Abstract
Chronic pain represents a substantial unmet medical need globally. In recent years, the quest for a new generation of novel, safe, mechanism-based analgesic treatments has focused on neurotrophic factors, a large group of secreted proteins that control the growth and survival of different populations of neurons, but that postnatally are involved in the genesis and maintenance of pain, with biological activity in both the periphery and the central nervous system. In this narrative review, we discuss the two families of neurotrophic proteins that have been extensively studied for their role in pain: first, the neurotrophins, nerve growth factor (NGF) and brain-derived growth factor (BDNF), and secondly, the GDNF family of ligands (GFLs). We provide an overview of the pain pathway, and the pain-producing effects of these different proteins. We summarize accumulating preclinical and clinical findings with a focus on musculoskeletal pain, and on osteoarthritis in particular, because the musculoskeletal system is the most prevalent source of chronic pain and of disability, and clinical testing of these novel agents – often biologics- is most advanced in this area.
Keywords: Neurotrophins, Pain, osteoarthritis, nerve growth factor, Trk
1. INTRODUCTION
While dramatic advances have been made in diagnostics and therapeutics in recent decades, acute and chronic pain remain substantial unmet needs in modern healthcare. Pain accounts for vast societal costs and morbidity across all societies, and is clearly inadequately controlled with current biomedical and psychosocial strategies [1]. Nonetheless, as our understanding of the physiology and neurobiology of pain has progressed, novel and innovative targets have been identified that hold great promise for a new generation of analgesic therapy. In recent years, neurotrophic factors, a large group of secreted proteins that promote growth and survival of neurons [2], have received abundant attention as novel targets for the treatment of chronic pain. Neurotrophic factors are often secreted by the innervated target tissues, and they control the growth and survival of different populations of neurons. It is now appreciated that postnatally, several neurotrophic factors are involved in the genesis of pain, both peripherally and in the central nervous system. Two families of neurotrophic proteins have been extensively studied for their role in pain, the neurotrophins and the GDNF family of ligands (GFLs) (Table 1), as discussed in detail below. It is recognized that a large number of additional neurotrophic factors exist, such as TGFβ, ephrins, etc., however these have not been a major focus in pain research.
Table 1.
Neurotrophic factors studied in pain research
| Neurotrophic factors | |
|---|---|
| Neurotrophins | GDNF family of ligands |
| nerve growth factor (NGF) | glial cell line-derived neurotrophic factor (GDNF) |
| brain-derived neurotrophic factor (BDNF) | neurturin |
| neurotrophin (NT)-3 | persephin |
| neurotrophin (NT)-4 | artemin (also known as neublastin and enovin) |
Pain affects all body systems, however musculoskeletal pain may serve as a representative surrogate for evaluating the utility of these novel agents, as it is the most prevalent source of pain and disability [3, 4] and clinical testing of these novel agents is most advanced in the area of musculoskeletal pain. This narrative review discusses the neurotrophic factors that appear to hold the greatest promise as therapeutic targets in the near future, and summarizes the accumulating preclinical and clinical data. For each topic, a PubMed search was performed using the relevant keywords, and this was supplemented by a Google search as well as a search of clinicaltrials.gov for registered clinical trials; these search strategies were not intended to be entirely comprehensive, but were meant to identify the most important and relevant information. A comprehensive systematic review with metaanalysis is beyond the scope of this review.
2. IMPACT OF MUSCULOSKELETAL PAIN
Acute and chronic musculoskeletal pain remain substantial unmet needs internationally. Systematic analyses of the prevalence and societal costs of pain have generally focused on musculoskeletal pain; in fact, the Global Burden of Disease project initiated by the World Bank and the WHO did not assess pain as a distinct condition [4–6]. Nonetheless, low back pain (LBP) has been the leading cause of “years lived with disability” identified by the Global Burden of Disease project through each of its iterations, and as the population ages across the world, musculoskeletal pain will have increased impact. Osteoarthritis (also often referred to as degenerative joint disease) and LBP are the most prevalent sources of musculoskeletal pain worldwide, and both are expected to continue to increase with the aging population [7]. For example, the National Health Interview Survey Data for the U.S. suggest that there were more than 54 million adults with physician-diagnosed symptomatic arthritis in the U.S. in 2013–2015 [8], but that number is estimated to reach more than 78 million, or 26% of the adult population, by 2040 [7]. The overwhelming majority of these have painful osteoarthritis. The economic impact is enormous. In the U.S. alone, annual direct costs and earnings losses for those with osteoarthritis are approximately US$11,502 per individual, and for those with chronic back pain the costs are US$8,622 [[http://meps.ahrq.gov/mepsweb/; Yelin EH, Cisternas M, Watkins-Castillo SI: BMUS, “Medical Care Expenditures for Select Musculoskeletal Diseases” The Burden of Musculoskeletal Diseases in the United States, 4th Edition, USBJI, 2019, https://www.boneandjointburden.org/fourth-edition/viiig0/medical-care-expenditures-select-musculoskeletal-diseases]]. Worldwide, it is estimated that 303 million people have clinical osteoarthritis[4] and 242 million have activity-limiting disease [9]. The most important impact on those with osteoarthritis or with LBP is pain, yet despite the availability of a variety of analgesics, treatment of musculoskeletal pain in general remains poor. For example, among all of the agents recommended by the non-surgical treatment guidelines for osteoarthritis of the knee and hip promulgated by the American College of Rheumatology [10] and by the Osteoarthritis Research Society International [11], none has an effect size that could normally be considered to be “large”, i.e., > 0.5–0.8; in fact, all of these agents have effect sizes less than 0.4 [12]. As such, there is an enormous unmet need to identify more effective strategies to manage acute and chronic pain in this setting. This need is underlined in the contemporaneous setting, where opiates have become increasingly recognized as having substantial morbidities when used for chronic non-malignant pain, especially among the elderly [13], and their long-term efficacy has been questioned in these populations [14].
3. PHYSIOLOGY OF PAIN IN MUSCULOSKELETAL CONDITIONS
The International Association for the Study of Pain defines pain as “An unpleasant sensory and emotional experience associated with actual or potential tissue damage” (iasp.org). Pain is vital to the organism, since it prompts behavior to avoid harmful situations (for example, withdrawal from a flame) and imposes immobilization of an injured tissue, which favors healing. Nociception is mediated by nociceptors, specialized afferent neurons that innervate peripheral tissues and detect damaging or potentially damaging stimuli of a mechanical, thermal, or chemical nature through an array of specialized receptors and channels [15]. The signals detected in the periphery are transmitted to the central nervous system (CNS) via the dorsal root ganglia (DRG), where the cell bodies of sensory neurons reside, to the dorsal horn of the spinal cord. There, peripheral afferents synapse with intermediate or secondary neurons that carry the signal further to the brainstem, thalamus, and cortex (Fig. 1). Under normal circumstances, painful stimuli and the ensuing neuronal responses are relatively transient in nature. However, when pain is experienced in the context of chronic pathology, the pain pathway undergoes numerous changes that result in hypersensitivity. This phenomenon is known as “sensitization”, and it occurs both in the peripheral nervous system (PNS) and in the CNS [16] [17]. Because of sensitization, chronic pain in many clinical syndromes loses its protective function and becomes pathological: the pain can arise spontaneously, can be elicited by normally innocuous stimuli (allodynia), is exaggerated and prolonged in response to noxious stimuli (hyperalgesia), and can spread beyond the site of injury (secondary hyperalgesia/allodynia). Chronic musculoskeletal pain is associated with plasticity of the PNS and the CNS, clinically manifest as quantitative sensory changes such as lowered pressure withdrawal thresholds in affected and in remote sites [18]. Peripheral and central sensitization may thus obscure the correlation between structural changes in the joint and pain and, in osteoarthritis, may be a factor in the documented discordance [19] between radiographic severity and pain [20].
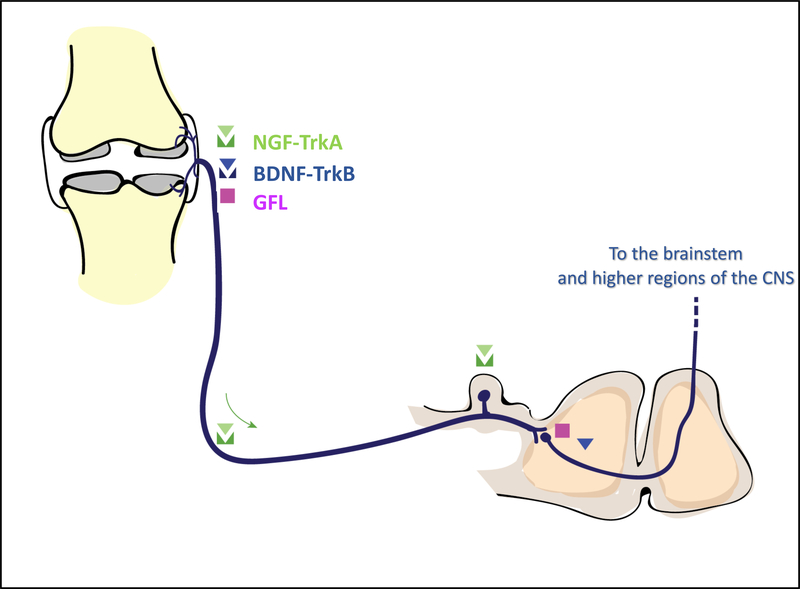
Fig. 1. Neuroanatomy of the pain pathway and sites of action of the different neurotrophic factors under investigation as targets for chronic musculoskeletal pain.
Sensory neurons, including nociceptors (medium sized Aδ-fibers and small unmyelinated slow-conducting C-fibers) innervate peripheral tissues. Nociceptors are equipped with a broad array of specialized receptors and channels to detect noxious signals in the innervated tissues and carry them to the dorsal horn (DH) of the spinal cord. To perform this function, nociceptors have a unique pseudo-unipolar morphology, with an axonal stalk that extends from the cell body in the dorsal root ganglia (DRG) and splits into two terminals. The peripheral terminal innervates the tissues, and the central terminal extends into the DH to synapse with second-order neurons. Injury or inflammation in the innervated tissues results in release of mediators by resident (damaged) tissue cells and infiltrating inflammatory cells (for example macrophages, mast cells), causing peripheral sensitization. At the same time, gene expression changes occur in the DRG, and inflammatory cells infiltrate the DRG. Ongoing nociceptive input from the periphery results in increased excitability of second order neurons in the spinal cord [16]. Neurotrophic factors can act at different sites to modify pain processing pathways (mostly to increase excitability and promote pain). Nerve growth factor (NGF), secreted by cells in the innervated tissues, is active in the periphery and binds TrkA expressed by afferents, resulting in sensitization. The NGF-TrkA complex can be internalized by neurons and retrogradely transported to cell bodies in the DRG, initiating gene expression changes that lead to synthesis of pro-algesic peptides and ion channels. Pain-producing effects of brain-derived neurotrophic factor (BDNF), which binds TrkB, have been documented in the periphery and in the dorsal horn. Members of the GDNF family of ligands (GFL) are active in the periphery as well as in the central nervous system, but their signaling in musculoskeletal pain and in the joint has been less studied.
Neurotrophic factors are expressed at different levels of the pain pathway, and can modulate nociceptive processing both in the periphery and the CNS (Fig. 1). Hence, several of them are being targeted for the development of novel therapies for musculoskeletal pain. Among them, the neurotrophins NGF and BDNF are the most advanced in clinical development. In addition, members of the GDNF family are also under investigation. The biology of these neurotrophic factors, as well as preclinical evidence and clinical development of neutralizing antibodies and compounds targeting them for musculoskeletal pain will be discussed below.
4. NEUROTROPHINS AND GDNF FAMILY AS CHRONIC PAIN TARGETS
4.1. Neurotrophins
Neurotrophins constitute a group of structurally related proteins that includes nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin (NT)-3, and NT-4. Neurotrophins signal through three types of tyrosine receptor kinases (Trk, also known as tropomyosin receptor kinases): TrkA, TrkB, or TrkC. TrkA is highly selective for NGF but also binds NT-3, while TrkB is selective for BDNF and NT-4, and TrkC for NT-3. In addition, all neurotrophins (including NGF) can also bind another receptor with low affinity, p75NTR, a member of the tumor necrosis factor receptor superfamily [21].
During development, neurotrophins play a key role in neuronal growth and specification of neuronal diversity in the peripheral nervous system [22, 23], which is outside the scope of this review. Postnatally, many neurotrophins are involved in the genesis of pain, and this is why they offer novel targets for development of analgesic therapies, especially using biologics. By far the most advanced strategy to target neurotrophic factors for musculoskeletal pain is the approach that uses neutralizing antibodies against the neurotrophin, NGF.
4.1.1. NERVE GROWTH FACTOR (NGF)
NGF became an important target for analgesia development because of its well documented role in pain and it has been found to be highly overexpressed in human pain states, including osteoarthritis [24]. These observations prompted development of neutralizing antibodies as therapeutic agents. Early clinical trials of anti-NGF for osteoarthritis sparked tremendous enthusiasm because patients appeared to experience remarkable pain relief [25]. However, unexpected safety concerns emerged, based on reports of rapidly progressive osteoarthritis (RPOA) in patients receiving anti-NGF, which have hampered clinical development of these antibodies. Below, we provide an in-depth discussion of the role of NGF in nociception and inflammatory pain. Because of the documented RPOA observed in clinical trials, we also summarized the literature on NGF in bone pain and in experimental models of osteoarthritis. Then, we discuss clinical development of strategies to block NGF.
4.1.1.a. NGF is a key peripheral pro-nociceptive mediator.
NGF was discovered for its role in promoting neuronal growth and survival in developing chick embryos [26] [27]. Two scientists at Washington University in St. Louis, Dr. Rita Levi-Montalcini and Dr. Stanley Cohen, isolated NGF from mouse tumors and reported that it caused nerve growth in chicken embryos, a discovery which laid the foundation for growth factor research and earned them the 1986 Nobel Prize in Medicine. The role of NGF in neuronal development has been reviewed elsewhere [24]. Notably, during development all nociceptors as well as sympathetic neurons express TrkA and are dependent on NGF for survival. Hence, mice lacking NGF display loss of sensory and sympathetic neurons, and cannot respond to noxious stimuli [28]. In humans, genetic mutations exist in the genes encoding NGF (NGF) or TrkA (NTRK1) that show a similar phenotype (Table 2). Several mutations have been described in NGF that result in Hereditary Sensory and Autonomic Neuropathy (HSAN) syndromes (reviewed in [29]). An extensively studied Swedish mutation (known as the Norrbottnian mutation) results in severe reduction of unmyelinated fibers and congenital insensitivity to pain, most compatible with HSAN type V, and accompanied by many musculoskeletal complications such as fractures and neuropathic joints [30, 31]. This NGFR100W mutation was recently engineered in mice, and found to mimic the human syndrome [32]. Furthermore, a rare heritable condition called congenital insensitivity to pain with anhidrosis (CIPA, also known as HSAN type IV), which is characterized by inability to feel pain and to sweat, was found to be caused by an autosomal recessive mutation in NTRK1 [33]. Until now, more than 100 loss-of-function mutations have been described in NTRK1. Each of these results in the inability to feel pain, along with the attendant complications such as severe injuries, fractures, and Charcot joints [34]. In addition to these mutations, single nucleotide polymorphisms (SNPs) have been reported in NTRK1 and in NGFR (the gene encoding p75NTR), with subtle effects on pain sensitivity [35] [36] (details in Table 2).
Table 2.
Neurotrophic factors: Human mutations and single nucleotide polymorphisms (SNP) associated with altered pain sensitivity.
| Neurotrophic factor | Mutation(s) reported | SNP(s) reported | Phenotype | Reference |
|---|---|---|---|---|
|
NGF Encoding NGF |
Point mutation located on chromosome 1p11.2-p13.2, resulting in R100W missense mutation in mature NGF | Severe reduction of unmyelinated fibers and congenital insensitivity to pain |
Einarsdottir 2004; Minde 2009 |
|
|
NTRK1 Encoding TrkA |
Autosomal recessive loss of function mutations | Congenital insensitivity to pain with anhydrosis (CIPA) | Indo 1996 | |
| nonsynonymous SNP NTRK1H604Y, with substitution of tyrosine for histidine | Children with the SNP experienced more post-surgical pain (subtle effect) | Mamie 2013 | ||
|
NGFR Encoding Low Affinity Neurotrophin Receptor P75NTR |
rs9908234, but did not replicate in independent cohorts | Genome-wide association study on migraine | Ligthart 2011 | |
|
BDNF Encoding BDNF |
Val66Met | Increased risk for chronic postoperative pain | Tian 2018 | |
| haploinsufficiency of theBDNFgene | Strong reduction in pain sensitivity in patients with Wilms tumor-aniridia (WAGR) syndrome | Sapio 2019 |
Because of its neurotrophic activities, recombinant NGF has been studied as a potential treatment for diseases of the PNS and the CNS, for example diabetic polyneuropathy [37] [38]. During clinical trials in healthy human adults, it became apparent that administration of exogenous NGF caused pain. When injected subcutaneously, NGF caused rapid onset hyperalgesia that could last for weeks [39]. One study found that intravenous and subcutaneous doses above 0.1 μg/kg induced diffuse myalgias, particularly in the neck and throat muscles, as well as mechanical and thermal hyperalgesia in the area of the subcutaneous injection site [39]. Intradermal injection of NGF (1 or 3 μg) also induced mechanical and thermal hypersensitivity in healthy subjects [40]. In clinical trials for diabetic neuropathy, painful side effects of rhNGF injection became dose limiting and resulted in termination of the development for this target [41].
The pro-algesic effects of NGF administered in vivo, both systemically and locally, have been extensively studied in experimental animals. A single systemic injection of NGF (1 μg/g, i.p.) induced rapid-onset mechanical and thermal hypersensitivity in rats [42, 43], while intraplantar administration resulted in local edema and thermal hyperalgesia [44], as well as immediate long-lasting mechanical hypersensitivity [45]. The effect of NGF injected intramuscularly has also been investigated. A single injection into the multifidus muscle in rats did not induce spinal neuron hyperexcitability, but a second injection 5 days later did, suggesting a type of nociceptive priming effect of NGF [46]. In addition, after two NGF injections, a large number of additional neurons had new receptive fields in the deep tissues (muscles, thoracolumbar fascia), which may be one explanation for the mechanism of the spread of myofascial low back pain.
Finally, NGF can induce pain-related behaviors when injected intra-articularly into the joints of healthy animals. NGF injected into healthy rat knees acutely induces weight-bearing asymmetry, mechanical allodynia of the hind paw, and knee swelling [47, 48], but it does not alter the firing of wide dynamic range dorsal horn neurons following extension or flexion of the knee [49]. Intra-articular injection into the cervical facet joints of the spine caused mechanical and thermal hypersensitivity in the forepaws that could be prevented by ablating substance P-expressing (i.e., peptidergic) C-fibers, but not IB4+ (i.e., non-peptidergic) C-fibers prior to injection [50]. One study examined the effects of injecting NGF into healthy rat hips, and found it caused mild synovitis as well as upregulation of the neuropeptide, calcitonin gene relate peptide (CGRP), in DRG neurons, but this study did not assess pain-related behaviors [51]. Collectively, these studies suggest that NGF-responsive neurons innervate healthy joints, but detailed information on the distribution of TrkA-positive neurons in joints is lacking. A recent study used immunohistochemistry to detect TrkA in healthy mouse femora and found it was restricted to nerve fibers located in the vicinity of NGF-positive blood vessels in the bone marrow [52].
4.1.1.b. Pro-nociceptive mechanisms of NGF.
One of the most fundamental mechanisms underlying clinical pain disorders is that nociceptors become hyperexcitable when the tissues they innervate become injured or inflamed. This hyperexcitability is brought about through biochemical and electrophysiological changes mediated by the changing tissue environment. Many studies have shown that NGF is a key contributor in this process, through distinct mechanisms with both rapid and slower onset effects (for a detailed review, see [24]). First, NGF has direct pro-algesic effects on nociceptors in a two-fold manner. Binding of NGF to TrkA on peripheral terminals of TrkA+ nociceptors has excitatory effects that result in sensitization of nociceptors. In the short term, TrkA signaling increases activity and/or expression of ion channels including transient receptor potential cation channel subfamily V member 1 (TRPV1), voltage-gated sodium channels, voltage-gated calcium channels, delayed rectifier potassium channels, and acid-sensing ion channels 2 and 3 [53]. This results in nociceptor depolarization and immediate sensitization. In addition to these rapid effects, the NGF-TrkA complex can be internalized by neurons and retrogradely transported to cell bodies in the DRG, initiating gene expression changes that lead to synthesis of pro-algesic peptides such as substance P, CGRP, and nociceptor-specific ion channels such as NaV1.8 [24].
In addition to these well documented directly sensitizing and pro-algesic effects of NGF on nociceptors, it is thought that NGF can also produce pain through stimulating neuronal sprouting at the injured site. This has been described in models of bone cancer and inflammation induced by intra-articular injection of complete Freund’s adjuvant (CFA), where neuronal sprouting caused formation of painful neuromas which can be inhibited by anti-NGF [54] [55].
While evidence mostly suggests that in disease states, the pro-nociceptive effects of NGF are largely mediated through TrkA, the exact mechanism by which NGF induces hyperalgesia through TrkA vs. p75NTR receptors in healthy animals remains unknown. Systemic injection of NGF into transgenic mice lacking p75NTR caused thermal and mechanical hyperalgesia similar to wild-type mice, suggesting that the TrkA receptor is sufficient to mediate the noxious action of NGF [56]. However, a 2013 study compared the effects of injecting either NGF or the p75NTR- selective agonist, pro-NGF, and found that either form of NGF could induce mechanical hyperalgesia when injected into the rat hind paw [57]. Furthermore, a blocking antibody to the p75 receptor inhibited mechanical hyperalgesia caused by both forms of NGF. The same group reported that thermal hyperalgesia, in contrast to mechanical hyperalgesia, is mediated through the TrkA pathway when NGF is injected into the hind paw of healthy rats [58]. Finally, another study compared the actions of NGF to the mutant NGFR100W that does not bind and activate the p75 signaling cascade. In this study, the mutant version did not induce thermal or mechanical hyperalgesia when injected into the rat hind paw [59]. Interestingly, both the wild-type and mutant forms of NGF can induce hyperalgesic priming: injection of PGE2 seven days after either NGF injection induced prolonged mechanical hyperalgesia, supporting the substantial evidence presented below for the role of TrkA in mediating inflammatory pain [59].
4.1.1.c. NGF mediates inflammatory pain. Evidence from experimental models and endogenous effects revealed through blockade of NGF.
NGF is a key mediator in the inflammatory response and is particularly essential for generating pain. Pain (dolor) is one of the 5 cardinal signs of inflammation- the other 4 are rubor (redness), calor (heat), tumor (swelling), and functio laesa (loss of function). As part of an inflammatory response following tissue injury, NGF is released by a variety of immune (macrophages, mast cells) and non-immune cells, resulting in pain [24, 60] and also contributing to edema.
An early study demonstrating a role for NGF in mediating inflammatory hypersensitivity was performed in the CFA model, in which CFA is injected into the hindpaw to induce local inflammation, including erythema, edema, and thermal and mechanical hypersensitivity [61]. Inflammation in this model was found to be accompanied by increased levels of NGF in the skin and upregulated levels of the neuropeptides, substance P and CGRP, in innervating afferents. Neutralizing NGF with systemically administered antibodies prevented hypersensitivity, upregulation of neuropeptides in sensory neurons, and the inflammation-induced expression of the immediate early gene c-fos in dorsal horn neurons, but anti-NGF did not affect tissue swelling. CFA can also be injected intra-articularly to induce joint inflammation and to model joint pain. When injected into the rat ankle, CFA causes monoarthritis with elevated NGF levels in the synovial fluid, and reduced weight-bearing on the affected limb, which can be partially rescued by systemic administration of either anti-NGF or a pan-Trk small molecule inhibitor [62]. Likewise, injecting CFA into mouse knee joints causes pain-related behaviors that can be blocked by anti-NGF [55]. In addition, in this mouse model, anti-NGF reduced ectopic sprouting of sensory and sympathetic neurons in the synovium.
In the carrageenan model of skin inflammation, innervating nociceptors are sensitized such that there is an increase in spontaneous activity as well as sensitivity to heat. Sequestering endogenous NGF by using a TrkA-IgG fusion protein prevented this sensitization, but not tissue swelling [63]. In addition, TrkA-IgG was able to block the thermal hyperalgesia that develops with this model [64]. In healthy animals, TrkA-IgG induces thermal and chemical hypoalgesia and downregulates CGRP in sensory neurons [64]. Systemic injection of the TrkA inhibitor, AR786, also inhibited mechanical hyperalgesia associated with the carrageen model as well as with the collagen-induced arthritis (CIA) model (a model of inflammatory arthritis), and simultaneously reduced joint swelling and joint damage [65]. Together, these studies suggest that under normal circumstances NGF acts to maintain nociception thresholds, while under inflammatory conditions an upregulation of NGF can induce hyperalgesia, likely through TrkA signaling.
In other pain models, anti-NGF has had mixed results in reversing hyperalgesia. In a study of painful neck injury caused by cervical facet joint injury in rats, intra-articular administration of anti-NGF immediately following the injury prevented mechanical hypersensitivity, but it was no longer effective after pain behaviors had already developed [66].
In addition to antibody strategies, vaccination against NGF has been explored as a strategy to sequester NGF and thereby treat chronic inflammatory pain. In one early study, NGF depletion through vaccination induced thermal hypoalgesia in otherwise healthy rats with high antibody serum titers [67]. More recently, mice vaccinated with recombinant murine NGF conjugated to virus-like particle induced anti-NGF-specific IgG antibodies capable of neutralizing NGF, and titers were sustained over a one year period with periodic boosting [68]. Vaccination reduced hyperalgesia in two models of inflammatory pain (CIA and zymosan A), but it did not impact paw volume in the zymosan model, nor did it affect average clinical score or disease progression in the hind paws in the CIA model [68]. Furthermore, vaccination did not impact sensory innervation patterns in the hind paw skin or sympathetic innervation of cerebral blood vessels. Vaccination has also been explored in the context of osteoarthritis, as discussed below (Section 4.1.1.f.).
Finally, another novel strategy that has been developed to target NGF-TrkA signaling is delivery of a phototoxic agent coupled to an engineered NGF ligand that does not elicit TrkA signaling [69]. Near-infrared illumination can thus selectively ablate TrkA-positive cells. In healthy mice, injection of this agent into the hind paw followed by exposure to near-infrared light reduced sensitivity to mechanical and thermal noxious stimuli. This NGF compound also effectively reduced mechanical pain behaviors in the CFA hind paw model of inflammatory pain, the monosodium iodoacetate (MIA) model of osteoarthritis pain, and the spared nerve injury model of neuropathic pain.
4.1.1.d. NGF blockade in models of bone pain.
NGF-TrkA signaling is important for bone pain [70]. The majority of sensory nerves that innervate bone express TrkA, including afferents in the periosteum, bone marrow, and mineralized bone [71]. Blocking NGF-TrkA signaling has been shown to provide pain relief in a variety of bone pain models. In an ovariectomy (OVX)-induced mouse model of osteoporosis, anti-NGF (Exalpha Biologicals Inc, 10 mg/kg, i.p.) improved mechanical and thermal hypersensitivity as well as deficits in grip force [72]. In a bone cancer model, a pan-Trk inhibitor (ARRY-470, 30 mg/kg, p.o., bid) reduced guarding behaviors when therapy was started early [55]. In addition, early treatment prevented the ectopic sprouting of sensory nerve fibers, but it did not have a significant effect on tumor growth or bone remodeling [55]. Similarly, in a model of prostate-cancer-induced bone pain, anti-NGF therapy (mAb911, 10 mg/kg, i.p.) reduced guarding behavior that developed in the late-stage of the model, and anti-NGF reduced both sensory and sympathetic nerve sprouting and neuroma formation [54]. In a model of fracture healing, anti-NGF therapy (mAb 911, 10 mg/kg, i.p.) delivered post fracture reduced guarding behaviors [73]. Similarly, in another model of fracture healing, anti-NGF (RN624, 10 mg/kg, i.p.) or anti-TrkA administered after osteotomy surgery improved locomotion levels [74]. In a rat model of complex regional pain syndrome type I induced by tibia fracture, NGF expression was increased in hindpaw skin and tibia bone. Anti-NGF given post-fracture rescued weight-bearing asymmetry, but it did not affect hindpaw edema [75]. In a closed femur fracture pain model in the mouse, anti-NGF (mAb911 at a dose of 10 mg/kg i.p.) reduced spontaneous guarding behaviors [76] and partially rescued locomotion deficits [77]. Finally, in a model of orthopedic surgery pain, induced by drilling and coring the trochlear groove of the mouse femur, anti-NGF injection (mAb911 at a dose of 10 mg/kg i.p.) prior to surgery attenuated the surgery-induced decline in locomotion [78].
While the above studies in fracture models did not detect an impact on bone healing with anti-NGF or TrkA blockade [73, 74, 76, 77], other reports support a role for NGF-TrkA signaling in the bone healing process (for review [79]). Indeed, recent studies have shown that NGF-TrkA signaling is necessary for skeletal development [80] and skeletal adaptation to mechanical loading in adult mice [81]. In addition, a recent study using an ulnar stress fracture mouse model suggested a role for NGF-TrkA signaling in fracture repair. Injury to bone acutely increased NGF levels, specifically in periosteal stromal progenitors and in fracture-associated macrophages, and this was associated with sprouting and arborization of CGRP+ TrkA+ sensory nerve fibers within the reactive periosteum in NGF-enriched cellular domains [82].
TrkA inhibition using a transgenic mouse reduced the numbers of sensory fibers and delayed ossification of the fracture callus [82]. The authors speculate that one reason why earlier studies testing anti-NGF in fracture models did not detect a change in fracture repair may be because the analgesic effects of anti-NGF may occur at lower doses than the effects needed to impair bone repair. Nonetheless, these studies emphasize the importance of understanding how NGF-TrkA signaling contributes to musculoskeletal homeostatic processes when considering long-term anti-NGF therapies for other indications such as osteoarthritis.
4.1.1.e. NGF in osteoarthritis. Expression and regulation.
While it is known that NGF is upregulated in animal models of osteoarthritis, there is a paucity of information on the precise expression patterns of NGF and TrkA in healthy joints and joints affected by osteoarthritis. It has been reported that Ngf expression is increased in the knee joint in the chronic phase of two surgical mouse models of osteoarthritis induced by either destabilization of the medial meniscus (DMM) [83] or by partial meniscectomy [84], particularly in cartilage [84]. Furthermore, Ngf expression has been shown to be increased in the L3-L5 DRG after DMM surgery, again in the late phase of the model when persistent pain is present [85]. In the rat MIA model, NGF protein is reportedly elevated in the synovium [86].
In addition, it remains unclear whether the response to NGF changes in the course of osteoarthritis, and whether the expression and distribution of TrkA in the joint changes. Injecting NGF intra-articularly into healthy rat knees caused transient weight-bearing asymmetry, but when injected in the chronic phase of rats with experimental osteoarthritis (induced by MIA or by surgical transection of the meniscus), there was a more persistent increase in weight-bearing asymmetry, and this was consistent with increased TrkA expression in the DRGs [47]. Interestingly, prophylactic treatment with indomethacin prevented NGF-induced increase in pain behavior in the MIA model and reduced TrkA upregulation in the DRG, suggesting that early inflammation in the model triggered these changes. Another study in the rat MIA model examined the effects on dorsal horn neurons of NGF injected into the knee [49]. This study reported that intra-articular administration of exogenous NGF increased both physiologic knee extension-evoked firing of spinal neurons and the size of the peripheral receptive fields of spinal neurons over the knee joint in MIA rats, but it did not exacerbate responses to noxious stimulation of the hind paw. Collectively, these studies support the idea that NGF heightens peripheral sensitization in osteoarthritis.
NGF has been detected in different tissues of human joints affected by osteoarthritis, but very few studies have attempted a systematic description of the expression patterns of NGF and its receptor. Osteoarthritis pathology affects all joint tissues, including articular cartilage, subchondral bone, synovium, menisci, and ligaments. Degradation of the articular cartilage and subchondral bone remodeling are hallmarks of osteoarthritis, and this is accompanied by formation of osteochondral vascular channels that breach the tidemark between the subchondral bone and articular cartilage. These osteochondral channels can contain CGRP+ C-fibers, as well as NGF. NGF has been reported in close association with osteoclasts, and NGF expression in these channels has been associated with pain in patients with knee osteoarthritis [87, 88]. In human synovium, increased levels of NGF were found mainly in fibroblasts and in some macrophages, and this was also associated with pain [89]. In addition, it has been reported that synovial fluid and serum from OA patients contain elevated NGF levels compared to controls (patients with minimal meniscal lesions who underwent routine arthroscopy) [90], and synovial fluid NGF levels have been correlated with poor knee function [91].
4.1.1.f. NGF blockade in experimental models of osteoarthritis.
Complementing the development of anti-NGF antibodies for the clinical treatment of osteoarthritis pain, pre-clinical studies have suggested that blocking NGF-TrkA signaling provides pain relief in experimental models of osteoarthritis (Table 3).
Table 3.
NGF blockade in experimental models of osteoarthritis
| Type of study | OA Model | Drug | Results | Reference |
|---|---|---|---|---|
| Therapeutic: One time injection | Destabilization of the medial meniscus (DMM); mouse | TrkA inhibitor (TrkAD5; 2 mg/kg, s.c.) | Reversed weight-bearing asymmetry for 3 days when given at the 16-week time point | McNamee 2010 |
| Therapeutic: One time injection | Monosodium iodoacetate (MIA); rat | anti- NGF (AS2886401–00; 0.3 or 1 mg/kg, i.v.) | Anti-NGF (0.3 or 1 mg/kg) given on day 3 or day 28 was able to suppress gait imbalances. Anti-NGF given on day 3 exacerbated knee swelling by day 35 of the model. | Ishikawa 2015 |
| Therapeutic: One time injection | Bilateral MIA; rat | anti-NGF (extracted from the patent application WO 2004/058184 A2; 9 mg/kg, s.c.) | Anti-NGF given on day 2 after MIA induction reversed burrowing deficits 24 hours later | Bryden 2015 |
| Therapeutic: One time injection | MIA; rat | anti-NGF (L148M (Exalpha Biological Inc.); 10mg/kg, i.p.) | Anti-NGF therapy given on day 21 inhibited gait changes for one week | Miyagi 2017 |
| Preventative or Therapeutic | MIA; rat | anti-NGF (muMab911; 10 mg/kg; s.c. weekly) |
Preventative (from day 0): Prevented development of weight-bearing asymmetry. Mechanical allodynia of the hind paw was reduced on day 28 only. There was a trend in increased cartilage damage score with treatment. Therapeutic (injections on days 14 and 21): Reversed weight-bearing asymmetry and hind paw mechanical allodynia through day 28. No change in cartilage damage score. |
Xu 2016 |
| Preventative or Therapeutic | Meniscal transection (MNX); rat | TrkA inhibitor (AR786; 30 mg/kg, orally, twice daily) |
Preventative (from 1 h before surgery): Prevented development of weight-bearing asymmetry and hind paw mechanical allodynia. Preventative (from 1 h before surgery – day 14): Withdrawal of treatment on day 14 continued to provide relief in weight-bearing asymmetry until day 24, but hind paw mechanical allodynia developed rapidly when treatment stopped and was fully developed by day 19. Therapeutic (day 14–21): Weight-bearing asymmetry and hind paw mechanical allodynia reduced after 5 days of treatment. |
Nwosu 2016 |
| Therapeutic | MIA; rat | Therapeutic (day 14–21): Weight-bearing asymmetry reduced after 3 days of treatment. Hind paw mechanical allodynia reduced after 5 days of treatment. Synovitis and cartilage degeneration were reduced with treatment. | ||
| Preventative or Therapeutic | Medial meniscal tear (MMT); rat | anti-NGF (tanezumab; 0.1, 1 or 10 mg/kg; s.c. once a week) |
Preventative (from day 0): All doses decreased gait changes observed at day 3 post surgery, but all doses increased cartilage damage scores in rats taken down at day 7, 14, or 28. Therapeutic (0.1 mg/kg, day 23–37): Cartilage damage score increased with treatment. Therapeutic (0.1 mg/kg, day 57–71): Cartilage damage score increased with treatment, but not significantly since damage was already severe by the time treatment was started. |
LaBranche 2017 |
| Preventative or Therapeutic | Male partial medial meniscectomy (PMX); mouse | anti-NGF vaccination |
Preventative (first immunization 2 weeks prior to surgery): Immunization with 3 boosters reduced weight-bearing asymmetry. No change in cartilage damage score. Therapeutic (first immunization week 10 after surgery): Immunization with 2 boosters reduced weight-bearing asymmetry until antibody titers decreased. |
von Loga 2019 |
| Therapeutic | Mechanical loading; mouse | anti-NGF (MedImmune, AstraZeneca; 3 mg/kg; i.p. every two weeks) | Therapeutic (week 2 and 4 post loading): Each anti-NGF injection reduced hind paw mechanical allodynia for 6 days until the allodynia began to return. Similarly, anti-NGF reduced weight bearing asymmetry for 1 week post injection but returned by week 2. | Ter Heegde 2019 |
An early study to test the effects of NGF/TrkA blockade in a widely used surgical mouse model found that a single injection of a small molecule TrkA inhibitor (TrkAD5; 2 mg/kg; s.c.) acutely reversed weight-bearing asymmetry when given 16 weeks after DMM surgery [83]. Other studies have also shown analgesic effects with single doses of anti-NGF antibodies. In the rat MIA model, a neutralizing NGF antibody (AS2886401–00; intravenous (i.v.); 0.3 or 1 mg/kg) given on day 3 or day 28 suppressed the development of gait imbalances assessed on day 35 [92]. However, notwithstanding its analgesic effects, anti-NGF given on day 3 resulted in exacerbated knee swelling by day 35 in this study [92]. In a murine MIA model, anti-NGF (L148 M; Exalpha Biological Inc., Shirley, MA) (10 mg/kg, i.p.) therapy given on day 21 was able to inhibit gait changes for one week [93]. In the bilateral MIA rat model, anti-NGF (variable domains were extracted from the patent application, WO 2004/058184 A2 (applicant: Rinat Neuroscience Corporation); 9 mg/kg, s.c.) reversed burrowing deficits on day 3 after MIA induction [94]. Finally, in a murine mechanical loading model, anti-NGF mAb treatment (3 mg/kg, i.p.; MedImmune, AstraZeneca) administered 2 and 4 weeks after injury inhibited weight-bearing asymmetry and mechanical allodynia in the hind paw [95].
Four studies have examined the effects of preventative vs. therapeutic blockade of NGF or TrkA on pain behaviors in models of osteoarthritis. Anti-NGF treatment (weekly subcutaneous injection of 10 mg/kg muMab 911) administered before or after (day 14) induction of osteoarthritis in the rat knee using MIA inhibited weight-bearing asymmetry and mechanical allodynia of the hind paw [96]. Both preventative and therapeutic anti-NGF treatment attenuated the number of tartrate resistant acid phosphatase (TRAP)-positive osteoclasts in the tibial plateau, which suggests that NGF contributes to the activation of osteoclasts in osteoarthritis. However, cartilage damage appeared to be increased when anti-NGF was given preventatively in this study. A study in the rat meniscectomy model tested a small molecule inhibitor of TrkA (AR786, oral doses (30 mg/kg) twice daily) and found that either preventative or therapeutic treatment beginning on day 14 was effective in reducing weight-bearing asymmetry and mechanical allodynia of the hind paw [97]. No effect on cartilage damage was observed in this study, but therapeutic treatment reduced knee swelling [97]. Vaccination against NGF, as described above, has also been tested in a mouse model of osteoarthritis (partial meniscectomy) [98]. Prophylactic and therapeutic vaccination reduced weight-bearing deficits in the operated limb, indicative of persistent pain, but had no impact on joint damage. As in the earlier study, boosting was required to maintain anti-NGF titers. Finally, a Pfizer-study used the rat medial meniscal tear (MMT) model to test the effects on joint damage of anti-NGF therapy initiated at different time points after surgery [99]. The results suggested that anti-NGF treatment starting prior to surgery or on day 23 of the model resulted in more severe cartilage damage compared to vehicle. As anti-NGF in clinical trials clearly resulted in adverse effects related to rapidly progressive osteoarthritis (as discussed below, Section 4.1.1.g), these animal studies highlight the need for detailed characterization of the mechanisms driving accelerated joint damage in experimental models of osteoarthritis.
A study in a murine MIA model examined neuro-immune mechanisms mediated by NGF-TrkA signaling in osteoarthritis pain by using mice with a gain-of-function mutation of TrkA [100]. Mice with increased TrkA activity developed more severe mechanical allodynia of the hind paw and more pronounced cellular changes in the dorsal horn of the spinal cord after MIA induction compared to wild-type mice. In addition, TrkA knock-in mice had more cellular infiltration in the joint after MIA, including higher numbers of leukocytes (CD45+ cells), macrophages (F4/80+CD11b+ cells), and mast cells (CD117+ and FCƐRI+). This study also demonstrated that TrkA knock-in mice had higher levels of prostaglandin D2 in the joint after MIA induction. Since mast cells exposed to NGF produce more prostaglandin D2, and prostaglandin D2 can promote pain by acting on nociceptors, this could be a potential mechanism for how NGF can indirectly contribute to the observed mechanical hypersensitivity through mast cells, in addition to its direct effects on neurons.
Veterinary studies have also tested the analgesic effects of anti-NGF for treatment of osteoarthritis in dogs and cats. NGF is upregulated in the synovial fluid of dogs with chronic lameness compared to healthy dogs [101]. A fully caninized anti-NGF (NV-01) reduced signs of lameness in an inflammatory model of pain induced by injection of kaolin into the footpad of the dog [102]. In a pilot study in dogs with osteoarthritis, anti-NGF (NV-01, 0.2 mg/kg, i.v.) reduced owner-assessed pain scores up to 4 weeks after administration [103]. A further study using a randomized, parallel group, stratified, double masked, placebo-controlled, proof of principle clinical pilot study design demonstrated that this anti-NGF antibody could also reduce pain in dogs with degenerative joint disease for 4 weeks [104]. The same company (Nexvet Australia Pty Ltd.) has also developed a felinized anti-NGF (NV-02), which has shown similar analgesic benefits in studies of cats using a model of inflammatory pain [105] and in cats with degenerative joint disease [106].
Finally, a safety study sponsored by Pfizer was conducted to investigate effects of tanezumab or muMab911 on select bone and joint endpoints and biomarkers in cynomolgus monkeys (4 to 30 mg/kg/week for 26 weeks), rats (0.2 to 10 mg/kg twice weekly for 28 days), and mice (10 mg/kg/week for 12 weeks). No deleterious effects were reported in this study [107].
4.1.1.g. Clinical relevance and development of drugs that target NGF-TrkA signaling
The documented role for NGF in pain, and the fact that its expression is markedly increased in human pain states, including osteoarthritis, prompted the development of neutralizing antibodies (Abs) as therapeutic agents. A search of the Clinicaltrials.gov registry (searched 24 December 2019) revealed 51 studies under the search terms “nerve growth factor” and “pain”, indicating the general interest in the target.
Strategies to directly inhibit the activity of NGF have focused largely on the biologicals rather than on the development of small molecule inhibitors. Several companies have developed humanized monoclonal antibodies that bind NGF with high specificity and affinity, preventing it from binding its receptor. Among these products are tanezumab (Pfizer and Eli Lilly) and fasinumab (Regeneron and Teva), which are still in clinical development. Development of fulranumab (Janssen and Amgen) was halted in 2016 (https://www.jnj.com/media-center/press-releases/janssen-announces-discontinuation-of-fulranumab-phase-3-development-program-in-osteoarthritis-pain), as was further work on MEDI-578 (AstraZeneca) and ABT-110 (Abbvie)[108]. Additional targets in the NGF signaling pathway that are under clinical development include the TrKs, especially TrkA, and in contrast to NGF, TrkA inhibition is mediated via small, orally available molecules. A search of Clinicaltrials.gov (completed 24 December 2019) for TrkA and osteoarthritis or pain revealed three studies, all completed. In addition, a pan-TrK inhibitor (which blocks TrkA, B, and C) has begun clinical testing for osteoarthritis [109].
Among musculoskeletal indications, targeted anti-NGF therapy has been most successful for osteoarthritis pain. The first Phase 2 randomized double blind controlled proof of concept trial of anti-NGF therapy evaluated tanezumab for osteoarthritis pain and was published in 2010 [25]. This trial demonstrated significantly improved pain palliation with tanezumab compared to placebo, and many participants were noted to experience dramatic benefit. Subsequently, positive findings were also reported using tanezumab for LBP [110]. Notwithstanding the apparent clinical benefit, however, careful evaluation of adverse events among patients treated with anti-NGF therapy, both in these trials and with the other anti-NGF agents under development, suggested a strong association with rapidly progressive osteoarthritis and osteonecrosis, including in joints that were thought to be clinically not involved. As a result, the US Food and Drug Administration (FDA) halted all clinical trials of NGF antagonists for non-malignant pain shortly after the primary publication date in 2010. Subsequently, due to concern about autonomic nervous system toxicity in preclinical models, a complete halt of clinical development was imposed [111]. After years of additional investigation and consultation between industry and the FDA, the hold was lifted in 2015, and clinical testing was permitted to resume, subject to new restrictions: lower treatment doses were mandated, and stringent risk mitigation strategies were imposed. These included strict pre-enrollment radiographic assessment of potential volunteers, to exclude those with pre-existing shoulder, hip, or knee abnormalities such as subchondral insufficiency fracture, atrophic or hypotrophic osteoarthritis, excessive malalignment of the knee, osteonecrosis, severe chondrocalcinosis, rheumatoid arthritis, systemic metabolic bone disease, tumors, fractures, and large cystic lesions [112]. In addition, close radiographic monitoring both during the treatment phase of the trials and for several months after completion was required [112]. Subsequently, in 2017, the FDA granted Fast Track designation to tanezumab for the treatment of chronic pain in patients with osteoarthritis and chronic LBP [113]. “Fast Track” (https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/fast-track) is a formal process to expedite the review of new therapies to treat FDA-defined serious conditions and to fill unmet medical needs.
Efficacy in osteoarthritis and back pain
Monoclonal antibody-mediated NGF inhibition has been consistently demonstrated to provide effective pain palliation for osteoarthritis of the knees or hips. Tanezumab is the best studied of the anti-NGF agents, is the furthest along in development, and is the closest of the agents to regulatory approval. In addition to the trials that were completed prior to the mandated dose reductions, several large randomized trials have recently been presented, and they consistently show a pain advantage with NGF inhibition. Two critical placebo controlled phase 3 trials of lower-dose subcutaneous tanezumab have recently been completed. The results of one have been published [114]; in that trial, subjects received either placebo or tanezumab 2.5 mg subcutaneously at day 1 and at week 8, or 2.5 mg on day 1 and 5.0 mg at week 8, and both pain and function were significantly improved at both doses vs. placebo at the primary outcome of 16 weeks [114]. The second trial is available only in abstract form at present [115]. This was a trial of placebo vs. subcutaneous tanezumab (2.5 mg or 5.0 mg) at baseline, week 8, and week 16. Reportedly, both doses provided significant improvements in pain and function over placebo, however the lower dose did not improve the patient global assessment, and hence did not meet that primary outcome criterion. Of note, the results of a trial comparing subcutaneous tanezumab with NSAID active comparators, rather than with placebo, have been presented in abstract form, but not in publications [116]. In this study, 3021 patients were randomized to receive either 2.5mg or 5mg of tanezumab subcutaneously every 8 weeks for 56 weeks, or an oral NSAID or coxib. According to the abstract, tanezumab was superior to NSAIDs for pain and function (but not patient global assessment) at the 5 mg dose, but was not superior in any of the outcomes at the 2.5 mg dose at the primary endpoint of 16 weeks.
Comparable results have been produced with fasinumab [117]. In a large placebo controlled trial, subjects were treated with placebo or subcutaneous fasinumab (1 mg, 3 mg, 6 mg, or 9 mg) every four weeks for 16 weeks; significant pain relief was observed at all doses, however there was no dose response relationship noted.
Overall, the magnitude of pain relief provided by the NGF inhibitors is clinically important, but may be less dramatic than had been anticipated. In studies of tanezumab monotherapy compared with either NSAIDs or with opiates, tanezumab in doses of 5 mg and 10 mg were statistically significantly superior to the active comparators, but had standardized effect sizes of only 0.22 to 0.24 [118, 119]. It should be noted that these effect sizes are not greater in magnitude than conventional osteoarthritis treatments [12], and are far lower than would be expected for dramatic relief. Moreover, as noted above, the tanezumab 2.5 mg dose was not superior to NSAIDs at 16 weeks.
In contrast to osteoarthritis, there are substantially fewer data of efficacy of the anti-NGF therapies for the treatment of chronic back pain than for osteoarthritis of the knees or hips. An older metaanalysis dating from prior to the lower doses mandated by the FDA found that whereas tanezumab may have some efficacy for pain and function, fulranumab (whose development was discontinued) did not [120]. Interestingly, in a small proof of concept study, fasinumab was ineffective for relieving pain in acute sciatica [121]. More recently, a long term Phase 3 trial of subcutaneous tanezumab 5mg or 10 mg dosed every 8 weeks for 56 weeks vs tramadol (ClinicalTrials.gov Identifier: NCT02528253) was completed, and reported as a press release; the sponsor claimed that the 10 mg dose met the primary endpoint and provided statistically significant pain relief relative to placebo, whereas the 5 mg dose had a trend towards benefit that was not statistically significant [116]. This trial, the so-called TANGO trial, has not yet been published in the peer reviewed literature, thus precluding a careful examination of the results. Similarly, a second Phase 3 trial of tanezumab for chronic low back pain was completed in the Japanese population in June 2019 (ClinicalTrials.gov Identifier: NCT02725411), but the results are not yet available.
Clinical development of the downstream signaling pathways from NGF is substantially less advanced than with the NGF inhibitors. Two early phase 2 trials of novel TrkA inhibitors have recently been published. In one, subjects were treated with a single intraarticular injection of the TrkA inhibitor, GZ389988A (Sanofi-Genzyme), or placebo, and followed for 12 weeks. There appeared to be some pain advantage in the TrkA inhibited group at four weeks, though it did not maintain statistical significance to the 12 weeks endpoint [122]. In the second trial, subjects were treated with oral ASP7962 (Astellas Pharma) 100 mg twice daily for four weeks, and compared to placebo and a NSAID group, however no benefit of the TrkA inhibitor was observed compared to placebo [123]. In addition, a recent report of the pan-Trk inhibitor, ONO-4474 (ONO Pharmaceuticals), administered orally 100 mg twice daily for four weeks suggests the possibility of a pain advantage with this agent in knee osteoarthritis [109].
Risks
Safety concerns led to the US FDA hold on clinical testing in 2010, based on reports of rapidly progressive osteoarthritis (RPOA) and of osteonecrosis among patients who had received anti-NGF therapy, including involvement of joints without known osteoarthritis. An expert adjudication committee funded by Pfizer performed detailed reviews of the adverse events reported during clinical trials with tanezumab and fulranumab. A dose-response relationship was noted between the serious adverse events (RPOA and reported osteonecrosis) and doses of tanezumab between 2.5 and 10 mg [124] or doses of fasinumab between 3 and 9 mg [125, 126]. Of note, the true incidence of osteonecrosis appears to be lower than initially feared. Of the 86 reported osteonecrosis cases prior to the FDA hold, the Pfizer-funded adjudication committee could demonstrate unambiguous osteonecrosis in only two (though eight had insufficient information to distinguish primary osteonecrosis and the committee failed to reach consensus on another five) [127]. Importantly, however, the risk of developing rapidly progressive osteoarthritis appeared to be substantially enhanced when NSAIDs were used in conjunction with tanezumab, compared to tanezumab monotherapy [124, 127]. As NSAIDs are ubiquitously available, this may have long-term implications for the widespread use of anti-NGF therapies.
As noted above, clinical trials resumed in 2015 with reduced doses, maximally 5 mg for tanezumab, and with strict limits on the duration of NSAID use during exposure to anti-NGF therapy. The effects of the newer restrictions have become apparent recently. In the recently published tanezumab trial, whereas there was a significant pain and function advantage relative to placebo, there was also a clearly elevated risk of developing RPOA relative to placebo, and a dose response effect on the risk of progression to endstage osteoarthritis requiring total joint replacement: whereas none of those receiving placebo developed RPOA by week 40, 1.2% of those receiving tanezumab did. More concerning, while 1.7% of placebo subjects progressed to joint replacement, 3.5% of those receiving low dose (2.5mg) tanezumab did so, and 7% receiving high dose (2.5mg followed by 5.0mg) progressed [114]. Almost identical results were reported in a separate abstract for the large active-comparator controlled trial whose results were released in abstract form but not yet published [128]: among the control group receiving only NSAIDs, 1.2% developed RPOA by 80 weeks vs. 3.2% of those receiving tanezumab 2.5mg, and 6.3% of those receiving 5.0mg. Regarding progression to joint replacements, a similar dose response relationship was observed: 2.6% of the NSAID controls vs. 5.3% of those receiving low dose (2.5mg) tanezumab and 8.0% of those receiving higher dose (5.0mg) tanezumab. There is evidence that even at the lower doses, these adverse events are a class effect and are not limited to a specific agent. For example, the recent large fasinumab trial demonstrated a similar dose response effect of RPOA, ranging from 0 in the placebo group to more than 8% in the fasinumab 9mg group[117]; there appeared to be a lower risk of joint replacement in this cohort, however. Thus, notwithstanding the stringent risk mitigation strategies adopted in 2015, the risks of rapid progression to joint replacement as a consequence of treatment with the anti-NGF agents remain. Finally, it is reasonable to expect that the side effect profile would be similar for indications beyond osteoarthritis, and indeed there appeared to be dose-related effects of RPOA in the trials of anti-NGF agents in back pain.
It is noteworthy that despite the risks of NGF-targeted therapy, cost-effectiveness analyses suggest that the pain palliation provided by these agents may be sufficiently significant that even a rate of RPOA occurring in up to 10% of patients would not nullify the overall improvement in quality-adjusted life years (QALY) achieved [129], and that anti-NGF therapy could be cost effective at up to $400 per dose [129].
4.1.2. BRAIN-DERIVED NEURONAL GROWTH FACTOR (BDNF)
BDNF is a neurotrophin that is key for neuronal growth and survival in the CNS [130]. It signals through TrkB, which is widely expressed throughout the brain and the spinal cord [131]. Both BDNF and its receptor have been found to be upregulated in chronic pain states; for example, BDNF has been reported to be upregulated in the CNS [132] as well as in the serum [133] of patients with fibromyalgia. A recent study in 1152 Han Chinese subjects reported a SNP in the gene encoding BDNF (Val66Met) that was associated with higher risk of chronic postoperative pain compared with the carriers of wild type A allele (Met/Met genotype) [134] (Table 2). Animal studies confirmed that BDNFMet/Met mice had less mechanical allodynia compared with BDNFVal/Val mice after plantar incision. A recent study reported that haploinsufficiency of the BDNF gene is associated with reduced pain sensitivity when assessed by quantitative sensory testing (both in humans and rats) [135].
4.1.2.a. BDNF is a key mediator of central sensitization.
BDNF can act at multiple levels of the nervous system (Fig.1): it is constitutively expressed in sensory neurons, and is produced by target tissues during an inflammatory response (for example, it can be produced by synovial fibroblasts in response to ATP [136]. It is transported to the spinal dorsal horn through anterograde axonal transport, and many animal studies suggest it increases spinal hyperexcitability, thus contributing to central sensitization (reviewed in [137]). Interestingly, systemic NGF treatment increased BDNF expression in the cell bodies and central terminals of sensory neurons and increased nociceptive spinal reflex excitability, and this could be reduced with TrkB-IgG, a BDNF-blocker [138]. In healthy mice, intrathecal injection of BDNF induces thermal hyperalgesia, and inhibition of TrkB expression through intrathecal injection of TrkB antisense oligonucleotides blocks BDNF-induced hyperalgesia [139].
Recently, the role of BDNF in sensory neurons was examined using mice in which Bdnf was deleted in adult sensory neurons by using Advillin-CreERT2 x Bdnf loxp mice [140]. These mice were protected from nocifensive responses in the second phase of formalin-induced inflammation. In addition, they recovered from mechanical hypersensitivity in a neuropathic pain model, and they did not develop prolonged mechanical hypersensitivity in a model of hyperalgesic priming. Taken together, these observations support the idea that BDNF produced by sensory neurons is important for the transition from acute to chronic pain.
4.1.2.b. BDNF in osteoarthritis pain: Preclinical evidence.
There is some evidence, mostly from small cohorts, that BDNF levels are associated with pain in osteoarthritis. One study reported that plasma levels (but not synovial fluid levels) of BDNF are correlated with self-reported pain in knee osteoarthritis [141]. A recent study performed transcriptome analysis on the synovium of knee osteoarthritis patients using next generation sequencing, and reported that TrkB mRNA levels were associated with pain [142]. Additionally, BDNF and TrkB mRNA and protein were recently detected in synovia from knee osteoarthritis patients, and expression was positively correlated with the pro-inflammatory chemokine, fractalkine [143].
Despite an extensive body of literature supporting the role of BDNF in pain, our literature search revealed just two interventional studies that have examined its role in mediating pain behaviors associated with experimental models of musculoskeletal pain. Cervical facet joint distraction in rats causes mechanical allodynia in both forepaws as early as one day after injury, and BDNF protein is upregulated in the cervical DRGs and in the dorsal horns of the spinal cord by day 7 after injury [144]. Intrathecal sequestration of BDNF with TrkB-Fc, a chimera consisting of the extracellular domain of TrkB and the Fc portion of IgG1, on day 5 after injury partially inhibited mechanical allodynia and decreased spinal expression of pERK1 and pERK2, suggesting that BDNF plays a role in facet joint pain. A recent study examined the role of BDNF in the periphery in two rat models of osteoarthritis pain [145]. When BDNF was injected intra-articularly into healthy rat knees, it had no effect. By contrast, when it was injected into the knees of rats with experimental osteoarthritis induced with MIA, it worsened weight-bearing deficits and mechanical allodynia in the hind paw. Furthermore, intra-articular injection of TrkB-Fc partially reversed established weight-bearing deficits and mechanical allodynia of the hind paw in rats with MIA-induced osteoarthritis, and even more so when osteoarthritis was induced with MNX surgery [145]. Because of the known link between NGF and BDNF expression, it will be interesting to further investigate the role of BDNF in driving chronic musculoskeletal pain and the interaction between BDNF and NGF in chronic pain, especially in osteoarthritis.
4.1.2.c. Development of drugs that target BDNF-TrkB signaling.
A search of the Clinicaltrials.gov registry (24 December 2019) revealed 34 studies for the search terms “BDNF” and “pain”. However, evaluation of those protocols reveals that none involve BDNF as a specific target. In each study, BDNF levels are followed after various minimally invasive interventions, such as transcranial direct current stimulation, acupuncture, yoga, etc. It appears that BDNF as a direct target for musculoskeletal pain (or any pain) has not yet been translated to human studies. In contrast, its receptor TrkB has been a specific target studied clinically. The pan-Trk inhibitor, Larotrectinib, is approved for anticancer treatments and has been extensively studied for the treatment of a variety of malignancies, but not yet for pain.
4.1.3. NEUROTROPHIN- 3 (NT-3)
Like NGF, NT-3 plays an important role in neuronal development, and NT-3 deletion in mice leads to loss of nociceptors and sympathetics, including spinal proprioceptive afferents, in a manner similar to NGF deletion (reviewed in [146]).
Studies in adult animal models of pain have revealed both pro- and antinociceptive effects of NT-3. Systemic injection of NT-3 in healthy rats induced mechanical hypoalgesia [147]. An antibody against NT-3 delivered by pump into the L5-DRG reversed mechanical allodynia of the hind paw in the chronic phase of neuropathic pain in a nerve injury model [148].
One study has investigated the role of NT-3 in muscle pain by using a model in which acid is injected into the gastrocnemius muscle to induce persistent mechanical hyperalgesia of the hindpaw [149]. Overexpression of NT-3 in the muscle using a genetic approach (myo/NT-3 mice), or intramuscular injection of exogenous NT-3 (but not BDNF or GDNF) at the time of acid injection abrogated development of persistent hyperalgesia. Interestingly, the route of injection of NT-3 was found to be important, since systemic (i.p.) or intrathecal injection of NT-3 was not effective. In addition, intramuscular injection of NT-3 was only effective when delivered before the emergence of hyperalgesia, suggesting an early role of NT-3 in this model.
4.2. GDNF FAMILY OF LIGANDS:
The GDNF family of ligands (GFLs) is a family of proteins that is distantly related to the TGFβ family [150]. It comprises glial cell line-derived neurotrophic factor (GDNF) [151], neurturin [152], persephin [153], and artemin (also known as neublastin and enovin) [154]. These proteins signal through the transmembrane receptor tyrosine kinase (RTK) rearranged during transfection (RET), after forming ternary complexes with a glycosyl phosphatidylinositol (GPI)-anchored co-receptor (GFR-α1, GFR-α2, GFR-α3, or GFR-α4). These co-receptors bind the ligand with high affinity and confer specificity (GDNF/GFR-α1, neurturin/GFR-α2, artemin/GFR-α3, and persephin/GFR-α4), although lower affinity alternative binding is also possible.
GDNF and neurturin are important for neuronal survival in the CNS. GDNF was first discovered in 1993 as a survival factor for midbrain dopaminergic neurons in culture [151]. Both GDNF and neurturin have reached the clinical trial stage for Parkinson’s disease, as a local protein or gene therapy delivered into the putamen [155, 156].
GDNF family members are also widely expressed in both the developing and the adult PNS. GDNF and neurturin stimulate survival of sensory and sympathetic neurons [150]. Artemin has been shown to support the growth of sensory neurons [157]. Subpopulations of sensory neurons express RET along with GFRα1–3. Small diameter unmyelinated neurons that bind the isolectin, IB4 (i.e., non peptidergic C-fibers) express RET, GFRα−1, and GFRα−2 [158] while GFRα3 is predominantly expressed in the TrkA positive subset of DRG neurons, although IB4 positive neurons can also express it [159].
4.2.1. GFLs in pain.
Because of their expression patterns, the role of the GDNF family in pain has been extensively studied, both in inflammatory and neuropathic pain models [160]. Early efforts focused on administering the GFRα3 ligand, artemin, in animals where a nerve injury was produced in order to model neuropathic pain. This approach was based on the trophic actions of artemin on sensory neurons and the restricted expression of GFRα3 on nociceptors. It was reported that intermittent systemic administration of artemin reversed morphological and neurochemical changes in the DRG caused by nerve injury (spinal nerve ligation) in rats, and this was accompanied by normalizing of associated pain-related behaviors (mechanical and thermal hypersensitivity). Interestingly, only a small fraction of DRG neurons in healthy rats express GFRα3, but this percentage increased after spinal nerve ligation, indicating that additional neurons had become responsive to artemin [161]. In addition to biological therapy approaches, there have been efforts to identify small molecule agonists. Specifically, a small molecule RET agonist, BT13, has been shown to promote neurite growth from sensory neurons and attenuate neuropathic pain in a rat spinal nerve ligation model [162].
A recent genome-wide association study suggested an association of chromosome 8p21.3, with new variants near GFRA2 with diabetic neuropathic pain [163] (Table 2). In contrast to the approach in neuropathic pain, where agonists are used, research in experimental models of inflammatory pain has focused on inhibiting GFL signaling in order to produce analgesic effects. A recent study examined the role of local GDNF, neurturin, and artemin in bone pain [164]. Injection of any of these factors directly into the marrow cavity of healthy rats caused a weight-bearing deficit in the ipsilateral limb, concordantly with their sensitizing effect on bone afferents to mechanical stimuli. The authors showed expression of GFRα1 or GFRα2 in approximately 20% of bone afferents, and these were largely non-peptidergic. In addition, they showed that 40% of afferents expressed GFRα3, the co-receptor for artemin. Interestingly, 80% of TrkA-positive sensory neurons innervating the marrow cavity of the tibia also expressed GFRα3. In addition, the authors tested the effect of neutralizing antibodies to either GDNF, neurturin, or artemin in a model of inflammatory pain induced by injecting CFA into the tibial marrow cavity. Antibodies against either neurturin or artemin prevented CFA-induced weight-bearing deficits, but sequestration of GDNF had no effect.
4.2.2. Development of drugs that target GFL signaling.
The clinical utility of targeting GFL signaling for musculoskeletal pain has not yet been demonstrated. Biogen-Idec developed an engineered artemin for clinical use, termed BG00010, and successfully completed Phase 1 trials in sciatica [165], which suggested that the agent was safe although there was no clear signal regarding pain relief. Larger Phase 2 trials subsequently failed to demonstrate convincing pain efficacy or any dose response for sciatica [166, 167], and the program was apparently discontinued since no further trials have been registered.
GFR-α3 remains an active target for clinical development. At present, there is at least one program evaluating a monoclonal antibody against GFR-α3 for pain. Regeneron is currently recruiting for a Phase 2 randomized placebo-controlled trial of their antibody, REGN5069, in osteoarthritis of the knee (https://clinicaltrials.gov/ct2/show/NCT03956550), with completion targeted for the end of 2021. To date, however, there is no literature in the public domain concerning possible efficacy and safety.
5. CONCLUSIONS
Musculoskeletal pain remains highly prevalent and is the source of vast morbidity and societal costs, notwithstanding the dramatic advances made in the areas of pharmacology and therapeutics during the past half century. Moreover, as the risks and societal impact of opiates as well as their inadequate efficacy for chronic non-cancer pain, have become apparent, novel approaches and improved therapies for musculoskeletal pain have become an urgent unmet medical need. Fortunately, recent advances in our understanding of the neurobiology of such pain have yielded multiple promising targets among the neurotrophins and their signaling pathways. It has been appreciated for decades that the neurotrophins mediate pain sensation, however it is only recently that these growth factors have been specifically targeted to relieve chronic pain. Among the neurotrophins, the best studied in humans, and the most advanced in clinical development, is NGF. Multiple monoclonal antibodies targeting NGF have been developed, Phase 3 trials have been successfully completed, and at least one agent is planned for submission to the US FDA for registration. The data are compelling that the NGF antagonists are effective for pain in knee and hip osteoarthritis, yet there are also convincing data to suggest that these agents carry a substantial risk of accelerating disease progression, with an apparent dose-related evolution to arthroplasty. Hence, even if the current anti-NGF antibodies are ultimately approved for clinical use, there will remain a need for additional effective approaches that entail less risk. A variety of other neurotrophin targets are in preclinical and clinical development, with extensive investigation both of small molecule therapeutics and of biologicals. Although several recent trials have failed to meet their pain endpoints, there is room for substantial optimism that the next decade will see dramatic improvement in the therapeutic armamentarium for the musculoskeletal pain conditions by leveraging the neurotrophin targets.
Acknowledgements
Anne-Marie Malfait (R01AR064251, R01AR060364, R61AR073576) and Rachel Miller (K01AR070328) are grateful for the supported by the US National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH/NIAMS). The funding sources had no role in the study.
List of Abbreviations
- BDNF
brain-derived neuronal growth factor
- CFA
complete Freund’s adjuvant
- CGRP
calcitonin gene-related peptide
- CIA
collagen-induced arthritis
- CNS
central nervous system
- DMM
destabilization of the medial meniscus
- DRG
dorsal root ganglion
- FDA
U.S. Food and Drug Administration
- GDNF
Glial cell-derived neurotrophic factor
- GFLs
GDNF family of ligands
- GFR-α
glycosyl phosphatidylinositol (GPI)-anchored co-receptor-α
- LBP
lower back pain
- MIA
monosodium iodoacetate
- MMT
medial meniscal tear
- NGF
nerve growth factor
- NSAID
non-steroidal anti-inflammatory drug
- NT-3
neurotrophin-3
- OA
osteoarthritis
- OVX
ovariectomy
- PNS
peripheral nervous system
- RET
receptor tyrosine kinase (RTK) rearranged during transfection
- QALY
quality-adjusted life years
- rhNGF
recombinant human NGF
- RPOA
rapidly progressive osteoarthritis
- SNP
single nucleotide polymorphism
- TGFβ
transforming growth factor beta
- TRAP
tartrate resistant acid phosphatase
- Trk
tyrosine receptor kinase (also known as tropomyosin receptor kinase)
- TRPV1
transient receptor potential cation channel subfamily V member 1
REFERENCES
- 1.Goldberg DS, McGee SJ: Pain as a global public health priority. BMC Public Health 2011, 11:770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henderson CE: Role of neurotrophic factors in neuronal development. Curr Opin Neurobiol 1996, 6(1):64–70. [DOI] [PubMed] [Google Scholar]
- 3.Malik KM, Beckerly R, Imani F: Musculoskeletal Disorders a Universal Source of Pain and Disability Misunderstood and Mismanaged: A Critical Analysis Based on the U.S. Model of Care. Anesth Pain Med 2018, 8(6):e85532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collaborators G: Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392(10159):1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray CJL, Lopez AD: Measuring the Global Burden of Disease. New England Journal of Medicine 2013, 369(5):448–457. [DOI] [PubMed] [Google Scholar]
- 6.Blyth FM, Briggs AM, Schneider CH, Hoy DG, March LM: The Global Burden of Musculoskeletal Pain—Where to From Here? American Journal of Public Health 2019, 109(1):35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hootman JM, Helmick CG, Barbour KE, Theis KA, Boring MA: Updated Projected Prevalence of Self-Reported Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation Among US Adults, 2015–2040. Arthritis Rheumatol 2016, 68(7):1582–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbour KE, Helmick CG, Boring M, Brady TJ: Vital Signs: Prevalence of Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation - United States, 2013–2015. MMWR Morb Mortal Wkly Rep 2017, 66(9):246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osteoarthritis, A serious disease. White Paper Submitted to the US Food and Drug Administration2016, Pre Competitive Consortium for Osteoarthritis Osteoarthritis Research Society International: https://wwwoarsiorg/sites/default/files/docs/2016/oarsi_white_paper_oa_serious_disease_121416_1pdf 2017.
- 10.Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, Callahan L, Copenhaver C, Dodge C, Felson D et al. : 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, Kraus VB, Lohmander LS, Abbott JH, Bhandari M et al. : OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage 2019, 27(11):1578–1589. [DOI] [PubMed] [Google Scholar]
- 12.Block JA: Osteoarthritis: OA guidelines: improving care or merely codifying practice? Nature Reviews Rheumatology 2014, 10(6):324–326. [DOI] [PubMed] [Google Scholar]
- 13.Ray WA, Chung CP, Murray KT, Hall K, Stein CM: Prescription of Long-Acting Opioids and Mortality in Patients With Chronic Noncancer Pain. Jama 2016, 315(22):2415–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krebs EE, Gravely A, Noorbaloochi S: Opioids vs Nonopioids for Chronic Back, Hip, or Knee Pain-Reply. Jama 2018, 320(5):508–509. [DOI] [PubMed] [Google Scholar]
- 15.Basbaum AI, Bautista DM, Scherrer G, Julius D: Cellular and molecular mechanisms of pain. Cell 2009, 139(2):267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woller SA, Eddinger KA, Corr M, Yaksh TL: An overview of pathways encoding nociception. Clinical and experimental rheumatology 2018, 36(1):172. [PubMed] [Google Scholar]
- 17.Woolf CJ: Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011, 152(3 Suppl):S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suokas AK, Walsh DA, McWilliams DF, Condon L, Moreton B, Wylde V, Arendt-Nielsen L, Zhang W: Quantitative sensory testing in painful osteoarthritis: a systematic review and meta-analysis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society 2012, 20(10):1075–1085. [DOI] [PubMed] [Google Scholar]
- 19.Bedson J, Croft PR: The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC musculoskeletal disorders 2008, 9:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finan PH, Buenaver LF, Bounds SC, Hussain S, Park RJ, Haque UJ, Campbell CM, Haythornthwaite JA, Edwards RR, Smith MT: Quantitative sensory tests of central sensitization are associated with discordance between pain and radiographic severity in knee osteoarthritis. Arthritis and rheumatism 2013, 65(2):363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao MV: Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 2003, 4(4):299–309. [DOI] [PubMed] [Google Scholar]
- 22.Lewin GR, Barde YA: Physiology of the neurotrophins. Annu Rev Neurosci 1996, 19:289–317. [DOI] [PubMed] [Google Scholar]
- 23.Marmigere F, Ernfors P: Specification and connectivity of neuronal subtypes in the sensory lineage. Nat Rev Neurosci 2007, 8(2):114–127. [DOI] [PubMed] [Google Scholar]
- 24.Denk F, Bennett DL, McMahon SB: Nerve Growth Factor and Pain Mechanisms. Annu Rev Neurosci 2017, 40:307–325. [DOI] [PubMed] [Google Scholar]
- 25.Lane NE, Schnitzer TJ, Birbara CA, Mokhtarani M, Shelton DL, Smith MD, Brown MT: Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med 2010, 363(16):1521–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levi-Montalcini R, Hamburger V: Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J Exp Zool 1951, 116(2):321–361. [DOI] [PubMed] [Google Scholar]
- 27.Cohen S, Levi-Montalcini R: Purification and properties of a nerve growth-promoting factor isolated from mouse sarcoma 180. Cancer Res 1957, 17(1):15–20. [PubMed] [Google Scholar]
- 28.Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, Ling LH, McMahon SB, Shelton DL, Levinson AD et al. : Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell 1994, 76(6):1001–1011. [DOI] [PubMed] [Google Scholar]
- 29.Capsoni S: From genes to pain: nerve growth factor and hereditary sensory and autonomic neuropathy type V. Eur J Neurosci 2014, 39(3):392–400. [DOI] [PubMed] [Google Scholar]
- 30.Einarsdottir E, Carlsson A, Minde J, Toolanen G, Svensson O, Solders G, Holmgren G, Holmberg D, Holmberg M: A mutation in the nerve growth factor beta gene (NGFB) causes loss of pain perception. Hum Mol Genet 2004, 13(8):799–805. [DOI] [PubMed] [Google Scholar]
- 31.Minde J, Andersson T, Fulford M, Aguirre M, Nennesmo I, Remahl IN, Svensson O, Holmberg M, Toolanen G, Solders G: A novel NGFB point mutation: a phenotype study of heterozygous patients. J Neurol Neurosurg Psychiatry 2009, 80(2):188–195. [DOI] [PubMed] [Google Scholar]
- 32.Testa G, Mainardi M, Morelli C, Olimpico F, Pancrazi L, Petrella C, Severini C, Florio R, Malerba F, Stefanov A et al. : The NGF(R100W) Mutation Specifically Impairs Nociception without Affecting Cognitive Performance in a Mouse Model of Hereditary Sensory and Autonomic Neuropathy Type V. J Neurosci 2019, 39(49):9702–9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Indo Y, Tsuruta M, Hayashida Y, Karim MA, Ohta K, Kawano T, Mitsubuchi H, Tonoki H, Awaya Y, Matsuda I: Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat Genet 1996, 13(4):485–488. [DOI] [PubMed] [Google Scholar]
- 34.Geng X, Liu Y, Ren X, Guan Y, Wang Y, Mao B, Zhao X, Zhang X: Novel NTRK1 mutations in Chinese patients with congenital insensitivity to pain with anhidrosis. Mol Pain 2018, 14:1744806918781140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mamie C, Rebsamen MC, Morris MA, Morabia A: First evidence of a polygenic susceptibility to pain in a pediatric cohort. Anesth Analg 2013, 116(1):170–177. [DOI] [PubMed] [Google Scholar]
- 36.Ligthart L, de Vries B, Smith AV, Ikram MA, Amin N, Hottenga JJ, Koelewijn SC, Kattenberg VM, de Moor MH, Janssens AC et al. : Meta-analysis of genome-wide association for migraine in six population-based European cohorts. Eur J Hum Genet 2011, 19(8):901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hefti F, Hartikka J, Knusel B: Function of neurotrophic factors in the adult and aging brain and their possible use in the treatment of neurodegenerative diseases. Neurobiol Aging 1989, 10(5):515–533. [DOI] [PubMed] [Google Scholar]
- 38.Apfel SC, Lipton RB, Arezzo JC, Kessler JA: Nerve growth factor prevents toxic neuropathy in mice. Ann Neurol 1991, 29(1):87–90. [DOI] [PubMed] [Google Scholar]
- 39.Petty BG, Cornblath DR, Adornato BT, Chaudhry V, Flexner C, Wachsman M, Sinicropi D, Burton LE, Peroutka SJ: The effect of systemically administered recombinant human nerve growth factor in healthy human subjects. Ann Neurol 1994, 36(2):244–246. [DOI] [PubMed] [Google Scholar]
- 40.Dyck PJ, Peroutka S, Rask C, Burton E, Baker MK, Lehman KA, Gillen DA, Hokanson JL, O’Brien PC: Intradermal recombinant human nerve growth factor induces pressure allodynia and lowered heat-pain threshold in humans. Neurology 1997, 48(2):501–505. [DOI] [PubMed] [Google Scholar]
- 41.Apfel SC: Nerve growth factor for the treatment of diabetic neuropathy: what went wrong, what went right, and what does the future hold? Int Rev Neurobiol 2002, 50:393–413. [DOI] [PubMed] [Google Scholar]
- 42.Lewin GR, Ritter AM, Mendell LM: Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J Neurosci 1993, 13(5):2136–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewin GR, Rueff A, Mendell LM: Peripheral and central mechanisms of NGF-induced hyperalgesia. Eur J Neurosci 1994, 6(12):1903–1912. [DOI] [PubMed] [Google Scholar]
- 44.Amann R, Schuligoi R, Herzeg G, Donnerer J: Intraplantar injection of nerve growth factor into the rat hind paw: local edema and effects on thermal nociceptive threshold. Pain 1996, 64(2):323–329. [DOI] [PubMed] [Google Scholar]
- 45.Mills CD, Nguyen T, Tanga FY, Zhong C, Gauvin DM, Mikusa J, Gomez EJ, Salyers AK, Bannon AW: Characterization of nerve growth factor-induced mechanical and thermal hypersensitivity in rats. Eur J Pain 2013, 17(4):469–479. [DOI] [PubMed] [Google Scholar]
- 46.Hoheisel U, Reuter R, de Freitas MF, Treede RD, Mense S: Injection of nerve growth factor into a low back muscle induces long-lasting latent hypersensitivity in rat dorsal horn neurons. Pain 2013, 154(10):1953–1960. [DOI] [PubMed] [Google Scholar]
- 47.Ashraf S, Mapp PI, Burston J, Bennett AJ, Chapman V, Walsh DA: Augmented pain behavioural responses to intra-articular injection of nerve growth factor in two animal models of osteoarthritis. Ann Rheum Dis 2014, 73(9):1710–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haywood AR, Hathway GJ, Chapman V: Differential contributions of peripheral and central mechanisms to pain in a rodent model of osteoarthritis. Sci Rep 2018, 8(1):7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sagar DR, Nwosu L, Walsh DA, Chapman V: Dissecting the contribution of knee joint NGF to spinal nociceptive sensitization in a model of OA pain in the rat. Osteoarthritis Cartilage 2015, 23(6):906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kras JV, Weisshaar CL, Pall PS, Winkelstein BA: Pain from intra-articular NGF or joint injury in the rat requires contributions from peptidergic joint afferents. Neurosci Lett 2015, 604:193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Omae T, Nakamura J, Ohtori S, Orita S, Yamauchi K, Miyamoto S, Hagiwara S, Kishida S, Takahashi K: A novel rat model of hip pain by intra-articular injection of nerve growth factor-characteristics of sensory innervation and inflammatory arthritis. Mod Rheumatol 2015, 25(6):931–936. [DOI] [PubMed] [Google Scholar]
- 52.Chartier SR, Mitchell SA, Majuta LA, Mantyh PW: Immunohistochemical localization of nerve growth factor, tropomyosin receptor kinase A, and p75 in the bone and articular cartilage of the mouse femur. Mol Pain 2017, 13:1744806917745465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fjell J, Cummins TR, Davis BM, Albers KM, Fried K, Waxman SG, Black JA: Sodium channel expression in NGF-overexpressing transgenic mice. J Neurosci Res 1999, 57(1):39–47. [DOI] [PubMed] [Google Scholar]
- 54.Jimenez-Andrade JM, Ghilardi JR, Castaneda-Corral G, Kuskowski MA, Mantyh PW: Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain 2011, 152(11):2564–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghilardi JR, Freeman KT, Jimenez-Andrade JM, Coughlin KA, Kaczmarska MJ, Castaneda-Corral G, Bloom AP, Kuskowski MA, Mantyh PW: Neuroplasticity of sensory and sympathetic nerve fibers in a mouse model of a painful arthritic joint. Arthritis Rheum 2012, 64(7):2223–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bergmann I, Reiter R, Toyka KV, Koltzenburg M: Nerve growth factor evokes hyperalgesia in mice lacking the low-affinity neurotrophin receptor p75. Neurosci Lett 1998, 255(2):87–90. [DOI] [PubMed] [Google Scholar]
- 57.Khodorova A, Nicol GD, Strichartz G: The p75NTR signaling cascade mediates mechanical hyperalgesia induced by nerve growth factor injected into the rat hind paw. Neuroscience 2013, 254:312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khodorova A, Nicol GD, Strichartz G: The TrkA receptor mediates experimental thermal hyperalgesia produced by nerve growth factor: Modulation by the p75 neurotrophin receptor. Neuroscience 2017, 340:384–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sung K, Ferrari LF, Yang W, Chung C, Zhao X, Gu Y, Lin S, Zhang K, Cui B, Pearn ML et al. : Swedish Nerve Growth Factor Mutation (NGF(R100W)) Defines a Role for TrkA and p75(NTR) in Nociception. J Neurosci 2018, 38(14):3394–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmelz M, Mantyh P, Malfait AM, Farrar J, Yaksh T, Tive L, Viktrup L: Nerve growth factor antibody for the treatment of osteoarthritis pain and chronic low-back pain: mechanism of action in the context of efficacy and safety. Pain 2019, 160(10):2210–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woolf CJ, Safieh-Garabedian B, Ma QP, Crilly P, Winter J: Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience 1994, 62(2):327–331. [DOI] [PubMed] [Google Scholar]
- 62.Angeby Moller K, Berge OG, Finn A, Stenfors C, Svensson CI: Using gait analysis to assess weight bearing in rats with Freunds complete adjuvant-induced monoarthritis to improve predictivity: Interfering with the cyclooxygenase and nerve growth factor pathways. Eur J Pharmacol 2015, 756:75–84. [DOI] [PubMed] [Google Scholar]
- 63.Koltzenburg M, Bennett DL, Shelton DL, McMahon SB: Neutralization of endogenous NGF prevents the sensitization of nociceptors supplying inflamed skin. Eur J Neurosci 1999, 11(5):1698–1704. [DOI] [PubMed] [Google Scholar]
- 64.McMahon SB, Bennett DL, Priestley JV, Shelton DL: The biological effects of endogenous nerve growth factor on adult sensory neurons revealed by a trkA-IgG fusion molecule. Nat Med 1995, 1(8):774–780. [DOI] [PubMed] [Google Scholar]
- 65.Ashraf S, Bouhana KS, Pheneger J, Andrews SW, Walsh DA: Selective inhibition of tropomyosin-receptor-kinase A (TrkA) reduces pain and joint damage in two rat models of inflammatory arthritis. Arthritis Res Ther 2016, 18(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kras JV, Kartha S, Winkelstein BA: Intra-articular nerve growth factor regulates development, but not maintenance, of injury-induced facet joint pain & spinal neuronal hypersensitivity. Osteoarthritis Cartilage 2015, 23(11):1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chudler EH, Anderson LC, Byers MR: Nerve growth factor depletion by autoimmunization produces thermal hypoalgesia in adult rats. Brain Res 1997, 765(2):327–330. [DOI] [PubMed] [Google Scholar]
- 68.Rohn TA, Ralvenius WT, Paul J, Borter P, Hernandez M, Witschi R, Grest P, Zeilhofer HU, Bachmann MF, Jennings GT: A virus-like particle-based anti-nerve growth factor vaccine reduces inflammatory hyperalgesia: potential long-term therapy for chronic pain. J Immunol 2011, 186(3):1769–1780. [DOI] [PubMed] [Google Scholar]
- 69.Nocchi L, Portulano C, Franciosa F, Doleschall B, Panea M, Roy N, Maffei M, Gargano A, Perlas E, Heppenstall PA: Nerve growth factor-mediated photoablation of nociceptors reduces pain behavior in mice. Pain 2019, 160(10):2305–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mantyh PW: Mechanisms that drive bone pain across the lifespan. Br J Clin Pharmacol 2019, 85(6):1103–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Castaneda-Corral G, Jimenez-Andrade JM, Bloom AP, Taylor RN, Mantyh WG, Kaczmarska MJ, Ghilardi JR, Mantyh PW: The majority of myelinated and unmyelinated sensory nerve fibers that innervate bone express the tropomyosin receptor kinase A. Neuroscience 2011, 178:196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suzuki M, Millecamps M, Ohtori S, Mori C, Stone LS: Anti-nerve growth factor therapy attenuates cutaneous hypersensitivity and musculoskeletal discomfort in mice with osteoporosis. Pain Rep 2018, 3(3):e652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koewler NJ, Freeman KT, Buus RJ, Herrera MB, Jimenez-Andrade JM, Ghilardi JR, Peters CM, Sullivan LJ, Kuskowski MA, Lewis JL et al. : Effects of a monoclonal antibody raised against nerve growth factor on skeletal pain and bone healing after fracture of the C57BL/6J mouse femur. J Bone Miner Res 2007, 22(11):1732–1742. [DOI] [PubMed] [Google Scholar]
- 74.Rapp AE, Kroner J, Baur S, Schmid F, Walmsley A, Mottl H, Ignatius A: Analgesia via blockade of NGF/TrkA signaling does not influence fracture healing in mice. J Orthop Res 2015, 33(8):1235–1241. [DOI] [PubMed] [Google Scholar]
- 75.Sabsovich I, Wei T, Guo TZ, Zhao R, Shi X, Li X, Yeomans DC, Klyukinov M, Kingery WS, Clark JD: Effect of anti-NGF antibodies in a rat tibia fracture model of complex regional pain syndrome type I. Pain 2008, 138(1):47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jimenez-Andrade JM, Martin CD, Koewler NJ, Freeman KT, Sullivan LJ, Halvorson KG, Barthold CM, Peters CM, Buus RJ, Ghilardi JR et al. : Nerve growth factor sequestering therapy attenuates non-malignant skeletal pain following fracture. Pain 2007, 133(1–3):183–196. [DOI] [PubMed] [Google Scholar]
- 77.Majuta LA, Mitchell SAT, Kuskowski MA, Mantyh PW: Anti-nerve growth factor does not change physical activity in normal young or aging mice but does increase activity in mice with skeletal pain. Pain 2018, 159(11):2285–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Majuta LA, Guedon JG, Mitchell SA, Ossipov MH, Mantyh PW: Anti-nerve growth factor therapy increases spontaneous day/night activity in mice with orthopedic surgery-induced pain. Pain 2017, 158(4):605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun S, Diggins NH, Gunderson ZJ, Fehrenbacher JC, White FA, Kacena MA: No pain, no gain? The effects of pain-promoting neuropeptides and neurotrophins on fracture healing. Bone 2020, 131:115109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tomlinson RE, Li Z, Zhang Q, Goh BC, Li Z, Thorek DLJ, Rajbhandari L, Brushart TM, Minichiello L, Zhou F et al. : NGF-TrkA Signaling by Sensory Nerves Coordinates the Vascularization and Ossification of Developing Endochondral Bone. Cell Rep 2016, 16(10):2723–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tomlinson RE, Li Z, Li Z, Minichiello L, Riddle RC, Venkatesan A, Clemens TL: NGF-TrkA signaling in sensory nerves is required for skeletal adaptation to mechanical loads in mice. Proc Natl Acad Sci U S A 2017, 114(18):E3632–E3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Z, Meyers CA, Chang L, Lee S, Li Z, Tomlinson R, Hoke A, Clemens TL, James AW: Fracture repair requires TrkA signaling by skeletal sensory nerves. J Clin Invest 2019, 129(12):5137–5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McNamee KE, Burleigh A, Gompels LL, Feldmann M, Allen SJ, Williams RO, Dawbarn D, Vincent TL, Inglis JJ: Treatment of murine osteoarthritis with TrkAd5 reveals a pivotal role for nerve growth factor in non-inflammatory joint pain. Pain 2010, 149(2):386–392. [DOI] [PubMed] [Google Scholar]
- 84.Driscoll C, Chanalaris A, Knights C, Ismail H, Sacitharan PK, Gentry C, Bevan S, Vincent TL: Nociceptive Sensitizers Are Regulated in Damaged Joint Tissues, Including Articular Cartilage, When Osteoarthritic Mice Display Pain Behavior. Arthritis Rheumatol 2016, 68(4):857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miller RE, Tran PB, Ishihara S, Syx D, Ren D, Miller RJ, Valdes AM, Malfait AM: Microarray Analyses of the Dorsal Root Ganglia Support a Role for Innate Neuro-Immune Pathways in Persistent Pain in Experimental Osteoarthritis. Osteoarthritis Cartilage 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Orita S, Ishikawa T, Miyagi M, Ochiai N, Inoue G, Eguchi Y, Kamoda H, Arai G, Toyone T, Aoki Y et al. : Pain-related sensory innervation in monoiodoacetate-induced osteoarthritis in rat knees that gradually develops neuronal injury in addition to inflammatory pain. BMC Musculoskelet Disord 2011, 12:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aso K, Shahtaheri SM, Hill R, Wilson D, McWilliams DF, Walsh DA: Associations of Symptomatic Knee Osteoarthritis With Histopathologic Features in Subchondral Bone. Arthritis Rheumatol 2019, 71(6):916–924. [DOI] [PubMed] [Google Scholar]
- 88.Walsh DA, McWilliams DF, Turley MJ, Dixon MR, Franses RE, Mapp PI, Wilson D: Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology (Oxford, England) 2010, 49(10):1852–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stoppiello LA, Mapp PI, Wilson D, Hill R, Scammell BE, Walsh DA: Structural associations of symptomatic knee osteoarthritis. Arthritis Rheumatol 2014, 66(11):3018–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Montagnoli C, Tiribuzi R, Crispoltoni L, Pistilli A, Stabile AM, Manfreda F, Placella G, Rende M, Cerulli GG: beta-NGF and beta-NGF receptor upregulation in blood and synovial fluid in osteoarthritis. Biol Chem 2017, 398(9):1045–1054. [DOI] [PubMed] [Google Scholar]
- 91.Nees TA, Rosshirt N, Zhang JA, Reiner T, Sorbi R, Tripel E, Walker T, Schiltenwolf M, Hagmann S, Moradi B: Synovial Cytokines Significantly Correlate with Osteoarthritis-Related Knee Pain and Disability: Inflammatory Mediators of Potential Clinical Relevance. J Clin Med 2019, 8(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ishikawa G, Koya Y, Tanaka H, Nagakura Y: Long-term analgesic effect of a single dose of anti-NGF antibody on pain during motion without notable suppression of joint edema and lesion in a rat model of osteoarthritis. Osteoarthritis Cartilage 2015, 23(6):925–932. [DOI] [PubMed] [Google Scholar]
- 93.Miyagi M, Ishikawa T, Kamoda H, Suzuki M, Inoue G, Sakuma Y, Oikawa Y, Orita S, Uchida K, Takahashi K et al. : Efficacy of nerve growth factor antibody in a knee osteoarthritis pain model in mice. BMC Musculoskelet Disord 2017, 18(1):428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bryden LA, Nicholson JR, Doods H, Pekcec A: Deficits in spontaneous burrowing behavior in the rat bilateral monosodium iodoacetate model of osteoarthritis: an objective measure of pain-related behavior and analgesic efficacy. Osteoarthritis Cartilage 2015, 23(9):1605–1612. [DOI] [PubMed] [Google Scholar]
- 95.Ter Heegde F, Luiz AP, Santana-Varela S, Chessell IP, Welsh F, Wood JN, Chenu C: Noninvasive Mechanical Joint Loading as an Alternative Model for Osteoarthritic Pain. Arthritis Rheumatol 2019, 71(7):1078–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu L, Nwosu LN, Burston JJ, Millns PJ, Sagar DR, Mapp PI, Meesawatsom P, Li L, Bennett AJ, Walsh DA et al. : The anti-NGF antibody muMab 911 both prevents and reverses pain behaviour and subchondral osteoclast numbers in a rat model of osteoarthritis pain. Osteoarthritis Cartilage 2016, 24(9):1587–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nwosu LN, Mapp PI, Chapman V, Walsh DA: Blocking the tropomyosin receptor kinase A (TrkA) receptor inhibits pain behaviour in two rat models of osteoarthritis. Ann Rheum Dis 2016, 75(6):1246–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.von Loga IS, El-Turabi A, Jostins L, Miotla-Zarebska J, Mackay-Alderson J, Zeltins A, Parisi I, Bachmann MF, Vincent TL: Active immunisation targeting nerve growth factor attenuates chronic pain behaviour in murine osteoarthritis. Ann Rheum Dis 2019, 78(5):672–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.LaBranche TP, Bendele AM, Omura BC, Gropp KE, Hurst SI, Bagi CM, Cummings TR, Grantham LE 2nd, Shelton DL, Zorbas MA: Nerve growth factor inhibition with tanezumab influences weight-bearing and subsequent cartilage damage in the rat medial meniscal tear model. Ann Rheum Dis 2017, 76(1):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sousa-Valente J, Calvo L, Vacca V, Simeoli R, Arevalo JC, Malcangio M: Role of TrkA signalling and mast cells in the initiation of osteoarthritis pain in the monoiodoacetate model. Osteoarthritis Cartilage 2018, 26(1):84–94. [DOI] [PubMed] [Google Scholar]
- 101.Isola M, Ferrari V, Miolo A, Stabile F, Bernardini D, Carnier P, Busetto R: Nerve growth factor concentrations in the synovial fluid from healthy dogs and dogs with secondary osteoarthritis. Vet Comp Orthop Traumatol 2011, 24(4):279–284. [DOI] [PubMed] [Google Scholar]
- 102.Gearing DP, Virtue ER, Gearing RP, Drew AC: A fully caninised anti-NGF monoclonal antibody for pain relief in dogs. BMC Vet Res 2013, 9:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Webster RP, Anderson GI, Gearing DP: Canine Brief Pain Inventory scores for dogs with osteoarthritis before and after administration of a monoclonal antibody against nerve growth factor. Am J Vet Res 2014, 75(6):532–535. [DOI] [PubMed] [Google Scholar]
- 104.Lascelles BD, Knazovicky D, Case B, Freire M, Innes JF, Drew AC, Gearing DP: A canine-specific anti-nerve growth factor antibody alleviates pain and improves mobility and function in dogs with degenerative joint disease-associated pain. BMC Vet Res 2015, 11:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gearing DP, Huebner M, Virtue ER, Knight K, Hansen P, Lascelles BD, Gearing RP, Drew AC: In Vitro and In Vivo Characterization of a Fully Felinized Therapeutic Anti-Nerve Growth Factor Monoclonal Antibody for the Treatment of Pain in Cats. J Vet Intern Med 2016, 30(4):1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gruen ME, Thomson AE, Griffith EH, Paradise H, Gearing DP, Lascelles BD: A Feline-Specific Anti-Nerve Growth Factor Antibody Improves Mobility in Cats with Degenerative Joint Disease-Associated Pain: A Pilot Proof of Concept Study. J Vet Intern Med 2016, 30(4):1138–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gropp KE, Carlson CS, Evans MG, Bagi CM, Reagan WJ, Hurst SI, Shelton DL, Zorbas MA: Effects of Monoclonal Antibodies against Nerve Growth Factor on Healthy Bone and Joint Tissues in Mice, Rats, and Monkeys: Histopathologic, Biomarker, and Microcomputed Tomographic Assessments. Toxicol Pathol 2018, 46(4):408–420. [DOI] [PubMed] [Google Scholar]
- 108.Bannwarth B, Kostine M: Nerve Growth Factor Antagonists: Is the Future of Monoclonal Antibodies Becoming Clearer? Drugs 2017, 77(13):1377–1387. [DOI] [PubMed] [Google Scholar]
- 109.Ishiguro N, Oyama S, Higashi R, Yanagida K: Efficacy, Safety, and Tolerability of ONO-4474, an Orally Available Pan-Tropomyosin Receptor Kinase Inhibitor, in Japanese Patients With Moderate to Severe Osteoarthritis of the Knee: A Randomized, Placebo-Controlled, Double-Blind, Parallel-Group Comparative Study. Journal of clinical pharmacology 2020, 60(1):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Katz N, Borenstein DG, Birbara C, Bramson C, Nemeth MA, Smith MD, Brown MT: Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain 2011, 152(10):2248–2258. [DOI] [PubMed] [Google Scholar]
- 111.Mullard A: Drug developers reboot anti-NGF pain programmes. Nat Rev Drug Discov 2015, 14(5):297–298. [DOI] [PubMed] [Google Scholar]
- 112.Roemer FW, Miller CG, West CR, Brown MT, Sherlock SP, Kompel AJ, Diaz L, Galante N, Crema MD, Guermazi A: Development of an imaging mitigation strategy for patient enrolment in the tanezumab nerve growth factor inhibitor (NGF-ab) program with a focus on eligibility assessment. Semin Arthritis Rheum 2017, In press. [DOI] [PubMed] [Google Scholar]
- 113.Miller RE, Block JA, Malfait AM: What is new in pain modification in osteoarthritis? Rheumatology (Oxford) 2018, 57(suppl_4):iv99–iv107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schnitzer TJ, Easton R, Pang S, Levinson DJ, Pixton G, Viktrup L, Davignon I, Brown MT, West CR, Verburg KM: Effect of Tanezumab on Joint Pain, Physical Function, and Patient Global Assessment of Osteoarthritis Among Patients With Osteoarthritis of the Hip or Knee: A Randomized Clinical Trial. Jama 2019, 322(1):37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Berenbaum F, Blanco FJ, Guermazi A, Vignon E, Miki K, Yamabe T, Viktrup L, Junor R, Carey W, Brown M et al. : LB0007 SUBCUTANEOUS TANEZUMAB FOR OSTEOARTHRITIS PAIN: A 24-WEEK PHASE 3 STUDY WITH A 24-WEEK FOLLOW UP. Annals of the Rheumatic Diseases 2019, 78(Suppl 2):262–264. [Google Scholar]
- 116.Hochberg M, Carrino J, Schnitzer T, Guermazi A, Vignon E, Walsh D, White A, Nakajo S, Fountaine R, Hickman A et al. : Subcutaneous Tanezumab vs NSAID for the Treatment of Osteoarthritis: Efficacy and General Safety Results from a Randomized, Double-Blind, Active-Controlled, 80-Week, Phase-3 Study [abstract]. Arthritis Rheum 2019, 71(suppl 10):1302. [Google Scholar]
- 117.Dakin P, DiMartino SJ, Gao H, Maloney J, Kivitz AJ, Schnitzer TJ, Stahl N, Yancopoulos GD, Geba GP: The Efficacy, Tolerability, and Joint Safety of Fasinumab in Osteoarthritis Pain: A Phase IIb/III Double-Blind, Placebo-Controlled, Randomized Clinical Trial. Arthritis Rheumatol 2019, 71(11):1824–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schnitzer TJ, Ekman EF, Spierings EL, Greenberg HS, Smith MD, Brown MT, West CR, Verburg KM: Efficacy and safety of tanezumab monotherapy or combined with non-steroidal anti-inflammatory drugs in the treatment of knee or hip osteoarthritis pain. Ann Rheum Dis 2015, 74(6):1202–1211. [DOI] [PubMed] [Google Scholar]
- 119.Schnitzer TJ, Marks JA: A systematic review of the efficacy and general safety of antibodies to NGF in the treatment of OA of the hip or knee. Osteoarthritis Cartilage 2015, 23 Suppl 1:S8–17. [DOI] [PubMed] [Google Scholar]
- 120.Leite VF, Buehler AM, El Abd O, Benyamin RM, Pimentel DC, Chen J, Hsing WT, Mazloomdoost D, Amadera JED: Anti-nerve growth factor in the treatment of low back pain and radiculopathy: a systematic review and a meta-analysis. Pain physician 2014, 17(1):E45–60. [PubMed] [Google Scholar]
- 121.Tiseo PJ, Ren H, Mellis S: Fasinumab (REGN475), an antinerve growth factor monoclonal antibody, for the treatment of acute sciatic pain: results of a proof-of-concept study. Journal of pain research 2014, 7:523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Krupka E, Jiang GL, Jan C: Efficacy and safety of intra-articular injection of tropomyosin receptor kinase A inhibitor in painful knee osteoarthritis: a randomized, double-blind and placebo-controlled study. Osteoarthritis and Cartilage 2019, 27(11):1599–1607. [DOI] [PubMed] [Google Scholar]
- 123.Watt FE, Blauwet MB, Fakhoury A, Jacobs H, Smulders R, Lane NE: Tropomyosin-related kinase A (TrkA) inhibition for the treatment of painful knee osteoarthritis: results from a randomized controlled phase 2a trial. Osteoarthritis and Cartilage 2019, 27(11):1590–1598. [DOI] [PubMed] [Google Scholar]
- 124.Hochberg MC: Serious joint-related adverse events in randomized controlled trials of anti-nerve growth factor monoclonal antibodies. Osteoarthritis Cartilage 2015, 23 Suppl 1:S18–21. [DOI] [PubMed] [Google Scholar]
- 125.Lane NE, Corr M: Osteoarthritis in 2016: Anti-NGF treatments for pain - two steps forward, one step back? Nat Rev Rheumatol 2017, 13(2):76–78. [DOI] [PubMed] [Google Scholar]
- 126.Maloney J, Kivitz A, Schnitzer TJ, Dakin P, Stehman-Breen C, Geba G: Efficacy and Safety of Fasinumab for Osteoarthritic Pain in Patients with Moderate to Severe Osteoarthritis of the Knees or Hips [abstract]. Arthritis Rheumatol 2016, 68(suppl 10). [Google Scholar]
- 127.Hochberg MC, Tive LA, Abramson SB, Vignon E, Verburg KM, West CR, Smith MD, Hungerford DS: When Is Osteonecrosis Not Osteonecrosis?: Adjudication of Reported Serious Adverse Joint Events in the Tanezumab Clinical Development Program. Arthritis Rheumatol 2016, 68(2):382–391. [DOI] [PubMed] [Google Scholar]
- 128.Hochberg M, Carrino J, Schnitzer T, Guermazi A, Walsh D, White A, Nakajo S, Fountaine R, Hickman A, Pixton G et al. : Subcutaneous Tanezumab versus NSAID for the Treatment of Osteoarthritis: Joint Safety Events in a Randomized, Double-Blind, Active-Controlled, 80-Week, Phase-3 Study [abstract]. Arthritis Rheum 2019, 71(suppl 10):2756. [Google Scholar]
- 129.Losina E, Michl G, Collins JE, Hunter DJ, Jordan JM, Yelin E, Paltiel AD, Katz JN: Model-based evaluation of cost-effectiveness of nerve growth factor inhibitors in knee osteoarthritis: impact of drug cost, toxicity, and means of administration. Osteoarthritis Cartilage 2016, 24(5):776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schwartz PM, Borghesani PR, Levy RL, Pomeroy SL, Segal RA: Abnormal cerebellar development and foliation in BDNF−/− mice reveals a role for neurotrophins in CNS patterning. Neuron 1997, 19(2):269–281. [DOI] [PubMed] [Google Scholar]
- 131.Yan Q, Radeke MJ, Matheson CR, Talvenheimo J, Welcher AA, Feinstein SC: Immunocytochemical localization of TrkB in the central nervous system of the adult rat. J Comp Neurol 1997, 378(1):135–157. [PubMed] [Google Scholar]
- 132.Sarchielli P, Mancini ML, Floridi A, Coppola F, Rossi C, Nardi K, Acciarresi M, Pini LA, Calabresi P: Increased levels of neurotrophins are not specific for chronic migraine: evidence from primary fibromyalgia syndrome. J Pain 2007, 8(9):737–745. [DOI] [PubMed] [Google Scholar]
- 133.Laske C, Stransky E, Eschweiler GW, Klein R, Wittorf A, Leyhe T, Richartz E, Kohler N, Bartels M, Buchkremer G et al. : Increased BDNF serum concentration in fibromyalgia with or without depression or antidepressants. J Psychiatr Res 2007, 41(7):600–605. [DOI] [PubMed] [Google Scholar]
- 134.Tian Y, Liu X, Jia M, Yu H, Lichtner P, Shi Y, Meng Z, Kou S, Ho IHT, Jia B et al. : Targeted Genotyping Identifies Susceptibility Locus in Brain-derived Neurotrophic Factor Gene for Chronic Postsurgical Pain. Anesthesiology 2018, 128(3):587–597. [DOI] [PubMed] [Google Scholar]
- 135.Sapio MR, Iadarola MJ, LaPaglia DM, Lehky T, Thurm AE, Danley KM, Fuhr SR, Lee MD, Huey AE, Sharp SJ et al. : Haploinsufficiency of the brain-derived neurotrophic factor gene is associated with reduced pain sensitivity. Pain 2019, 160(5):1070–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Klein K, Aeschlimann A, Jordan S, Gay R, Gay S, Sprott H: ATP induced brain-derived neurotrophic factor expression and release from osteoarthritis synovial fibroblasts is mediated by purinergic receptor P2X4. PLoS One 2012, 7(5):e36693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nijs J, Meeus M, Versijpt J, Moens M, Bos I, Knaepen K, Meeusen R: Brain-derived neurotrophic factor as a driving force behind neuroplasticity in neuropathic and central sensitization pain: a new therapeutic target? Expert Opin Ther Targets 2015, 19(4):565–576. [DOI] [PubMed] [Google Scholar]
- 138.Kerr BJ, Bradbury EJ, Bennett DL, Trivedi PM, Dassan P, French J, Shelton DB, McMahon SB, Thompson SW: Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. J Neurosci 1999, 19(12):5138–5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Groth R, Aanonsen L: Spinal brain-derived neurotrophic factor (BDNF) produces hyperalgesia in normal mice while antisense directed against either BDNF or trkB, prevent inflammation-induced hyperalgesia. Pain 2002, 100(1–2):171–181. [DOI] [PubMed] [Google Scholar]
- 140.Sikandar S, Minett MS, Millet Q, Santana-Varela S, Lau J, Wood JN, Zhao J: Brain-derived neurotrophic factor derived from sensory neurons plays a critical role in chronic pain. Brain 2018, 141(4):1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Simao AP, Mendonca VA, de Oliveira Almeida TM, Santos SA, Gomes WF, Coimbra CC, Lacerda AC: Involvement of BDNF in knee osteoarthritis: the relationship with inflammation and clinical parameters. Rheumatol Int 2014, 34(8):1153–1157. [DOI] [PubMed] [Google Scholar]
- 142.Bratus-Neuenschwander A, Castro-Giner F, Frank-Bertoncelj M, Aluri S, Fucentese SF, Schlapbach R, Sprott H: Pain-Associated Transcriptome Changes in Synovium of Knee Osteoarthritis Patients. Genes (Basel) 2018, 9(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gowler PRW, Li L, Woodhams SG, Bennett AJ, Suzuki R, Walsh DA, Chapman V: Peripheral brain-derived neurotrophic factor contributes to chronic osteoarthritis joint pain. Pain 2020, 161(1):61–73. [DOI] [PubMed] [Google Scholar]
- 144.Kras JV, Weisshaar CL, Quindlen J, Winkelstein BA: Brain-derived neurotrophic factor is upregulated in the cervical dorsal root ganglia and spinal cord and contributes to the maintenance of pain from facet joint injury in the rat. J Neurosci Res 2013, 91(10):1312–1321. [DOI] [PubMed] [Google Scholar]
- 145.Gowler PRW, Li L, Woodhams SG, Bennett AJ, Suzuki R, Walsh DA, Chapman V: Peripheral brain-derived neurotrophic factor contributes to chronic osteoarthritis joint pain. Pain 2019. [DOI] [PubMed] [Google Scholar]
- 146.Wood JN, Sikandar S, Sommer C: Neurotrophins, Cytokines, and Pain. In.: Oxford University Press; 2019. [Google Scholar]
- 147.Malcangio M, Garrett NE, Cruwys S, Tomlinson DR: Nerve growth factor- and neurotrophin-3-induced changes in nociceptive threshold and the release of substance P from the rat isolated spinal cord. J Neurosci 1997, 17(21):8459–8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhou XF, Deng YS, Xian CJ, Zhong JH: Neurotrophins from dorsal root ganglia trigger allodynia after spinal nerve injury in rats. Eur J Neurosci 2000, 12(1):100–105. [DOI] [PubMed] [Google Scholar]
- 149.Gandhi R, Ryals JM, Wright DE: Neurotrophin-3 reverses chronic mechanical hyperalgesia induced by intramuscular acid injection. J Neurosci 2004, 24(42):9405–9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Airaksinen MS, Saarma M: The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 2002, 3(5):383–394. [DOI] [PubMed] [Google Scholar]
- 151.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F: GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993, 260(5111):1130–1132. [DOI] [PubMed] [Google Scholar]
- 152.Kotzbauer PT, Lampe PA, Heuckeroth RO, Golden JP, Creedon DJ, Johnson EM Jr., Milbrandt J: Neurturin, a relative of glial-cell-line-derived neurotrophic factor. Nature 1996, 384(6608):467–470. [DOI] [PubMed] [Google Scholar]
- 153.Milbrandt J, de Sauvage FJ, Fahrner TJ, Baloh RH, Leitner ML, Tansey MG, Lampe PA, Heuckeroth RO, Kotzbauer PT, Simburger KS et al. : Persephin, a novel neurotrophic factor related to GDNF and neurturin. Neuron 1998, 20(2):245–253. [DOI] [PubMed] [Google Scholar]
- 154.Masure S, Geerts H, Cik M, Hoefnagel E, Van Den Kieboom G, Tuytelaars A, Harris S, Lesage AS, Leysen JE, Van Der Helm L et al. : Enovin, a member of the glial cell-line-derived neurotrophic factor (GDNF) family with growth promoting activity on neuronal cells. Existence and tissue-specific expression of different splice variants. Eur J Biochem 1999, 266(3):892–902. [DOI] [PubMed] [Google Scholar]
- 155.Kordower JH: AAV2-Neurturin for Parkinson’s Disease: What Lessons Have We Learned? Methods Mol Biol 2016, 1382:485–490. [DOI] [PubMed] [Google Scholar]
- 156.Whone AL, Boca M, Luz M, Woolley M, Mooney L, Dharia S, Broadfoot J, Cronin D, Schroers C, Barua NU et al. : Extended Treatment with Glial Cell Line-Derived Neurotrophic Factor in Parkinson’s Disease. J Parkinsons Dis 2019, 9(2):301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Baloh RH, Tansey MG, Lampe PA, Fahrner TJ, Enomoto H, Simburger KS, Leitner ML, Araki T, Johnson EM Jr., Milbrandt J: Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFRalpha3-RET receptor complex. Neuron 1998, 21(6):1291–1302. [DOI] [PubMed] [Google Scholar]
- 158.Bennett DL, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, McMahon SB, Priestley JV: A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci 1998, 18(8):3059–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Orozco OE, Walus L, Sah DW, Pepinsky RB, Sanicola M: GFRalpha3 is expressed predominantly in nociceptive sensory neurons. Eur J Neurosci 2001, 13(11):2177–2182. [DOI] [PubMed] [Google Scholar]
- 160.Merighi A: Targeting the glial-derived neurotrophic factor and related molecules for controlling normal and pathologic pain. Expert Opin Ther Targets 2016, 20(2):193–208. [DOI] [PubMed] [Google Scholar]
- 161.Gardell LR, Wang R, Ehrenfels C, Ossipov MH, Rossomando AJ, Miller S, Buckley C, Cai AK, Tse A, Foley SF et al. : Multiple actions of systemic artemin in experimental neuropathy. Nat Med 2003, 9(11):1383–1389. [DOI] [PubMed] [Google Scholar]
- 162.Sidorova YA, Bespalov MM, Wong AW, Kambur O, Jokinen V, Lilius TO, Suleymanova I, Karelson G, Rauhala PV, Karelson M et al. : A Novel Small Molecule GDNF Receptor RET Agonist, BT13, Promotes Neurite Growth from Sensory Neurons in Vitro and Attenuates Experimental Neuropathy in the Rat. Front Pharmacol 2017, 8:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Meng W, Deshmukh HA, van Zuydam NR, Liu Y, Donnelly LA, Zhou K, Wellcome Trust Case Control C, Surrogate Markers for M, Macro-Vascular Hard Endpoints for Innovative Diabetes Tools Study G, Morris AD et al. : A genome-wide association study suggests an association of Chr8p21.3 (GFRA2) with diabetic neuropathic pain. Eur J Pain 2015, 19(3):392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nencini S, Ringuet M, Kim DH, Greenhill C, Ivanusic JJ: GDNF, Neurturin, and Artemin Activate and Sensitize Bone Afferent Neurons and Contribute to Inflammatory Bone Pain. J Neurosci 2018, 38(21):4899–4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rolan PE, O’Neill G, Versage E, Rana J, Tang Y, Galluppi G, Aycardi E: First-In-Human, Double-Blind, Placebo-Controlled, Randomized, Dose-Escalation Study of BG00010, a Glial Cell Line-Derived Neurotrophic Factor Family Member, in Subjects with Unilateral Sciatica. PLoS One 2015, 10(5):e0125034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Backonja M, Williams L, Miao X, Katz N, Chen C: Safety and efficacy of neublastin in painful lumbosacral radiculopathy: a randomized, double-blinded, placebo-controlled phase 2 trial using Bayesian adaptive design (the SPRINT trial). Pain 2017, 158(9):1802–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Okkerse P, Hay JL, Versage E, Tang Y, Galluppi G, Ravina B, Verma A, Williams L, Aycardi E, Groeneveld GJ: Pharmacokinetics and pharmacodynamics of multiple doses of BG00010, a neurotrophic factor with anti-hyperalgesic effects, in patients with sciatica. Br J Clin Pharmacol 2016, 82(1):108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]