Abstract
Background
Recovery after colonic surgery is invariably delayed by disturbed gut motility. It is commonly assumed that colonic motility becomes quiescent after surgery, but this hypothesis has not been evaluated rigorously. This study quantified colonic motility through the early postoperative period using high-resolution colonic manometry.
Methods
Fibre-optic colonic manometry was performed continuously before, during and after surgery in the left colon and rectum of patients undergoing right hemicolectomy, and in healthy controls. Motor events were characterized by pattern, frequency, direction, velocity, amplitude and distance propagated.
Results
Eight patients undergoing hemicolectomy and nine healthy controls were included in the study. Colonic motility became markedly hyperactive in all operated patients, consistently dominated by cyclic motor patterns. Onset of cyclic motor patterns began to a minor extent before operation, occurring with increasing intensity nearer the time of surgery; the mean(s.d.) active duration was 12(7) per cent over 3 h before operation and 43(17) per cent within 1 h before surgery (P = 0.024); in fasted controls it was 2(4) per cent (P < 0·001). After surgery, cyclic motor patterns increased markedly in extent and intensity, becoming nearly continuous (active duration 94(13) per cent; P < 0·001), with peak frequency 2–4 cycles per min in the sigmoid colon. This postoperative cyclic pattern was substantially more prominent than in non-operative controls, including in the fed state (active duration 27(20) per cent; P < 0·001), and also showed higher antegrade velocity (P < 0·001).
Conclusion
Distal gut motility becomes markedly hyperactive with colonic surgery, dominated by cyclic motor patterns. This hyperactivity likely represents a novel pathophysiological aspect of the surgical stress response. Hyperactive motility may contribute to gut dysfunction after surgery, potentially offering a new therapeutic target to enhance recovery.
May contribute to gut dysfunction
Introduction
The period following gastrointestinal surgery is associated with disturbed gut motility, prolonging hospital stay and increasing morbidity, conferring a resource-intensive burden on healthcare institutions1. This dysfunction is thought to stem from a combination of inflammatory cell activation, electrolyte imbalance, autonomic dysfunction and activation of peripheral opioid receptors, and varies in severity from a mild delay in the return of gut function to prolonged postoperative ileus2.
Considerable progress has been made in recent years in defining the mechanisms that underpin postoperative gut dysfunction, complemented by advances in prevention and management2–4. However, the mechanisms by which pathophysiological disturbances translate into symptoms remain incompletely understood. It is presumed that abnormal motility is a key intermediary step, but it is unclear whether this constitutes dysmotility, hypomotility or atony. The colon is often the slowest gut segment to recover after surgery and postoperative quiescence is typically considered to be a key contributing factor5.
One barrier to understanding has been technological limitation in investigating human colonic motor activity. Although colonic manometry has been a prominent method for evaluating motility in vivo, the sparse sensor resolution of previous devices prevented reliable assessment of the frequency and polarity of motor patterns such as cyclic motor activity, which propagate over relatively short distances6,7. The recent development of high-resolution (HR) colonic manometry is now enabling more accurate quantification of colonic motor patterns in health and disease states8,9. In particular, these recordings have revealed the prominence of cyclic motor patterns in the distal colon7,10.
The aim of this study was to use HR fibre-optic manometry to accurately define postoperative colorectal motility in patients undergoing elective right hemicolectomy, in comparison to normal motility in healthy controls. Right hemicolectomy was chosen because this operation appears to be accompanied by slower functional recovery and higher rates of postoperative ileus compared with left-sided colorectal resections, for reasons that are currently unknown11.
Methods
Ethical approval was obtained from the New Zealand Health and Disability Ethics Committee and Flinders University Human Research Ethics Committee. All participants provided informed consent.
Patients
Patients presenting to Auckland City Hospital for elective right hemicolectomy were screened for eligibility. Included were patients aged 18–75 years scheduled to undergo right-sided colonic resection for any indication using a laparoscopic or open approach. Exclusion criteria were: a current or previous functional motility disorder, coexisting medical illness known to affect colonic motility, ASA fitness grade IV or greater, anticipation of requirement for high-dependency or intensive care, epidural use, pregnancy and previous gastrointestinal surgery. Patients using laxatives or antidiarrhoeal medication were also excluded.
High-resolution manometry
All recordings were made using a 36-sensor fibre-optic manometry catheter with an intersensor spacing of 1 cm, designed and validated for HR gastrointestinal recordings8. The catheters were attached to a spectral interrogator unit (FBG-scan 804D; FBGS International, Geel, Belgium) and intraluminal pressure events were recorded using a LabVIEW© interface (National Instruments, Austin, Texas, USA).
Participants presented to the endoscopy suite on the morning of the scheduled colectomy. Two Fleet® (Lynchburg, Virginia, USA) enemas were administered before insertion of the catheter under colonoscopic guidance, without use of sedation or other medication. The fibre-optic catheter was clipped to the mucosa of the descending colon using Resolution™ Clips (Boston Scientific, Marlborough, Massachusetts, USA) such that the sensory portion was situated entirely in the rectum, sigmoid and descending colon. Patients returned to the recovery area with the bed inclined to 30°, and were asked to restrict movement. Baseline recordings were performed for minimum of 2 h with patients fasted, before transfer to the operating room. Care was taken to avoid movement or displacement of the catheter on patient transfer.
Intraoperative manometric recordings began before induction and were continued uninterrupted until emergence from anaesthesia. After operation, data acquisition was continued in recovery and on the ward with interruption only during patient transfers. Care conformed to an enhanced recovery after surgery (ERAS) pathway, encompassing no oral bowel preparation, routine transverse abdominis plane blocks, opiate minimization and stepwise oral analgesia progression, restricted intravenous fluids, and early postoperative feeding. The catheter was removed after midday on day 1, allowing data acquisition for minimum of 16 h after operation.
Data analysis
Analytical methods for HR colonic manometry have been published in full elsewhere7,12. The primary analysis was performed in PlotHRM (Flinders University, Adelaide, South Australia, Australia). Artefacts were identified as abnormal events simultaneously spanning all channels, and were removed. Fiducial markers were then placed by visual inspection for propagating or simultaneous activities, defined as pressure events occurring in four or more adjacent channels (greater than 3 cm) with an overlapping time course. These sequences were classified within five distinct patterns in accordance with a recently-devised scheme: high-amplitude propagating sequences (HAPS), cyclic motor patterns (repetitive propagating events with a frequency range between 2 and 6 cycles per min (c.p.m.)), short single motor events, long single motor events and retrograde slowly propagating motor events7.
Amplitudes were calculated in PlotHRM by averaging the pressure at all channels within each sequence. The frequency of cyclic motor patterns was analysed separately in MATLAB R.2015a (MathWorks, Natick, Massachusetts, USA). Raw data were filtered using a second-order Butterworth filter with a bandpass of 0·01–2·5 Hz. The dominant frequency of the filtered data from each sensor was then calculated using the fast Fourier transform (FFT) method over an interval of 240 s with a 40-s overlap between successive periods analysed. Detected dominant frequencies lying within a range of 0·001–8 c.p.m. were displayed on a colour plot. For visualization, these data were also mapped to the centre line of a three-dimensional colonic model generated using data from the Visible Human Project® (US National Library of Medicine, Bethesda, Maryland, USA), modified for right hemicolectomy, then projected to the surface of the model, as described previously13.
Control data
Preoperative data served as a baseline for the intraoperative and postoperative motility data. In addition, control data were obtained from healthy volunteers using similar methods7. The rationale for using this group as controls was to provide a direct physiological comparison of cyclic motor activity in subjects who were not undergoing surgery, using the HR manometry technique. These subjects underwent 2 h of preprandial and postprandial recordings (700-kcal meal challenge) following oral bowel preparation, using fibre-optic catheters with 72 sensors at 1-cm intervals. Motor events were analysed as described above; however, evaluated data were limited to motor events within the descending colon, sigmoid and rectum, to allow direct comparison with the patient data for the present study. The fasted control data were used for comparisons with patients during the preoperative period, who were also fasted. The postprandial control data were used for comparisons with patients during the postoperative period, as these patients were free to eat, in accordance with the ERAS protocol.
Statistical analysis
Frequency, amplitude, velocity and distance of propagation were determined for each motility pattern and recording period; these data are presented as mean(s.d.). Repeated-measures ANOVA was used to compare data across the preoperative, intraoperative and postoperative periods. The rate of cyclic motor events across each period was expressed as per cent active duration (the percentage of each recording period occupied by cyclic motor patterns); these values were compared using Mann–Whitney U or Freidman tests as appropriate. Statistical tests were undertaken in Prism® version 5 (GraphPad Software, La Jolla, California, USA) and SPSS® version 19 (IBM, Armonk, New York, USA), with a significance threshold of P < 0·050.
Results
A total of 29 patients were assessed for eligibility. Thirteen declined or were unable to consent, and eight were excluded owing to a need for high-dependency or intensive care support (2), epidural (1), operative complexity (1), previous anterior resection (1), or because catheter placement could not be coordinated with surgery (3). The remaining eight patients (7 men), of median age 70 (range 25–83) years, underwent HR manometry. Indications for surgery were neoplasia (7) or chronic stricture secondary to ileocolic Crohn's disease (1).
The median recording duration was 320 (range 190–432) min before, 151 (88–207) min during and 1001 (947–1278) min after operation. One patient had the HR manometry catheter inserted under general anaesthetic in the operating theatre immediately before surgery, so did not have preoperative recordings. Six patients underwent laparoscopic right hemicolectomy as planned, one had a limited ileocolic resection, and one patient underwent an open trial right-sided dissection (lasting more than 1 h) followed by defunctioning ileostomy owing to unresectable locally infiltrative malignant disease. No patient had an epidural. All patients received some doses of opiate (mean(s.d.) fentanyl dose 350(156) μg; mean morphine dose 4·8(4·5) mg) during surgery, and opiate patient-controlled analgesia (PCA) during the postoperative period. Five patients tolerated a single light meal on day 1, but none of the patients passed flatus or stool during the postoperative recording period.
The controls comprised nine healthy volunteers (3 men), of median age 51 (range 30–69) years. All had complete 4-h recordings across 2-h preprandial and postprandial periods.
Cyclic motor patterns
Perioperative motility patterns were highly consistent in all eight patients, demonstrating the onset of marked motility responses that were hyperactive compared with non-operative controls. A progressive increase in the cyclic motor pattern began before surgery, increased substantially after the incision, and dominated the entire postoperative recording period.
The frequency of preoperative cyclic motor patterns was counted in five patients, who were fasted during this time, received no premedication, and were not subject to any other interventions. Two patients had a preoperative carbohydrate drink 2 h before surgery, and one patient did not have preoperative recordings. Cyclic motor patterns in the five patients occurred for a mean(s.d.) of 22(5) per cent of the recording time, which was a substantially greater intensity of activity than was registered in non-operative fasted controls (2(4) per cent active duration; P < 0·001). The intensity of the preoperative cyclic activity correlated closely with proximity to surgery, increasing from 12(7) per cent of the recording duration at more than 3 h before surgery to 32(17) per cent at 1–2 h and 43(17) per cent within 1 h before operation (P = 0·024) (Fig. 1).
Fig. 1.
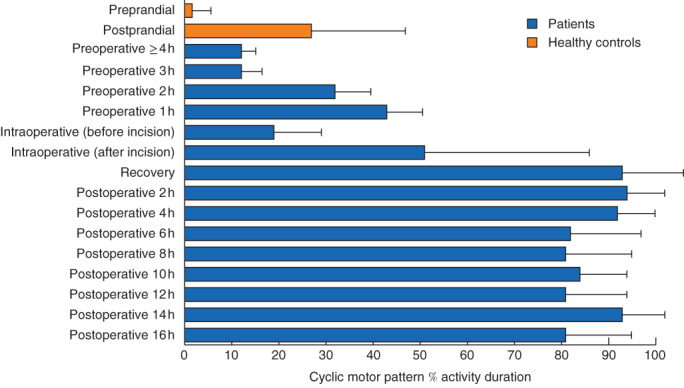
Mean(s.d.) percentage of recording time in which cyclic motor patterns were active in controls and patients. In all patients, there was a marked increase in the occurrence of cyclic motor patterns that began before operation, increased during surgery and was largely maintained after operation (P < 0·001, repeated-measures ANOVA)
After induction of anaesthesia, but before the start of surgery, the intensity of cyclic motor events initially dropped to 19(10) per cent active duration, before markedly increasing in all patients to 51(35) per cent after the incision, and increasing further to 93(5) per cent in postoperative recovery (P < 0·001). After operation, cyclic motor activity was then sustained at about the same intensity (range 81–98 per cent), occupying 94(13) per cent of the recorded duration by 2 h; this represented a significant increase compared with both the preoperative and intraoperative percentage active durations (P < 0·001). This intensity of postoperative cyclic motor patterns was significantly greater than was observed in postprandial healthy controls (27(20) per cent; P < 0·001). These data are summarized in Fig. 1.
A representative example of the hyperactive cyclic motor pattern response to surgery is shown for one patient in Fig. 2a, with the activity transition occurring over the preoperative, intraoperative and postoperative periods. Fig. 2b shows traces of the pressure waveforms from this patient, over a representative 12-min time window after surgery. Examples from two further patients are provided in Fig. 3, again showing intense cyclic motor pattern activity in the postoperative period. For comparison, cyclic motor events occurring as part of a normal postprandial response in the non-operative controls are shown in Fig. 4.
Fig. 2.
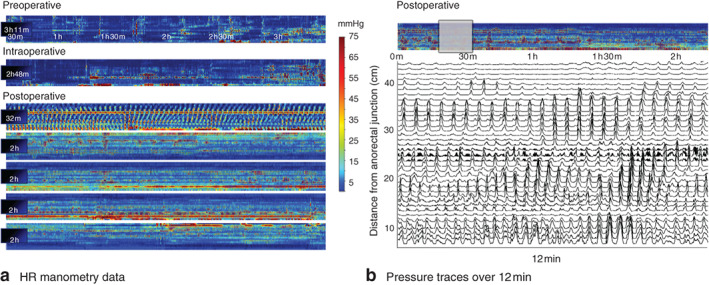
a Representative high-resolution (HR) colonic manometry data from one patient, demonstrating pressure profiles across the preoperative, intraoperative and postoperative periods (total 14·5 h of data shown). The colour plots demonstrate pressure (scale bar) across the 36-channel manometry device, arranged from proximal (top of plot) to distal (bottom) bowel. An intense cyclic motor pattern hyperactivity is seen to arise in the preoperative period, increasing during the procedure and sustained throughout a larger area of the colon across the entire postoperative period. b Pressure traces during a representative 12-min postoperative window in the same patient. The selected period is indicated by the grey box above the pressure plots. Each pressure trace relates to a single manometry channel, arranged according to distance from the anorectal junction (top channel proximal)
Fig. 3.
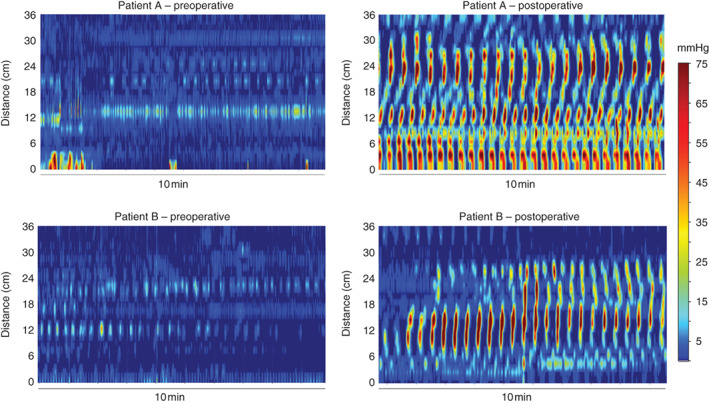
Representative examples of preoperative and postoperative colonic motor activity from two further patients (A and B). Preoperative colonic motility was relatively quiescent (data shown 3–4 h before surgery). By contrast, postoperative motility (data shown 8–10 h after surgery) was characterized by a continuous hyperactivity of cyclic motor patterns occurring at a frequency near to 3 c.p.m.
Fig. 4.
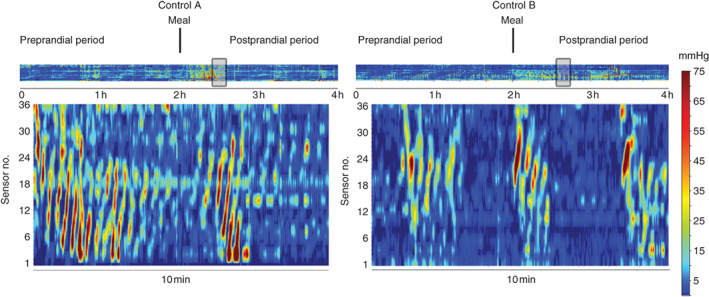
Comparison of cyclic motor patterns in two healthy controls (A and B). The colour plots are arranged as shown in Figs 2 and 3; the grey box in the top panel indicates the selected time window for the main display. In contrast to the postoperative cyclic motor pattern response, cyclic motor patterns in healthy controls occurred mainly in the postprandial period and in periodic bursts, rather than continuously, and at a lower velocity (reduced event slope)
The frequency of cyclic motor patterns identified by the FFT method is illustrated in Fig. 5. Frequency trends are shown for six patients and all channels across the three recording periods, with the dominant frequency range lying between 2 and 4 c.p.m. during active periods of motility. The average event rate per min (including periods of motor quiescence) increased across the three periods from a median of 1·3 per min before surgery, to 1·6 per min during the operation to 1·9 per min after surgery (P < 0·001). Within these data, consistent regional differences in the event rate per minute were identified. The peak frequency occurred between sensors 18 and 30 during all recording periods, with a decline in frequency in the proximal and distal directions away from this zone (Fig. 6a). This region of maximal frequency corresponded to the sensors located in the general proximity of the sigmoid colon (Fig. 6b).
Fig. 5.
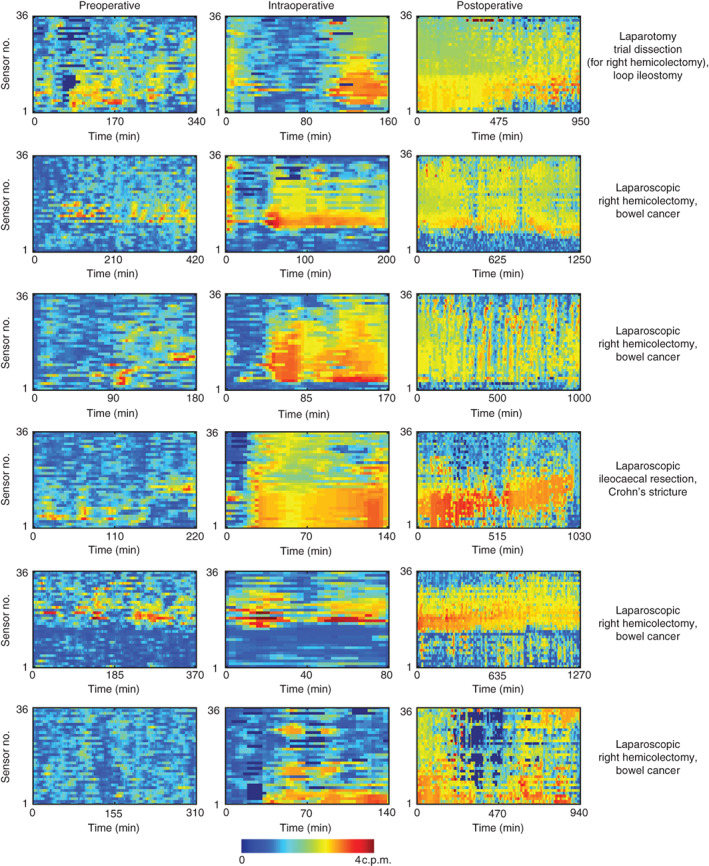
Frequency maps for six representative patients across the preoperative, intraoperative and postoperative recording periods, generated by fast Fourier transform analysis. Frequency (colour scale) is shown for each manometry channel (top proximal; bottom distal). Cyclic motor events generally occurred within a range of 2–4 c.p.m. and were consistent in all patients regardless of the operation
Fig. 6.
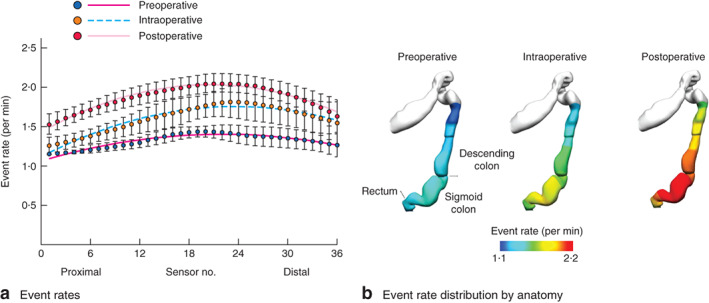
a Event rates (including periods of quiescence) of cyclic motor patterns detected across catheter sensors during the preoperative, intraoperative and postoperative periods. Mean(s.d.) values are shown for each sensor, along with the trend line for each period generated by non-linear regression. Event rates were maximal in the mid-region of the catheter for all periods (between sensors 18 and 30), declining in the proximal and distal directions. b Anatomical registration of the event rate distribution in a to a colonic geometry model, based on the estimated catheter insertion position. The colours represent the mean number of propagating events per minute over the entire recording period. The cyclic motor pattern was most active in the sigmoid colon
Table 1 summarizes data on the amplitude, velocity and extent of propagation for cyclic motor events. The amplitude of the cyclic motor events peaked during the intraoperative phase, at a mean(s.d.) of 33·4(17·9) mmHg for antegrade contractions and 34·2(17·6) mmHg for retrograde contractions, and was substantially lower before operation (P = 0·045 for antegrade events, P = 0·015 for retrograde events). The velocity of cyclic contractions was similar across all three periods for antegrade (P = 0·909) and retrograde (P = 0·359) sequences, whereas extent of propagation was slightly shorter before surgery (P = 0·003 for antegrade and P = 0·054 for retrograde sequences). The postoperative data showed a similar amplitude to that in non-operative postprandial controls, a higher antegrade velocity (appearing with almost no delay in some traces; Fig. 2), with a shorter distance of propagation.
Table 1.
Summary data for cyclic motor patterns
| Preoperative | Intraoperative | Postoperative | P * | Controls | |
|---|---|---|---|---|---|
| Antegrade | |||||
| Amplitude (mmHg) | 18·5(3·2) | 33·4(17·9) | 31·1(16·0) | 0·045 | 24·7(7·4) |
| Velocity (cm/s) | 1·6(0·2) | 1·5(0·5) | 1·5(0·1) | 0·909 | 0·9(0·1)‡ |
| Extent (cm) | 5·0(0·4) | 6·5(1·3) | 7·1(0·9) | 0·003 | 8·8(0·2)† |
| Retrograde | |||||
| Amplitude (mmHg) | 20·5(4·9) | 34·2(17·6) | 31·2(12·0) | 0·015 | 31·5(10·2) |
| Velocity (cm/s) | 1·4(0·4) | 1·5(0·3) | 1·4(0·3) | 0·359 | 1·5(0·9) |
| Extent (cm) | 5·4(0·5) | 7·4(2·5) | 7·5(0·9) | 0·054 | 11·3(3·7)† |
Values are mean(s.d.).
Comparison of preoperative, intraoperative and postoperative periods;
P < 0·050,
P < 0·001, postoperative versus control postprandial data (normal baseline colonic activity in its active non-operative state) (repeated-measures ANOVA).
Other motor patterns
A small number of other motor patterns were also observed during the recording periods in operated patients. A total of 123 retrograde and 60 antegrade short single motor patterns were observed. These patterns occurred before operation in six patients at a mean(s.d.) of 5·3(17·4) events/h; during surgery in two patients at 2·2(2·4) events/h; and after operation in three patients at 0·4(0·9) events/h. A reduction in the extent of antegrade short single motor events (P = 0·033), and an increased velocity (P < 0·001) and reduced amplitude (P = 0·029) of retrograde short single motor events, were identified after the start of surgery (Table S1, supporting information). Five long single motor patterns were observed in one postoperative recording in a single participant, but no further analysis of this pattern was undertaken.
Discussion
Delayed recovery of gut function remains common after surgery, influencing duration of hospital stay, morbidity and the cost of care1,14. Despite extensive research into the mechanisms of ileus, and the advent of enhanced recovery care2,3, there have been few reliable data informing the actual patterns of gut motility after surgery. Consequently, there is a commonly held view that colorectal motility becomes quiescent in the postoperative period owing to hypomotility and/or atonia. The present findings refute this theory as colonic motility in the early postoperative period was predominantly characterized by a marked increase in the cyclic motor pattern for most of the recording time. The increase in cyclic activity began before surgery, increased markedly in area and amplitude after the surgical incision, then continued largely unabated throughout the postoperative period in all patients.
Cyclic motor patterns are known to be a normal feature of human colonic motor activity7,15. In previous studies7,10,15,16 under non-operative conditions, cyclic motor patterns were shown to occur in the distal colon and rectum, primarily in response to a meal, and mainly propagated in a retrograde direction, potentially contributing to a ‘rectosigmoid brake’ that limits rectal filling. The postoperative cyclic activity had both similarities and differences to the normal postprandial cyclic pattern. The postoperative event rate was much higher (nearly continuous), with a faster antegrade velocity and occurring nearly synchronously in some traces, but events were comparable in frequency and amplitude. The authors consider cyclic motor patterns to be synonymous with what others have variously termed ‘periodic rectal motor activity’ or ‘rectal motor complexes’16, and these have notably been observed to occur at 2–4 c.p.m. in low-resolution studies of the healthy human colon. The cyclic motor activity in the present study is also concordant with previous findings17 in that it appears sporadically in ‘bursts’ every 20–40 min in the preoperative period.
It is therefore hypothesized that this postoperative increase in the cyclic motor pattern represents overexpression of a normal intrinsic colonic motility pattern. Specifically, the observed pattern and frequency is concordant with bioelectrical slow-wave activity generated by networks of interstitial cells of Cajal (ICCs) at the submucosal plexus, which are known to depolarize adjacent circular smooth muscle rhythmically at a frequency of around 2–4 c.p.m. in humans18,19. An extrinsic co-regulatory stimulus might be required for the expression of these motor patterns, modulating ICC networks and/or responses in smooth muscle. Co-regulation of gut smooth muscle by the autonomic nervous system and ICCs is evidenced by close synaptic contacts between vagal nerve endings and ICCs in the upper gut20, and vagal stimulation can initiate pacemaker responses in gastric ICCs21. Under normal circumstances it is plausible that parasympathetic excitation might evoke ICC-mediated cyclic motor activity in the colorectum, explaining why these motor patterns are expressed preferentially in the postprandial state, and why they can also be promoted by sacral nerve stimulation10,12,22.
It is currently unknown, however, why cyclic motor patterns appear to become intensely overexpressed after surgery, when parasympathetic outflow generally decreases and sympathetic outflow increases2. Sympathetic innervation of the gastrointestinal smooth musculature is sparse; adrenergic fibres instead interface with intramural ganglion cells within the enteric plexi, where they are perceived to play an indirect role in motility inhibition23,24. It is theoretically possible that the sympathetic drive of surgery inhibits intrinsic nerves that are themselves inhibitory, thereby releasing expression of this motor pattern. Other postoperative stress-state factors could also be responsible, such as catecholamine release, which can influence ICC and smooth muscle contractility2,25, or the release of vasopressin, which promotes colonic motility26. Interestingly, Welgan and colleagues27 reported a strong increase in sigmoid colonic motor and spike potential activity at approximately 3 c.p.m. in response to anger, another physiological state associated with parasympathetic withdrawal, sympathetic arousal and adrenal responses28. The present preoperative finding of increasing cyclic motor activity intensity with proximity to the time of surgery implies an additional role for anxiety. Consistent with this hypothesis, in another study by Welgan and co-workers29 it was shown that a fear stressor increased the rate of rectosigmoid electrical spike potentials and the amplitude of colonic motor activity.
An alternative explanation for the present results, which may be safely discounted, is the impact of segmental resection and formation of a new anastomosis. Gastrointestinal transection has been shown to disrupt ICCs and slow-wave coupling acutely, with potential to affect downstream motility30–32. However, this mechanism cannot have been responsible for the hyperactive cyclic motor pattern observed here, because the length of colorectum investigated was separated from the resection site by 60–90 cm of intact bowel, and the heightened motility rapidly increased during the procedure before resection. Furthermore, one patient who underwent bowel handling without resection had the same hyperactivity profile.
Previous studies on postoperative colonic motor activity using other technologies have shown inconsistent results. Roberts and colleagues30 studied left-sided resections using a system of strain gauge transducers at 10-cm resolution, and reported relative colonic inactivity after surgery, although 3-c.p.m. motor complexes were identified. Other low-resolution studies5,33,34, applying strain gauges or electrodes to various parts of the gastrointestinal tract, also reported that colonic segments became relatively quiescent after surgery, contributing to the traditional notions of postoperative colonic inactivity. The discrepancy between these findings and those of the present investigation may reflect differences in methodology, and particularly the superior sensitivity of HR fibre-optic manometry for detecting cyclic motor patterns6. In another related study, Huge et al.35 studied postoperative motility using a combined barostat and low-resolution manometry device. Although only left-sided resections were studied, they reported an increase in colonic tone 1 day after surgery, which decreased over the following 2 days, and concluded that ‘it seems the colon is contracted or even spastic after surgery’. These barometric findings may be consistent with those of the present study.
More research is needed to determine the exact functional significance of these manometry results. One specific area of possible relevance to the present cohort is the suggested slower recovery of patients undergoing right hemicolectomy versus left-sided colectomy, with the former patients showing slower return of bowel function and higher rates of prolonged ileus11. Resection of distal bowel regions generating hyperactive motility could theoretically contribute to faster recovery after left- compared with right-sided resections. This hypothesis is supported by the recent finding that the length of bowel resected correlated positively with speed of bowel recovery for left-sided resections, but not for right-sided resections11.
HAPS were not observed in the patients who underwent surgery in the present study. These relatively infrequent propulsive events originate in the proximal colon and are responsible for mass movement of intraluminal content36. Although it is acknowledged that only the left and distal colon were studied here, it is likely that HAPS would be suppressed by preoperative fasting, resection of the right hemicolon and by the autonomic shift after surgery, and their absence may also contribute to postoperative bowel inactivity. Little inference can be made on the significance of short and long single motor patterns in recovery, given the relative scarcity of these events in the present cohort. It is also important to consider the potential influence of opiate analgesia. Frantzides and colleagues37,38 studied the colonic effects of opiates, including in humans, and showed that opiates could stimulate colonic spike-burst electrical activity, notably in the sigmoid. However, these events were not observed in the first 2 days after operation, and their frequency was different from that of the cyclic motor patterns in the present study, suggesting a different mechanism of effect38. In addition, patients in this study were treated according to an ERAS protocol, and received relatively small doses of opiates during surgery and in the hours after laparoscopic procedures as they had transverse abdominis plane blocks. It is therefore proposed that opiates were not responsible for the hyperactivity. However, it should be noted that real-time opioid use from PCA equipment was not recorded in the postoperative period; it would be of value to study the effect of opiates on colonic motility using colonic HR manometry.
The use of non-operative controls allowed a useful physiological comparison of cyclic motor activity, but it should be acknowledged that these patients were not age- or sex-matched, were studied with oral bowel preparation, and probably ate a larger meal than patients would usually manage after operation. Other comparative populations could usefully be studied in future, such as those undergoing abdominal surgery without bowel handling, or subjects receiving opiates but not having surgery. More research is also needed to define the duration of the hyperactive motor response, its correlation with clinical recovery, and potential interventions. It would also be worthwhile to investigate motility changes occurring in other parts of the gastrointestinal tract after surgery using HR manometry. Although perioperative care conformed to an ERAS pathway, it is acknowledged that the relatively short duration of postoperative recording in the context of inpatient convalescence spanning many days meant that the effects of many aspects of ERAS protocols, such as early opiate minimization, restricted intravenous fluids and early postoperative feeding, were not reflected in the present data.
Hyperactive cyclic motor patterns arise in the distal colon and rectum as a response to surgery. It is postulated that this sustained abnormal pattern interferes with normal colonic motility, potentially delaying gut function recovery after right hemicolectomy. These findings may indicate a novel therapeutic target for enhancing surgical recovery.
Supplementary Material
Table S1 Summary data for short single motor patterns
Acknowledgements
The authors thank the patients and staff at Auckland City Hospital for their invaluable contributions, and M. Costa for helpful comments. R.V. was funded by the Royal Australasian College of Surgeons Foundation for Surgery Research Fellowship. Device funding was provided by the Colorectal Surgical Society of Australia and New Zealand and the Auckland Medical Research Foundation. Salary support was from the New Zealand Health Research Council, the National Institutes of Health (R01 DK64775), a Rutherford Discovery Fellowship administered by the Royal Society of New Zealand (P.D.) and the James Ramsay Project Grant from the Royal Australasian College of Surgeons (C.I.W.).
J.A. designed and manufactured the HR fibre-optic manometry catheters, and is a director and major shareholder in Arkwright Technologies Ltd, a company making fibre-optic sensors. No commercial funding or support was granted for this work.
Disclosure: The authors declare no other conflict of interest.
References
- 1. Goldstein JL, Matuszewski KA, Delaney CP, Senagore A, Chiao EF, Shah Met al. Inpatient economic burden of postoperative ileus associated with abdominal surgery in the United States. Pharmacology and Therapeutics 2007; 32: 82−90. [Google Scholar]
- 2. Vather R, O'Grady G, Bissett IP, Dinning PG. Postoperative ileus: mechanisms and future directions for research. Clin Exp Pharmacol Physiol 2014; 41: 358–370. [DOI] [PubMed] [Google Scholar]
- 3. Holte K, Kehlet H. Postoperative ileus: a preventable event. Br J Surg 2000; 87: 1480–1493. [DOI] [PubMed] [Google Scholar]
- 4. Traut U, Brügger L, Kunz R, Pauli-Magnus C, Haug K, Bucher HCet al. Systemic prokinetic pharmacologic treatment for postoperative adynamic ileus following abdominal surgery in adults. Cochrane Database Syst Rev 2008; (1)CD004930. [DOI] [PubMed] [Google Scholar]
- 5. Graber JN, Schulte WJ, Condon RE, Cowles VE. Relationship of duration of postoperative ileus to extent and site of operative dissection. Surgery 1982; 92: 87−92. [PubMed] [Google Scholar]
- 6. Dinning PG, Wiklendt L, Gibbins I, Patton V, Bampton P, Lubowski DZet al. Low-resolution colonic manometry leads to a gross misinterpretation of the frequency and polarity of propagating sequences: initial results from fiber-optic high-resolution manometry studies. Neurogastroenterol Motil 2013; 25: e640−e649. [DOI] [PubMed] [Google Scholar]
- 7. Dinning PG, Wiklendt L, Maslen L, Patton V, Bampton P, Lubowski DZet al. Quantification of in vivo colonic motor patterns in healthy humans before and after a meal revealed by high-resolution fiber-optic manometry. Neurogastroenterol Motil 2014; 26: 1443−1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arkwright JW, Blenman NG, Underhill ID, Maunder SA, Szczesniak MM, Dinning PGet al. In-vivo demonstration of a high resolution optical fiber manometry catheter for diagnosis of gastrointestinal motility disorders. Opt Express 2009; 17: 4500−4508. [DOI] [PubMed] [Google Scholar]
- 9. Dinning PG, Wiklendt L, Maslen L, Patton V, Bampton P, Lubowski DZet al. Colonic motor abnormalities in slow transit constipation defined by high resolution, fibre-optic manometry. Neurogastroenterol Motil 2015; 27: 379−388. [DOI] [PubMed] [Google Scholar]
- 10. Lin AY, Du P, Dinning PG, Arkwright JW, Kamp JP, Cheng LKet al. High-resolution anatomic correlation of cyclic motor patterns in the human colon: evidence of a rectosigmoid brake. Am J Physiol Gastrointest Liver Physiol 2017; 312: G508–G515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yuan L, O'Grady G, Milne T, Jaung R, Vather R, Bissett IP. Prospective comparison of return of bowel function after left versus right colectomy. ANZ J Surg 2016; DOI: 10.1111/ans.13823. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 12. Vather R, O'Grady G, Arkwright JW, Rowbotham DS, Cheng LK, Dinning PGet al. Restoration of normal colonic motor patterns and meal responses after distal colorectal resection. Br J Surg 2016; 103: 451–461. [DOI] [PubMed] [Google Scholar]
- 13. Davidson JB, O'Grady G, Arkwright JW, Zarate N, Scott SM, Pullan AJet al. Anatomical registration and three-dimensional visualization of low and high-resolution pan-colonic manometry recordings. Neurogastroenterol Motil 2011; 23: 387–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wolthuis AM, Bislenghi G, Fieuws S, de Buck van Overstraeten A, Boeckxstaens G, D'Hoore A. Incidence of prolonged postoperative ileus after colorectal surgery: a systematic review and meta-analysis. Colorectal Dis 2016; 18: O1–O9. [DOI] [PubMed] [Google Scholar]
- 15. Rao SS, Welcher K. Periodic rectal motor activity: the intrinsic colonic gatekeeper? Am J Gastroenterol 1996; 91: 890–897. [PubMed] [Google Scholar]
- 16. Lin AY, Dinning PG, Milne T, Bissett IP, O'Grady G. The ‘rectosigmoid brake’: review of an emerging neuromodulation target for colorectal functional disorders. Clin Exp Pharmacol Physiol 2017; 44: 719–728. [DOI] [PubMed] [Google Scholar]
- 17. Rao SS, Sadeghi P, Beaty J, Kavlock R, Ackerson K. Ambulatory 24-h colonic manometry in healthy humans. Am J Physiol Gastrointest Liver Physiol 2001; 280: G629–G639. [DOI] [PubMed] [Google Scholar]
- 18. Taylor I, Duthie HL, Smallwood R, Linkens D. Large bowel myoelectrical activity in man. Gut 1975; 16: 808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen JH, Yu Y, Yang Z, Yu WZ, Chen WL, Yu Het al. Intraluminal pressure patterns in the human colon assessed by high-resolution manometry. Sci Rep 2017; 7: 41436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huizinga JD, Zarate N, Farrugia G. Physiology, injury, and recovery of interstitial cells of Cajal: basic and clinical science. Gastroenterology 2009; 137: 1548–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hirst GD, Dickens EJ, Edwards FR. Pacemaker shift in the gastric antrum of guinea-pigs produced by excitatory vagal stimulation involves intramuscular interstitial cells. J Physiol 2002; 541: 917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patton V, Wiklendt L, Arkwright JW, Lubowski DZ, Dinning PG. The effect of sacral nerve stimulation on distal colonic motility in patients with faecal incontinence. Br J Surg 2013; 100: 959–968. [DOI] [PubMed] [Google Scholar]
- 23. Lundgren O Sympathetic input into the enteric nervous system. Gut 2000; 47(Suppl 4): iv33–iv35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gillis RA, Dias Souza J, Hicks KA, Mangel AW, Pagani FD, Hamilton BLet al. Inhibitory control of proximal colonic motility by the sympathetic nervous system. Am J Physiol 1987; 253: G531–G539. [DOI] [PubMed] [Google Scholar]
- 25. Owyang C, Hasler WL. Physiology and pathophysiology of the interstitial cells of Cajal: from bench to bedside. VI. Pathogenesis and therapeutic approaches to human gastric dysrhythmias. Am J Physiol Gastrointest Liver Physiol 2002; 283: G8–G15. [DOI] [PubMed] [Google Scholar]
- 26. Schang JC, Dapoigny M, Devroede G. Stimulation of colonic peristalsis by vasopressin: electromyographic study in normal subjects and patients with chronic idiopathic constipation. Can J Physiol Pharmacol 1987; 65: 2137–2141. [DOI] [PubMed] [Google Scholar]
- 27. Welgan P, Meshkinpour H, Beeler M. Effect of anger on colon motor and myoelectric activity in irritable bowel syndrome. Gastroenterology 1988; 94: 1150–1156. [DOI] [PubMed] [Google Scholar]
- 28. Kop WJ, Synowski SJ, Newell ME, Schmidt LA, Waldstein SR, Fox NA. Autonomic nervous system reactivity to positive and negative mood induction: the role of acute psychological responses and frontal electrocortical activity. Biol Psychol 2011; 86: 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Welgan P, Meshkinpour H, Hoehler F. The effect of stress on colon motor and electrical activity in irritable bowel syndrome. Psychosom Med 1985; 47: 139–149. [DOI] [PubMed] [Google Scholar]
- 30. Roberts JP, Benson MJ, Rogers J, Deeks JJ, Williams NS. Characterization of distal colonic motility in early postoperative period and effect of colonic anastomosis. Dig Dis Sci 1994; 39: 1961–1967. [DOI] [PubMed] [Google Scholar]
- 31. Yanagida H, Yanase H, Sanders KM, Ward SM. Intestinal surgical resection disrupts electrical rhythmicity, neural responses, and interstitial cell networks. Gastroenterology 2004; 127: 1748–1759. [DOI] [PubMed] [Google Scholar]
- 32. Du P, Hameed A, Angeli TR, Lahr C, Abell TL, Cheng LKet al. The impact of surgical excisions on human gastric slow wave conduction, defined by high-resolution electrical mapping and in silico modeling. Neurogastroenterol Motil 2015; 27: 1409–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Woods JH, Erickson LW, Condon RE, Schulte WJ, Sillin LF. Postoperative ileus: a colonic problem. Surgery 1978; 84: 527–533. [PubMed] [Google Scholar]
- 34. Waldhausen JH, Shaffrey ME, Skenderis BS, Jones RS, Schirmer BD. Gastrointestinal myoelectric and clinical patterns of recovery after laparotomy. Ann Surg 1990; 211: 777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huge A, Kreis ME, Zittel TT, Becker HD, Starlinger MJ, Jehle EC. Postoperative colonic motility and tone in patients after colorectal surgery. Dis Colon Rectum 2000; 43: 932–939. [DOI] [PubMed] [Google Scholar]
- 36. Bharucha AE. High amplitude propagated contractions. Neurogastroenterol Motil 2012; 24: 977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Frantzides CT, Condon RE, Schulte WJ, Cowles V. Effects of morphine on colonic myoelectric and motor activity in subhuman primates. Am J Physiol 1990; 258: G247–G252. [DOI] [PubMed] [Google Scholar]
- 38. Frantzides CT, Cowles V, Salaymeh B, Tekin E, Condon RE. Morphine effects on human colonic myoelectric activity in the postoperative period. Am J Surg 1992; 163: 144–148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Summary data for short single motor patterns


