Abstract
The emergence and worldwide spread of SARS-CoV-2 raises new concerns and challenges regarding possible environmental contamination by this virus through spillover of human sewage, where it has been detected. The coastal environment, under increasing anthropogenic pressure, is subjected to contamination by a large number of human viruses from sewage, most of them being non-enveloped viruses like norovirus. When reaching coastal waters, they can be bio-accumulated by filter-feeding shellfish species such as oysters. Methods to detect this viral contamination were set up for the detection of non-enveloped enteric viruses, and may need optimization to accommodate enveloped viruses like coronaviruses (CoV).
Here, we aimed at assessing methods for the detection of CoV, including SARS-CoV-2, in the coastal environment and testing the possibility that SARS-CoV-2 can contaminate oysters, to monitor the contamination of French shores by SARS-CoV-2 using both seawater and shellfish.
Using the porcine epidemic diarrhea virus (PEDV), a CoV, as surrogate for SARS-CoV-2, and Tulane virus, as surrogate for non-enveloped viruses such as norovirus, we assessed and selected methods to detect CoV in seawater and shellfish. Seawater-based methods showed variable and low yields for PEDV. In shellfish, the current norm for norovirus detection was applicable to CoV detection. Both PEDV and heat-inactivated SARS-CoV-2 could contaminate oysters in laboratory settings, with a lower efficiency than a calicivirus used as control. Finally, we applied our methods to seawater and shellfish samples collected from April to August 2020 in France, where we could detect the presence of human norovirus, a marker of human fecal contamination, but not SARS-CoV-2.
Together, our results validate methods for the detection of CoV in the coastal environment, including the use of shellfish as sentinels of the microbial quality of their environment, and suggest that SARS-CoV-2 did not contaminate the French shores during the summer season.
Keywords: SARS-CoV-2, Coastal environment, Seawater, Shellfish, Detection method, Genomic detection
Graphical abstract
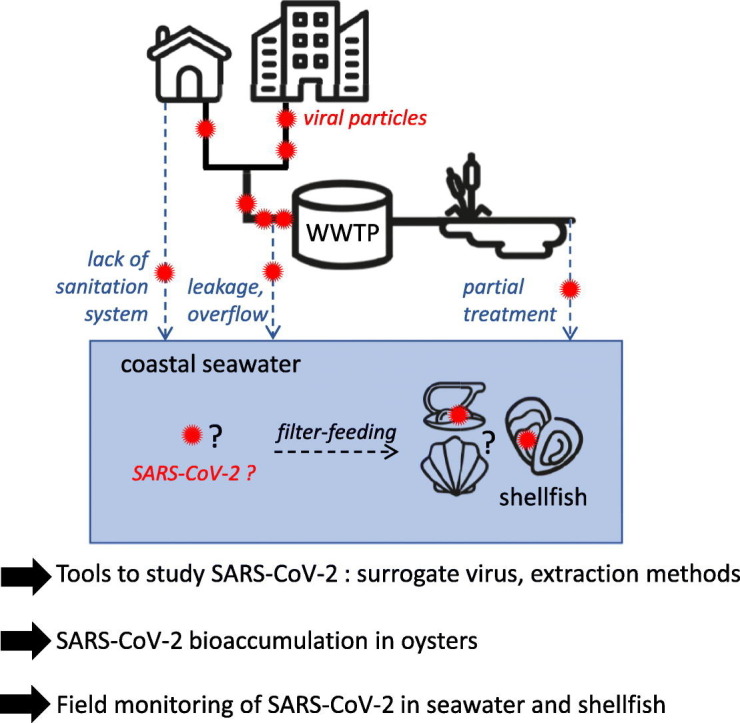
1. Introduction
The emergence and global spread of Severe-Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), responsible for the COVID-19 pandemics, poses an overwhelming challenge to health policies worldwide and has stirred many initiatives to investigate the circulation of this virus in the human population. SARS-CoV-2 belongs to the Coronaviridae family, which is characterized by a 30 kb, positive-sense, single-stranded RNA genome and enveloped virions of around 120-nm in diameter (Gorbalenya et al., 2020). Five genera of CoV have been described, among which alpha- and beta- coronavirus (CoV) comprise coronaviruses infecting humans (HCoV). SARS-CoV-2 is grouped among the betaCoV genus with other HCoV, SARS-CoV, MERS-CoV and the seasonal HKU1 and OC43 (Gorbalenya et al., 2020). Two other HCoV, the seasonal NL63 and 229E, belong to the alphaCoV genus (Gorbalenya et al., 2020). Other known CoV infect vertebrates hosts, and some were used as surrogates for HCoV, such as the alphaCoV Porcine Epidemic Diarrhea Virus (PEDV) and Transmissible Gastroenteritis Virus in pigs; the betaCoV Murine Hepatitis Virus in mice and Bovine coronavirus in cattle; and gammaCoV in birds (Ahmed et al., 2020; Randazzo et al., 2020; Saif, 2004).
HCoV are respiratory viruses mainly transmitted from person to person, through exposure to droplets generated by coughing, sneezing or breathing, either directly in the airways, or through hand-mediated contact (Zhang et al., 2020). Yet, other transmission routes have been described for HCoV and especially SARS-CoV-2: aerosol-borne and the fecal-oral route (reviewed in (Arslan et al., 2020)). Indeed, the presence of HCoV RNA in feces of infected people has been reported several times (reviewed in (Jones et al., 2020)). SARS-CoV-2 was detected in stool samples from infected individuals, even in the absence of symptoms. Viral RNA concentration in feces was lower than in saliva or sputum but could reach 107 genome copies (gc)/mL (Jones et al., 2020).
Following its shedding in body fluids, SARS-CoV-2 is drained into wastewaters, where its genome has been detected now in many countries (reviewed in (Kitajima et al., 2020)). Genome concentration of SARS-CoV-2 in sewage paralleled the number of human cases in the corresponding population (Peccia et al., 2020; Wurtzer et al., 2020) and could reach 106 gc/L (Jones et al., 2020). Thus, wastewater-based epidemiology (WBE) is now proposed as an efficient strategy to monitor SARS-CoV-2 dynamics in the human population (Kitajima et al., 2020). Yet this promising approach still faces many challenges, especially in areas where wastewater networks are not implemented (Arslan et al., 2020; Street et al., 2020).
The contamination of aquatic environments by human sewage has long been recognized as an important transmission route for enteric pathogens, such as human enteric viruses, either through direct exposure to contaminated waters, or through their use for food production and consumption of contaminated foods. (Bosch et al., 2018; Sano et al., 2016). In the case of HCoV, sewage or fecal-borne outbreaks through aerosols generation were suspected occasionally for SARS-CoV and SARS-CoV-2 (Kang et al., 2020; McKinney et al., 2006; Yuan et al., 2020), but foodborne outbreaks were never reported (Jones et al., 2020). However, SARS-CoV-2 has been detected occasionally in treated sewage (Westhaus et al., 2020; Wurtzer et al., 2020) and in rivers (Guerrero-Latorre et al., 2020; Rimoldi et al., 2020), albeit at lower levels than in raw sewage. This re-inforces the hypothesis that SARS-CoV-2 can reach the aquatic environment, due to insufficient wastewater treatment (Guerrero-Latorre et al., 2020; Wurtzer et al., 2020) or sewage spillover before treatment (Rimoldi et al., 2020). Coastal marine waters are also submitted to anthropogenic pollution and sewage contamination, but, to our knowledge, the presence of SARS-CoV-2 in coastal water remains unstudied to date.
Upon contamination of these waters by sewage containing human pathogens, shellfish can become contaminated in turn and transmit these pathogens back to human hosts (Iwamoto et al., 2010). Indeed, filter-feeding bivalve molluscan shellfish are known to concentrate in their tissues pollutants or micro-organisms that are present in the surrounding waters. As such, they can be used as sentinels of the seawater quality (Donia et al., 2012; Fiorito et al., 2019; Metcalf et al., 1980; Winterbourn et al., 2016). In the recent years, shellfish have been monitored mainly considering the risk for human consumption as illustrated by the recent study performed in Europe on prevalence of norovirus (NoV) in oysters (EFSA, 2019). Thus, studying the microbiological contamination of shellfish has a dual purpose: monitoring the presence of micro-organisms in the aquatic environment, and assessing the sanitary risks posed to consumers.
Many families of human enteric viruses, such as Astroviridae, Reoviridae (human rotavirus A), Picornaviridae (aichivirus, enterovirus, hepatovirus) and especially Caliciviridae (human NoV, sapovirus) can be detected in sewage-contaminated marine shellfish, leading to human infection upon consumption (Benabbes et al., 2013; Fusco et al., 2019; Le Guyader et al., 2008). Conversely, the occurrence of Coronaviridae in shellfish has never been reported. This could be due to the absence of CoV in the marine environment, to the lack of studies pertaining to this question, or to the inadequacy of current detection methods which were mainly optimized for non-enveloped enteric viruses (La Rosa et al., 2020). Following the emergence and spread of SARS-CoV-2, and its detection in sewage in France, we undertook this study to validate detection methods for Coronaviridae in samples from the coastal environment, assess the ability of bivalve shellfish to accumulate these viruses, and monitor the presence of SARS-CoV-2 on the French shores using shellfish and seawater samples.
2. Material and methods
2.1. Virus stocks and cell lines
Tulane virus (TuV) strain M033, kindly provided by T. Farkas (Louisiana State University, Baton Rouge, USA) was produced on the LLC-mk2 cell line as described previously (Polo et al., 2018). Porcine Epidemic Diarrhea Virus (PEDV) strain CV777 was produced in vero-E6 cells as described previously (Bigault et al., 2020). The heat inactivated SARS-CoV-2 was kindly provided by Dr. C. Bressolette-Bodin (Nantes Université, Centre de Recherche en Transplantation et Immunologie, UMR 1064, ITUN, Nantes, France). Mengovirus (MgV) strain pMC0 (kindly provided by A. Bosch, University of Barcelona, Spain) was propagated in HeLa cells as previously described (Martin et al., 1996).
When specified, viruses were inactivated for 15 s. at 60 °C (Abraham et al., 2020). For SARS-CoV-2, inactivation was verified by TCID50 assay.
2.2. Artificial contamination of seawater and oysters (bioaccumulation)
For protocol validation, 1 L of coastal water sampled in November 2019 and February 2020 were spiked with PEDV and TuV (Table 1 ). This was repeated two or three times to ensure replicate extractions for each sample and method.
Table 1.
Characteristics of artificially contaminated samples.
| Sample | Matrix | Collection date | Viral inoculum (genome copies) |
|||
|---|---|---|---|---|---|---|
| TuV | PEDV | Inactivated PEDV | Inactivated SARS-CoV-2 | |||
| E1980 | Coastal seawater site O | Oct. 2019 | 1.8 × 109 | 2 × 109 | ||
| E1982 | Coastal seawater site G | Oct. 2019 | 1.8 × 109 | 2 × 109 | ||
| E1989 | Coastal seawater site O | Feb. 2020 | 2 × 108 | 3.7 × 1010 | ||
| E1990 | Coastal seawater site G | Feb. 2020 | 2 × 108 | 3.7 × 1010 | ||
| B1109 | 36 commercial oysters | Jun. 2020 | 2 × 109 | 3.7 × 1010 | ||
| B1112 | 12 wild oysters | Jul. 2020 | 2.3 × 109 | 2 × 109 | 6.4 × 108 | |
| B1113 | 18 commercial oysters | Jul. 2020 | 2.3 × 109 | 2 × 109 | 6.4 × 108 | |
| B1114 | 18 commercial oysters | Aug. 2020 | 3.5 × 109 | 3.7 × 109 | 5.5 × 109 | |
| B1110 | 9 commercial oysters | Jul. 2020 | 2.3 × 109 | 2 × 109 | ||
| B1111 | 9 commercial oysters | Jul. 2020 | 2.3 × 109 | 4 × 109 | ||
| B1117 | 9 commercial oysters | Sep. 2020 | 3.1 × 109 | 7.9 × 108 | ||
| B1118 | 9 commercial oysters | Sep. 2020 | 3.1 × 109 | 1.2 × 109 | ||
Oysters (Crassostrea gigas) were either purchased live from a producer (commercial oysters), or harvested on the French shore (wild oysters), and kept overnight at 4 °C. Artificial contaminations were carried out by bioaccumulation of oysters for 24 h at room temperature (18–20 °C) in aerated seawater seeded with known concentrations of the viruses (Table 1). The volume of seawater was adjusted to the number of animals in the tank (Table 1), with a ratio of 1 L/6 animals for commercial oysters, and 1.5 L/6 animals for wild oysters which were twice bigger based on the weight of digestive tissues (DT) recovered. For each experiment, a fraction of the viral inoculum was titrated in parallel by qRT-PCR to calculate the total amount of each virus used for bio-accumulation. After 24 h of bioaccumulation, oysters were open, shucked and dissected to collect the DT, the gills and the mantle. Tissues from all oysters were pooled by type, minced, and stored as 2 g-aliquotes at −20 °C before analysis.
2.3. Environmental sampling
Along the French coastline, 21 sites were selected based on exposure to human sewage contamination as demonstrated by Escherichia coli (Piquet et al., 2019) or NoV contamination (data not shown) (Fig. 1 , black dots). The sites were selected to cover the different French coastal areas (Fig. 1). From each site, one shellfish sample was collected bi-monthly, when possible, from mid-April 2020 to end of August. Only shellfish present onsite for at least 6 months or from wild populations were harvested, so that they could reflect the local viral contamination. Most collected samples were cupped oysters (Crassostrea gigas), two samples were mussels (Mytilus spp.) and one, clams (Ruditapes philippinarum). One sample was constituted of at least of 12 oysters, 20 mussels or 20 clams. Shellfish samples were shipped on ice to the laboratory, where they were dissected and the DT from 10 animals pooled, minced, and stored at −20 °C as 2 g-aliquotes.
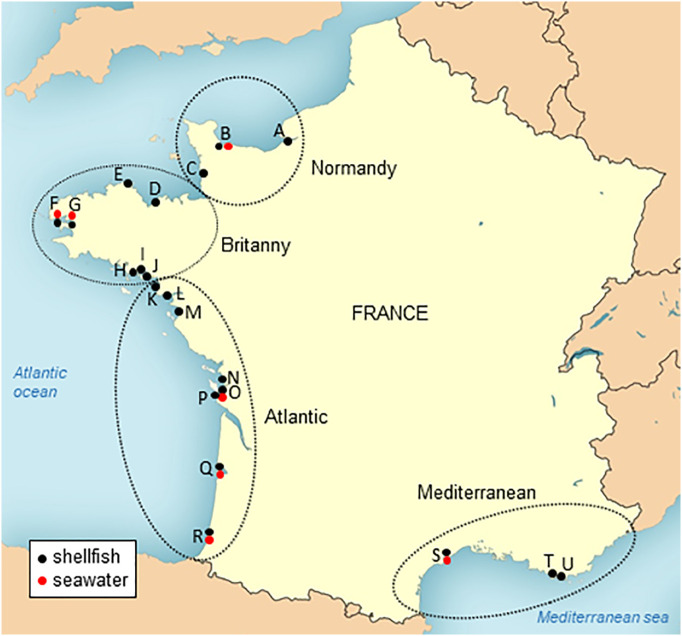
Fig. 1.
Localization of the sampling points for SARS-CoV-2 monitoring along the French coasts. Shellfish (black dots) and coastal seawater (red dots) were sampled bimonthly in 21 sites distributed along the French coasts and belonging to 4 geographical areas: Normandy (sites A to C), Brittany (sites D to J), Atlantic (sites K to R) and Mediterranean (sites S to U).
Coastal water (1 L) was sampled together with shellfish from seven sites (Fig. 1, red dots), sent on ice to the laboratory, where they were stored at −20 °C until processing.
Besides this scheduled sampling, additional shellfish samples were collected on an ad-hoc basis in other coastal sites upon alerts of microbiological contamination characterized by increased E. coli concentrations in shellfish flesh (Piquet et al., 2019). A total of 18 shellfish samples linked to alerts were collected (eleven oyster samples, four mussel samples and three cockle samples), as well as seven water samples.
2.4. Extraction of viral nucleic acids from coastal water
Samples of coastal water (1 L) were analyzed by two methods based on negative-charged membrane filtration (MF) (Katayama et al., 2002) and FeCl3 flocculation (FF) (John et al., 2011). For method MF, coastal water samples were directly filtered on a negative-charged HA-type membrane with a 47 mm diameter and 0.45 μm pores (Millipore, Burlington, MA, USA) placed on a vacuum sterile bottle. Filters were rinsed with 100 mL of 0.5 mM H2SO4 (pH 3) prior to viral elution with 1 mM NaOH (pH 10.5). After pH neutralization, 10 mL of viral suspension were concentrated using a 50 kda Centriprep ultrafiltration device (Millipore) to obtain 2 mL of viral concentrate. In parallel, for method FF, 200 μL of 10 g/L FeCL3 solution was added to the filtrate from method MF (kept at 4 °C), and incubated 2 h at 10 °C under gentle agitation, in the dark. A flocculate was then collected on a 0.8 μm pore-size polycarbonate filter (Whatman, Maidstone, UK). Virus resuspension was achieved with 2 mL of ascorbate-oxalate–EDTA buffer during a 30 min incubation at 4 °C under agitation. Viral suspensions (method FF) and concentrates (method MF) were extracted using the NucliSens kit (bioMérieux, Lyon, France) with 10 mL of lysis buffer and 140 L of magnetic silica, and eluted in 100 μL of the kit's elution buffer.
2.5. Extraction of viral nucleic acids from shellfish
Three methods were tested on 2 g-aliquotes of oyster tissues. The PK-ISO method was applied as described in the norm for Hepatitis A and NoV detection in shellfish (ISO 15216-1:2017). Briefly, tissues were incubated with 2 mL of a 3000 U/L solution of proteinase K (PK) for 1 h at 37 °C and 15 min at 60 °C, centrifuged for 5 min at 3500 ×g at 4 °C, and 500 μL of supernatant was used for extraction directly using the NucliSens kit (bioMérieux). The remaining supernatant (2.5–3 mL) was used for the PK-PEG extraction method, for which it was sonicated 3 × 1 min at full power with a Sonopuls sonicator equipped with a cup-horn (Bandelin, Berlin, Germany), with 1-min resting on ice between each sonication. Pyrophosphate (100 mM) was added 1:10 in the supernatant, which was then incubated at 4 °C for 40 min with agitation and further treated as described previously (Strubbia et al., 2020) until concentration by poly-ethylene-glycol (PEG)-6000 precipitation. For the chloroform:butanol/PEG method (CB-PEG), tissues were homogenized with a pestle in a potter with 2 mL glycine buffer (glycine 3.75 g/L, NaCl 9 g/L, pH 9.5). Additional 3 mL of glycine buffer were used to rinse the pestle and potter, and added to the tissue homogenate before adding 6 mL of chloroform:butanol (50% vol:vol) solvent and mixing by 30 s on vortex. Cat-Floc T (Calgon, Ellwood City, PA) was added (173 L per tube), the mixture agitated for 5 min at room temperature, before being centrifuged for 15 min at 13,500 ×g at 4 °C (Atmar et al., 1995). The supernatant was collected, 3 mL of PEG-6000 (24%) – NaCl (7%) were added and incubated 1-2 h at 4 °C with agitation, before a final centrifugation for 20 min at 11,000 ×g at 4 °C. For both the PK-PEG and the CB-PEG methods, the pellet was resuspended in 1 mL ddH2O pre-heated at 56 °C, by vortexing and pipetting. All viral eluates/concentrates were extracted using the NucliSens kit (bioMérieux) following the manufacturer's instruction, with 2 mL lysis buffer and 50 μL magnetic silica, and eluted in 100 μL of the kit's elution buffer.
2.6. Process control
The MgV, a murine picornavirus, was used as a process control for nucleic acid extraction from shellfish, as described in (ISO15216-1,2017). Briefly, 100 μL of MgV solution were added to each tissue aliquot just before extraction, and an extraction control was carried out with 100 μL of pure MgV solution in each series of extraction. MgV concentration in nucleic acids extracted from shellfish tissues were compared to the extraction control to calculate the efficiency of each series of extraction. For the environmental screening, samples whose extraction efficiency was below 1% were not considered for the final analysis, since any absence of virus detection could be due to extraction issues (ISO15216-1,2017). The extraction efficiency was not evaluated for water samples collected in the environmental screening.
2.7. Detection of viral genomes by one-step quantitative RT-PCR
The Ultrasens one step quantitative RT-PCR kit (Life technologies, Carlsbad, CA, USA) was used for all qRT-PCR reactions, following the manufacturer's indications, using an Aria Mx or MxP3000 real-time PCR system (Agilent Technologies, Santa Clara, CA, USA). For SARS-CoV-2, two sets of primers and probes were used: IP4, targeting the polymerase gene (Etievant et al., 2020) and E, targeting the envelope gene (Corman et al., 2020). Cycling were adapted to comply with the qRT-PCR kit requirements: reverse-transcription for 15 min at 55 °C, first denaturation and Taq polymerase activation for 5 min at 95 °C, and 45 cycles of denaturation (94 °C, 15 s), annealing (58 °C, 30 s) and extension (65 °C, 30 s) followed by fluorescence acquisition. The MgV, TuV and NoV genogroup I (GI) and II (GII) qRT-PCR were carried out as described previously (Drouaz et al., 2015; Le Guyader et al., 2009). For PEDV, previously described primers (Bigault et al., 2020) and probe (Kim et al., 2007) were used based on the same cycling conditions as NoV GII.
For quantification, duplicate 6-points standard curves were made with TuV synthetic DNA (Drouaz et al., 2015), PEDV in-vitro transcript T171 (Bigault et al., 2020) and SARS-CoV-2 RNA transcript (CNR des virus respiratoires, Pasteur Institute), and the synthetic ssRNA-EURM-019 (European Commission Joint Research Center).
Considering the sensitivity of our qRT-PCR assays, the theoretical detection limit was set as 1 genome copy per 5 L of nucleic acid that were assessed. For shellfish samples, this means 50 gc/g of tissue analyzed using the PK-ISO method, 10 gc/g for the CB-PEG method, and 13 gc/g for the PK-PEG method. For seawater, this equals to 20 gc/L for both methods.
For virus detection in shellfish field samples, after verification of extraction efficiency and absence of inhibitors, triplicates of undiluted nucleic acid extracts were assessed and for water samples amplifications were performed on duplicate of undiluted extracts and 1/10 dilutions in molecular grade water. For their quantification in seeded or bioaccumulated contaminated samples, duplicates of undiluted, 1/10 and 1/100-diluted extracts were used. Good laboratory practices were observed throughout the analysis process, with dedicated separate rooms for oyster bioaccumulation, shellfish dissection, viral elution from shellfish, seawater processing, nucleic acid (NA) extraction, preparation of reaction mixtures, template addition, positive controls addition, and amplification. No-template controls were included in all qRT-PCR assays and proved always negative.
2.8. Statistics
GraphPad Prism version 8.4.3 was used for statistical analysis of the data by 2-way ANOVA with Tukey's multiple comparisons test. In some instance, the viral concentrations in oyster tissues were below the theoretical limit of detection, or even non-detected. This was observed before with other viral targets, and may be due to the complex matrix in oyster extracts. We chose to keep these values for statistical analysis.
3. Results
To validate protocols for the extraction of SARS-CoV-2, we used a surrogate coronavirus, the porcine epidemic diarrhea virus (PEDV) to mimic the behavior of SARS-CoV-2 (which requires access to a BSL3 facility). In addition, we used the TuV, a simian calicivirus often used as a surrogate for human NoV, as a non-enveloped control virus known to be bio-accumulated by oyster (Drouaz et al., 2015; Polo et al., 2018).
3.1. Assessment of extraction methods for CoV in seawater
Several protocols were previously described allowing the concentration and extraction of viruses from environmental waters, including seawater. We selected two methods that were found efficient for the recovery of enteric viruses (John et al., 2011; Katayama et al., 2002) and applied them to coastal water samples spiked with PEDV and TuV (Table 1). The first method (MF) allowed to recover the PEDV and TuV genomes with a mean yield of 0.981% and 1.33% respectively (Table 2 ), but with high inhibition of RT-PCR enzymes necessitating at least 2-log dilutions of nucleic acid extracts. The second method (FF) was applied to two samples, where it allowed the recovery of 1.78% and 0.23% of PEDV and TuV, respectively (Table 2). Both methods showed a high variability of recovery on both viruses across the different samples, and statistical comparison were not significant (Table 2, p > 0.05). As they present complementary approaches, we chose to apply both methods on environmental seawater samples for SARS-CoV-2 monitoring. Besides, given the low viral recovery in seawater samples, another approach was tested with the use of shellfish to concentrate the contamination.
Table 2.
Yields in PEDV and TuV using two methods for virus extraction from coastal waters.
| Method |
Method MF |
Method FF |
ANOVA |
||||
|---|---|---|---|---|---|---|---|
| Virus | Sample | N | Mean recovery (%) | SD (%) | Mean recovery (%) | SD (%) | p value |
| PEDV | E1980 | 3 | 0.0754 | 0.126 | 3.55 | 3.38 | p = 0.0004 |
| E1982 | 3 | 0.687 | 0.600 | 0.0112 | 0.00899 | p = 0.5707 | |
| E1989 | 2 | 1.61 | 0.339 | ND | |||
| E1990 | 2 | 1.55 | 0.979 | ND | |||
| Mean | 0.981 | 0.736 | 1.78 | 2.50 | ns | ||
| TuV | E1980 | 3 | 0.0777 | 0.0818 | 0.471 | 0.0750 | p = 0.2575 |
| E1982 | 3 | 0.471 | 0.472 | 0.00513 | 0.00449 | p = 0.0511 | |
| E1989 | 2 | 0.948 | 0.247 | ND | |||
| E1990 | 2 | 3.84 | 1.09 | ND | |||
| Mean | 1.33 | 1.71 | 0.238 | 0.329 | ns | ||
ND: not done.
3.2. Assessment of extraction methods for CoV in shellfish
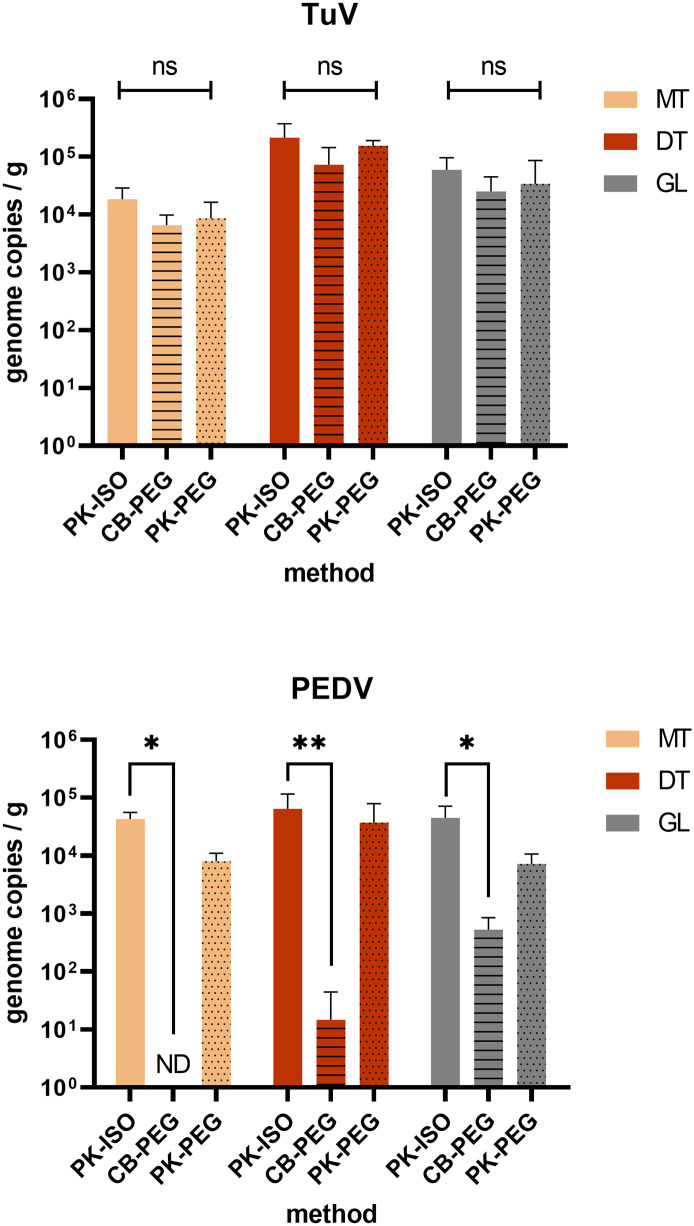
The current preconized method for the detection of NoV or hepatitis A virus in shellfish relies on a simple protocol based on proteinase K (PK) digestion to release viruses from DT (PK-ISO) (ISO 15216-1). It was compared to the original protocol set up to detect enteric viruses in shellfish, which uses chloroform-butanol to elute viruses and PEG to concentrate them (CB-PEG) (Atmar et al., 1995). A third protocol, combining PK elution and PEG concentration, able to recover a high diversity of viruses from shellfish (Strubbia et al., 2020) was also tested here (PK-PEG). We used three tissues dissected from PEDV/TuV-bioaccumulated oysters to compare these methods: the mantle (MT), the digestive tissues (DT) and the gills (GL) (Fig. 2 ). Three to four series of extraction were performed. Their efficiencies were calculated for each method and tissue using the MgV process control, and were comprised between 0.4 and 10% for PK-ISO, 0.03 and 4% for CB-PEG, and 0.3 and 5% for PK-PEG. The three methods allowed to recover TuV to similar levels (p > 0.05, Fig. 2) and this virus was more concentrated in the DT than in other tissues (p = 0.0002, Fig. 2). PEDV was recovered from the three shellfish tissues using PK-based methods, when the CB-PEG was poorly efficient, allowing PEDV detection only in the gills at a very low concentration (Fig. 2). Although it used more PK eluate, the PK-PEG method was not significantly more efficient at recovering both viruses. The simpler PK-ISO method was the most efficient on all tissues for PEDV recovery (p < 0.05 or 0.01), (Fig. 2). Finally, all tissues appeared equally suited for PEDV detection (p > 0.05, Fig. 2).
Fig. 2.
Assessment of extraction methods for CoV in oysters. Oysters (C. gigas) were incubated in presence of TuV and PEDV for 24 h, and the concentration of each virus was measured in three tissues – the mantle (MT, beige), the digestive tissues (DT, brown) and the gills (GL, grey) – by qRT-PCR following repeated extractions by three different methods – PK-ISO (plain, n = 4), CB-PEG (horizontal lines, n = 3), PK-PEG (dots, n = 4). *: p < 0.05, **: p < 0.01, ns: non-significant (ANOVA). Theoretical limits of detection: PK-ISO, 50 gc/g; CB-PEG, 10 cg/g; PK-PEG, 13 cg/g.
3.3. Oysters bioaccumulation with inactivated SARS-CoV-2
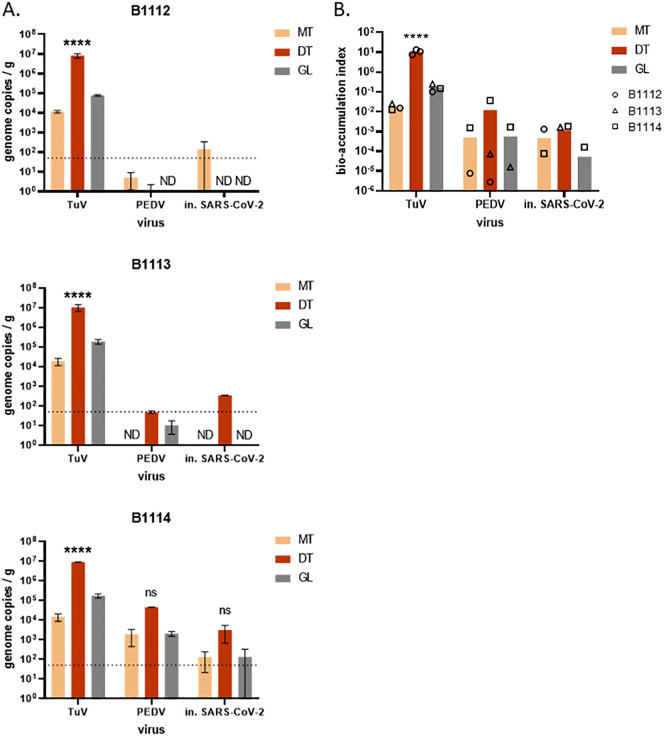
Oysters are known to bio-accumulate very efficiently some enteric viruses, such as human NoV (Maalouf et al., 2011), while other viruses may be poorly uptaken or kept in their tissues, like bovine NoV (Zakhour et al., 2010). To test the bio-accumulation of SARS-CoV-2 by oysters, and validate the PK-ISO protocol on the target virus, we used SARS-CoV-2 from cell culture, heat-inactivated (in.) for safety reasons. Three different batches of C. gigas oysters were incubated with in. SARS-CoV-2, and with TuV and PEDV as controls. Using the PK-ISO method, the concentration in viral genomes was then quantified in three tissues (Fig. 3 ). TuV was highly concentrated in oyster tissues, and most concentrated in the DT (p < 0.0001, Fig. 3, A), as expected, with similar levels of contamination for the three batches. In the two first batches (B1112 and B1113), PEDV and in. SARS-CoV-2 were detected mainly in the gills and the DT, respectively, at very low levels (Fig. 3, A). In the third batch, higher quantities of in. SARS-CoV-2 (Table 1) were used to contaminate oysters, and CoV were detected in the three tissues at intermediate levels, with apparent highest concentration in the DT that did not reach statistical significance (p > 0.05) (Fig. 3, A). Variability of results across the three oyster batches can be explained by a slight inhibition of PCR and lower extraction efficiencies for the first batch (2–4%), while the last batch was contaminated with more inactivated SARS-CoV-2, and also showed the highest extraction efficiencies (1–21%), which may have resulted in higher amounts of CoV detected. Importantly, PEDV and in. SARS-CoV-2 displayed very similar distributions and concentrations in each oyster batch (Fig. 3, A), which supports the use of PEDV as a surrogate for SARS-CoV-2 in shellfish.
Fig. 3.
Bio-accumulation of heat-inactivated SARS-CoV-2 in oysters. Three batches of C. gigas oysters (B1112, B1113, B1114) were incubated for 24 h in presence of TuV, PEDV and heat-inactivated (in.) SARS-CoV-2. A. The viral concentration was quantified in three tissues - mantle (MT), digestive tissues (DT) and gills (GL) - by duplicate extractions using the PK-ISO method. ****: p < 0.0001, ns: non-significant (ANOVA), n = 2 series of extractions. In B1112 and B1113, PEDV or SARS-CoV-2 were not detected (ND) in some tissues. Theoretical limit of detection: 50 gc/g (dotted line). B. The virus concentration of in each tissue was divided by the initial virus concentration in the seawater to calculate the bio-accumulation index. Each oyster batch is plotted as a black symbol (circle, B1112; triangle, B1113; square, B1114) when the virus was detected in the corresponding tissue, missing symbols corresponding to undetected virus. The arithmetic mean values of the three experiments are plotted as columns, for the three tissues. ****: p < 0.0001, ns: non-significant (ANOVA), n = 3 experiments with different oyster batches.
To compare the data more easily regarding the initial amount of virus used for oyster contamination, the viral concentration in oyster tissues was divided by the initial viral concentration in seawater (Fig. 3, B) (Maalouf et al., 2011). TuV bioaccumulation index reached a mean value of 10.6 in oyster DT and was highly reproducible across the three oyster batches. For PEDV and inactivated SARS-CoV-2, the mean bioaccumulation index was highest in DT (0.012 and 0.0017 respectively), and varied between oyster batches. Together, our data show that CoV can contaminate oyster tissues but are not as efficiently bio-accumulated as a calicivirus like the TuV.
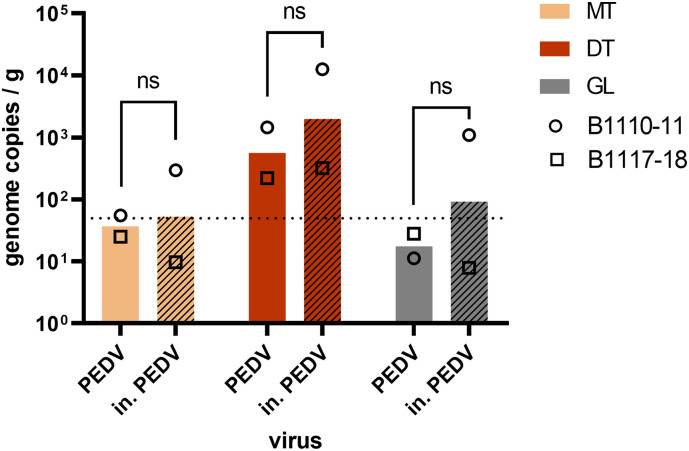
For safety reasons, we could not use native, infectious SARS-CoV-2 to contaminate oysters, and had to rely on heat-inactivated SARS-CoV-2. To check that heat inactivation does not impact the bioaccumulation efficiency and tissue distribution of CoV, we contaminated oysters with TuV and native PEDV or TuV and heat-inactivated PEDV (in. PEDV), in two separate aquariums with the same batch of oysters at the same time. Two independent experiments using different batches were conducted (Fig. 4 ). For both, TuV displayed the expected distribution and was equally concentrated in each tissue between oysters from the two aquariums (data not shown). The MgV extraction efficiencies were also similar, with respective mean values of 5.5% (range 1–22%) and 4,6% (1–11%). In the first experiment (B1110-11), inactivated PEDV appeared more concentrated than native PEDV in the oyster tissues (Fig. 4, circles). In the second experiment (B1117-18), native and inactivated PEDV exhibited the same levels of concentration (Fig. 4, triangles). Considering both experiments, the mean concentration of native and inactivated PEDV did not differ significantly (p > 0.05, Fig. 4), and their tissue distribution were similar, suggesting that heat inactivation does not impair CoV bioaccumulation by oysters, and validating our results with in. SARS-CoV-2.
Fig. 4.
Impact of heat inactivation on CoV bioaccumulation in oysters. Oysters (C. gigas) from two batches (B1110-11 and B1117-18) were incubated in presence of native PEDV (plain columns) or heat-inactivated (in.) PEDV (hatched columns) for 24 h. The concentration of viral genome was quantified in three tissues - the mantle (MT), the digestive tissue (DT) and the gills (GL) - following duplicate extractions with the ISO-PK method and qRT-PCR. Columns represent geometrical means and error bars, geometrical standard deviations. ****: p < 0.0001, ns: nonsignificant (ANOVA), n = 2 experiments with different oyster batches. Theoretical limit of detection: 50 gc/g (dotted line).
3.4. Screening of environmental samples for the presence of SARS-CoV-2
A total of 187 samples were collected from 37 sites, including 21 sites regularly sampled (monitoring, Fig. 1) and 16 sites sampled upon alerts on microbiological contamination (alerts). All these samples were processed by the PK-ISO method. Among these, three samples (one from Normandy, and two from Brittany area) provided extraction efficiencies lower than 1% despite repeated extractions, and thus were excluded of the analysis.
Among the 166 samples collected during the monitoring survey, 141 were oyster samples, 17 mussel samples and 8 clam samples. None of these samples were found contaminated by SARS-CoV-2 using any of the two primer sets (Table 3 ). NoVs searched to confirm human sewage contamination were detected in 35 samples (21%), 69% of these positive samples being detected at the beginning of the study (from mid-April to end of May). Four sampling sites (L, J, P, R) were devoid of NoV contamination and NoV were detected once in nine sites (F to I, O to U). Most of NoV-contaminated samples were detected in eight sites including three sites (A, L and N) located close to the mouth of large rivers which displayed the highest contamination frequency and highest concentrations.
Table 3.
Results obtained on water and shellfish samples collected during the monitoring study or the microbiological alerts.
| Area | Shellfish |
Water |
|||||
|---|---|---|---|---|---|---|---|
| monitor. | Alert | Total | monitor. | Alert | Total | ||
| Normandy | Nb of sampling sites | 3 | 3 | 6 | 1 | 1 | 2 |
| Nb of samples collected | 23 | 3 | 26 | 8 | 1 | 9 | |
| SARS-CoV-2 positive samples | 0 | 0 | 0 | 0 | 0 | 0 | |
| NoV positive samples | 6 | 0 | 6 | 2 | 0 | 2 | |
| NoV positive sites | 2 | 0 | 2 | 1 | 0 | 1 | |
| Brittany | Nb of sampling sites | 7 | 9 | 16 | 2 | 3 | 5 |
| Nb of samples collected | 59 | 11 | 70 | 18 | 4 | 22 | |
| SARS-CoV-2 positive samples | 0 | 0 | 0 | 0 | 0 | 0 | |
| NoV positive samples | 8 | 3 | 11 | 3 | 0 | 3 | |
| NoV positive sites | 6 | 3 | 9 | 1 | 0 | 1 | |
| Atlantic | Nb of sampling sites | 8 | 3 | 11 | 3 | 1 | 4 |
| Nb of samples collected | 57 | 3 | 60 | 14 | 2 | 16 | |
| SARS-CoV-2 positive samples | 0 | 0 | 0 | 0 | 0 | 0 | |
| NoV positive samples | 18 | 0 | 18 | 3 | 0 | 3 | |
| NoV positive sites | 6 | 0 | 6 | 2 | 0 | 2 | |
| Mediterranea | Nb of sampling sites | 3 | 1 | 4 | 1 | 0 | 1 |
| Nb of samples collected | 27 | 1 | 28 | 9 | 0 | 9 | |
| SARS-CoV-2 positive samples | 0 | 0 | 0 | 0 | 0 | 0 | |
| NoV positive samples | 3 | 0 | 3 | 2 | 0 | 2 | |
| NoV positive sites | 3 | 0 | 3 | 1 | 0 | 1 | |
| Total | Nb of sampling sites | 21 | 16 | 37 | 7 | 5 | 12 |
| Nb of samples collected | 166 | 18 | 184 | 52 | 7 | 59 | |
| SARS-CoV-2 positive samples | 0 | 0 | 0 | 0 | 0 | 0 | |
| NoV positive samples | 35 | 3 | 38 | 10 | 0 | 10 | |
| NoV positive sites | 19 | 3 | 22 | 5 | 0 | 5 | |
monitor.: samples collected during regular monitoring; alert: samples collected following alerts of microbiological contamination in additional locations.
Among the 18 shellfish samples collected following microbiological alerts suspected to be linked to sewage contaminations events, none were found contaminated by SARS-CoV-2. They were collected mainly in May and August. Three samples (two collected in May and one in June) were found contaminated by NoVs confirming the human fecal contamination.
None of the water samples were found contaminated by SARS-CoV-2, however NoV were detected in 10 samples. Both methods gave positive results with two samples being positives for both methods, two with the MF method and 6 with the FF method. NoV were not detected in site G, while they were detected twice or three times in all the other sampling sites (concentrations ranged from 20 to 300 RNAc/L). On one occasion (site F, sampled on May 5) both water and oyster samples were found positive for NoV.
4. Discussion
Most existing protocols for the detection of viruses in environmental samples are optimized for non-enveloped, enteric viruses such as gastroenteritis or hepatitis viruses (Bosch et al., 2018). The emergence and possible environmental spread of the SARS-CoV-2, an enveloped virus, raised new challenges to environmental virologists (La Rosa et al., 2020). Our first aim was to select a method to detect CoV, in samples from the coastal environment, using real-time, quantitative RT-PCR, which is one of the most sensitive and robust techniques available for virus detection in environmental samples (Haramoto et al., 2018). As manipulating infectious SARS-CoV-2 required working in a biosafety level 3 laboratory, we first selected a surrogate virus allowing to assess detection methods without this safety considerations. Important points to select a surrogate are the genetic proximity to the target virus, the physical and chemical characteristics but also the absence of human pathogenicity, and/or easy way of production (Cromeans et al., 2014). In this study, to use this surrogate with seawater and oysters, the lack of natural contamination was another constraint. Phages are good surrogate for some eukaryotic viruses but their presence in environmental samples may complicate their use (Flannery et al., 2012). Usually a virus from the same family is preferred so that target and surrogate viruses share a similar size, structure, and other characteristics. For example, the TuV, prototype strain of the genus Recovirus within the Caliciviridae family, is used to mimic NoV behavior (Drouaz et al., 2015). Among the Coronaviridae family, we selected PEDV, a porcine enteric CoV which belongs to a different group of CoV than SARS-CoV-2 (alpha and beta-CoV, respectively). The first one is an enteric virus while the second is respiratory, which could imply differences in environmental stability. Nevertheless, porcine enteric CoV have been used in the past to as surrogates for HCoV, including SARS-CoV-2 (Randazzo et al., 2020), and in a recent study, all tested CoV (including PEDV) fitted in the same model regarding their sensitivity to temperature in fomites (Guillier et al., 2020). Altogether with the TuV, it allowed us to control the efficacy of our methods on a target, non-enveloped virus, and to compare with enveloped coronavirus data.
As the aim of this work was to evaluate the possible coastal contamination by SARS-CoV-2 shed by infected people, we first evaluated methods for SARS-CoV-2 detection and quantification in seawater. In human feces and in sewage, which are the sources of human viruses in the coastal environment, viruses are rarely free but adsorbed onto particles. Thus, we selected a combination of two complementary methods, one recovering large particles (membrane filtration, MF) and the other one, smaller aggregates and free viruses (FeCl3 floculation, FF). When applied on seawater samples spiked with the TuV and the PEDV, these methods allowed to detect both viruses, however at low yield and with high variability between water samples. These very low yields could be explained by the use of coastal marine waters, which were turbid and contained PCR inhibitors (Hata et al., 2020). Surprisingly, results were similar for TuV and PEDV for each sample, which suggest that the yield of the methods is mostly influenced by parameters of the seawater matrix (presumably particulate material, PCR inhibitors) and not by the nature of the virus. Considering that the two methods showed similar ranges of yields, they were both applied on naturally contaminated seawater samples during environmental monitoring, where NoV, but not SARS-CoV-2, were detected. These results underline that virus detection from environmental waters is not an easy process. In the ISO15216:1–2017 norm, as low as 1% recovery rate is considered an acceptable quality parameter. A recovery of 11% for PEDV and MgV in raw sewage using aluminum hydroxide adsorption-precipitation was achieved, but the recovery of PEDV was down to 3% in treated sewage (Randazzo et al., 2020). Here, the filtration of one-liter samples was difficult to achieve while still being too small for the detection of SARS-CoV-2 that is likely present at very low concentrations (if present) in the environment. Even if the detection of some NoV confirmed the efficacy of these methods in the field, a grab sample of such a small volume is also not representative of the whole water present in a site. Given these limitations for direct seawater analysis, we proposed to use shellfish, which are filter-feeding animals known to concentrate chemical and microbial contaminants, as sentinel for the detection of SARS-CoV-2 in the coastal environment.
Like was done for seawater, we first evaluated different methods to detect CoV in oysters contaminated with TuV and PEDV. Two methods used proteinase K (PK) for viral elution from the oyster tissues, and one used lipophilic solvents (chloroform/butanol). The latter method was inefficient on PEDV, with only traces of this CoV detected in one tissue, while the non-enveloped TuV was detected in high concentrations in all tissues. Lipophilic solvents disrupt lipid membranes like viral envelopes, and chloroform was already shown to dramatically alter the recovery of CoV (Conceição-Neto et al., 2015). Contrarily, the PK-based elution methods allowed the detection and quantification of both TuV and PEDV in three oyster tissues. We thus chose to apply the current recommended ISO15216:1–2017 method for NoV and hepatitis A virus detection in shellfish for the next experiments. Indeed, using the ISO method allows for comparisons with more studied viruses (such as NoV). It is also a simple protocol, that could be easily implemented in laboratories for routine analysis if this becomes needed for SARS-CoV-2.
Using PEDV and inactivated SARS-CoV-2, we show that CoV can contaminate oysters. To our knowledge, this is the first demonstration that oysters can bioaccumulate a CoV. PEDV and heat-inactivated SARS-CoV-2 displayed very similar distributions and levels of contaminations in three oyster batches. In addition, we show that heat inactivation does not impair the distribution of PEDV in oyster tissues nor negatively impact its bio-accumulation by oysters. These results validate our observations with inactivated SARS-CoV-2 and reinforce our confidence that PEDV can be used as a surrogate for SARS-CoV-2 in oysters. The low impact of thermal inactivation on CoV bioaccumulation by oysters also suggest that partially degraded SARS-CoV-2 present in sewage may still be able to contaminate shellfish when reaching the coastal environment. These observations are encouraging for the use of shellfish as sentinel of human contamination. However, given the expected low levels and low stability of CoV in the environment, the persistence of CoV RNA in shellfish tissues needs to be investigated to estimate how long after contamination the virus could still be detected.
Both PEDV and inactivated SARS-CoV-2 were less efficiently bio-accumulated by oysters than TuV, a calicivirus, which could indeed be due to a lower stability in seawater and oysters, and/or to a lower affinity for oyster tissues. The tissue distribution pattern of CoV does not show a marked concentration in DT, contrarily to TuV, and high concentrations of viruses were needed to contaminate oysters, as previously shown for mengovirus, from the Picornaviridae family (Drouaz et al., 2015). Bioaccumulation efficiency may vary from one virus to another or depend on the shellfish species. If for NoV the impact of ligands and their seasonal expression has been demonstrated, this is still unclear for other human enteric viruses (Grodzki et al., 2012; Maalouf et al., 2010; Zakhour et al., 2010).
In the coastal environment, expected concentrations of enteric viruses are usually much lower than those used for artificial bioaccumulation (Gentry et al., 2009; Keller et al., 2019), and may be even lower for SARS-CoV-2. Yet, repeated exposures to the virus in the open environment, where larger volumes of seawater are filtered by shellfish, may still lead to their contamination. C. gigas oysters are present on all French shores and in many countries worldwide (Europe, North Africa, China, Japan, Korea, Australia, Pacific coast of USA and Canada) as a farmed animal and/or an invasive species (Herbert et al., 2016), and is thus suitable for use as sentinel in many settings. As mentioned above, other filter-feeding shellfish species may exhibit differences in bioaccumulation efficiency and should be tested in further work, such as Dreissena polymorpha proposed as a biomonitoring tool in fresh water (Géba et al., 2020).
Considering that seawater sampling and analysis is complicated and unlikely to be positive for SARS CoV-2, and our results showing a possible bioaccumulation of SARS-CoV-2 in oysters, we set up a monitoring survey that begun at the end of the first wave of infections in France to evaluate the possible contamination of coastal areas before the summer season, using shellfish as sentinels. We used mostly oyster samples, as it was the species in which methods were tested, but some samples consisted in mussels or clams in areas where oysters were not available. Sites known for their sensitivity to human sewage contamination were sampled, hypothesizing that if SARS-CoV-2 could contaminate the coastal environment, these sites should be positive. Indeed, the observed prevalence in NoV (20.5%) was high compared to previous surveys, especially considering the low epidemic burden of NoV in summertime (EFSA, 2019; Schaeffer et al., 2013). Several water samples were also found contaminated with NoV showing that in some instance this approach can be complementary to shellfish sampling, although technical improvements are necessary to increase the recovery rate.
Conversely, all samples (shellfish and seawater) were negative for SARS-CoV-2. The survey period covered the end of the French lock-down (until may 11th, 2020) and the summer season when tourism results in a larger population on the French coastline. During the first wave of SARS-CoV-2 in France (March to May 2020), most cases occurred in the north-eastern part of France, and viral concentrations were likely very low in sewage from the rest of the territory, including western and southern coasts. After the lock-down, although some Covid-19 clusters were reported in seaside communities, the overall prevalence of SARS-CoV-2 remained low in France throughout the survey period (“Taux d'incidence de 458 l'épidémie de COVID-19 (SI-DEP) - data.gouv.fr”, n.d.) which was carried out between the two first waves of Covid-19 (Spaccaferri et al., 2020). Although we cannot rule out a transient contamination, or contamination outside the study sites, these results suggest that SARS-CoV-2 did not reach the French coastal environment during summer 2020 at significant levels. Environmental monitoring should be continued during the winter season, where the risk of viral spread in the environment is likely to increase due to the second wave of Covid-19 in the French population, cold temperatures stabilizing the virus and heavy rainfalls resulting in sewage spillover.
This pandemic raises many questions, including some technical issues regarding CoV detection in different types of environmental samples. As mentioned above, environmental virology in the past has tended to consider mainly non-enveloped viruses. After the first emergence of SARS-CoV, a study demonstrated the persistence of some strains in environmental waters (Casanova et al., 2009). Recently, if many papers have been published regarding sewage contamination by SARS-CoV-2, to our knowledge none report on its detection in seawater and/or shellfish. In developed countries with efficient sewage treatment systems, the risk of coastal contamination may be limited, and linked to accidental contamination with untreated sewage. Yet, in some settings, using shellfish as sentinels for viral diffusion in the environment may be useful, and we show here that two CoV, including SARS-CoV-2, can contaminate oysters under experimental conditions. The demonstration that a surrogate porcine CoV, PEDV, may be used to mimic SARS-CoV2 in oysters, suggest that it could be used in other matrices and, to some extent, to evaluate the stability of infectious particles. Infectious SARS-CoV-2 was isolated from several, but not all, stool or urine samples from Covid-19 patients (Jones et al., 2020; Sun et al., 2020; Xiao et al., 2020). Although in two outbreaks, sewage was suspected as a SARS-CoV-2 contamination source (Kang et al., 2020; Yuan et al., 2020), attempts at isolating infectious SARS-CoV-2 from raw or treated sewage, or freshwater, remains unsuccessful to date (Rimoldi et al., 2020; Wang et al., 2020). A recent study reports the infection of non-human primates through gastrointestinal inoculation with a high inoculum of SARS-CoV-2 (Jiao et al., 2020). Yet, in humans, the fecal-oral route of transmission has never being observed for SARS-CoV-2 (Zuber and Brüssow, 2020). The sanitary risk posed by potential contamination of shellfish by SARS-CoV-2 is likely very low but having a method to detect this virus in a food matrix known to be at risk for virus transmission is important to anticipate questions that may raise with environmental or food contamination by this virus.
To conclude, we believe that surveying shellfish may help to monitor the viral diffusion in seaside communities, and may be especially suited for countries lacking centralized sewage collection infrastructures, in which environmental contamination is also more likely (Guerrero-Latorre et al., 2020). Further work is needed to evaluate and adapt existing methods for the detection of SARS-CoV-2 in the environment, that may also be suited for other emerging enveloped viruses such as Influenza, Ebola, or Nipah viruses, should we face another emerging viral pandemic.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank Audrey Rodallec, Virginie Ferré, Berthe-Marie Imbert-Marcille (Service de Virology, Centre Hospitalier Universitaire & Université de Nantes, France) for technical advice and helpful discussions. We thank the Obepine network for helpful discussions.
We are grateful for the help and expertise of our colleagues from the Laboratoires Environnement Ressource (LER, Coastal Unit, Ifremer) who participated in the environmental sampling and sample logistics: Sylviane Boulben and Aourégan Terre-Tillon (LER/BO), Julien Chevé, Théodore Marie Lepoittevin and Manuel Rouquette (LER/BN), Camille Gianaroli (LER/LR), James Grizon and Jonathan Deborde (LER/PC), Myriam Perrière-Rumebe, Florence d'Amico and Elvire Antajan (LER/AR), and all members of the LER/BO, BN, MPL, N, LR, PC and PAC.
Funding
This work is supported by the Agence Nationale de la Recherche and the Fondation de France (ANR RA-Covid wave 5, n°00109676), the Région Pays de la Loire (order n°2020-12887), by an internal funding from Ifremer General Direction (SARS-CoV-2 action plan) and the European project VEO (H2020, SC1-2019-874735).
CRediT authorship contribution statement
MD: Conceptualization; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Visualization; Roles/Writing - original draft; Writing - review & editing. JCP: Conceptualization; Formal analysis; Funding acquisition; Investigation; Methodology; Supervision; Visualization; Writing - review & editing. CW: Investigation; Writing - review & editing. CLM: Investigation; Writing - review & editing. SP: Formal analysis; Investigation; Methodology; Writing - review & editing. SJ: Investigation; Writing - review & editing. SR: Investigation; Methodology; Writing - review & editing. LB: Investigation; Methodology; Writing - review & editing. MC: Investigation; Methodology; Writing - review & editing. PG: Conceptualization; Funding acquisition; Project administration; Writing - review & editing. FC: Investigation; Writing - review & editing. RG: Investigation; Writing - review & editing. LLa: Investigation; Writing - review & editing. LLe: Investigation; Writing - review & editing. PLG: Investigation; Writing - review & editing. CM: Investigation; Writing - review & editing. AS: Investigation; Writing - review & editing. JLS, Investigation; Writing - review & editing. OS, Investigation; Writing - review & editing. CP, Investigation; Writing - review & editing. CBB, Investigation; Writing - review & editing. YB: Funding acquisition; Project administration; Supervision; Writing - review & editing. FLG: Conceptualization; Formal analysis; Funding acquisition; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Roles/Writing - original draft; Writing - review & editing.
Editor: Damia Barcelo
References
- Abraham J.P., Plourde B.D., Cheng L. Using heat to kill SARS-CoV-2. Rev. Med. Virol. 2020;30 doi: 10.1002/rmv.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bibby K., Haramoto E., Hewitt J., Huygens F., Gyawali P., Korajkic A., Riddell S., Sherchan S.P., Simpson S.L., Sirikanchana K., Symonds E.M., Verhagen R., Vasan S.S., Kitajima M., Bivins A. Decay of SARS-CoV-2 and surrogate murine hepatitis virus RNA in untreated wastewater to inform application in wastewater-based epidemiology. Environ. Res. 2020;191:110092. doi: 10.1016/j.envres.2020.110092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan M., Xu B., Gamal El-Din M. Transmission of SARS-CoV-2 via fecal-oral and aerosols-borne routes: environmental dynamics and implications for wastewater management in underprivileged societies. Sci. Total Environ. 2020;743:140709. doi: 10.1016/j.scitotenv.2020.140709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmar R.L., Neill F.H., Romalde J.L., Guyader O.L.E., Woodley C.M., Metcalf T.G., Estes M.K. Detection of Norwalk virus and hepatitis a virus in shellfish tissues with the PCR. Appl. Environ. Microbiol. 1995;61:3014–3018. doi: 10.1128/aem.61.8.3014-3018.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabbes L., Ollivier J., Schaeffer J., Parnaudeau S., Rhaissi H., Nourlil J., Le Guyader F.S. Norovirus and other human enteric viruses in Moroccan shellfish. Food Environ. Virol. 2013;5:35–40. doi: 10.1007/s12560-012-9095-8. [DOI] [PubMed] [Google Scholar]
- Bigault L., Brown P., Bernard C., Blanchard Y., Grasland B. Porcine epidemic diarrhea virus: viral RNA detection and quantification using a validated one-step real time RT-PCR. J. Virol. Methods. 2020;283:113906. doi: 10.1016/j.jviromet.2020.113906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch A., Gkogka E., Le F.S., Loisy-Hamon F., Lee A., Lieshout L.V., Marthi B., Myrmel M., Sansom A., Schultz A.C., Winkler A., Zuber S., Phister T. Foodborne viruses: detection, risk assessment, and control options in food processing. Int. J. Food Microbiol. 2018;285:110–128. doi: 10.1016/j.ijfoodmicro.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova L., Rutala William A., Weber David J., Sobsey Mark D. Survival of surrogate coronaviruses in water. Water Res. 2009;43(7):1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conceição-Neto N., Zeller M., Lefrère H., De Bruyn P., Beller L., Deboutte W., Yinda C.K., Lavigne R., Maes P., Ranst M.V., Heylen E., Matthijnssens J. Modular approach to customise sample preparation procedures for viral metagenomics: a reproducible protocol for virome analysis. Sci. Rep. 2015;5:16532. doi: 10.1038/srep16532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.-L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromeans T., Park G.W., Costantini V., Lee D., Wang Q., Farkas T., Lee A., Vinjé J. Comprehensive comparison of cultivable norovirus surrogates in response to different inactivation and disinfection treatments. Appl. Environ. Microbiol. 2014;80:5743–5751. doi: 10.1128/AEM.01532-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donia D., Dell’Amico M.C., Petrinca A.R., Martinucci I., Mazzei M., Tolari F., Divizia M. Presence of hepatitis E RNA in mussels used as bio-monitors of viral marine pollution. J. Virol. Methods. 2012;186:198–202. doi: 10.1016/j.jviromet.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Drouaz N., Schaeffer J., Farkas T., Le Pendu J., Le Guyader F.S. Tulane virus as a potential surrogate to mimic norovirus behavior in oysters. Appl. Environ. Microbiol. 2015;81:5249–5256. doi: 10.1128/AEM.01067-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Analysis of the European baseline survey of norovirus in oysters. EFSA J. 2019;17:1–99. doi: 10.2903/j.efsa.2019.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etievant S., Bal A., Escuret V., Brengel-Pesce K., Bouscambert M., Cheynet V., Generenaz L., Oriol G., Destras G., Billaud G., Josset L., Frobert E., Morfin F., Gaymard A. Performance assessment of SARS-CoV-2 PCR assays developed by WHO referral laboratories. J. Clin. Med. 2020;9:1871. doi: 10.3390/jcm9061871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorito F., Amoroso M.G., Lambiase S., Serpe F.P., Bruno T., Scaramuzzo A., Maglio P., Fusco G., Esposito M. A relationship between environmental pollutants and enteric viruses in mussels (Mytilus galloprovincialis) Environ. Res. 2019;169:156–162. doi: 10.1016/j.envres.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Flannery J., Keaveney S., Rajko-Nenow P., O’Flaherty V., Dor?? W. Concentration of norovirus during wastewater treatment and its impact on oyster contamination. Appl. Environ. Microbiol. 2012;78:3400–3406. doi: 10.1128/AEM.07569-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco G., Anastasio A., Kingsley D.H., Amoroso M.G., Pepe T., Fratamico P.M., Cio B., Rossi R., Rosa G.L., Boccia F. 2019. Detection of Hepatitis A Virus and Other Enteric Viruses in Shellfish Collected in the Gulf of Naples, Italy 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Géba E., Aubert D., Durand L., Escotte S., La Carbona S., Cazeaux C., Bonnard I., Bastien F., Palos Ladeiro M., Dubey J.P., Villena I., Geffard A., Bigot-Clivot A. Use of the bivalve Dreissena polymorpha as a biomonitoring tool to reflect the protozoan load in freshwater bodies. Water Res. 2020;170:115297. doi: 10.1016/j.watres.2019.115297. [DOI] [PubMed] [Google Scholar]
- Gentry J., Vinje J., Guadagnoli D., Lipp E.K. Norovirus distribution within an estuarine environment. Appl. Environ. Microbiol. 2009;75:5474–5480. doi: 10.1128/AEM.00111-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J., Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome-related coronavirus classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzki M., Ollivier J., Le Saux J.C., Piquet J.C., Noyer M., Le Guyader F.S. Impact of Xynthia tempest on viral contamination of shellfish. Appl. Environ. Microbiol. 2012;78:3508–3511. doi: 10.1128/AEM.07604-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Latorre L., Ballesteros I., Villacrés-Granda I., Granda M.G., Freire-Paspuel B., Ríos-Touma B. SARS-CoV-2 in river water: implications in low sanitation countries. Sci. Total Environ. 2020;743:140832. doi: 10.1016/j.scitotenv.2020.140832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillier L., Martin-Latil S., Chaix E., Thébault A., Pavio N., Le Poder S., Batéjat C., Biot F., Koch L., Schaffner D.W., Sanaa M., Covid-19 Emergency Collective Expert Appraisal Group Modeling the inactivation of viruses from the Coronaviridae family in response to temperature and relative humidity in suspensions or on surfaces. Appl. Environ. Microbiol. 2020;86 doi: 10.1128/AEM.01244-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Kitajima M., Hata A., Torrey J.R., Masago Y., Sano D., Katayama H. A review on recent progress in the detection methods and prevalence of human enteric viruses in water. Water Res. 2018;135:168–186. doi: 10.1016/j.watres.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Hata A., Furumai H., Katayama H. Sequential treatment using a hydrophobic resin and gel fi ltration to improve viral gene quanti fi cation from highly complex environmental concentrates. Water Res. 2020;174:115652. doi: 10.1016/j.watres.2020.115652. [DOI] [PubMed] [Google Scholar]
- Herbert R.J.H., Humphreys J., Davies Clare J., Roberts C., Fletcher S., Crowe Tasman P. Ecological impacts of non-native Pacific oysters (Crassostrea gigas) and management measures for protected areas in Europe. Biodivers. Conserv. 2016;25:2835–2865. doi: 10.1007/s10531-016-1209-4. [DOI] [Google Scholar]
- Iwamoto M., Ayers T., Mahon B.E., Swerdlow D.L. Epidemiology of seafood-associated infections in the United States. Clin. Microbiol. Rev. 2010;23:399–411. doi: 10.1128/CMR.00059-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao L., Li H., Xu J., Yang M., Ma C., Li J., Zhao S., Wang H., Yang Y., Yu W., Wang J., Yang J., Long H., Gao J., Ding K., Wu D., Kuang D., Zhao Y., Liu J., Lu S., Liu H., Peng X. The gastrointestinal tract is an alternative route for SARS-CoV-2 infection in a nonhuman primate model. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John S.G., Mendez C.B., Deng L., Poulos B., Kauffman A.K.M., Kern S., Brum J., Polz M.F., Boyle E.A., Sullivan M.B. Vol. 3. 2011. A Simple and Efficient Method for Concentration of Ocean Viruses by Chemical Flocculation; pp. 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.L., Baluja M.Q., Graham D.W., Corbishley A., McDonald J.E., Malham S.K., Hillary L.S., Connor T.R., Gaze W.H., Moura I.B., Wilcox M.H., Farkas K. Shedding of SARS-CoV-2 in feces and urine and its potential role in person-to-person transmission and the environment-based spread of COVID-19. Sci. Total Environ. 2020;749:141364. doi: 10.1016/j.scitotenv.2020.141364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M., Wei J., Yuan J., Guo J., Zhang Y., Hang J., Qu Y., Qian H., Zhuang Y., Chen X., Peng X., Shi T., Wang J., Wu J., Song T., He J., Li Y., Zhong N. Probable evidence of fecal aerosol transmission of SARS-CoV-2 in a high-rise building. Ann. Intern. Med. 2020 doi: 10.7326/M20-0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama H., Shimasaki A., Ohgaki S. Development of a virus concentration method and its application to detection of enterovirus and Norwalk virus from coastal seawater. Appl. Environ. Microbiol. 2002;68:1033–1039. doi: 10.1128/AEM.68.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R., Pratte-Santos R., Scarpati K., Martins S.A., Loss S.M., Fumian T.M., Miagostovich M.P., Cassini S.T. Surveillance of enteric viruses and thermotolerant coliforms in surface water and bivalves from a mangrove estuary in southeastern Brazil. Food Environ. Virol. 2019;11:288–296. doi: 10.1007/s12560-019-09391-3. [DOI] [PubMed] [Google Scholar]
- Kim S.-H., Kim I.-J., Pyo H.-M., Tark D.-S., Song J.-Y., Hyun B.-H. Multiplex real-time RT-PCR for the simultaneous detection and quantification of transmissible gastroenteritis virus and porcine epidemic diarrhea virus. J. Virol. Methods. 2007;146:172–177. doi: 10.1016/j.jviromet.2007.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Haramoto E., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020;739:139076. doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods - a scoping review. Water Res. 2020;115899 doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guyader F.S., Le Saux J.-C., Ambert-Balay K., Krol J., Serais O., Parnaudeau S., Giraudon H., Delmas G., Pommepuy M., Pothier P., Atmar R.L. Aichi virus, Norovirus, Astrovirus, Enterovirus, and Rotavirus involved in clinical cases from a French oyster-related gastroenteritis outbreak. J. Clin. Microbiol. 2008;46:4011–4017. doi: 10.1128/JCM.01044-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guyader F.S., Parnaudeau S., Schaeffer J., Bosch A., Loisy F., Pommepuy M., Atmar R.L. Detection and quantification of noroviruses in shellfish. Appl. Environ. Microbiol. 2009;75:618–624. doi: 10.1128/AEM.01507-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf H., Zakhour M., Pendu J.L., Le Saux J.C., Atmar R.L., Le Guyader F.S. Distribution in tissue and seasonal variation of norovirus genogroup I and II ligands in oysters. Appl. Environ. Microbiol. 2010;76:5621–5630. doi: 10.1128/AEM.00148-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf H., Schaeffer J., Parnaudeau S., Le Pendu J., Atmar R.L., Crawford S.E., Le Guyader F.S. Strain-dependent norovirus bioaccumulation in oysters. Appl. Environ. Microbiol. 2011;77:3189–3196. doi: 10.1128/AEM.03010-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L.R., Duke G.M., Osorio J.E., Hall D.J., Palmenberg A.C. Mutational analysis of the mengovirus poly(C) tract and surrounding heteropolymeric sequences. J. Virol. 1996;70:2027–2031. doi: 10.1128/JVI.70.3.2027-2031.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney K.R., Gong Y.Y., Lewis T.G. Environmental transmission of SARS at Amoy Gardens. J. Environ. Health. 2006;68:26–30. (quiz 51–52) [PubMed] [Google Scholar]
- Metcalf T.G., Moulton E., Eckerson D. Improved method and test strategy for recovery of enteric viruses from shellfish. Appl. Environ. Microbiol. 1980;39:141–152. doi: 10.1128/aem.39.1.141-152.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A., Ko A.I., Malik A.A., Wang D., Wang M., Warren J.L., Weinberger D.M., Arnold W., Omer S.B. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020;38:1164–1167. doi: 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piquet J.-C., Boulben S., Cheve J., Derrien A., Lamort L., Marco-Miralles F., Marzin A., Meteigner C., Morin D., Orsoni V., Treguier C., Verin F., Amouroux I., Catherine M., Miossec L. 2019. REMI Dataset: The French Microbiological Monitoring Program of Mollusc Harvesting Areas. [DOI] [Google Scholar]
- Polo D., Schaeffer J., Teunis P., Buchet V., Le Guyader F.S. Infectivity and RNA persistence of a norovirus surrogate, the Tulane virus, in oysters. Front. Microbiol. 2018;9:1–8. doi: 10.3389/fmicb.2018.00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., Maresca M., Longobardi C., Mancon A., Romeri F., Pagani C., Cappelli F., Roscioli C., Moja L., Gismondo M.R., Salerno F. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020;744:140911. doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif L.J. Animal coronaviruses: what can they teach us about the severe acute respiratory syndrome? Rev. Sci. Tech. Int. Off. Epizoot. 2004;23:643–660. doi: 10.20506/rst.23.2.1513. [DOI] [PubMed] [Google Scholar]
- Sano D., Amarasiri M., Hata A., Watanabe T., Katayama H. Risk management of viral infectious diseases in wastewater reclamation and reuse: review. Environ. Int. 2016;91:220–229. doi: 10.1016/j.envint.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer J., Le Saux J.C., Lora M., Atmar R.L., Le Guyader F.S. Norovirus contamination on French marketed oysters. Int. J. Food Microbiol. 2013;166:244–248. doi: 10.1016/j.ijfoodmicro.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaccaferri G., Larrieu S., Pouey J., Calba C., Benet T., Sommen C., Lévy-Bruhl D., Smaili S., Che D., Filleul L., Caserio-Schönemann C., Ait-El-Belghiti F., Haeghebaert S., Desenclos J.-C., Huiart L., Laporte A., Rolland P. Early assessment of the impact of mitigation measures to control COVID-19 in 22 French metropolitan areas, October to November 2020. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.50.2001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street R., Malema S., Mahlangeni N., Mathee A. Wastewater surveillance for Covid-19: an African perspective. Sci. Total Environ. 2020;743:140719. doi: 10.1016/j.scitotenv.2020.140719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strubbia S., Schaeffer J., Besnard A., Wacrenier C., Le Mennec C., Garry P., Desdouits M., Le Guyader F.S. Metagenomic to evaluate norovirus genomic diversity in oysters: impact on hexamer selection and targeted capture-based enrichment. Int. J. Food Microbiol. 2020;323:108588. doi: 10.1016/j.ijfoodmicro.2020.108588. [DOI] [PubMed] [Google Scholar]
- Sun J., Zhu A., Li H., Zheng K., Zhuang Z., Chen Z., Shi Y., Zhang Z., Chen S.-B., Liu X., Dai J., Li X., Huang S., Huang X., Luo L., Wen L., Zhuo J., Li Y., Wang Y., Zhang L., Zhang Y., Li F., Feng L., Chen X., Zhong N., Yang Z., Huang J., Zhao J., Li Y.-M. Isolation of infectious SARS-CoV-2 from urine of a COVID-19 patient. Emerg. Microbes Infect. 2020;9:991–993. doi: 10.1080/22221751.2020.1760144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taux d'incidence de l'épidémie de COVID-19 (SI-DEP) - data.gouv.fr [WWW Document], n.d. URL /fr/datasets/taux-dincidence-de-lepidemie-de-covid-19/ (accessed Nov. 9, 2020).
- Wang J., Feng H., Zhang S., Ni Z., Ni L., Chen Y., Zhuo L., Zhong Z., Qu T. SARS-CoV-2 RNA detection of hospital isolation wards hygiene monitoring during the Coronavirus Disease 2019 outbreak in a Chinese hospital. Int. J. Infect. Dis. 2020;94:103–106. doi: 10.1016/j.ijid.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhaus S., Weber F.-A., Schiwy S., Linnemann V., Brinkmann M., Widera M., Greve C., Janke A., Hollert H., Wintgens T., Ciesek S. Detection of SARS-CoV-2 in raw and treated wastewater in Germany - suitability for COVID-19 surveillance and potential transmission risks. Sci. Total Environ. 2020;751:141750. doi: 10.1016/j.scitotenv.2020.141750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn J.B., Clements K., Lowther J.A., Malham S.K., Mcdonald J.E., Jones D.L. Use of Mytilus edulis biosentinels to investigate spatial patterns of norovirus and faecal indicator organism contamination around coastal sewage discharges. Water Res. 2016;105:241–250. doi: 10.1016/j.watres.2016.09.002. [DOI] [PubMed] [Google Scholar]
- Wurtzer S., Marechal V., Mouchel J.-M., Maday Y., Teyssou R., Richard E., Almayrac J.L., Moulin L. Evaluation of lockdown impact on SARS-CoV-2 dynamics through viral genome quantification in Paris wastewaters (preprint) Epidemiology. 2020 doi: 10.1101/2020.04.12.20062679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Sun J., Xu Y., Li F., Huang X., Li H., Zhao Jingxian, Huang J., Zhao Jincun. Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerg. Infect. Dis. 2020;26:1920–1922. doi: 10.3201/eid2608.200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Chen Z., Gong C., Liu H., Li B., Li K., Chen X., Xu C., Jing Q., Liu G., Qin P., Liu Y., Zhong Y., Huang L., Zhu B., Yang Z. Sewage as a possible transmission vehicle during a coronavirus disease 2019 outbreak in a densely populated community: Guangzhou, China, April 2020. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakhour M., Maalouf H., Di Bartolo I., Haugarreau L., Le Guyader F.S., Ruvoën-Clouet N., Le Saux J.C., Ruggeri F.M., Pommepuy M., Le Pendu J. Bovine norovirus: carbohydrate ligand, environmental contamination, and potential cross-species transmission via oysters. Appl. Environ. Microbiol. 2010;76:6404–6411. doi: 10.1128/AEM.00671-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Li Y., Zhang A.L., Wang Y., Molina M.J. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc. Natl. Acad. Sci. 2020;117:14857–14863. doi: 10.1073/pnas.2009637117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber S., Brüssow H. COVID 19: challenges for virologists in the food industry. Microb. Biotechnol. 2020;13:1689–1701. doi: 10.1111/1751-7915.13638. [DOI] [PMC free article] [PubMed] [Google Scholar]