Abstract
Patients with type 2 diabetes display an exaggerated pressor response to exercise. However, evidence supporting the association between the magnitude of the pressor response to exercise and insulin resistance-related factors including hemoglobin A1c (HbA1c) or homeostatic model assessment of insulin resistance (HOMA-IR) in nondiabetic subjects has remained sparse and inconclusive. Thus we investigated the relationship between cardiovascular responses to exercise and insulin resistance-related factors in nondiabetic healthy men (n = 23) and women (n = 22) above 60 yr old. We measured heart rate (HR) and blood pressure (BP) responses during: isometric handgrip (IHG) exercise of 30% maximal voluntary contraction, a period of skeletal muscle ischemia (SMI) induced by tourniqueting the arm after IHG, and rhythmic dynamic handgrip (DHG) exercise during SMI. Greater diastolic BP (DBP) responses to DHG with SMI was associated with male sex (r = 0.44, P = 0.02) and higher HbA1c (r = 0.33, P = 0.03), heart-ankle pulse wave velocity (haPWV) (r = 0.45, P < 0.01), and resting systolic BP (SBP) (r = 0.36, P = 0.02). HbA1c persisted as a significant determinant explaining the variance in the DBP response to DHG with SMI in multivariate models despite adjustment for sex, haPWV, and resting SBP. It was also determined that the DBP response to DHG with SMI in a group in which HOMA-IR was abnormal (Δ33 ± 3 mmHg) was significantly higher than that of groups in which HOMA-IR was at intermediate (Δ20 ± 4 mmHg) and normal (Δ23 ± 2 mmHg) levels. These data suggest that even in nondiabetic older adults, insulin resistance is related to an exaggerated pressor response to exercise especially when performed under ischemic conditions.
NEW & NOTEWORTHY The diastolic blood pressure response to rhythmic dynamic handgrip exercise under ischemic conditions was demonstrated to be correlated with insulin resistance-related factors in nondiabetic older adults. This finding provides important insight to the prescription of exercise in this particular patient population as the blood pressure response to exercise, especially under ischemic conditions, could be exaggerated to nonsafe levels.
Keywords: exercise pressor reflex, muscle mechanoreflex, muscle metaboreflex, postexercise muscle ischemia, prediabetic state
INTRODUCTION
Type 2 diabetes mellitus (T2DM), one of the pathological consequences of insulin resistance, is a risk factor for the development of micro- and macrovascular diseases (12) as well as for peripheral and autonomic neuropathies (6). Increasing evidence suggests that physical training improves symptoms of T2DM (for review, see Refs. 33, 35). However, T2DM is characterized by an exaggerated arterial blood pressure (BP) response to exercise (7, 16, 19, 29). Since an abnormally large BP surge during physical activity could increase the risk for an adverse cardiovascular event (18, 30, 37, 41), exercise prescription for patients with T2DM is often limited in quality and/or quantity.
The sympathetic nervous system plays a crucial role in the regulation of the cardiovascular system during exercise. For example, increases in muscle sympathetic nerve activity (MSNA) contribute to elevations in BP by increasing peripheral vascular resistance during exercise (31). It has been reported that MSNA during mild-intensity exercise in patients with T2DM is associated with insulin resistance-related factors including fasting blood glucose concentration, hemoglobin A1c (HbA1c), and homeostatic model assessment of insulin resistance (HOMA-IR) (19). An earlier study demonstrated that even in nondiabetic young to middle-aged men, the BP response to low-intensity cycling exercise was correlated with HOMA-IR (7). However, it remains to be fully elucidated whether insulin resistance is associated with the magnitude of the BP response to constrictive exercise using small muscle mass, especially in nondiabetic older individuals. Understanding the relationship between insulin resistance and BP responsiveness during small muscle mass exercise in older individuals could be beneficial, because much of the physical activity performed in daily life by this population requires utilization of a small muscle mass to be completed, e.g., holding heavy groceries bags for several minutes. This is particularly applicable to a number of Western and Asian countries in which the older population is steadily growing. In addition, increasing our understanding of this relationship could guide the safe prescription of exercise. The latter could prove especially beneficial given that exercise training is known to slow the progression of disease in prediabetic individuals (24), resulting in reductions in mortality rates associated with T2DM. The purpose of this study was, therefore, to determine the relationship between the BP responses to handgrip exercise and insulin resistance-related factors in nondiabetic older adults. It was hypothesized that the BP response to handgrip exercise is significantly related to indices of insulin resistance such as HbA1c or HOMA-IR in nondiabetic older adults.
Two distinct neural control mechanisms, a feed-forward neural drive originating in higher brain centers (central command) and a peripheral reflex originating in exercising muscle (the exercise pressor reflex), are known to contribute to autonomic cardiovascular control during exercise (11). The latter is generated by activation of mechanically (muscle mechanoreflex) and chemically sensitive (muscle metaboreflex) muscle afferents (47). To test our hypothesis, we investigated the BP and heart rate (HR) responses to isometric handgrip exercise (IHG) followed by a period of experimentally-induced skeletal muscle ischemia (SMI). To ensure robust activation of the muscle mechanoreflex and metaboreflex, BP and HR responses to passive wrist movement (PAS) or rhythmic dynamic handgrip exercise (DHG) during SMI were likewise assessed.
MATERIALS AND METHODS
Subjects
Healthy female (n = 23) and male (n = 22) Japanese adults above 60 yr of age without diabetes, chronic heart, kidney, or liver disease, or peripheral arterial disease volunteered to participate in the study. All subjects completed a medical health history questionnaire. A physician examined their medical condition, medication, and family history. In addition, subjects also underwent screening that included a BP measurement by auscultation, an ECG, and an echocardiogram. Subjects with blood glucose levels above 126 mg/dL and HbA1c levels higher than 6.5% were excluded from the study as they were clinically diagnosed as T2DM individuals according to the Treatment Guide for Diabetes 2018–2019 by the Japan Diabetes Society (25). After the exclusion of subjects with both glucose and HbA1c levels above these limits, there were no subjects with blood glucose levels above 126 mg/dL although there remained 10 subjects with HbA1c levels higher than 6.5% (3). The latter were treated as non-diabetic because they had not been diagnosed as diabetics nor had they received medical treatment for diabetes despite periodic health examinations by licensed health professionals.
In the present study, 25 subjects were classified as hypertensive as they met the guidelines established by the 2019 Guidelines for the Management of Hypertension by the Japanese Society of Hypertension [systolic BP (SBP) ≥ 140 and/or diastolic BP (DBP) ≥ 90 mmHg] (53) and/or they took antihypertensive drugs (ANG II receptor blocker, n = 7; ANG II receptor blocker + DHP calcium antagonist, n = 2; ANG II receptor blocker + diuretic, n = 1; DHP calcium antagonist, n = 6; α-blocker, n = 1).
All subjects provided written consent after being informed of the experimental protocol and possible risks involved with their participation. Approval of this study was given by the Ethics Committee of Chubu University (290077–2).
Protocol
During the 24 h before experimentation, the subjects were asked to avoid alcohol and caffeine intake, as well as unusually long or heavily intense exercise. On the day of the experiments, subjects came to the laboratory between 9:00 AM and 9:30 AM after at least 12.5-h fasting and without taking any morning medications. First, a physician or nurse withdrew ~5 mL of blood from the antecubital vein to evaluate insulin resistance-related factors, blood lipids, renal function, and oxidative stress. Second, resting BP and HR were measured, and heart-ankle pulse wave velocity (haPWV) was assessed to ascertain arterial stiffness. Third, BP and HR responses to handgrip exercise was obtained to elucidate the relationships between insulin resistance-related factors and exercise BP. Finally, minute ventilation (V̇e) and oxygen uptake (V̇o2) were measured during incremental cycling exercise to calculate the oxygen uptake efficiency slope (OUES) (4), which was subsequently used as an index of cardiorespiratory fitness. Before beginning the experimental sessions, subjects were familiarized with all equipment and procedures. Room temperature was kept at ~25°C for all experiments.
Assessment of the Cardiovascular Response to Handgrip Exercise
Systolic and diastolic BP measurements (SBP and DBP) were obtained from the subject’s right upper arm every minute using an electrosphygmomanometer (Tango+, Sun Tech Medical Instruments). The Sun Tech Tango+ is an oscillometric device so SBP and DBP are directly measured. An ECG recording was acquired continuously to assess HR (Tango+).
Sitting in a comfortable chair, the subjects performed IHG for 1 min using their left hand after baseline measurements were obtained. Subjects maintained gripping force at ~30% of maximal voluntary contraction (MVC) by utilizing a visual feedback system. Force measurements were processed by a hand dynamometer (MLT004/ST, ADInstruments, New Zealand), A/D converter, and application software (PowerLab ML4856 26T and Chart v8.1, ADInstruments). About 5 s before terminating IHG, a tourniquet-like cuff (SC5, Hokanson) was inflated using a rapid cuff inflator and air source (E20 and AG101, Hokanson) on the left upper arm with a pressure of 250 mmHg to evoke a 3-min period of skeletal muscle ischemia (SMI). During the first 1 min of SMI, the subject rested to isolate the effects of muscle metaboreflex activation. During the next 1 min of SMI, the experimenter fully flexed and extended the subject’s left wrist one time per second. This passive movement (PAS) was designed to stimulate the muscle mechanoreflex (39). During the last 1 min of SMI, the subject performed low-intensity rhythmic dynamic handgrip exercise (DHG) to further engage the muscle mechanoreflex. In this final paradigm, subjects were asked to simply flex and extend fully their left hand once per second with minimal force development. The rationale for combining PAS or DHG with SMI was to facilitate activation of the muscle mechanoreflex (see Methodological Considerations for more detailed information).
The Borg scale (5) was used to obtain ratings of perceived exertion (RPE) immediately after the end of IHG and SMI + DHG.
Hemanalysis and Measurement of haPWV and OUES
Hemanalysis.
By using whole blood samples, blood glucose level and HbA1c were measured by Antsense Duo (Horiba, Japan) and HbA1c 501 blood analysis system (HemoCue, Sweden), respectively.
The blood sample was allowed to clot for 20 min at room temperature and then centrifuged at 2,000 rpm for 10 min at room temperature to obtain serum. To evaluate oxidative stress level, we determined diacron-reactive oxygen metabolites (d-ROMs), the main measurement target of which is hydroperoxide (2), and biological antioxidant potential (BAP) that has the capacity to reduce iron from Fe3+ to Fe2+ (13). These were measured by spectrophotometric assay using a free radical analyzer (FREE Carrio Duo, WISMERLL, Japan). BAP and d-ROMs were expressed to reflect systemic redox status, i.e., BAP/d-ROMs (52).
The rest of serum sample was stored at −20°C, then sent to a commercial laboratory (SRL Inc., Japan) to measure insulin, LDL and HDL cholesterol, triglyceride, and creatinine. The homeostatic model assessment of insulin resistance (HOMA-IR) was used to assess insulin resistance. HOMA-IR was calculated as (blood glucose level × insulin)/22.5.
Arterial stiffness.
Pulse wave velocity from the aortic annulus (e.g., heart) to ankle (i.e., haPWV), an index of systemic arterial stiffness, was measured with a semiautomated vascular screening system (Vasera 1500 N, Fukuda Denshi, Japan) in the supine position after resting, as previously reported (50, 51). Briefly, an ECG, a phonocardiogram via a microphone placed on the left edge of the sternum (PCG), and arterial pressure waveforms of right brachial and both posterior-tibial arteries via air-plethysmographic sensors were recorded at the same instant. The characteristic points of ECG, PCG, and waveforms of arterial pressure were automatically detected. haPWV was calculated by dividing arterial path length from the aortic annulus to the midpoint of the right ankle cuff by the sum of two time intervals: 1) between the commencement of the second heart sound and the dicrotic notch on the right brachial arterial pressure wave; and 2) between brachial and posterior-tibial arterial pressure waves. Mean values of both ankles’ haPWV were used for assessment.
Cardiorespiratory fitness.
Using an ergometer (75XL III, Konami Co Ltd., Japan), subjects performed a leg cycling exercise test with an incremental ramp protocol. After a 3-min warm-up stage at 0 W, the load was increased by 20 W every minute until the subject’s HR exceeded ~80% of maximal predicted HR. The subjects maintained ~60 rpms throughout. In two subjects, the test was terminated by the attending physician, using the appropriate ACC/AHA guidelines (15), before reaching 80% of maximal predicted HR. During the test, V̇e and V̇o2 were measured continuously on a breath-by-breath basis with a metabolic analyzer (AE-310S, Minato Medical Science, Japan).
During incremental exercise testing, the following regression equation was used to determine OUES: V̇o2 = alog10V̇e + b, where a and b were constants, and a was defined as OUES according to methods described previously (4). The reason OUES was adopted as an index of cardiorespiratory fitness was that it has been reported to be correlated with maximal V̇o2 (V̇o2max) even when calculated from data measured at submaximal intensities. In other words, fitness levels can be assessed accurately without necessarily obtaining a true V̇o2max during testing (4).
Statistical Analysis
An a priori statistical power analysis was performed to determine the sample size needed for the study. Based on a previous investigation (19), we estimated that the correlation coefficients (r) of the relationships between the pressor responses to handgrip exercise and insulin resistance-related factors were 0.6–0.7. From this analysis, it was determined that a minimal sample size of 41 participants was needed to achieve the power of more than 80% (1 − β) required to reject the null hypothesis when r was 0.65 at an α of 0.05 using a Pearson’s correlation.
We assessed the relationships between the pressor response to exercise and clinical variables using Pearson’s correlations, except for the relationship with sex where a Spearman’s correlation was used. If a significant correlation between the pressor response to exercise and insulin resistance-related factors was detected, a multivariate analysis was performed by adjusting all indices that were significantly correlated with the pressor response in the univariate model. One-way ANOVAs with or without repeated measures, as appropriate, were also performed. If a significant F-value was observed with ANOVA, a post hoc Bonferroni test was used to identify specific differences. For ratio comparisons, a chi-square test was used. For power analysis, we used G* Power 3.1.9.7. All data were expressed as means ± SD. Statistical analyses were performed using StatView 5.0 and SPSS 24.0 software. The level of significance was set at P < 0.05.
RESULTS
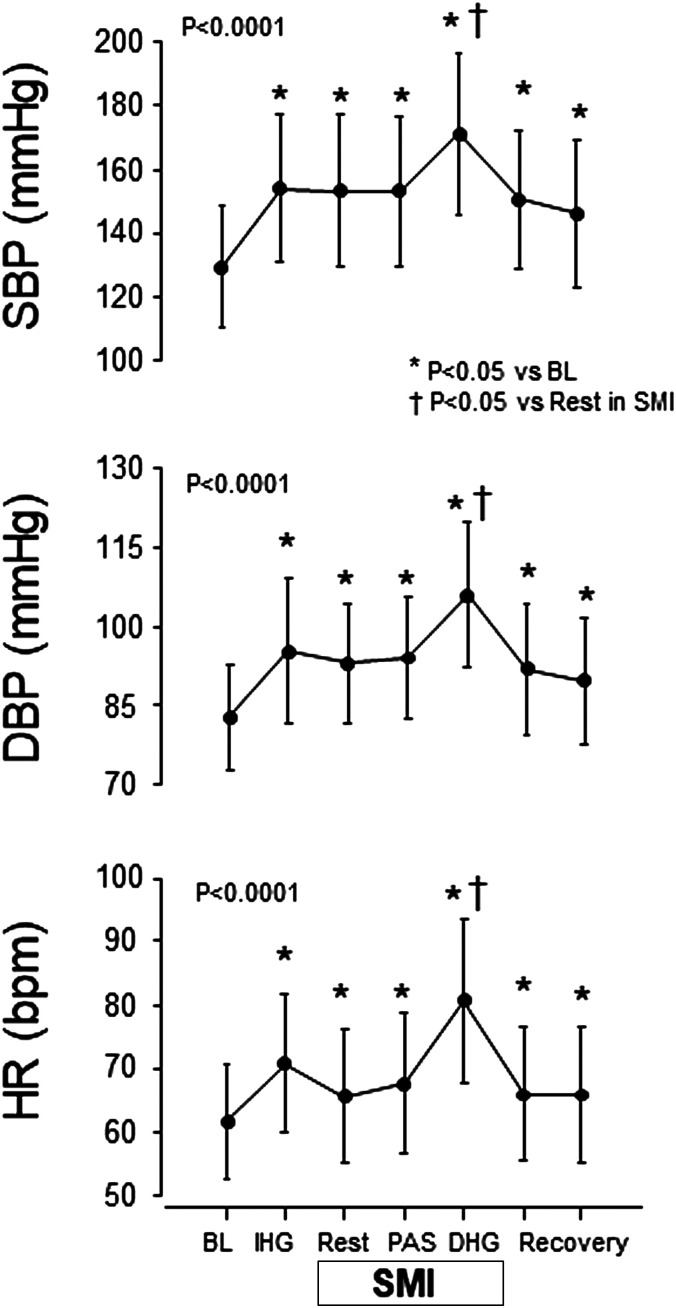
In comparison with baseline, SBP, DBP, and HR during IHG, SMI, SMI + PAS, and SMI + DHG significantly increased (Fig. 1). PAS (SMI+ PAS) did not significantly increase SBP, DBP, or HR compared with SMI alone; however, DHG (SMI + DHG) significantly augmented these parameters compared with SMI alone (Fig. 1). Mean MVC force of the subjects was 263 ± 97 N. Mean absolute force during IHG was 78 ± 29 N (30 ± 1%MVC) and during SMI + DHG was 22 ± 9 N (9 ± 4%). Mean RPE during IHG or SMI + DHG was 12.3 ± 1.4 and 15.1 ± 2.0, respectively.
Fig. 1.
Systolic blood pressure (SBP), diastolic blood pressure (DBP) and heart rate (HR) responses to isometric handgrip exercise (IHG), skeletal muscle ischemia (SMI), passive wrist movement (PAS) during SMI, and rhythmic dynamic handgrip exercise (DHG) during SMI. A one-way ANOVA with repeated measures resulted in a significant F-value (P < 0.001). *P < 0.05 compared with baseline (BL). †P < 0.05 compared with SMI alone. Values are means and SD.
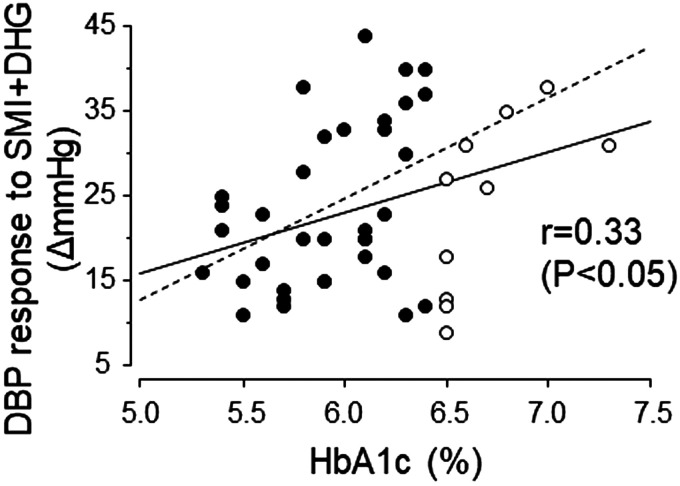
Table 1 shows single correlations between exercise BP and clinical parameters. The DBP response (i.e., the change in DBP evoked by SMI + DHG from baseline) was positively correlated with HbA1c (Fig. 2). With regard to the latter, even when using data only from subjects with an HbA1c less than 6.5% (3), a significant correlation was achieved (Fig. 2). The DBP response to SMI + DHG was also significantly correlated with sex, haPWV, and resting SBP. We used these parameters as adjusted variables for multivariate models. Importantly, even if we adjusted all combinations of these factors, HbA1c persisted as a significant determinant explaining the variance in the DBP response to SMI + DHG (Table 2).
Table 1.
Results of single correlation analysis between exercise blood pressure and clinical variables
| SBP Response (Δ mmHg) |
DBP Response (Δ mmHg) |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IHG |
SMI |
SMI+PAS |
SMI+DHG |
IHG |
SMI |
SMI+PAS |
SMI+DHG |
||||||||||||
| Variables | Mean | SD | r | P | r | P | r | P | r | P | r | P | r | P | r | P | r | P | |
| Age, yr | 70.0 | 6.1 | 0.13 | 0.39 | 0.04 | 0.79 | −0.05 | 0.75 | 0.24 | 0.11 | 0.17 | 0.27 | −0.18 | 0.24 | −0.08 | 0.62 | 0.17 | 0.25 | |
| Sex | F = 0 (51%), | 0.17 | 0.75 | 0.03 | 0.74 | 0.12 | 0.95 | 0.08 | 0.72 | 0.42 | 0.03 | 0.18 | 0.67 | 0.08 | 0.68 | 0.44 | 0.02 | ||
| M = 1 (49%) | |||||||||||||||||||
| Cigarettes, number/day | 1.2 | 4.2 | −0.03 | 0.86 | −0.01 | 0.94 | −0.03 | 0.82 | −0.08 | 0.58 | 0.12 | 0.42 | 0.29 | 0.06 | 0.01 | 0.97 | −0.01 | 0.97 | |
| BMI, kg/m2 | 22.5 | 2.4 | −0.15 | 0.33 | −0.05 | 0.76 | −0.01 | 0.94 | 0.07 | 0.66 | −0.08 | 0.59 | 0.03 | 0.87 | 0.27 | 0.08 | 0.22 | 0.14 | |
| Triglycerides, mg/dL | 109.5 | 62.5 | −0.14 | 0.37 | 0.02 | 0.88 | −0.04 | 0.80 | −0.02 | 0.89 | −0.16 | 0.28 | −0.04 | 0.77 | 0.09 | 0.54 | 0.10 | 0.50 | |
| LDL cholesterol, mg/dL | 128.9 | 39.5 | −0.17 | 0.27 | 0.00 | 0.98 | 0.18 | 0.24 | 0.07 | 0.64 | −0.19 | 0.22 | 0.29 | 0.05 | 0.37 | 0.01 | 0.05 | 0.72 | |
| HDL cholesterol, mg/dL | 64.8 | 16.7 | 0.40 | <0.01 | 0.29 | 0.05 | 0.00 | 0.98 | 0.09 | 0.54 | 0.11 | 0.46 | 0.08 | 0.61 | −0.12 | 0.43 | −0.21 | 0.17 | |
| Creatinine, mg/dL | 0.8 | 0.2 | 0.14 | 0.37 | −0.09 | 0.54 | 0.03 | 0.83 | −0.03 | 0.82 | 0.23 | 0.12 | −0.03 | 0.85 | −0.16 | 0.29 | 0.22 | 0.15 | |
| BAP/d-ROMs, (μmol/L)/(U.CARR) | 6.9 | 1.7 | 0.08 | 0.60 | −0.03 | 0.84 | 0.03 | 0.82 | −0.02 | 0.91 | 0.27 | 0.07 | −0.04 | 0.80 | −0.24 | 0.11 | 0.15 | 0.32 | |
| OUES, L/min | 1610.6 | 505.5 | 0.04 | 0.78 | 0.05 | 0.75 | 0.21 | 0.17 | 0.03 | 0.87 | 0.15 | 0.34 | 0.18 | 0.24 | 0.21 | 0.16 | 0.28 | 0.06 | |
| haPWV, cm/ms | 0.8 | 0.1 | 0.41 | <0.01 | 0.30 | 0.04 | 0.26 | 0.09 | 0.27 | 0.07 | 0.52 | <0.01 | 0.05 | 0.76 | −0.02 | 0.87 | 0.45 | <0.01 | |
| Resting circulatory variables | |||||||||||||||||||
| SBP, mmHg | 139.4 | 18.2 | 0.14 | 0.36 | 0.19 | 0.21 | 0.22 | 0.15 | 0.32 | 0.03 | 0.25 | 0.10 | −0.01 | 0.93 | 0.18 | 0.24 | 0.36 | 0.02 | |
| DBP, mmHg | 88.0 | 10.8 | 0.02 | 0.88 | 0.06 | 0.69 | 0.13 | 0.40 | 0.18 | 0.25 | 0.14 | 0.34 | 0.01 | 0.95 | 0.02 | 0.90 | 0.10 | 0.53 | |
| HR, beats/min | 61.9 | 9.5 | 0.21 | 0.17 | 0.11 | 0.45 | 0.07 | 0.67 | −0.01 | 0.95 | 0.08 | 0.61 | 0.07 | 0.63 | 0.09 | 0.56 | −0.03 | 0.85 | |
| Insulin resistance-related factors | |||||||||||||||||||
| Blood glucose, mg/dL | 96.4 | 12.9 | −0.07 | 0.64 | −0.06 | 0.67 | 0.02 | 0.88 | 0.06 | 0.69 | 0.01 | 0.95 | −0.18 | 0.25 | −0.11 | 0.48 | 0.17 | 0.26 | |
| HbA1c, % | 6.1 | 0.4 | 0.01 | 0.92 | 0.04 | 0.81 | 0.01 | 0.94 | 0.05 | 0.72 | −0.15 | 0.33 | 0.00 | 1.00 | −0.06 | 0.70 | 0.33 | 0.03 | |
| Insulin, μIU/mL | 5.9 | 4.0 | −0.18 | 0.23 | −0.23 | 0.13 | −0.17 | 0.25 | −0.22 | 0.14 | −0.03 | 0.87 | 0.01 | 0.96 | 0.09 | 0.58 | 0.21 | 0.16 | |
| HOMA-IR | 1.4 | 1.0 | −0.20 | 0.18 | −0.25 | 0.10 | −0.17 | 0.25 | −0.19 | 0.21 | −0.04 | 0.80 | −0.04 | 0.78 | 0.04 | 0.79 | 0.25 | 0.10 | |
Overall P values were assessed by the Spearman’s or Pearson’s correlation coefficient. The number of current smokers was four (8.9%). F, female; M, male; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; BAP, biological antioxidant potential; d-ROMs, diacron-reactive oxygen metabolites; BAP/d-ROMs, index of system redox states; OUES, oxygen uptake efficiency slope, index of cardiorespiratory fitness; haPWV, heart-ankle pulse wave velocity, index of arterial stiffness; HR, heart rate; HbA1c, glycated hemoglobin A1c, HOMA-IR, homeostasis model assessment-estimated insulin resistance. U.CARR, Carratelli Units (1 U = 0.08 mg/dL H2O2). Boldface values represent significant difference.
Fig. 2.
Association between the diastolic blood pressure (DBP) response to rhythmic dynamic handgrip exercise (DHG) during skeletal muscle ischemia (SMI) and glycated hemoglobin A1c (HbA1c). The regression line for data from all subjects (solid line) is Y = 7.1X − 19.7 (R2 = 0.11). The regression line for data from subjects with an HbA1c of less than 6.5% (dotted line) (r = 0.41, P = 0.01) is Y = 11.9X − 47.0 (R2 = 0.17). ○, HbA1c greater than or equal to 6.5%; ●, HbA1c less than 6.5%.
Table 2.
Association between glycated hemoglobin A1c (HbA1c) and the change in diastolic blood pressure (ΔDBP) in response to rhythmic dynamic handgrip exercise (DHG) during skeletal muscle ischemia (SMI) after adjustment for covariates
| Model | Variables Included in the Model | β | SE | P Value | Model R2 |
|---|---|---|---|---|---|
| Unadjusted | 7.122 | 3.109 | 0.027 | 0.088 | |
| 1 | Sex | 8.364 | 2.842 | 0.005 | 0.252 |
| 2 | Sex, haPWV | 7.062 | 2.763 | 0.014 | 0.322 |
| 3 | Sex, haPWV, SBPrest | 7.064 | 2.761 | 0.014 | 0.323 |
β represents unstandardized regression coefficient, and R2 represents a measure for the model prediction. Adjusted βs (SE) is ΔDBP in mmHg that is associated with a 1% increase in HbA1c. haPWV, heart-ankle pulse wave velocity; SBPrest, systolic blood pressure at rest. The adjusted factors were chosen because significant single correlations were detected with DBP response to SMI + DHG (Table 1).
Mean relative force during IHG did not significantly correlate with SBP (r = 0.05, P = 0.75) or DBP responses (r = −0.15, P = 0.33). Likewise, relative force during SMI + DHG was not associated with SBP (r = 0.14, P = 0.36) or DBP (r = 0.17, P = 0.26). There was no significant relationship between RPE during IHG or SMI + DHG and SBP (r = 0.17, P = 0.27; r = 0.10, P = 0.50, respectively) or DBP (r = −0.03, P = 0.84; r = 0.22, P = 0.14, respectively).
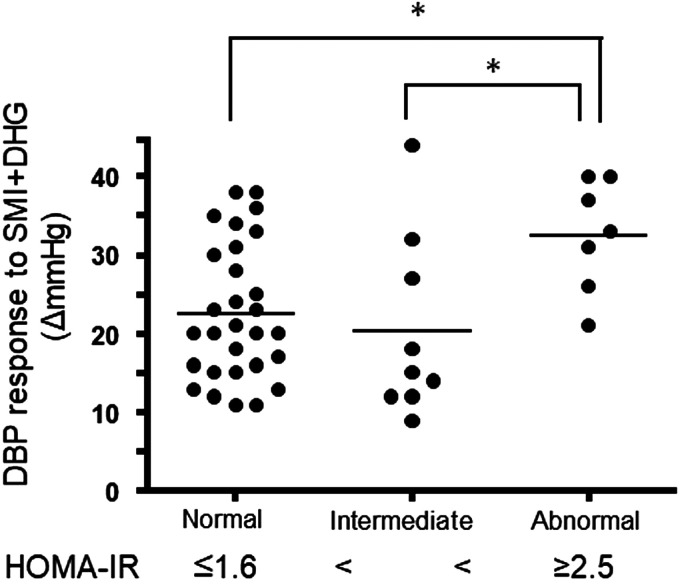
Although not significant, the DBP response to SMI + DHG tended to be correlated with HOMA-IR (r = 0.25, P = 0.10) (Table 1). Given this trend, further analysis was performed. Subjects were divided into three groups according to HOMA-IR classifications defined in the Treatment Guide for Diabetes 2018–2019 by the Japan Diabetes Society (25) as well as previous studies in Japanese subjects (45, 55): normal group (≤1.6, n = 29); intermediate or borderline group (>1.6–<2.5, n = 9); and abnormal or insulin-resistant group (≥2.5, n = 7) (Fig. 3). There was no significant differences in age, mean relative force, and RPE during SMI + DHG among the groups (P = 0.93, 0.93, and 0.65, respectively). Further, there were no significant differences in male to female ratio, percentage of hypertensive subjects or smokers among groups (P = 0.32, 0.50, and 0.16, respectively). Importantly, however, the DBP response to SMI + DHG in the abnormal insulin-resistant group was significantly higher than in the normal and intermediate groups (Fig. 3).
Fig. 3.
Comparison of the diastolic blood pressure (DBP) response to rhythmic dynamic handgrip exercise (DHG) during skeletal muscle ischemia (SMI) between three homeostasis model assessment-estimated insulin resistance (HOMA-IR) groups. A one-way ANOVA resulted in a significant F-value (P = 0.02). *Significant difference between groups (P < 0.05). The dots and bars illustrate individual and mean values, respectively.
DISCUSSION
The major findings of the present study are as follows. First, HbA1c was a significant factor accounting for the variance in the DBP response to SMI + DHG. Second, the DBP response to SMI + DHG in the group with abnormally high HOMA-IR was significantly greater than that of groups with normal and intermediate HOMA-IR. Collectively, the present study is the first to identify a significant positive correlative relationship between insulin resistance and the BP response to dynamic exercise during ischemic conditions in nondiabetic older adults. Importantly, the relationship suggests that as insulin resistance becomes greater so too does the exaggeration in the pressor response to ischemic exercise.
Potential Mechanisms that May Contribute to the Relationship between Insulin Resistance and the Augmented Pressor Response to Ischemic Exercise
Although causation cannot be inferred from correlative relationships, it is tempting to speculate as to the potential mechanisms that could underlie an augmented DBP response to ischemic exercise in nondiabetic insulin-resistant individuals. It has been well documented that the cardiovascular response to exercise is significantly regulated by central command and the exercise pressor reflex (i.e., the muscle mechano- and metaboreflexes) (11, 47). In the present study, the pressor responses to IHG (a condition in which both central command and the exercise pressor reflex were engaged) or SMI (a paradigm in which the muscle metaboreflex was isolated) were not correlated with any insulin resistance-related factors. As such, it would seem logical to conclude that insulin-resistant states in nondiabetic older adults likely do not influence these neural regulatory mechanisms under conditions in which muscle is normally perfused.
In contrast, pressor responses to SMI + DHG (an ischemic condition in which both the muscle metaboreflex and mechanoreflex were selectively engaged) were significantly related to HbA1c as well as HOMA-IR in the current study. Speculatively, this suggests that insulin-resistant states in nondiabetic older adults may facilitate ischemia-induced augmentations in the expression of the exercise pressor reflex. In support of this concept, recent in vitro and ex vivo studies in healthy, nondiabetic rodents demonstrated that insulin augments the mechanical responsiveness of small dorsal root ganglion neurons (soma of thin fiber afferents) while also sensitizing group IV muscle afferents to mechanical stimuli (20). An additional in vitro study reported that extracellular fluid high in glucose lowered the mechanical threshold of group IV afferent fibers and increased their responsiveness to mechanical stimulation in streptozotocin-induced diabetic rats (49).
It is possible that the augmented BP response to ischemic handgrip exercise observed in insulin-resistant subjects was related to acute ischemic muscle pain, although this was not evaluated in the present study. A previous study has shown an association between insulin resistance and mechanical hyperalgesia in rats (14). As such, it is possible that the degree of ischemic pain may have been altered by the level of insulin resistance in the current investigation even in nondiabetic subjects absent of chronic neuropathic pain. Because at least some thin-fiber muscle afferents may play a dual role in the expression of the exercise pressor reflex serving as both an ergoreceptor (transmitting metabolic and mechanical information) and a nociceptor (transmitting pain information) (16, 42, 48), it is feasible that the pressor responses to SMI + DHG could have been significantly influenced by insulin resistance-related factors. Further studies are warranted to address this possibility.
It is reasonable to ask whether central command may also be augmented in nondiabetic older adults during SMI + DHG trials. Historically, it has been generally accepted that the degree of central command engagement is related to an individual’s effort sense during exercise (54). In the present study, RPE as well as mean relative force during SMI + DHG were not significantly correlated with the DBP response to SMI + DHG. These findings suggest that it is unlikely that the central command associated with volitional effort significantly contributed to the heightened BP responses observed in the present study. Evidence also suggests that insulin resistance is positively related to the level of oxidative stress (10). Moreover, others have suggested that central command is affected by the levels of oxidative stress within the cardiovascular centers that regulate its function (34). In a related fashion, oxidative stress is likewise known to contribute to the exaggerated pressor response to exercise (16, 34). In the present study, BAP/d-ROMs were measured as an index of redox status. However, the pressor response to SMI + DHG was not correlated with BAP/d-ROMs. Given these factors, it is unlikely that insulin resistance in nondiabetic older adults contributes significantly to the augmentation in the pressor response to ischemic exercise via alterations in either central command or oxidative stress.
Although it is not unusual in itself that DBP and SBP relationships become uncoupled (in the case of the current study only DBP responses to ischemic exercise were demonstrated to be related to insulin resistance) (7), the reasons are not readily apparent in the present investigation. One possibility is that the DBP response to exercise could have been influenced by changes in peripheral vascular resistance more so than SBP (44). It is plausible, although highly speculative, that insulin-resistant states contributed to the enhanced activation of the sympathetic nervous system via ischemia-induced augmentations in exercise pressor reflex activity in the present study. As a result, increases in peripheral vascular resistance might have influenced the relationship between the DBP response to SMI+DHG and insulin resistance-related factors. Alternatively, an acknowledged technical limitation may have played a role. In this study, BP was measured every 1 min by the electrosphygmomanometer. It is possible that SBP was detected before being influenced by the continuous physiological changes induced by SMI + DHG and thus SBP responses might have been underestimated. Future studies utilizing beat-to beat BP measurements are warranted to clarify this issue.
Methodological Considerations
Since the pressor response to passive wrist movement has been reported to be small in healthy subjects (39), it is technically difficult to fully activate and isolate the muscle mechanoreflex in humans. Animal studies suggest that thin-fiber muscle afferents might be sensitized to mechanical stimuli by accumulated metabolites and/or acidosis produced by muscle contractions (1, 17, 21, 22, 43). As such, to intensify the expression of the muscle mechanoreflex, we combined PAS with SMI utilizing a method previously described in humans (9). Unexpectedly, BP and HR responses to SMI + PAS did not statistically increase from those of SMI alone in the present study. This may have occurred for the following reasons. The PAS utilized was not truly passive muscle stretch but rather passive muscle movement. Moreover, the method of PAS used did not cause flexion-extension of the hand but rather the wrist. Use of this method might not have generated a mechanical stimulus sufficient enough to activate the muscle mechanoreflex even under ischemic condition. Given these possibilities, it is acknowledged that the finding is inconsistent with a previous study that demonstrated increases in MSNA and BP, compared with rest, in response to passive stretch of the wrist during postexercise muscle ischemia (9). In the latter study (9), the maximal force evoked without inducing pain during stretch was utilized. Unfortunately, in the current study the maximal force generated during PAS was not determined. Alternatively, the SMI + PAS protocol utilized might not have stimulated the muscle mechanoreflex to the extent needed to overcome the changes in BP induced by SMI alone.
Commonly, IHG is performed for 2 min to evaluate changes in BP and MSNA in response to the exercise. This 2 min period is likewise utilized to sufficiently engage the muscle metaboreflex which is then often isolated during postexercise SMI (19). In contrast, the period of IHG utilized in the current investigation was only 1 min. The rationale for this modification of the more standard 2-min period of IHG was to minimize the risk of an adverse cardiovascular event occurring in the older adult population studied. This caution was precipitated by preliminary findings that 1 min of IHG and SMI following 1 min of IHG induced markedly exaggerated increases in BP in representatives from the subject population investigated. Regardless, it is acknowledged that the reduction in exercise time may have mitigated the expression of the sympathoexcitatory response as well as limited the production of metabolites (19).
It is also acknowledged that some participants in the study were active smokers while others were hypertensive. Clearly, in such individuals the cardiovascular responses noted could have been affected by these factors (38, 40). That being stated, statistical analysis determined that smoking was not significantly related to the pressor responses observed in the present study. This finding is consistent with an earlier report demonstrating that DBP changes during cycling exercise were positively correlated with insulin resistance (7). Moreover, in agreement with a previous study (36), resting BP was significantly associated with the pressor response to exercise in the current investigation. Importantly, even when BP at rest was taken into account and adjusted, the contribution ratio of HbA1c explaining the DBP response to SMI + DHG was unchanged in multivariate analyses. Hence, it is reasonable to conclude that the finding of the present study in which insulin resistance affects the pressor response to ischemic exercise is unaffected by the possibility that smoking and/or hypertension may likewise impact blood pressure responses to physical activity (38, 40).
Clinical Implications
Evidence suggests that physical exercise reduces the risk of disease in individuals with prediabetes (24) whereas the chances for development of T2DM are enhanced with a more sedentary lifestyle. Aerobic exercise such as jogging or walking is more commonly prescribed in clinical practice rather than forms of resistance exercise (46). However, diabetes is an independent risk factor for low muscular strength. As such, a position statement by the American Diabetes Association recommends resistance training (e.g., static/ischemic) as well for T2DM patients (8). Combining endurance exercise with resistance exercise may provide greater improvements (46), and high-intensity interval training may be superior to continuous aerobic training in adults with diabetes (26). More interestingly, engaging in ischemic exercise could be an effective strategy to mitigate insulin resistance. For instance, previous studies have demonstrated that traditional high-intensity resistance training, low-intensity exercise with blood flow restriction, and high-intensity interval training reduce insulin resistance (23, 27, 28). However, it has also been suggested that abnormally large increases in BP during exercise in insulin resistant individuals could contribute to the development of hypertension (7) and dementia (32). Moreover, it is well known that exaggerated BP responses to physical activity increase the risk of exercise-related adverse cardiac and/or cerebrovascular events (18, 37). It is suggested that these findings, along with those of the current investigation, be considered when prescribing exercise training programs for insulin-resistant nondiabetic older adults.
Conclusion
The data demonstrate that insulin resistance-related factors are positively related to the pressor response to ischemic dynamic exercise in nondiabetic older adults. This relationship is important as it suggests that the prescription of exercise for older adults should be made taking their insulin-resistant status into account even if T2DM has yet to manifest.
GRANTS
This work was supported in part by JSPS KAKENHI JP17K01769 (to N. Hotta), Health Science Center Foundation (to N. Hotta), Short-term Research Project from the Research Institute of Life and Health Sciences of Chubu University (to N. Hotta), Nakatomi Foundation (to N. Hotta), the Lawson & Rogers Lacy Research Fund in Cardiovascular Disease (to J. H. Mitchell), Southwestern School of Health Professions Interdisciplinary Research Grant Program (to M. Mizuno), and the National Heart, Lung, and Blood Institute (R01HL-151632 to M. Mizuno).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.H. and M.M. conceived and designed research; N.H., A.H., Y.O., R.B., and H.W. performed experiments; N.H., A.H., J.S., J.W., and M.M. analyzed data; N.H., A.H., Y.O., R.B., H.W., J.S., W.V., H.-K.K., R.I., G.A.I., J.H.M., S.A.S., and M.M. interpreted results of experiments; N.H., A.H., and M.M. prepared figures; N.H. and M.M. drafted manuscript; J.S., W.V., J.W., H.-K.K., R.I., G.A.I., J.H.M., S.A.S., and M.M. edited and revised manuscript; N.H., A.H., Y.O., R.B., H.W., J.S., W.V., J.W., H.-K.K., R.I., G.A.I., J.H.M., S.A.S., and M.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Kimie Kato, Mai Sugie, Miho Makino, Daichi Okumura, and Tatsuya Kato for their assistance.
REFERENCES
- 1.Adreani CM, Kaufman MP. Effect of arterial occlusion on responses of group III and IV afferents to dynamic exercise. J Appl Physiol (1985) 84: 1827–1833, 1998. doi: 10.1152/jappl.1998.84.6.1827. [DOI] [PubMed] [Google Scholar]
- 2.Alberti A, Bolognini L, Macciantelli D, Caratelli M. The radical cation of N,N-diethl-para-phenylendiamine: A possible indicator of oxidative stress in biological samples. Res Chem Intermed 26: 253–267, 2000. doi: 10.1163/156856700X00769. [DOI] [Google Scholar]
- 3.American Diabetes Association . Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care 43, Suppl 1: S14–S31, 2020. doi: 10.2337/dc20-S002. [DOI] [PubMed] [Google Scholar]
- 4.Baba R, Nagashima M, Goto M, Nagano Y, Yokota M, Tauchi N, Nishibata K. Oxygen uptake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J Am Coll Cardiol 28: 1567–1572, 1996. doi: 10.1016/S0735-1097(96)00412-3. [DOI] [PubMed] [Google Scholar]
- 5.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Boulton AJM, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D; American Diabetes Association . Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 28: 956–962, 2005. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 7.Brett SE, Ritter JM, Chowienczyk PJ. Diastolic blood pressure changes during exercise positively correlate with serum cholesterol and insulin resistance. Circulation 101: 611–615, 2000. doi: 10.1161/01.CIR.101.6.611. [DOI] [PubMed] [Google Scholar]
- 8.Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, Horton ES, Castorino K, Tate DF. Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care 39: 2065–2079, 2016. doi: 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui J, Mascarenhas V, Moradkhan R, Blaha C, Sinoway LI. Effects of muscle metabolites on responses of muscle sympathetic nerve activity to mechanoreceptor(s) stimulation in healthy humans. Am J Physiol Regul Integr Comp Physiol 294: R458–R466, 2008. doi: 10.1152/ajpregu.00475.2007. [DOI] [PubMed] [Google Scholar]
- 10.Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal 7: 1040–1052, 2005. doi: 10.1089/ars.2005.7.1040. [DOI] [PubMed] [Google Scholar]
- 11.Fadel PJ. Reflex control of the circulation during exercise. Scand J Med Sci Sports 25, Suppl 4: 74–82, 2015. doi: 10.1111/sms.12600. [DOI] [PubMed] [Google Scholar]
- 12.Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes 26: 77–82, 2008. doi: 10.2337/diaclin.26.2.77. [DOI] [Google Scholar]
- 13.Fukui T, Yamauchi K, Maruyama M, Yasuda T, Kohno M, Abe Y. Significance of measuring oxidative stress in lifestyle-related diseases from the viewpoint of correlation between d-ROMs and BAP in Japanese subjects. Hypertens Res 34: 1041–1045, 2011. doi: 10.1038/hr.2011.76. [DOI] [PubMed] [Google Scholar]
- 14.García G, Gutiérrez-Lara EJ, Centurión D, Granados-Soto V, Murbartián J. Fructose-induced insulin resistance as a model of neuropathic pain in rats. Neuroscience 404: 233–245, 2019. doi: 10.1016/j.neuroscience.2019.01.063. [DOI] [PubMed] [Google Scholar]
- 15.Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, Mark DB, McCallister BD, Mooss AN, O’Reilly MG, Winters WL, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Russell RO, Smith SC; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Committee to Update the 1997 Exercise Testing Guidelines . ACC/AHA 2002 guideline update for exercise testing: Summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to update the 1997 exercise testing guidelines). J Am Coll Cardiol 40: 1531–1540, 2002. doi: 10.1016/S0735-1097(02)02164-2. [DOI] [PubMed] [Google Scholar]
- 16.Grotle A-K, Stone AJ. Exaggerated exercise pressor reflex in type 2 diabetes: Potential role of oxidative stress. Auton Neurosci 222: 102591, 2019. doi: 10.1016/j.autneu.2019.102591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayes SG, Kindig AE, Kaufman MP. Cyclooxygenase blockade attenuates responses of group III and IV muscle afferents to dynamic exercise in cats. Am J Physiol Heart Circ Physiol 290: H2239–H2246, 2006. doi: 10.1152/ajpheart.01274.2005. [DOI] [PubMed] [Google Scholar]
- 18.Hoberg E, Schuler G, Kunze B, Obermoser A-L, Hauer K, Mautner H-P, Schlierf G, Kübler W. Silent myocardial ischemia as a potential link between lack of premonitoring symptoms and increased risk of cardiac arrest during physical stress. Am J Cardiol 65: 583–589, 1990. doi: 10.1016/0002-9149(90)91034-4. [DOI] [PubMed] [Google Scholar]
- 19.Holwerda SW, Restaino RM, Manrique C, Lastra G, Fisher JP, Fadel PJ. Augmented pressor and sympathetic responses to skeletal muscle metaboreflex activation in type 2 diabetes patients. Am J Physiol Heart Circ Physiol 310: H300–H309, 2016. doi: 10.1152/ajpheart.00636.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hotta N, Katanosaka K, Mizumura K, Iwamoto GA, Ishizawa R, Kim HK, Vongpatanasin W, Mitchell JH, Smith SA, Mizuno M. Insulin potentiates the response to mechanical stimuli in small dorsal root ganglion neurons and thin fibre muscle afferents in vitro. J Physiol 597: 5049–5062, 2019. doi: 10.1113/JP278527. [DOI] [PubMed] [Google Scholar]
- 21.Hotta N, Kubo A, Mizumura K. Effect of protons on the mechanical response of rat muscle nociceptive fibers and neurons in vitro. Neurosci Res 92: 46–52, 2015. doi: 10.1016/j.neures.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Hotta N, Kubo A, Mizumura K. Chondroitin sulfate attenuates acid-induced augmentation of the mechanical response in rat thin-fiber muscle afferents in vitro. J Appl Physiol (1985) 126: 1160–1170, 2019. doi: 10.1152/japplphysiol.00633.2018. [DOI] [PubMed] [Google Scholar]
- 23.Hwang C-L, Yoo J-K, Kim H-K, Hwang M-H, Handberg EM, Petersen JW, Christou DD. Novel all-extremity high-intensity interval training improves aerobic fitness, cardiac function and insulin resistance in healthy older adults. Exp Gerontol 82: 112–119, 2016. doi: 10.1016/j.exger.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jadhav RA, Hazari A, Monterio A, Kumar S, Maiya AG. Effect of physical activity intervention in prediabetes: A systematic review with meta-analysis. J Phys Act Health 14: 745–755, 2017. doi: 10.1123/jpah.2016-0632. [DOI] [PubMed] [Google Scholar]
- 25.Japan Diabetes Society . Treatment Guide for Diabetes 2018–2019. Tokyo: Bunkodo, 2018. [Google Scholar]
- 26.Jelleyman C, Yates T, O’Donovan G, Gray LJ, King JA, Khunti K, Davies MJ. The effects of high-intensity interval training on glucose regulation and insulin resistance: a meta-analysis. Obes Rev 16: 942–961, 2015. doi: 10.1111/obr.12317. [DOI] [PubMed] [Google Scholar]
- 27.Kadoglou NPE, Fotiadis G, Kapelouzou A, Kostakis A, Liapis CD, Vrabas IS. The differential anti-inflammatory effects of exercise modalities and their association with early carotid atherosclerosis progression in patients with type 2 diabetes. Diabet Med 30: e41–e50, 2013. doi: 10.1111/dme.12055. [DOI] [PubMed] [Google Scholar]
- 28.Kambič T, Novaković M, Tomažin K, Strojnik V, Jug B. Blood flow restriction resistance exercise improves muscle strength and hemodynamics, but not vascular function in coronary artery disease patients: A pilot randomized controlled trial. Front Physiol 10: 656, 2019. doi: 10.3389/fphys.2019.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karavelioglu Y, Karapinar H, Gul İ, Kucukdurmaz Z, Yilmaz A, Akpek M, Kaya MG. Blood pressure response to exercise is exaggerated in normotensive diabetic patients. Blood Press 22: 21–26, 2013. doi: 10.3109/08037051.2012.701045. [DOI] [PubMed] [Google Scholar]
- 30.Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension 56: 765–773, 2010. doi: 10.1161/HYPERTENSIONAHA.110.157149. [DOI] [PubMed] [Google Scholar]
- 31.Katayama K, Saito M. Muscle sympathetic nerve activity during exercise. J Physiol Sci 69: 589–598, 2019. doi: 10.1007/s12576-019-00669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kennelly SP, Lawlor BA, Kenny RA. Blood pressure and the risk for dementia: a double edged sword. Ageing Res Rev 8: 61–70, 2009. doi: 10.1016/j.arr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Kirwan JP, Sacks J, Nieuwoudt S. The essential role of exercise in the management of type 2 diabetes. Cleve Clin J Med 84, Suppl 1: S15–S21, 2017. doi: 10.3949/ccjm.84.s1.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koba S, Hisatome I, Watanabe T. Central command dysfunction in rats with heart failure is mediated by brain oxidative stress and normalized by exercise training. J Physiol 592: 3917–3931, 2014. doi: 10.1113/jphysiol.2014.272377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marwick TH, Hordern MD, Miller T, Chyun DA, Bertoni AG, Blumenthal RS, Philippides G, Rocchini A; Council on Clinical Cardiology, American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; Council on Nutrition, Physical Activity, and Metabolism; Interdisciplinary Council on Quality of Care and Outcomes Research . Exercise training for type 2 diabetes mellitus: impact on cardiovascular risk: a scientific statement from the American Heart Association. Circulation 119: 3244–3262, 2009. doi: 10.1161/CIRCULATIONAHA.109.192521. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell JH. Abnormal cardiovascular response to exercise in hypertension: contribution of neural factors. Am J Physiol Regul Integr Comp Physiol 312: R851–R863, 2017. doi: 10.1152/ajpregu.00042.2017. [DOI] [PubMed] [Google Scholar]
- 37.Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE; Determinants of Myocardial Infarction Onset Study Investigators . Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion. N Engl J Med 329: 1677–1683, 1993. doi: 10.1056/NEJM199312023292301. [DOI] [PubMed] [Google Scholar]
- 38.Mizuno M, Murphy MN, Mitchell JH, Smith SA. Antagonism of the TRPv1 receptor partially corrects muscle metaboreflex overactivity in spontaneously hypertensive rats. J Physiol 589: 6191–6204, 2011. doi: 10.1113/jphysiol.2011.214429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park J, Middlekauff HR. Abnormal neurocirculatory control during exercise in humans with chronic renal failure. Auton Neurosci 188: 74–81, 2015. doi: 10.1016/j.autneu.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park W, Miyachi M, Tanaka H. Does aerobic exercise mitigate the effects of cigarette smoking on arterial stiffness? J Clin Hypertens (Greenwich) 16: 640–644, 2014. doi: 10.1111/jch.12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pierdomenico SD, Pierdomenico AM, Coccina F, Lapenna D, Porreca E. Circadian blood pressure changes and cardiovascular risk in elderly-treated hypertensive patients. Hypertens Res 39: 805–811, 2016. doi: 10.1038/hr.2016.74. [DOI] [PubMed] [Google Scholar]
- 42.Queme LF, Ross JL, Lu P, Hudgins RC, Jankowski MP. Dual modulation of nociception and cardiovascular reflexes during peripheral ischemia through P2Y1 receptor-dependent sensitization of muscle afferents. J Neurosci 36: 19–30, 2016. doi: 10.1523/JNEUROSCI.2856-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rotto DM, Schultz HD, Longhurst JC, Kaufman MP. Sensitization of group III muscle afferents to static contraction by arachidonic acid. J Appl Physiol (1985) 68: 861–867, 1990. doi: 10.1152/jappl.1990.68.3.861. [DOI] [PubMed] [Google Scholar]
- 44.Rowell LB. Human Cardiovascular Control. New York: Oxford University Press, 1993. [Google Scholar]
- 45.Sato T, Takeda H, Sasaki Y, Kawata S. Increased homeostasis model assessment-insulin resistance is a risk factor for colorectal adenoma in Japanese males. Tohoku J Exp Med 223: 297–303, 2011. doi: 10.1620/tjem.223.297. [DOI] [PubMed] [Google Scholar]
- 46.Sigal RJ, Kenny GP, Boulé NG, Wells GA, Prud’homme D, Fortier M, Reid RD, Tulloch H, Coyle D, Phillips P, Jennings A, Jaffey J. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med 147: 357–369, 2007. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- 47.Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol 91: 89–102, 2006. doi: 10.1113/expphysiol.2005.032367. [DOI] [PubMed] [Google Scholar]
- 48.Smith SA, Williams MA, Mitchell JH, Mammen PPA, Garry MG. The capsaicin-sensitive afferent neuron in skeletal muscle is abnormal in heart failure. Circulation 111: 2056–2065, 2005. doi: 10.1161/01.CIR.0000162473.10951.0A. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki Y, Sato J, Kawanishi M, Mizumura K. Tissue glucose level modulates the mechanical responses of cutaneous nociceptors in streptozotocin-diabetic rats but not normal rats in vitro. Pain 99: 475–484, 2002. doi: 10.1016/S0304-3959(02)00244-0. [DOI] [PubMed] [Google Scholar]
- 50.Tomoto T, Maeda S, Sugawara J. Relation between arterial stiffness and aerobic capacity: Importance of proximal aortic stiffness. Eur J Sport Sci 17: 571–575, 2017. doi: 10.1080/17461391.2016.1277787. [DOI] [PubMed] [Google Scholar]
- 51.Tomoto T, Sugawara J, Hirasawa A, Imai T, Maeda S, Ogoh S. Impact of short-term training camp on arterial stiffness in endurance runners. J Physiol Sci 65: 445–449, 2015. doi: 10.1007/s12576-015-0383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsubone H, Hanafusa M, Endo M, Manabe N, Hiraga A, Ohmura H, Aida H. Effect of treadmill exercise and hydrogen-rich water intake on serum oxidative and anti-oxidative metabolites in serum of thoroughbred horses. J Equine Sci 24: 1–8, 2013. doi: 10.1294/jes.24.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, Horio T, Hoshide S, Ikeda S, Ishimitsu T, Ito M, Ito S, Iwashima Y, Kai H, Kamide K, Kanno Y, Kashihara N, Kawano Y, Kikuchi T, Kitamura K, Kitazono T, Kohara K, Kudo M, Kumagai H, Matsumura K, Matsuura H, Miura K, Mukoyama M, Nakamura S, Ohkubo T, Ohya Y, Okura T, Rakugi H, Saitoh S, Shibata H, Shimosawa T, Suzuki H, Takahashi S, Tamura K, Tomiyama H, Tsuchihashi T, Ueda S, Uehara Y, Urata H, Hirawa N. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res 42: 1235–1481, 2019. doi: 10.1038/s41440-019-0284-9. [DOI] [PubMed] [Google Scholar]
- 54.Williamson JW, McColl R, Mathews D, Mitchell JH, Raven PB, Morgan WP. Brain activation by central command during actual and imagined handgrip under hypnosis. J Appl Physiol (1985) 92: 1317–1324, 2002. doi: 10.1152/japplphysiol.00939.2001. [DOI] [PubMed] [Google Scholar]
- 55.Yamada C, Mitsuhashi T, Hiratsuka N, Inabe F, Araida N, Takahashi E. Optimal reference interval for homeostasis model assessment of insulin resistance in a Japanese population. J Diabetes Investig 2: 373–376, 2011. doi: 10.1111/j.2040-1124.2011.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]