Abstract
Background
Pain is a common debilitating symptom in pancreatic adenocarcinoma. This cohort study examined the use of, and factors associated with, pain-directed interventions for a high pain score in patients with non-curable pancreatic adenocarcinoma.
Methods
Administrative databases were linked and patients with non-resected pancreatic adenocarcinoma diagnosed between 2010 and 2016, who reported one or more Edmonton Symptom Assessment System (ESAS) score, were identified. A high pain score was defined as an ESAS score of at least 4. Outcomes were pain-directed interventions: opiates (in patients aged 65 years or more with universal drug coverage), nerve block and radiation therapy for a high pain score. Reduction in pain score of at least 1 point after pain-directed intervention was also evaluated. Modified Poisson regression was used to examine factors associated with pain-directed intervention.
Results
Among 2623 patients with a median age of 67 years, 1223 (46·6 per cent) were women, and 1621 (61·8 per cent) reported a high pain score at a median of 38 days after diagnosis. Of those with a high pain score, 75·6 per cent (688 of 910) received opiates, 13·5 per cent (219 of 1621) radiation and 1·2 per cent (19 of 1621) nerve block. The pain score decreased in 62·1 per cent of patients after administration of opiates, 73·4 per cent after radiation and all patients after nerve block. In multivariable analysis, no patient factor (age, sex, co-morbidity burden, rurality, income quintile) was associated with receipt of non-opiate pain-directed intervention for a high pain score. In patients aged at least 65 years, advanced age was associated with lower odds of opiate use.
Conclusion
Opiates are the most common pain-directed intervention for non-curable pancreatic adenocarcinoma, whereas radiation therapy and nerve blocks are seldom used. The lack of association between pain-directed interventions and patient factors points toward practice-driven patterns.
Graphical Abstract
Patient-reported outcomes were linked to healthcare databases to examine patterns of care for patients with non-curative pancreatic adenocarcinoma reporting high pain scores. Opiates are currently the most commonly used pain-directed intervention, whereas non-opiate strategies such as radiation and nerve blocks are potentially underused, despite being effective in reducing pain scores. ESAS, Edmonton Symptom Assessment System.
Graphical Abstract.
Pain management an issue
Resumen
Antecedentes
El dolor es un síntoma debilitante frecuente en el adenocarcinoma de páncreas. Este estudio de cohortes examinó el uso de las intervenciones dirigidas para el tratamiento del dolor y los factores asociados a las mismas en pacientes con adenocarcinoma pancreático incurable que presentaban puntuaciones altas de dolor.
Métodos
Se revisaron las bases de datos administrativas y se identificaron los pacientes con adenocarcinoma pancreático no resecado diagnosticados entre 2010-2016 con puntuaciones > 1 del Sistema de Evaluación de Síntomas de Edmonton (Edmonton Symptom Assessment System, ESAS). La puntuación alta de dolor se definió como ESAS > 4. Los resultados evaluados fueron las intervenciones dirigidas contra el dolor: opiáceos (en pacientes mayores de 65 años con cobertura universal de medicamentos), bloqueo nervioso y radioterapia en el caso de puntuación alta del dolor. También se evaluó la reducción en la puntuación del dolor (> 1 punto) después de la intervención dirigida contra el dolor. Los factores asociados a la intervención contra el dolor se analizaron mediante una regresión de Poisson modificada.
Resultados
De los 2.623 pacientes con una mediana de edad de 67 años, 1.223 (46,6%) eran mujeres, y 1.621 (61.8%) presentaron una puntuación alta de dolor con una mediana de 38 días desde el momento del diagnóstico. De aquellos con puntuación alta de dolor, el 75,6% recibió opiáceos (n = 688/910), el 13,5% radiación (n = 219/1.621) y el 1,2% bloqueo nervioso (n = 19/1.621). La puntuación del dolor disminuyó en el 62,2% después del tratamiento con los opiáceos, en el 73,8% después de la radiación y en el 100% después del bloqueo nervioso. En el análisis multivariable, ningún factor relacionado con el paciente (edad, sexo, comorbilidades, vivir en una zona rural, quintil de ingresos) se asoció con una intervención dirigida contra dolor sin opiáceos en los casos de puntuación alta del dolor. En pacientes mayores de 65 años, la edad avanzada se asoció con menor probabilidad de uso de opiáceos.
Conclusión
Mientras que los opiáceos son la intervención dirigida contra dolor más común para el adenocarcinoma pancreático no resecable, la radioterapia y el bloqueo nervioso rara vez se usan. La falta de asociación de las intervenciones dirigidas contra el dolor con los factores del paciente apunta hacia el uso de patrones terapéuticos basados en la práctica clínica.
Introduction
Pancreatic adenocarcinoma is a high-fatality cancer representing a considerable societal and healthcare burden; it affects up to 55 000 people and results in 44 000 deaths per year in the USA1. The majority of patients present with advanced or metastatic disease and are not eligible for surgery with curative intent2. For patients with non-curable disease, survival remains limited at a median of 7 months, despite recent advances in systemic therapy3. In addition to systemic therapy, symptom control and optimization of quality of life is particularly important in this population4.
Pain is one of the cardinal symptoms experienced by patients with pancreatic adenocarcinoma, with up to 80 per cent of patients affected5,6. Clinical practice guidelines7,8 focus on pharmacological therapy, with opiates as the dominant modality used for treatment. Although historical concerns focused on undertreating cancer pain5,9–10, more recently new concerns have been raised about high and chronic use of opiates in patients with cancer, despite metastatic status11. Frequent use of opiates has been suggested as a chemical coping mechanism for patients with cancer, whereby use of opiates may mask other needs that cannot be addressed adequately11. Therefore, the use of opiate-sparing treatments is important12.
Patient-reported outcome measures (PROMs) have become a growing focus in oncology13,14. RCTs demonstrated improvements in patient engagement, outcome and satisfaction with use of PROMs, which led to their routine use in clinical practice15,16. In 2007, the province of Ontario initiated population-level routine prospective screening with the Edmonton Symptom Assessment System (ESAS) during outpatient oncology visits17,18. However, information regarding the usefulness and actionability of these data to support patients in clinical practice outside of controlled trial settings is limited19,20. Therefore, a population-based study was undertaken to examine the use of, and factors associated with, pain-directed interventions for patient-reported high pain scores in the management of non-curable pancreatic adenocarcinoma, and to assess changes in pain scores with intervention.
Methods
A population-based cohort study was undertaken using data linked from prospectively maintained administrative databases stored at the ICES in Ontario, Canada. Under the Canada Health Act, the Ontario population benefits from universally accessible and publicly funded healthcare though the Ontario Health Insurance Plan (OHIP). All residents of Ontario are eligible for OHIP after they have resided in the province for 3 months.
The study was approved by the Sunnybrook Health Sciences Centre Research Ethics Board, and met the data confidentiality and privacy guidelines of ICES. It was conducted and reported according to the STROBE statement21.
Study population and cohort
The study included patients with a valid OHIP number diagnosed from 2010 to 2016. Patients with a new diagnosis of pancreatic adenocarcinoma were identified in the Ontario Cancer Registry (OCR) using ICD-O.3 codes C25.0–C25.9. Those who did not undergo pancreatectomy with curative intent at any time up to 31 December 2017, and who had contact at a Registered Cancer Centre and reported at least one ESAS score in the 6 months after the date of diagnosis, were retained (Table S1, supporting information). Patients who met the following criteria were excluded: invalid or missing unique identification number; date of death missing; death before or on the date of diagnosis; date of last contact missing; another cancer diagnosis before or after the pancreatic adenocarcinoma diagnosis; or aged less 18 years at the time of diagnosis.
Follow-up
Patients were followed from the time of diagnosis to the end of follow-up, defined as the date of death, last clinical encounter, or end of the study on 31 March 2018, whichever came first.
Data sources
This study used several linked administrative data sets. The OCR includes all patients diagnosed with cancer (excluding non-melanoma skin cancer) in Ontario since 196422. The reliability of its data has been reported previously23,24. The Registered Persons Database (RPDB) contains vital status and demographic data on all individuals covered under OHIP23. Information regarding health services is included in the Canadian Institute of Health Information Discharge Abstract Database for acute inpatient hospital admissions; the National Ambulatory Care Reporting System for same-day surgery admissions, emergency room visits and oncology clinic visits; and the OHIP Claims Database for billing from healthcare providers, including physicians, groups, laboratories and out-of-province providers. The Cancer Activity Level Reporting (ALR) database is maintained by the OCR, and includes chemotherapy drugs and medications administered to patients with cancer. These databases have been validated for a variety of diagnoses and services25.
The data sets were linked using unique encoded identifiers and analysed at the ICES. The research team's analyst had complete access to all data sets used in this study in order to create the study cohorts, proceed to linkage and perform the analyses.
Exposure
The main exposure was a patient-reported high pain score, defined as a moderate-to-severe pain score on the ESAS. The ESAS is a validated and reliable patient-reported outcome assessing the severity of nine common cancer-associated symptoms, including pain17,18. Patients are asked to rate each symptom on a 11-point numeric scale, from 0 (absence of symptom) to 10 (worst possible symptom)17 (Fig. S1, supporting information). The first ESAS score of 4 or higher within the first 6 months after diagnosis26 was captured to avoid reflecting high pain scores at the end of life.
Outcome measures
The primary outcome of interest was receipt of a pain-directed intervention, subdivided into receipt of radiation therapy, nerve block and opiates. Receipt of radiation therapy was defined by ALR codes, and nerve block by OHIP physician claims. Opiate medication use was defined by filling of a prescription for opiates according to the Ontario Drug Benefit (ODB), using drug identification numbers. The ODB covers all patients in Ontario aged at least 65 years with OHIP. Therefore, for assessment of opiate use the cohort was restricted patients aged 65 years or older.
Considering the opportunistic nature of ESAS collection, it was possible that patients may have been experiencing pain before their index assessment and so, because of possible delays in initiating therapy, receipt of therapy was measured during time windows around the date on which the high pain score was registered (Fig. 1a)27. Alternative time windows were tested and did not alter the proportion of patients receiving the intervention. The use of opiates was captured from 30 days before to 7 days after the date of the high pain score (Fig. 1a). This approach has been validated previously, and other time windows have been tested with no change in the results28.
Fig. 1.
Time windows used for measurement of outcomes and change in pain scores
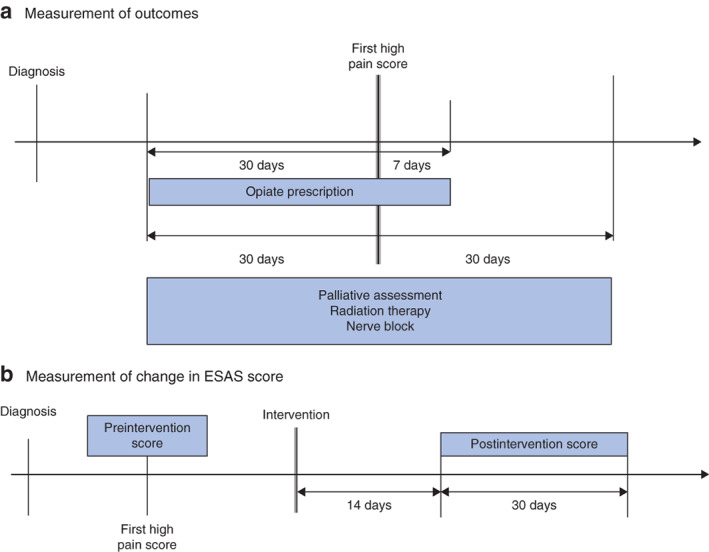
a Measurement of outcomes and b measurement of change in Edmonton Symptom Assessment System (ESAS) scores.
The secondary outcomes were: palliative care assessment; and change in ESAS score following receipt of pain-directed intervention, categorized as increased, stable or decreased. Palliative care assessments were defined by OHIP billing codes and examined for the entire cohort of patients with a high pain score (Table S1, supporting information). The change in ESAS score was examined in a subgroup analysis of patients with a high pain score who received pain-directed therapy. A clinically significant increase or decrease was defined as a change in score of at least 1 of 10 compared with the preintervention score29. The postintervention ESAS pain score was captured during a 30-day time window starting from 14 days after the intervention, to allow time for the treatment to take effect (Fig. 1b). In this analysis, the denominator was the number of patients receiving the interventions and recording an ESAS score during the postintervention score observation time window.
Co-variables
Age and sex were abstracted from the RPDB. Rural living was determined based on postal code of residence30. Income quintile was assessed by means of an ecological measure based on the median income of a patient's postal code of residence using national census data25. The co-morbidity burden was measured using the Johns Hopkins Adjusted Clinical Groups system score. The 32 Aggregated Diagnosis Groups were summed to create a total score, then dichotomized, with a cut-off of 10 indicative of a high co-morbidity burden, consistent with previous reports31. Patients who received chemotherapy were identified as those with at least one chemotherapy infusion billed from the date of diagnosis to the end of follow-up; this strategy was demonstrated previously to have 90 per cent concordance with patient medical records (ALR)32.
Finally, the prevalence of concomitant high patient-reported scores (score at least 4) for ESAS symptoms other than pain at the time the high pain score was recorded was analysed.
Statistical analysis
Categorical variables are reported as numbers with percentages, and continuous variables as median (i.q.r.). Comparisons were undertaken using the χ2 test for categorical variables and the Kruskal–Wallis or t test for continuous variables. Median survival from the time of diagnosis was computed as actual survival.
Predictors of receipt of pain-directed intervention for a high pain score were examined using modified Poisson regression with robust error variance. Relevant demographic and clinical characteristics were identified a priori as potential predictors of pain-directed intervention based on clinical relevance (markers of complexity of cancer care) and existing literature (known relationship with symptom burden in pancreatic adenocarcinoma). The following variables were included: age (categorical), sex, co-morbidity burden, income, rural living and time interval of diagnosis (categorical). As opiate data were available only for patients aged 65 years or older, three models were constructed. The first included all patients with a high pain score and examined predictors of receiving radiation therapy and nerve blocks (opiate-sparing interventions). The second model was restricted to patients aged at least 65 years with a high pain score for whom opiate prescription data were available, and assessed predictors of combined radiation therapy, nerve block and opiates (all interventions). The third model was restricted to patients aged at least 65 years with a high pain score, and examined predictors of opiate use (opiate-only intervention). These models were designed to elucidate the different patterns of patient selection for opiate and non-opiate interventions for a high pain score. Results are reported as relative risks with 95 per cent confidence intervals.
All analyses were two-sided and statistical significance was set at P ≤ 0·050. Analyses were conducted using SAS® Enterprise Guide® 6.1 (SAS Institute, Cary, North Carolina, USA).
Results
A total of 3286 patients diagnosed with pancreatic adenocarcinoma between 2010 and 2016, and reporting at least one ESAS score in the 6 months following diagnosis, were identified (Fig. 2). Of these, 2623 patients, with a median age of 67 years, were included in the analysis. A high pain score was reported by 1621 patients (61·8 per cent), of whom 910 were aged 65 years or older. Overall, 64·1 per cent of patients received chemotherapy, and the remainder received best supportive care. The characteristics of patients with and without a high pain score are shown in Table 1. Younger patients and those with a higher co-morbidity burden were more likely to report a high pain score. Median follow-up from the time of diagnosis was 263 (117–453) days for patients without and 185 (96–329) days for patients with a high pain score. Median survival from the time of diagnosis was 8 (4–14) and 6 (3–11) months respectively (Fig. 3). The median time from diagnosis to first registering a high pain score was 38 (21–69) days and the median time from recording a high pain score to death was 119 (49–258) days. The proportions of moderate-to-severe patient-reported symptoms concomitant with the index high pain score are shown in Fig. 4.
Fig. 2.
Flow diagram of cohort inclusion and exclusion
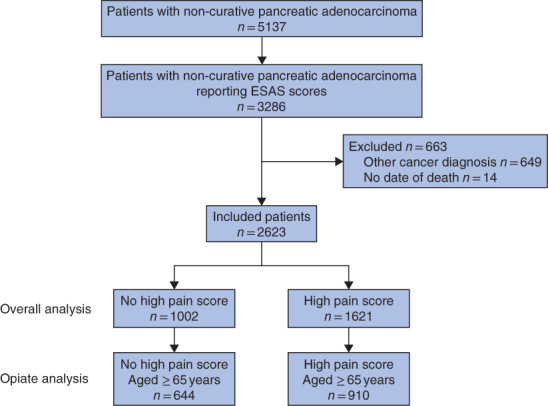
ESAS, Edmonton Symptom Assessment System.
Table 1.
Demographic and clinical characteristics of included patients, stratified by reporting of a high pain score
| No high pain score (n = 1002) | High pain score (n = 1621) | P * | |
|---|---|---|---|
| Age (years) | < 0·001 | ||
| < 65 | 358 (35·7) | 711 (43·9) | |
| 65–70 | 189 (18·9) | 351 (21·7) | |
| 71–80 | 299 (29·8) | 396 (24·4) | |
| ≥ 81 | 156 (15·6) | 163 (10·1) | |
| Sex ratio (F : M) | 457 : 545 | 766 : 855 | 0·410 |
| Rural residence | 104 (10·4) | 155 (9·6) | 0·490 |
| High co-morbidity burden (ADG ≥ 10) | 269 (26·8) | 544 (33·6) | 0·002 |
| Income quintile | 0·060 | ||
| 1 (lowest) | 159 (15·9) | 285 (17·6) | |
| 2 | 169 (16·9) | 307 (18·9) | |
| 3 | 210 (21·0) | 348 (21·5) | |
| 4 | 229 (22·9) | 373 (23·0) | |
| 5 (highest) | 235 (23·5) | 308 (19·0) | |
| Time interval of diagnosis | 0·410 | ||
| 2010–2013 | 494 (49·3) | 826 (51·0) | |
| 2014–2016 | 508 (50·7) | 795 (49·0) | |
| Receipt of chemotherapy | 0·606 | ||
| No | 366 (36·5) | 576 (35·6) | |
| Yes | 636 (63·5) | 1045 (64·4) |
Values in parentheses are percentages. ADG, Aggregated Diagnosis Group.
χ2 test.
Fig. 3.
Overall survival after diagnosis of non-curable pancreatic adenocarcinoma, stratified by patient-reporting of high pain score
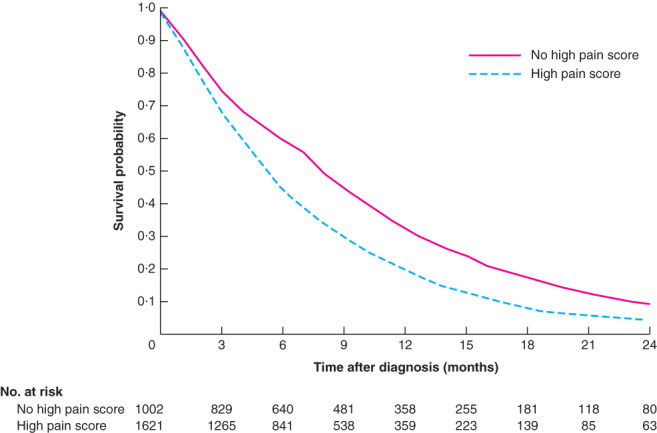
P < 0·001 (log rank test).
Fig. 4.
Proportion of patients reporting moderate-to-severe symptoms at the time of recording a high pain score
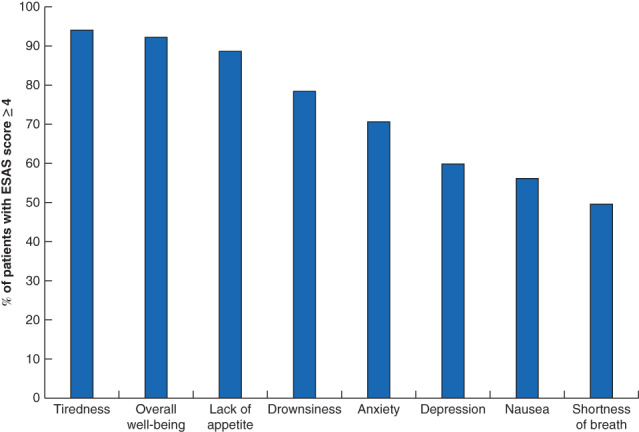
Moderate-to-severe symptoms were defined as those with an Edmonton Symptom Assessment System (ESAS) score of at least 4.
Of all patients with a high pain score, 13·5 per cent (219 of 1621) received radiation therapy and 1·2 per cent (19 of 1621) nerve block around the time they reported a high pain score. Of patients aged at least 65 years, 75·6 per cent (688 of 910) filled a prescription for opiates (Fig. 5). Overall, 74·0 per cent of patients had a palliative care assessment around the time the high pain score was registered.
Fig. 5.
Change in pain score in patients stratified by receipt of opiate prescription and radiation therapy, among patients with a high pain score
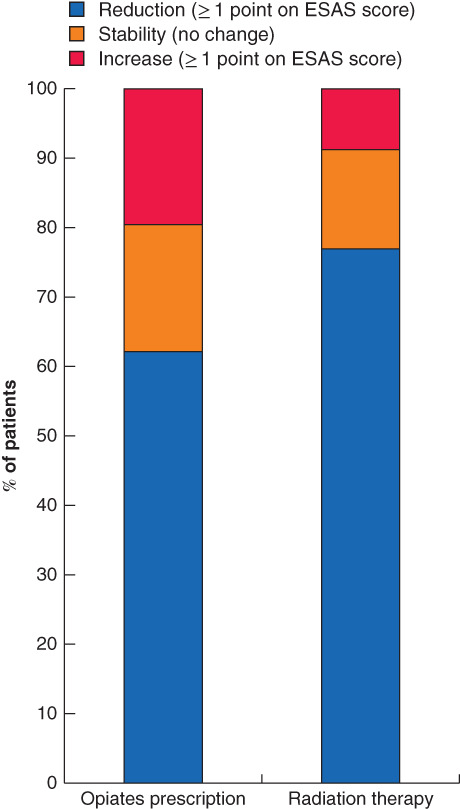
Change in Edmonton Symptom Assessment System (ESAS) pain score stratified by receipt of opiate prescription (354 patients) and radiation therapy (109).
Changes in ESAS pain score following a pain-directed intervention for a high pain score are shown in Fig. 5. A reduction in ESAS pain score was identified in 73·4 per cent of all patients receiving radiation therapy (80 of 109) and 62·1 per cent of those aged 65 years or more who received opiates (220 of 354). All nine patients who received nerve blocks and reported a postintervention score had a reduction in ESAS pain score (not shown in figure owing to small numbers).
The results of the multivariable analyses examining factors associated with receipt of pain-directed interventions are detailed in Table 2. There was no patient factor associated with receipt of non-opiate intervention. The only patient-level factor associated with receipt of opiate-based intervention was older age; patients aged 81 years or older had a lower odds of receiving a composite of radiation, nerve block and opiates, or opiates alone, compared with patients aged 65–70, in an analysis restricted to patients aged 65 years or older.
Table 2.
Predictors of receipt of pain-directed intervention in patients with a high pain score
| Relative risk | |||
|---|---|---|---|
| Radiation therapy and nerve block (all patients) | Radiation therapy, nerve block and opiates (aged ≥ 65 years) | Opiates only aged ≥ 65 years) | |
| Age (years) | |||
| < 65 | 1·00 (reference) | – | – |
| 65–70 | 1·31 (0·98, 1·75) | 1·00 (reference) | 1·00 (reference) |
| 71–80 | 0·81 (0·58, 1·14) | 0·92 (0·86, 1·00) | 0·92 (0·85, 1·00) |
| ≥ 81 | 1·36 (0·94, 1·97) | 0·89 (0·79, 0·99) | 0·89 (0·79, 1·00) |
| Female sex (versus male) | 0·90 (0·71, 1·13) | 0·96 (0·89, 1·03) | 0·94 (0·87, 1·02) |
| High co-morbidity burden (ADG ≥ 10 versus < 10) | 0·97 (0·90, 1·05) | 0·97 (0·90, 1·10) | 0·96 (0·88, 1·04) |
| Rural residence (versus urban) | 1·02 (0·68, 1·51) | 0·96 (0·84, 1·10) | 0·93 (0·81, 1·08) |
| Income quintile | |||
| 1 (lowest) | 0·79 (0·52, 1·20) | 1·01 (0·89, 1·14) | 1·02 (0·90, 1·15) |
| 2 | 1·22 (0·85, 1·74) | 1·01 (0·90, 1·14) | 0·99 (0·88, 1·12) |
| 3 | 1·01 (0·70, 1·45) | 1·06 (0·96, 1·18) | 1·05 (0·94, 1·17) |
| 4 | 0·89 (0·61, 1·29) | 1·01 (0·90, 1·13) | 0·97 (0·86, 1·10) |
| 5 (highest) | 1·00 (reference) | 1·00 (reference) | 1·00 (reference) |
| Diagnosis in 2014–2016 (versus 2010–2013) | 0·63 (0·50, 0·81) | 1·02 (0·95, 1·10) | 1·04 (0·96, 1·12) |
Values in parentheses are 95 per cent confidence intervals. ADG, Aggregated Diagnosis Group. All factors shown are included in the model as potential predictors (multivariable modified Poisson regression).
Discussion
This study provides insight into patient-reported pain for non-curable pancreatic adenocarcinoma based on population-based, validated, prospectively collected data. Opiates are the most common pain-directed intervention for non-curable pancreatic adenocarcinoma, whereas radiation therapy and nerve blocks are seldom used. Decision-making for pain-directed interventions appears to be dependent on provider and practice patterns, rather than patient factors. These findings are important to raise awareness about the need to optimize use of opiates and guide oncology practice, to increase the use of non-opiate interventions where appropriate33. This is highly relevant to surgeons, who are often the first specialists to see patients with non-curable pancreatic adenocartcinoma34. As one of the key specialists orienting patients for both curative and palliative care, participating in multidisciplinary case conferences, and having the ability to perform nerve blocks during staging or exploratory procedures, surgeons have a unique role to play in improving the multimodal management of patient-reported pain for non-curable pancreatic adenocarcinoma.
Patients with non-curable pancreatic adenocarcinoma experience a high symptom burden, with pain as one of the cardinal symptoms6,9,35. Pain is one of the most distressing symptoms for patients and has a significant impact on quality of life36. Routine screening with PROMs has been integrated into clinical practice to improve the care experience and symptom management for patients with cancer37,38. However, the value of such symptom screening is contingent on following up with interventions, which has been an issue in implementing routine screening programmes; a minority of physicians look at symptom scores or use them to direct management20.
Beyond describing the frequency of high pain scores, the authors connected this knowledge to a detailed analysis of patterns of care for pain. The literature focuses mostly on the effectiveness of isolated pain strategies, and there are no data on whether or not such therapies are actually delivered to patients in practice39–41. The present study provides a real-life assessment of how PROM information can be leveraged to gain insight into patient care. It provides an important understanding of the management of a high level of pain associated with non-curable pancreatic adenocarcinoma so that routine PROM screening can be followed effectively by intervention, multimodal management improved, and patients better supported.
The present results indicate that current management of pain in pancreatic adenocarcinoma is dominated by opiate therapy. Opiates are highly effective analgesics, but can represent a health concern, even in patients with non-curable cancer. There might be a tendency to over-rely on the use of opiates owing to traditional practice patterns, comfort and knowledge with this therapy, and ease of use, but data suggest that cancer pain may remain undertreated with opiates42,43. Additional issues pertain to chemical coping, referring to non-medical opioid use by patients with cancer as a means of coping with the stresses of their cancer journey, including psychological or spiritual distress11. It affects up to one in five patients with cancer and can lead to addictive behaviours as well opioid misuse11. It may also mask undertreatment of pain as well as inadequate management of other cancer-related symptoms. Therefore, non-opiate or opiate-sparing pain interventions should be used when available and feasible, to optimize pain and overall management of patients with cancer.
Although their prognosis remains guarded, patients with non-curable pancreatic adenocarcinoma now live longer with the disease, and symptom palliation should take into account opiate-related side-effects and chemical coping11. Non-opiate pain interventions showed a reduction in patient-reported pain scores in this study. Of note, the results regarding nerve blocks should be interpreted with caution as they relate to a small sample. Coeliac nerve blocks have been shown to provide effective and sustained pain relief in phase III trials39–41. They can also improve sleep and appetite disturbances44. Radiation therapy has also been established as an effective pain treatment in pancreatic adenocarcinoma, with results sustained up to a median of 6 months and a concomitant reduction in need for opiates45–47. Despite their effectiveness, these pain interventions were used rarely for patients with a high pain score in the present study. Enhanced use of nerve blocks and radiation therapy could result in better pain control overall for more patients, while optimizing the use of opiates. It may also result in better management of other cancer-related symptoms, by concomitant management pathways or avoidance of chemical coping.
Factors associated with the use of pain interventions for a high pain score were examined to understand the selection process for pain management. There were no patient factors associated with receipt of non-opiate pain-directed intervention. Among patients aged at least 65 years, advanced age was associated with a lower odds of opiate interventions, probably owing to the different risk profile of older patients with regard to opiates. This suggests that decision-making is more practice- or provider-driven than patient-based. Additional work is warranted to understand the reasons underlying the underuse of non-opiate pain interventions in non-curable pancreatic adenocarcinoma; this fell beyond the scope of the present study.
The study has some limitations. This retrospective cohort study used healthcare administrative data sets that were not collected specifically to address the research question. As such, some patient and disease details were lacking. The opiate analysis included only patients aged 65 years or older who benefited from drug coverage under the ODB, which could have led to underestimation or overestimation of the actual use of opiates in the entire cohort. However, the rates reported are consistent with previous studies investigating the use of opiates in cancer care48. In addition, there is variation in rates of patient-reported symptom screening in the population, which may limit the generalizability of the results to patients well enough to visit outpatient cancer clinics. The reporting of ESAS scores is opportunistic; although a high pain score is captured on a specific date, manifestations of pain and reporting to healthcare providers might happen before this time. Therefore, interventions were assessed during time windows encompassing the period before and after ESAS score acquisition28. Finally, it was not possible to determine the details of the patient's pain experience to decipher the eligibility for each pain intervention. Some patients may not have been candidates for radiation or nerve block. Nevertheless, the number of patients receiving non-opiate interventions was very small; even if this represents a worst-case scenario in patterns of care, it highlights a potential underuse rather than simply a lack of an appropriate indication.
Supplementary Material
Table S1 Databases and associated codes utilized to define and abstract variables
Fig. S1 Edmonton Symptom Assessment Score Form.
Acknowledgements
This study was funded by a grant from the Canadian Institute of Health Research Operating Grant: Partnerships for Health System Improvement for Cancer Control (FRN 154131). J.H. has received speaking honoraria from Novartis Oncology and Ipsen Biopharmaceuticals Canada. N.G.C. receives salary support from Cancer Care Ontario as the Clinical Lead of Patient Reported Outcomes and Symptom Management.
Disclosure: The authors declare no other conflict of interest.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet 2016; 388: 73–85. [DOI] [PubMed] [Google Scholar]
- 3. Sun H, Ma H, Hong G, Sun H, Wang J. Survival improvement in patients with pancreatic cancer by decade: a period analysis of the SEER database, 1981–2010. Sci Rep 2014; 4: 6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes 2009; 7: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fisch MJ, Lee JW, Weiss M, Wagner LI, Chang VT, Cella Det al. Prospective, observational study of pain and analgesic prescribing in medical oncology outpatients with breast, colorectal, lung, or prostate cancer. J Clin Oncol 2012; 30: 1980–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. High prevalence of pain in patients with cancer in a large population-based study in The Netherlands. Pain 2007; 132: 312–320. [DOI] [PubMed] [Google Scholar]
- 7. Caraceni A, Hanks G, Kaasa S, Bennett MI, Brunelli C, Cherny Net al.; European Palliative Care Research Collaborative (EPCRC); European Association for Palliative Care (EAPC). Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 2012; 13: e58–e68. [DOI] [PubMed] [Google Scholar]
- 8. Fallon M, Giusti R, Aielli F, Hoskin P, Rolke R, Sharma Met al. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann Oncol 2018; 29(Suppl 4): iv166–iv191. [DOI] [PubMed] [Google Scholar]
- 9. Breivik H, Cherny N, Collett B, de Conno F, Filbet M, Foubert AJet al. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol 2009; 20: 1420–1433. [DOI] [PubMed] [Google Scholar]
- 10. Cleeland CS, Gonin R, Hatfield AK, Edmonson JH, Blum RH, Stewart JAet al. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med 1994; 330: 592–596. [DOI] [PubMed] [Google Scholar]
- 11. Del Fabbro E. Assessment and management of chemical coping in patients with cancer. J Clin Oncol 2014; 32: 1734–1738. [DOI] [PubMed] [Google Scholar]
- 12. Brown MR, Ramirez JD, Farquhar-Smith P. Pain in cancer survivors. Br J Pain 2014; 8: 139–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eaton AA, Gonen M, Karanicolas P, Jarnagin WR, D'Angelica MI, DeMatteo Ret al. Health-related quality of life after pancreatectomy: results from a randomized controlled trial. Ann Surg Oncol 2016; 23: 2137–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reilly CM, Bruner DW, Mitchell SA, Minasian LM, Basch E, Dueck ACet al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer 2013; 21: 1525–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini Pet al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 2016; 34: 557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kotronoulas G, Kearney N, Maguire R, Harrow A, Di Domenico D, Croy Set al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol 2014; 32: 1480–1501. [DOI] [PubMed] [Google Scholar]
- 17. Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 1991; 7: 6–9. [PubMed] [Google Scholar]
- 18. Richardson LA, Jones GW. A review of the reliability and validity of the Edmonton Symptom Assessment System. Curr Oncol 2009; 16: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carli Buttenschoen D, Stephan J, Watanabe S, Nekolaichuk C. Health care providers' use and knowledge of the Edmonton Symptom Assessment System (ESAS): is there a need to improve information and training? Support Care Cancer 2014; 22: 201–208. [DOI] [PubMed] [Google Scholar]
- 20. Pereira JL, Chasen MR, Molloy S, Amernic H, Brundage MD, Green Eet al. Cancer care professionals' attitudes toward systematic standardized symptom assessment and the Edmonton Symptom Assessment System after large-scale population-based implementation in Ontario, Canada. J Pain Symptom Manage 2016; 51: 662–672.e8. [DOI] [PubMed] [Google Scholar]
- 21. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007; 335: 806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robles SC, Marrett LD, Clarke EA, Risch HA. An application of capture–recapture methods to the estimation of completeness of cancer registration. J Clin Epidemiol 1988; 41: 495–501. [DOI] [PubMed] [Google Scholar]
- 23. Iron K, Zagorski B, Sykora K, Manuel DG. Living and Dying in Ontario: an Opportunity for Improved Health Information. ICES Investigative Report. Institute for Clincial Evaluative Sciences: Toronto, 2008. [Google Scholar]
- 24. Booth CM, Nanji S, Wei X, Peng Y, Biagi JJ, Hanna TPet al. Use and effectiveness of adjuvant chemotherapy for stage III colon cancer: a population-based study. J Natl Compr Canc Netw 2016; 14: 47–56. [DOI] [PubMed] [Google Scholar]
- 25. Alter DA, Naylor CD, Austin P, Tu JV. Effects of socioeconomic status on access to invasive cardiac procedures and on mortality after acute myocardial infarction. N Engl J Med 1999; 341: 1359–1367. [DOI] [PubMed] [Google Scholar]
- 26. Selby D, Cascella A, Gardiner K, Do R, Moravan V, Myers J, Chow E. A single set of numerical cutpoints to define moderate and severe symptoms for the Edmonton Symptom Assessment System. J Pain Symptom Manage 2010; 39: 241–249. [DOI] [PubMed] [Google Scholar]
- 27. Barbera L, Seow H, Husain A, Howell D, Atzema C, Sutradhar Ret al. Opioid prescription after pain assessment: a population-based cohort of elderly patients with cancer. J Clin Oncol 2012; 30: 1095–1099. [DOI] [PubMed] [Google Scholar]
- 28. Barbera L, Sutradhar R, Chu A, Seow H, Howell D, Earle CCet al. Opioid prescribing among cancer and non-cancer patients: time trend analysis in the elderly using administrative data. J Pain Symptom Manage 2017; 54: 484–492.e1. [DOI] [PubMed] [Google Scholar]
- 29. Hui D, Shamieh O, Paiva CE, Perez-Cruz PE, Kwon JH, Muckaden MAet al. Minimal clinically important differences in the Edmonton Symptom Assessment Scale in cancer patients: a prospective, multicenter study. Cancer 2015; 121: 3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kralj B. Measuring Rurality – RIO2008_BASIC: Methodology and Results. Ontario Medical Association: Toronto, 2009. [Google Scholar]
- 31. Reid RJ, MacWilliam L, Verhulst L, Roos N, Atkinson M. Performance of the ACG case-mix system in two Canadian provinces. Med Care 2001; 39: 86–99. [DOI] [PubMed] [Google Scholar]
- 32. Nam RK, Cheung P, Herschorn S, Saskin R, Su J, Klotz LHet al. Incidence of complications other than urinary incontinence or erectile dysfunction after radical prostatectomy or radiotherapy for prostate cancer: a population-based cohort study. Lancet Oncol 2014; 15: 223–231. [DOI] [PubMed] [Google Scholar]
- 33. Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PAet al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999; 282: 1458–1465. [DOI] [PubMed] [Google Scholar]
- 34. Mavros MN, Coburn NG, Davis LE, Mahar AL, Liu Y, Beyfuss Ket al. Low rates of specialized cancer consultation and cancer-directed therapy for noncurable pancreatic adenocarcinoma: a population-based analysis. CMAJ 2019; 191: E574–E580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer 2011; 11: 695–707. [DOI] [PubMed] [Google Scholar]
- 36. Mantyh PW. Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci 2006; 7: 797–809. [DOI] [PubMed] [Google Scholar]
- 37. Basch E, Jia X, Heller G, Barz A, Sit L, Fruscione Met al. Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst 2009; 101: 1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Basch E. Patient-reported outcomes – harnessing patients' voices to improve clinical care. N Engl J Med 2017; 376: 105–108. [DOI] [PubMed] [Google Scholar]
- 39. Polati E, Finco G, Gottin L, Bassi C, Pederzoli P, Ischia S. Prospective randomized double-blind trial of neurolytic coeliac plexus block in patients with pancreatic cancer. Br J Surg 1998; 85: 199–201. [DOI] [PubMed] [Google Scholar]
- 40. Yan BM, Myers RP. Neurolytic celiac plexus block for pain control in unresectable pancreatic cancer. Am J Gastroenterol 2007; 102: 430–438. [DOI] [PubMed] [Google Scholar]
- 41. Wong GY, Schroeder DR, Carns PE, Wilson JL, Martin DP, Kinney MOet al. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: a randomized controlled trial. JAMA 2004; 291: 1092–1099. [DOI] [PubMed] [Google Scholar]
- 42. Jandhyala R, Fullarton JR, Bennett MI. Efficacy of rapid-onset oral fentanyl formulations vs. oral morphine for cancer-related breakthrough pain: a meta-analysis of comparative trials. J Pain Symptom Manage 2013; 46: 573–580. [DOI] [PubMed] [Google Scholar]
- 43. Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol 2008; 19: 1985–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu S, Fu W, Liu Z, Liu M, Ren R, Zhai Het al. MRI-guided celiac plexus neurolysis for pancreatic cancer pain: efficacy and safety. J Magn Reson Imaging 2016; 44: 923–928. [DOI] [PubMed] [Google Scholar]
- 45. Ceha HM, van Tienhoven G, Gouma DJ, Veenhof CH, Schneider CJ, Rauws EAet al. Feasibility and efficacy of high dose conformal radiotherapy for patients with locally advanced pancreatic carcinoma. Cancer 2000; 89: 2222–2229. [DOI] [PubMed] [Google Scholar]
- 46. Morganti AG, Trodella L, Valentini V, Barbi S, Macchia G, Mantini Get al. Pain relief with short-term irradiation in locally advanced carcinoma of the pancreas. J Palliat Care 2003; 19: 258–262. [PubMed] [Google Scholar]
- 47. Soliman H, Ringash J, Jiang H, Singh K, Kim J, Dinniwell Ret al. Phase II trial of palliative radiotherapy for hepatocellular carcinoma and liver metastases. J Clin Oncol 2013; 31: 3980–3986. [DOI] [PubMed] [Google Scholar]
- 48. Koyyalagunta D, Bruera E, Aigner C, Nusrat H, Driver L, Novy D. Risk stratification of opioid misuse among patients with cancer pain using the SOAPP-SF. Pain Med 2013; 14: 667–675. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Databases and associated codes utilized to define and abstract variables
Fig. S1 Edmonton Symptom Assessment Score Form.


