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material