Abstract
Rewarming patients after profound hypothermia may result in acute heart failure and high mortality (50–80%). However, the underlying pathophysiological mechanisms are largely unknown. We characterized cardiac contractile function in the temperature range of 15–30°C by measuring the intracellular Ca2+ concentration ([Ca2+]i) and twitch force in intact left ventricular rat papillary muscles. Muscle preparations were loaded with fura-2 AM and electrically stimulated during cooling at 15°C for 1.5 h before being rewarmed to the baseline temperature of 30°C. After hypothermia/rewarming, peak twitch force decreased by 30–40%, but [Ca2+]i was not significantly altered. In addition, we assessed the maximal Ca2+-activated force (Fmax) and Ca2+ sensitivity of force in skinned papillary muscle fibers. Fmax was decreased by ∼30%, whereas the pCa required for 50% of Fmax was reduced by ∼0.14. In rewarmed papillary muscle, both total cardiac troponin I (cTnI) phosphorylation and PKA-mediated cTnI phosphorylation at Ser23/24 were significantly increased compared with controls. We conclude that after hypothermia/rewarming, myocardial contractility is significantly reduced, as evidenced by reduced twitch force and Fmax. The reduced myocardial contractility is attributed to decreased Ca2+ sensitivity of force rather than [Ca2+]i itself, resulting from increased cTnI phosphorylation.
Keywords: calcium sensitivity, troponin I phosphorylation, rat
rewarming patients from accidental hypothermia is often complicated by cardiovascular instability ranging from minor cardiac output depression to fatal circulatory collapse, the latter termed “rewarming shock.” However, the pathophysiological mechanisms of posthypothermic cardiovascular instability remain elusive, leaving mortality after accidental hypothermia between 50% and 80% (21, 23).
Experimental hypothermia research has disclosed that hypothermia may induce cardiac dysfunction (4), and, depending on level of severity, cardiac contractile dysfunction will be an essential determinant in cardiovascular instability during rewarming. Thus, the determination of the pathophysiological mechanisms underlying hypothermia-induced cardiac dysfunction will be critical to comprehensively treating hypothermia-induced myocardial failure in the clinical setting.
The present study focused on possible hypothermia-induced changes in regulatory processes at the level of cardiac sarcomeric proteins. It is well known that the functional dynamics of cardiac troponin C (cTnC) is not only dominated by the reception of Ca2+ but also the transduction of this Ca2+-binding signal by cardiac troponin I (cTnI) and cardiac troponin T (cTnT) (28, 29). Phosphorylation of these sarcomeric proteins tunes the intensity and dynamics of cardiac contraction and relaxation independent of membrane Ca2+ fluxes to meet physiological demands (28, 29). The phosphorylation of cTnI at Ser23/24 represents a well-established physiological mechanism for reduced myofilament Ca2+ sensitivity after β-adrenergic stimulation and the activation of PKA. Previous studies (7, 10, 11, 34, 41, 43–45) have reported that changes in the cTnI phosphorylation status of myofibrillar proteins underlie myocardial contractile dysfunction under various pathophysiological conditions, such as ischemia-reperfusion injury, hypertrophy, sepsis, and heart failure. A prevailing notion is that physiological states reflect a homeostatic distribution of phosphorylation of sarcomeric proteins, whereas pathophysiological states reflect a disturbance in this homeostasis with maladaptive distribution of these phosphorylations (27). Accordingly, we hypothesized that hypothermia and rewarming lead at least to changes in cardiac myofilament Ca2+ sensitivity and/or in the phosphorylation level of cTnI, thus contributing to reduced contractility.
To test this hypothesis, intact left ventricular (LV) rat papillary muscles were loaded with fura-2 AM to follow changes in the intracellular Ca2+ concentration ([Ca2+]i), and twitch force was evaluated in papillary muscle preparations maintained at 15°C for 1.5 h before being rewarmed to baseline temperature (30°C). Hypothermia at 15°C was chosen to mimic the core body temperature (15°C) of rats used in our previous in vivo rewarming shock experiments (16, 17, 37). In skinned papillary muscle fibers, we assessed maximal Ca2+-activated force (Fmax) and Ca2+ sensitivity of force. PKA-mediated phosphorylation of cTnI at Ser23/24 and the total phosphorylation of cTnI were determined using Western blot analysis and two-dimensional (2-D) gel electrophoresis, respectively.
MATERIALS AND METHODS
The Institutional Animal Care and Use Committee of Mayo Clinic approved the experimental protocols and care of the animals. Adult male Sprague-Dawley rats (250–350 g) were injected intramuscularly with ketamine (60 mg/kg) and xylazine (2.5 mg/kg) for anesthesia.
Isolation of LV papillary muscle.
Hearts from anesthetized rats were quickly removed and placed in a custom-made dish continuously circulated with aerated Krebs-Henseleit (KH) solution containing (in mM) 118.4 NaCl, 4.7 KCl, 2.0 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, and 10 glucose. Papillary muscles from the LV were gently excised and immediately mounted between a force transducer and a length change manipulator in a quartz cuvette continuously perfused with aerated KH solution (Guth Muscle Research System) (14). The average muscle length was 3 mm and diameter was ∼1.2 mm, allowing for proper gas exchange with the perfusate (26). Force normalized to cross-sectional area (CSA) was expressed as milliNewtons per millimeter squared.
Simultaneous measurement of intracellular Ca2+ transients and twitch force.
The horizontally mounted papillary muscle was loaded with 10 μM fura-2 AM for 1.5 h in oxygenated KH solution containing Pluronic F-127 [0.01% (wt/vol)], which facilitated the loading of fura-2 AM into the muscle fibers at room temperature (20–22°C) (26, 42). After being loaded, the muscle was washed out for 10 min and continuously perfused with KH solution aerated with 95% O2-5% CO2 throughout the experiment. Afterward, the muscle was electrically stimulated at 0.5 Hz at the baseline temperature of 30°C, during which muscle length was adjusted for maximal twitch force. During hypothermia at 15°C, the papillary muscle was stimulated at 0.25 Hz to imitate the hypothermia-induced change in heart rate of the intact heart at this temperature. Twitch force and the corresponding ratio signals from fura-2 fluorescences at 340- and 380-nm excitation were recorded using a real-time data-acquisition program (LabVIEW, National Instruments, Austin, TX). To minimize photobleaching, the excitation shutter was only opened during data recording. The emission fluorescence signal from the fura-2-loaded muscle was filtered through a 510-nm filter and collected by a photomultiplier tube. Background fluorescence from the unloaded muscle was subtracted from the corresponding fura-2 fluorescence signals at 340 and 380 nm. [Ca2+]i was calculated from the ratio of fluorescence signals (ratio = 340/380 nm) using the equation described by Grynkiewcz et al. (13).
Experimental protocol.
All experiments started at baseline temperature (30°C), which kept the progressive fura-2 loss small enough to carry out the 2.5-h experimental protocol (26). Twitch force and [Ca2+]i transients were recorded at the following specific steps: baseline temperature before the tissue was cooled (30°C, step 1), initial hypothermia (15°C, 0 h, step 2), hypothermia maintained for 1.5 h (15°C, 1.5 h, step 3), and after the tissue was rewarmed to the baseline temperature (30°C, step 4). The duration of cooling and rewarming the muscle tissue was 30 min. Time-matched controls were maintained at 30°C for 2.5 h. The cooling/rewarming protocol is shown in Fig. 1. Hypothermia at 15°C was chosen to mimic the core body temperature (15°C) of rats used in our previous in vivo rat model of rewarming shock (16, 17, 37).
Fig. 1.
Experimental protocol showing the four experimental steps. CTL, control.
Permeabilized papillary muscle fibers.
In a separate set of experiments, LV papillary muscles from the control and rewarming groups were teased into thin strips (∼1 mm in length and <200 μm in diameter) in relaxing solution containing the following: 85 mM K+, 5 mM MgATP, 1 mM Mg2+, 7 mM EGTA, and 70 mM imidazole, with a total ionic strength of 150 mM and pH 7.0 with propionic acid (HPr). The activating solution contained the following: 0.1 mM CaPr2, 85 mM K+, 5 mM MgATP, 1 mM Mg2+, 7 mM EGTA, and 70 mM imidazole, with a total ionic strength of 150 mM and pH 7.0 with HPr. A computer program was used to calculate the free Ca2+ concentration for the activating and relaxing solutions. Dissected thin fibers were permeabilized in relaxing solution containing an additional 1% (vol/vol) Triton X-100 for 1 h at room temperature (20–22°C). Subsequently, the permeabilized fibers were mounted in the Guth Muscle Research System (14) and rinsed for 10 min with relaxing solution. The sarcomere length of the fibers was set to ∼2.2 μm using a calibrated monocular microscope (×20 long focal length lens for positioning the whole fiber strip inside the quartz cuvette and ×40 lens for adjusting the sarcomere length, respectively) at 30°C. Varied pCa solutions (6.2, 6.0, 5.8, 5.6, 5.4, 5.2, 5.0, 4.5, and 4.0) were made by mixing the activating solution with the relaxing solution on the basis of a computer program-determined ratio (9). [Ca2+] in the cuvette perfusing a permeabilized muscle strip was sequentially varied from pCa 8.0 to 4.0 to obtain force-pCa relationships. Force-pCa relationships were fitted with the following Hill equation:
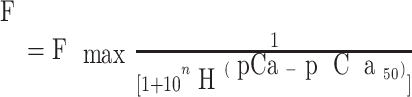 |
where F is the steady-state force at various pCa, pCa50 is the pCa required for 50% of Fmax, and nH is the Hill coefficient. pCa50 and nH were used as an index of myofibrillar Ca2+ sensitivity on the force development and an index of cooperativity of myofibrillar proteins, respectively. Fibers were subjected to a test activation/relaxation cycle (at pCa 4.0 and 8.0, respectively) before the measurement of force-pCa relationships.
Western blot analysis.
To analyze the phosphorylation of cTnI, a separate set of experiments was performed using LV papillary muscle samples (5–8 mg) from time-matched control and rewarmed groups. All steps were carried out at 4°C to avoid protein degradation. Tissue samples were homogenized in buffer solution containing the following: 20 mM Tris (pH 8.0), 0.15 mM NaCl; 1% Nonidet P-40, 1% Triton X-100, 1% sodium deoxycholate, 10 mM EDTA, and 10 mM sodium azide. Homogenized samples were solubilized in a sample rotator, placed in a cold room for 20 min, and spun in a refrigerated centrifuge at 10,000 g for 10 min. Protein content was assayed by a Lowry assay (Bio-Rad DC protein Assay). Protein (50 μg) was denatured in 1× sample buffer at 100°C for 3 min, fractionated by SDS-PAGE, and transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad). Transferred proteins on separate PVDF membranes were detected with specific antibodies for either total cTnI [polyclonal antibodies produced with the following epitope: NH2-terminal (102–110) and COOH-terminal (170–180), Cell Signaling, Santa Cruz, CA] or phospho-cTnI at Ser23/24 (Cell Signaling) and visualized by a highly sensitive enhanced chemiluminescent substrate for detecting horseradish peroxidase on immunoblots (Pierce Biotechnology, Rockford, IL). The bands were quantitatively analyzed with molecular imaging software (version 4.0.1, Kodak).
2-D gel electrophoresis.
To complement the Western blot analysis results, which only showed cTnI phosphorylation at specific Ser23/24 residues, total cTnI phosphorylation in time-matched control and rewarming groups was determined using 2-D gel electrophoresis (isoelectric point for cTnI: 9.6). The spots were quantitatively analyzed with ImageQuant software (version 5.2, Amersham Bioscience, Piscataway, NJ). The detailed protocols for 2-D gel electrophoresis were previously described (25). In brief, LV papillary muscle samples (5–8 mg) from control and rewarmed groups were extracted with 7 M urea, 2 M thiourea, 4% CHAPS, 0.5% immobilized pH gradient (IPG) buffer (pH 7–11), and 40 mM DTT. Extracts were treated with a 2-D clean-up kit (Amersham Biosciences), loaded with rehydration buffer solution containing 7 M urea, 2 M thiourea, 2% CHAPS, 0.5% IPG buffer, and 0.002% bromophenol blue, and then kept in a freezer (−70°C). pH 7–11NL IPG dry gel strips (Amersham Biosciences) laid on rehydration buffer were rehydrated overnight. After rehydration, proteins were isoelectrically focused in a Protein IEF Cell system (Bio-Rad) equipped with a cup loading tray. After isoelectric point separation or isoelectric focusing (IEF), gel strips were equilibrated in 6 M urea, 75 mM Tris·HCl (pH 8.8), 30% glycerol, 2% (wt/vol) SDS, and 0.002% bromophenol blue with 130 mM DTT and 135 mM iodoacetamide before undergoing SDS-PAGE for protein separations by molecular weight. After SDS-PAGE using 12.5% Tris·HCl criterion precast gels (Bio-Rad), the gels were silver stained for the analysis of cTnI phosphorylation or transferred to a PVDF membrane for Western blot analysis to identify TnI phosphorylation spots.
Statistical analysis.
All results are presented as means ± SE. To statistically analyze the changes in twitch force and [Ca2+]i in the time-matched control group and in the hypothermia/rewarming groups within experimental steps, one-way ANOVA was used. When significant differences were observed, Student's t-test with the Bonferroni correction was used as a post hoc analysis. For the comparison of the time-matched control group versus hypothermia/rewarming groups, unpaired two-tailed Student's t-test was used. Differences were considered significant at P < 0.05.
RESULTS
Time-matched control group maintained at 30°C for 2.5 h.
A general decline (<12% of initial control values) in the peak [Ca2+]i transient and peak twitch force was found in time-matched controls at baseline temperature (30°C), but there were no significant differences in the peak [Ca2+]i transient and peak twitch force between initial (0 h) and final (2.5 h) control values (Table 1). Furthermore, there were no significant changes in control values of time to peak (TTP) and time to 50% decay (TT50%) of [Ca2+]i, whereas there were significant decreases in TTP (∼20% at 2 and 2.5 h) and TT50% (20% at 0.5 h, 30% at 2 h, and 40% at 2.5 h) of twitch force compared with the initial control values (0 h; Table 1).
Table 1.
Amplitude, kinetic parameters, and time-matched control values for peak [Ca2+]i transients and twitch force at each experimental step
| Amplitude |
Time to Peak, ms | Time to 50% Decay, ms | ||
|---|---|---|---|---|
| [Ca2+]i, nM | Force, mN/mm2 | |||
| Step 1: baseline (0 h) | ||||
| [Ca2+]i transient | 812.41 ± 133.82 | 57.03 ± 3.06 | 177.19 ± 5.32 | |
| Control | 925.20 ± 51.65 | 52.01 ± 2.83 | 204.50 ± 9.71 | |
| Twitch force | 7.71 ± 1.29 | 118.13 ± 7.82 | 86.04 ± 6.53 | |
| Control | 8.28 ± 1.30 | 116.02 ± 6.99 | 101.80 ± 4.45 | |
| Step 2: initial hypothermia (0.5 h) | ||||
| [Ca2+]i transient | 1,025.85 ± 192.91* | 162.09 ± 8.55* | 681.89 ± 30.23* | |
| Control | 910.31 ± 135.25 | 45.50 ± 1.72 | 178.50 ± 29.50 | |
| Twitch force | 13.23 ± 2.22* | 407.97 ± 13.36* | 324.51 ± 22.62* | |
| Control | 7.57 ± 0.98 | 103.40 ± 5.84 | 81.80 ± 7.22† | |
| Step 3: maintained hypothermia (2.0 h) | ||||
| [Ca2+]i transient | 1,190.66 ± 205.17* | 166.99 ± 8.48* | 625.98 ± 19.30* | |
| Control | 917.24 ± 42.89 | 50.02 ± 2.84 | 192.05 ± 15.34 | |
| Twitch force | 13.43 ± 2.30* | 382.51 ± 11.38* | 279.00 ± 18.41* | |
| Control | 7.63 ± 0.75 | 92.20 ± 4.37† | 70.80 ± 3.34† | |
| Step 4: rewarming to baseline temperature (2.5 h) | ||||
| [Ca2+]i transient | 973.99 ± 154.89 | 56.29 ± 3.07 | 162.14 ± 4.41* | |
| Control | 815.86 ± 78.49 | 54.50 ± 9.19 | 171.50 ± 19.50 | |
| Twitch force | 4.73 ± 0.68* | 96.31 ± 4.46* | 62.77 ± 2.97* | |
| Control | 7.31 ± 0.93 | 91.40 ± 4.46† | 59.60 ± 2.23† | |
Results are presented as means ± SE; n = 7 rats for experimental steps and 4 rats for time-matched control at 30°C. [Ca2+]i, intracellular Ca2+ concentration.
P < 0.05 vs. baseline temperature (30°C);
P < 0.05 vs. initial control values (0 h).
Changes in [Ca2+]i transients and twitch force after tissues were cooled to 15°C (15°C, 0 h).
Compared with values at the baseline temperature (30°C), the following characteristics of both the peak [Ca2+]i transient and twitch force were significantly increased: amplitude, by 26% and 72%, respectively; TTP, by 184% and 245%, respectively; and TT50%, by 285% and 277%, respectively (Fig. 2, A and B, and Table 1). These significant increases in the [Ca2+]i transient and/or force during tissue cooling to 15°C were comparable with those presented in previous studies (12, 18, 20, 32, 37).
Fig. 2.

Representative traces of twitch forces (A) and intracellular Ca2+ concentration ([Ca2+]i) transients (B) in intact papillary muscles undergoing the four experimental steps. Muscles were continuously perfused with Krebs-Henseleit solution during an electrical stimulation at 1 Hz at 30°C and 0.5 Hz at 15°C. Trace a, baseline temperature (30°C); trace b, initial cooling at 15°C (0 h); trace c, cooling maintained at 15°C (1.5 h); trace d, rewarming to baseline temperature.
Changes in [Ca2+]i transients and twitch force during stable hypothermia for 1.5 h at 15°C.
At the end of 1.5 h at 15°C, peak [Ca2+]i increased by 16%, whereas peak force remained unchanged, compared with values obtained after tissue was cooled to 15°C (0 h at 15°C). TTP in peak [Ca2+]i transients remained unchanged, whereas TTP in force was significantly decreased (6.1%). There were significant reductions in TT50% in both peak [Ca2+]i transients and force (6% and 13%, respectively) compared with those at 0 h at 15°C (Fig. 2, A and B, and Table 1).
Changes in [Ca2+]i transients and twitch force after tissue was rewarmed to baseline temperature (30°C).
After the tissue was rewarmed, the peak [Ca2+]i transient was increased by 13%, whereas peak force was reduced by 36%, compared with baseline temperature (30°C). TTP in peak [Ca2+]i transients returned to within prehypothermic control values, whereas TTP in force decreased by 20%. TT50% in both peak [Ca2+]i transients and force was decreased by 10% and 30%, respectively, compared with baseline (Fig. 2, A and B, and Table 1).
Intact myofilament Ca2+ sensitivity.
By plotting phase-plane loops (the relationship between twitch force and [Ca2+]i), which represent a coarse dynamic index of myofilament Ca2+ sensitivity during the relaxation phase of twitch force (3, 8, 31), we observed a rightward shift in the relaxation phase during stable hypothermia (15°C) as well as after rewarming in intact papillary muscle, indicating a decrease in myofilament Ca2+ sensitivity due to hypothermia and rewarming (Fig. 3).
Fig. 3.
Typical phase-plane loops demonstrating the changes in Ca2+ sensitivities during cooling at 15°C and after rewarming (RW) to baseline temperature (30°C).
Force-pCa relationship in permeabilized muscle fibers.
Fmax was significantly decreased in rewarmed muscle fibers (32.1 ± 3.2 vs. 45.9 ± 3.9 mN/mm2 in controls; Fig. 4A). In Fig. 4B, the data for Fmax are normalized to maximal values and plotted against increasing pCa concentrations. In rewarmed muscle fibers, pCa50 was significantly reduced (5.57 ± 0.03 vs. 5.71 ± 0.06 in controls). However, nH remained unchanged (3.19 ± 0.13 in rewarmed muscle fibers vs. 3.04 ± 0.27 in controls).
Fig. 4.

Force-pCa relationships in the CTL and RW groups. A: all the data of Ca2+-activated force in absolute values (ordinate) were plotted as a function of pCa (abscissa). B: normalized force-pCa relationships in the CTL and RW groups are shown. Data are means ± SE; n = 21–22 muscle fibers from 8 rats in the CTL and RW groups, respectively.
Western blot analysis of cTnI.
The bands of cTnI phosphorylated at Ser23/24 residues (top) as well as total cTnI protein expression (bottom), used as a reference from time-matched control and rewarmed LV papillary muscles, are shown in Fig. 5A. In rewarmed papillary muscle, cTnI phosphorylation was significantly increased compared with that of time-matched control (114.33 ± 7.64 vs. 93.02 ± 3.27 arbitrary units; Fig. 5B).
Fig. 5.
Analysis of troponin I (TnI) phosphorylation in the CTL and RW samples. A: TnI protein expression (bottom) was used as a reference for the determination of TnI phosphorylation (top). Bands from the Western blot were quantified using densitometry. B: results were quantified as a ratio of phosphorylated (p-)TnI intensity to TnI intensity. Quantified results are shown in the bar graph. Data are means ± SE; n = 5 from the CTL and RW groups, respectively. au, Arbitrary units. *P < 0.05 vs. the CTL group (considered as a significant difference).
2-D gel electrophoresis analysis.
Figure 6A shows typical spots of proteins separated by IEF based on the isoelectric points of specific proteins. Total cTnI phosphorylation in rewarmed papillary muscles was significantly increased compared with that of time-matched controls (51.63 ± 5.19 vs. 33.96 ± 4.69 arbitrary units; Fig. 6B).
Fig. 6.

Analysis of the total TnI phosphorylation in the CTL and RW samples using two-dimensional gel electrophoresis (A). P1, a single phosphorylated spot of TnI; P0, an unphosphorylated spot of TnI. B: phosphorylated spots of TnI were quantified as the ratio of P1 to the sum of P1 and P0. Quantified results are shown in the bar graph. Data are means ± SE; n = 6 from the CTL and RW groups, respectively. MW, molecular weight. *P < 0.05 vs. the CTL group (considered as a significant difference).
DISCUSSION
The major findings in the present study are that rewarming after 1.5 h of stable hypothermia increased PKA-mediated TnI phosphorylation as well as the total phosphorylation of cardiac TnI and decreased the Ca2+ sensitivity of force in myocardial contractile tissue. These novel data, in conjunction with a substantial reduction in contractile force after rewarming, add to our understanding of the mechanisms underlying hypothermia-induced cardiac contractile dysfunction and also point to a potential therapeutic target to counteract rewarming-induced cardiac failure.
Cooling and rewarming alter myocardial contractile function.
After rewarming, papillary muscle twitch force was reduced by ∼35% despite a ∼15% increase in the [Ca2+]i transient, indicating that reduced contractile force after rewarming is related to a decrease in the myofibrillar Ca2+ sensitivity of force rather than an impairment in [Ca2+]i regulation. Significant increases in the temporal parameters of contraction and relaxation in twitch force after rewarming likely reflect a change in myosin/actin cross-bridge cycling kinetics (Table 1).
Despite the fact that in the present study we did not measure cross-bridge rate constants or stiffness, these findings support another explanation for reduced force after hypothermia/rewarming on the basis of a two-state model (5). According to the two-state model, maximum isometric force is defined as follows: maximum isometric force = nFαfs, where n is the number of cross-bridges within the half sarcomere, F is the average force per cross-bridge, and αfs is the fraction of cross-bridges in a strongly bound force-generating state. Furthermore, αfs is defined as follows: αfs = fapp/(fapp + gapp), where fapp and gapp are the apparent rate constants for cross-bridge attachment and detachment, respectively. Thus, the reduced force development after rewarming may be attributable to the decrease in cross-bridge recruitment (αfs) resulting from increased gapp during hypothermia/rewarming. This assumption is supported by our results showing that after tissue rewarming, there were significant decreases in TT50% of the [Ca2+]i transient as well as twitch force (∼10% and 30%, respectively; Table 1).
Decreased myofilament Ca2+ sensitivity as an underlying mechanism of posthypothermic contractile dysfunction.
Ca2+ activates cardiac muscle in a graded manner, which makes the relationship between [Ca2+]i and force development of functional importance (2). Using intact papillary muscle, we found a significant increase in both the twitch force and peak [Ca2+]i transient at 15°C (Table 1 and Fig. 2, A and B). These findings are comparable with those in previous studies reporting that contractility is greater at 23–25°C than at normothermia in the papillary muscle of various species (20). It is known that temperature change is one of the major factors that alters cardiac myofilament Ca2+ sensitivity (1), but controversy exists whether cardiac myofilament Ca2+ sensitivity will increase or decrease during hypothermia. Stowe et al. (32) reported that in isolated hearts, mild hypothermia (27°C) increases myofilament Ca2+ sensitivity, whereas moderate hypothermia (17°C) decreases it. These findings support our observations that twitch force in intact papillary muscle reaches a maximum at 25°C and then declines as temperature is reduced to 15°C. However, it needs to be emphasized that the significantly increased peak [Ca2+]i transient at 15°C in the present experiments (Table 1) may counteract an expected decrease in twitch force due to reduced Ca2+ sensitivity at this temperature. By plotting phase-plane loops (the relationship between twitch force and [Ca2+]i), which represent a coarse dynamic index of myofilament Ca2+ sensitivity during the relaxation phase of twitch force (3, 8, 31), we observed a rightward shift in the relaxation phase during stable hypothermia (15°C) as well as after rewarming in intact papillary muscle, indicating a decrease in myofilament Ca2+ sensitivity due to hypothermia and rewarming (Fig. 3). We used skinned papillary muscle fibers to better describe the Ca2+ sensitivity and contractile force after rewarming and found that both Fmax and myofilament Ca2+ sensitivity were significantly decreased in rewarmed cardiac muscle fibers. These data suggest that hypothermia decreases cardiac myofilament Ca2+ sensitivity, which is not reversed by rewarming, thus contributing to posthypothermic cardiac contractile dysfunction.
Increased cTnI phosphorylation and cardiac contractile dysfunction but maintained relaxation characteristics after tissue rewarming.
Numerous studies (6, 7, 19, 24, 33, 44) have demonstrated that increased PKA-mediated phosphorylation of the sarcomeric protein cTnI reduces myofilament Ca2+ sensitivity and shifts the force-pCa relationship rightward under normothermic circumstances. To the best of our knowledge, the present study is the first to show that rewarming after stable hypothermia simultaneously increases cTnI phosphorylation and reduces myofilament Ca2+ sensitivity and Fmax. In principle, during systole, Ca2+ moves into the cytoplasmic space and binds to the myofilaments, and contraction starts. One way to increase the contractile force of the sarcomere is to increase cytosolic Ca2+; another is to regulate how the sarcomeres respond to Ca2+. The unique position of cTnI to regulate and modulate cardiac function under the influence of adrenergic signaling is well documented (30). In more detail, cTnI has a NH2-terminal extension of some 30 amino acids that houses Ser23/24, which is phosphorylated by PKA to depress sarcomere sensitivity to Ca2+ and increase cross-bridge kinetics (15). In the present study, we found that after tissues were rewarmed, there was a significant increase in cTnI phosphorylation at PKA sites (Ser23/24), as shown by Western blot analysis (Fig. 5A) and 2-D gel (Fig. 6A), which likely included all phospohorylatable sites on cTnI (Ser23/24, Ser43/45, Thr144, and Ser149). Therefore, we could not exclude other possible phospohorylatable sites on cTnI (Ser43/45, Thr144, and Ser149) that might be phosphorylated by hypothermia/rewarming, even though Ser23 and Ser24 are known to be the dominant phospohorylatable sites on cTnI. Those possible sites on cTnI to be identified in future studies would require the use of the mass spectrometry technique.
Phosphorylation of cTnI is mediated by several kinases [PKA, PKC, PKG, and PAK3 (p21-activated kinases)] (19), but PKA-mediated phosphorylation via β-adrenergic stimulation plays a major physiological role in meeting the increased circulatory demand in the physiological state (19). Interestingly, unlike other pathological clinical conditions, such as ischemia-reperfusion injury, hypertrophy, sepsis, and heart failure (7, 10, 11, 34, 41, 43–45), where a slower relaxation rate is a common finding, a faster relaxation rate after rewarming was observed despite a significant reduction of contractile force in the present experiments. Furthermore, we noticed that in time-matched controls at baseline temperature, TTP and TT50% of twitch force were significantly reduced after 2.5 h (Table 1), whereas TTP and TT50% of [Ca2+]i remained statistically unchanged. Therefore, we speculate that fapp and gapp may be altered in response to the baseline temperature in this time-matched control group.
Yet, it is a challenge to understand the exact mechanism of the direct effects of hypothermia and rewarming on β-adrenergic stimulation leading to PKA-mediated cTnI phosphorylation. In general, changes in the cTnI phosphorylation status, as reported in the failing human heart (7, 10, 11, 34, 41, 43–45), are considered a change in the balance between kinase and phosphatase activities, and the optimal balance is to be found during normal cardiac function. Thus, hypothermia and rewarming, depending on severity and depth, appear to tip the balance between kinase and phosphatase acitivities, leading to alterations in myofilament Ca2+ sensitivity and myocardial contractility.
Considering the similarities in the changes between cardiac contractile functional variables of in vitro papillary muscle preparations and those of the whole intact heart after experimental hypothermia and rewarming previously studied by our group (16, 17, 36, 38, 39), it is tempting to make a direct comparison between them. However, such a comparison has to be made with great caution. Nevertheless, previous reports (22, 35) have shown that rewarming intact hearts from stable hypothermia (25–15°C) resulted in a significant (∼50%) reduction in systolic function but maintained diastolic cardiac function. Thus, similarities in cardiac contractile functional variables between in vitro papillary muscle and the intact heart may be ascribed to a hypothermia-induced decrease in Ca2+ sensitivity that persists after rewarming. PKA-dependent phosphorylation of cTnI, induced by hypothermia and rewarming, may explain both the reduction in systolic functional characteristics and proper maintenance in diastolic function in the rewarmed intact heart. Furthermore, we (16) have reported a lack of effect of pharmacological agents to support cardiac contractile function mediated via the β-receptor-cAMP-PKA pathway during as well as after rewarming. Likewise, if hypothermia and rewarming per se activate the β-receptor-cAMP-PKA pathway, one may interpret that an additional pharmacological stimulus under these circumstances of the β-receptor would bring about much less of cardiac inotropic effect. As already outlined, these assumptions cannot be applied to draw any conclusions but may be used as a plausible rationale for future research protocols.
Although rewarming shock is considered a variant of irreversible circulatory shock, cardiac contractile dysfunction after rewarming may range from cardiac stunning to severe acute cardiac failure. The present findings may also have relevance to rewarming hypothermic patients after heart surgery as well as to therapeutic hypothermia used to treat patients after being resuscitated after acute cardiac standstill. However, there are still limitations in extrapolating experimental results using small animals to human medicine, and we also have an experimental limitation in the present experiments: rewarming of the muscle fibers before the initiation of the experimental protocol, although care was taken to minimize this additional rewarming. The isolation of the papillary muscle from the LV, the dye loading, and the muscle skinning preparation were all performed at 22°C, whereas the baseline temperature was 30°C. This temperature of 22°C was chosen due to the fact that fura-2 AM loading into muscle fibers is optimal at this temperature, minimizing the compartmentalization that is usually more pronounced at higher loading temperatures.
Conclusions.
After rewarming, myocardial contractility is significantly reduced, as determined by the reduced twitch force in LV intact papillary muscle and also by the decreased myofibrillar Ca2+ sensitivity and Fmax in permeabilized muscle fibers. The reductions in twitch force, myofibrillar Ca2+ sensitivity, and Fmax likely result from increased cTnI phosphorylation in the present model. An increase in cTnI phosphorylation possibly involves not only PKA but also other kinases activated by hypothermia and rewarming.
GRANTS
This work was supported by the Mayo Foundation and Graduate School (Rochester, MN).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGEMENTS
The authors thank Dr. Frank Brozovich and Dr. Ozgur Ogut for helping to set up the 2-D gel electrophoresis procedure for this study.
REFERENCES
- 1.Bers D, Dridge J, Spitzer K. Intracellular Ca2+ transients during rapid cooling contractures in guinea-pig ventricular myocytes. J Physiol 417: 537–553, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Bers DM. Cardiac inotropy and Ca mismanagment. In: Excitation-Contraction Coupling and Cardiac Contraction Force (2nd ed.). Dordrecht, The Netherlands: Kluwer Academic, 2003.
- 4.Blair E, Montgomery AV, Swan H. Posthypothermic circulatory failure. I. Physiologic observations on the circulation. Circulation 13: 990–915, 1956. [DOI] [PubMed] [Google Scholar]
- 5.Brenner B. Effects of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers:implications for regulation of muscle contraction. Proc Natl Acad Sci USA 85: 3265–3269, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burkart EM, Sumanda MP, Kobayashi T, Nili M, Martin AF, Homsher E, Solaro RJ. Phosphorylation or glutamic acid substitution at protein kinase C sites on cardiac troponin I differentially depress myofilament tension and shortening velocity. J Biol Chem 278: 11265–11272, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Chen F, Ogut O. Decline of contractility during ischemia-reperfusion injury: actin glutathionylation and its effect on allosteric interaction with tropomyosin. Am J Physiol Cell Physiol 290: C719–C727, 2006. [DOI] [PubMed] [Google Scholar]
- 8.DeSantiago J, Li Y, Schlotthauer K, Bers DM. Phenylephrine induced inotropy is not associated with IP3 induced Ca release in ventricular myocytes (Abstract). Biophys J 74: A57, 1998. [Google Scholar]
- 9.Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol 75: 463–505, 1979. [PubMed] [Google Scholar]
- 10.Gao WD, Atar D, Backx PH, Marban E. Relationship between intracellular calcium and contractile force in stunned myocardium. Circ Res 76: 1036–1048, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Gao WD, Liu Y, Mellgren R, Marban E. Intrinsic myofilament alterations underlying the decreased contractility of stunned myocardium. A consequence of Ca2+-dependent proteolysis? Circ Res 78: 455–465, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Groban L, Zapata-Sudo G, Lin M, Nelson TE. Effects of moderate and deep hypothermia on Ca2+ signalling in rat ventricular myocytes. Cell Physiol Biochem 12: 101–110, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescent properties. J Biol Chem 260: 3440–3450, 1985. [PubMed] [Google Scholar]
- 14.Guth K, Wojciechowski R. Perfusion cuvette for the simultaneous measurement of mechanical, optical and energetic parameters of skinned muscle fibers. Pflügers Arch 407: 552–557, 1986. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi T, Solaro RJ. Calcium, thin filaments, and integrative biology of cardiac contractility. Annu Rev Physiol 67: 39–60, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Kondratiev T, Myhre E, Simonsen O, Nymark T, Tveita T. Cardiovascular effects of epinephrine during rewarming from hypothermia in an intact animal model. J Appl Physiol 100: 457–464, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Kondratiev T, Wold RM, Assum E, Tveita T. Myocardial mechanical dysfunction and calcium overload following rewarming from experimental hypothermia in vivo. Cryobiology 56: 15–21, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Langer GA, Brady AJ. The effects of temperature upon contraction and ionic exchange in rabbit ventricular myocardium. Relation to control of active state. J Gen Physiol 52: 682–713, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res 66: 12–21, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Liu B, Wang LCH, Belke DD. Effect of low-temperature on the cytosolic free Ca2+ in rat ventricular myocytes. Cell Calcium 12: 11–18, 1991. [DOI] [PubMed] [Google Scholar]
- 21.McLean D, Emslie-Smith D. Accidental Hypothermia. Melbourne, Australia: Blackwell Scientific, 1977.
- 22.Meissner A, Szymanska G, Morgan JP. Effects of dantrolene sodium on intracellular Ca2+-handling in normal and Ca2+-overloaded cardiac muscle. Eur J Pharmacol 316: 333–342, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Murray P, Hall J. Hypothermia. In: Principles of Critical Care. New York: McGraw-Hill, 1998.
- 24.Pi YQ, Kemnitz KR, Zhang D, Kranias EG, Walker JW. Phosphorylation of troponin I controls cardiac twitch dynamics: evidence from phosphorylation site expressed on a troponin I-null background in mice. Circ Res 90: 649–656, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Rabilloud T. Use of thiourea to increase the solubility of membrane proteins in two-dimmensional electrophoresis. Electrophoresis 19: 758–760, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Reilly AM, Williams DA, Dusting GJ. Dexamethasone inhibits endotoxin-induced changes in calcium and contractility in rat isolated papillary muscle. Cell Calcium 26: 1–8, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Solaro RJ, de Tombe PP. Review focus series: sarcomeric proteins as key elements in integrated control of cardiac function. Cardiovasc Res 77: 616–618, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Solaro RJ. The cardiovascular system. In: The Heart. Oxford: Oxford Univ. Press, 2001, p. 264–300.11179261
- 29.Solaro RJ, Rarick HM. Troponin and tropomyosin. Proteins that switch on and tune in the activity of cardiac myofilaments. Circ Res 83: 471–480, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Solaro RJ, Rosevear P, Kobayashi T. The unique functions of cardiac troponin I in the control of cardiac muscle contraction and relaxation. Biochem Biophys Res Commun 25: 82–87, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spurgeon HA, duBell WH, Stern MD, Sollott SJ, Ziman BD, Silverman HS, Capogrossi MC, Talo A, Lakatta EG. Cytosolic calcium and myofilaments in single rat cardiac myocytes achieve a dynamic equilibrium during twitch relaxation. J Physiol 447: 83–102, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stowe DF, Fujita S, An J, Paulsen RA, Varadarajan SG, Smart S. Modulation of myocardial function and [Ca2+] sensitivity by moderate hypothermia in guinea pig isolated hearts. Am J Physiol Heart Circ Physiol 277: H2321–H2332, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Tavernier B, Li JM, El-Omar MM, Lanone S, Yang ZK, Trayer IP, Shak AM. Cardiac contractile impairment associoated with increased phosphorylation of tronponin I in endotoxemic rats. FASEB J 15: 294–296, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Tavernier B, Mebazaa A, Mateo P, Sys S, Ventura-Clapier R, Veksler V. Phosphorylation-dependent alteration in myofilament Ca2+ sensitivity but normal mitochondrial function in septic heart. Am J Respir Crit Med 163: 362–367, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Tveita T. Rewarming from hypothermia. Newer aspects on the pathophysiology of rewarming shock. Int J Circumpolar Health 59: 260–266, 2000. [PubMed] [Google Scholar]
- 36.Tveita T, Mortensen E, Hevroy E, Refsum H, Ytrehus K. Experimental hypothermia: effects of core cooling and rewarming on hemodynamics, coronary blood flow, and myocardial metabolism in dogs. Anesth Analg 79: 212–218, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Tveita T, Skandfer M, Refsum H, Ytrehus K. Experimental hypothermia and rewarming: changes in mechanical function and metabolism of rat hearts. J Appl Physiol 80: 291–297, 1996. [DOI] [PubMed] [Google Scholar]
- 38.Tveita T, Traasdal E, Ytrehus K. Alteration of autonomous cardiovascular control after rewarming from experimental hypothermia in rat. Acta Anaesthesiol Scand Suppl 43: 104, 1999. [Google Scholar]
- 39.Tveita T, Ytrehus K, Myhre ESP, Hevroy O. Left ventricular dysfunction following rewarming from experimental hypothermia. J Appl Physiol 85: 2135–2139, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Tveita T, Ytrehus K, Skandfer M, Oian P, Helset E, Myhre ES, Larsen TS. Changes in blood flow distribution and capillary function after deep hypothermia in rat. Can J Physiol Pharmacol 74: 376–381, 1996. [PubMed] [Google Scholar]
- 41.van der Velden J, Papp Z, Zaremba R, Boontje NM, de Jong JW, Owen VJ, Burton PB, Goldmann P, Jaquet K, Stienen GJ. Increased Ca2+-sensitivity of the contractile apparatus in end-stage human heart failure results from altered phosphorylatioin of contractile proteins. Cardiovasc Res 57: 37–47, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Xu Y, Kerrick WGL, Wang YC, Guzman G, Diaz-Perez Z, Szczesna-Cordary D. Prolonged Ca2+ and force transients in myosin RLC transgenic mouse fibers expressing malignant and benign FHC mutations. J Mol Biol 361: 286–299, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Wolfgang S, Kogler H. Altered phosphorylation and Ca2+-sensitivity of myofilaments in human heart failure. Cardiovasc Res 57: 5–7, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Wu LL, Tang C, Liu MS. Altered phosphorylation and calcium sensitivity of cardiac myofibrillar proteins during sepsis. Am J Physiol Regul Integr Comp Physiol 281: R408–R416, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Yasuda S, Lew WY. Lipopolysaccharide depresses cardiac contractility and β-adrenergic contractile response by decreasing myofilament response to Ca2+ in cardiac myocytes. Circ Res 81: 1011–1020, 1997. [DOI] [PubMed] [Google Scholar]





