Abstract
Background
Since the very early days of surgical practice, surgeons have recognized the importance of considering that intestinal microbes might have a profound influence on recovery from surgical diseases such as appendicitis and peritonitis. Although the pathogenesis of surgical diseases such as cholelithiasis, diverticulosis, peptic ulcer disease and cancer have been viewed as disorders of host biology, they are emerging as diseases highly influenced by their surrounding microbiota.
Methods
This is a review of evolving concepts in microbiome sciences across a variety of surgical diseases and disorders, with a focus on disease aetiology and treatment options.
Results
The discovery that peptic ulcer disease and, in some instances, gastric cancer can now be considered as infectious diseases means that to advance surgical practice humans need to be viewed as superorganisms, consisting of both host and microbial genes. Applying this line of reasoning to the ever-ageing population of patients demands a more complete understanding of the effects of modern-day stressors on both the host metabolome and microbiome.
Conclusion
Despite major advances in perioperative care, surgeons today are witnessing rising infection-related complications following elective surgery. Many of these infections are caused by resistant and virulent micro-organisms that have emerged as a result of human progress, including global travel, antibiotic exposure, crowded urban conditions, and the application of invasive and prolonged medical and surgical treatment. A more complete understanding of the role of the microbiome in surgical disease is warranted to inform the path forward for prevention.
More important than many think
Introduction
The discovery of Helicobacter pylori as a cause of peptic ulcer disease was truly disruptive to how surgeons viewed their approach to surgical diseases. Once the domain of the surgeon-scientist, peptic ulcer disease was described by Schwartz as following the dictum ‘no acid, no ulcer’. When Barry Marshall's experiments to fulfil Koch's postulates required that he ingest the bacterium himself in order to prove causality between H. pylori and ulcer disease, the idea that an infectious agent can cause a surgical phenomenon was born. Yet, as experienced surgeons and gastroenterologists who regularly care for patients with ulcer diatheses are aware, such tidy stories are rarely complete explanations for the clinical manifestations they encounter when treating complex conditions such as peptic ulcer disease.
Although the discovery of the link between H. pylori and peptic ulcer disease was remarkable, the implications have led to many conflicting observations. For example, it is difficult to reconcile the observation that in developing countries the prevalence of colonization with H. pylori approaches 80 per cent (whereas its prevalence in industrialized nations has been estimated at 20–50 per cent), yet most of these patients never develop ulcer disease. Clearly, other lines of defence must break down before H. pylori is able to damage the epithelium. Among these are the cytoprotective mucous layer and the normal microbiota, which themselves are critical for the maintenance of a thick mucous coat. This controversy has led scientists to develop a specific lexicon to describe causality between a given factor (infectious agent) and a host phenotype (peptic ulcer), using the qualifier that, in order to implicate a particular factor in a disease phenotype, the factor must be both ‘necessary’ and ‘sufficient’ to cause the disease. In many patients H. pylori alone is not sufficient to cause ulcer disease and, similarly, it is not necessary, as ulcers can develop in the absence of H. pylori.
Unfortunately, the H. pylori story was wholly dismissive of the potential role of the microbiome in the pathogenesis of ulcer disease that is now coming to light1. The presence of an abundant and diverse microbiome within the stomach and duodenum may be an unrecognized defence factor in the pathogenesis of ulcer disease2. This may even include the more common non-steroidal anti-inflammatory drug (NSAID)-mediated ulcers that are rapidly becoming the most common cause of ulcer disease. For example, mice chronically fed NSAIDs will develop spontaneous intestinal ulcers and perforations, whereas mice treated with antibiotics or raised in germ-free conditions are protected against these NSAID-induced effects3–6. The clinical context of these experimental observations may be significant7. For instance, surgeons occasionally manage ulcers (gastric, duodenal, anastomotic, small intestinal) that do not heal despite maximal medical therapy. Often, like an infected extremity ulcer, these lesions can be treated only with excision. Is the persistence of such ulcers a function of infection with a yet-to-be identified pathogen or community of pathogens (a pathobiome)? Or alternatively, do some patients lack the microbial composition and abundance to heal the ulcer? Such questions beg a more precise description of when a microbiome becomes a pathobiome and the conditions under which a pathobiome produces disease8,9.
Definition of the microbiome
In this review a microbiome is defined as all of the microbial consortia (both commensal and pathogenic bacteria, viruses and fungi), their genes, their gene products (proteins, metabolites), their community structure (distribution, diversity, evenness) and the particulars of the environment in which they reside. The microbiome is the microbial ecosystem of the body. The scientific community has moved from speaking about a cultured microbial species as a causative pathogen, to this expanded description of the diversity of a human microbial ecosystem, moving beyond simple culture and antibiotic sensitivity. This transition has been driven by rapid advances in DNA and RNA sequencing, mass spectrometry and proteomics, which allow the measurement of multiple dynamic components of that ecosystem within a given sample. In this way, understanding of the microbiome is the same whether samples from the bottom of the ocean10, our homes11 or at an anastomotic tissue site are being analysed12. Metagenomic sequencing and mass spectrometry can describe not only who is there, but also what they are doing, who they are communicating with, and how they are interacting metabolically with one another. In fact, these tools are so powerful that it is possible to predict whether the local environment (pH, redox state, phosphate, nitrogen, carbon, etc.) is sufficient to support the growth and complexity of a microbial community13. This explosion in the ability to measure the microbiome across multiple environments has led to the emergence of what might be identified as a ‘health-promoting microbiome’ versus a ‘disease-promoting microbiome’ or pathobiome9 (https://youtu.be/QRynQinhABw?list=PLOWlK6maMFyywrvzx2HJNEOd9nCK65LKS). When microbiome measurements do not conform to a defined normal configuration, such as when diversity, abundance or evenness is lost, or when known harmful pathogens are identified to predominate in the community, the term dysbiosis is often used. A developing concept in microbiome science is that a highly diverse and abundant microbiome provides resilience to the host, much along the lines that the normal microbial flora of the gut provide colonization resistance to invading pathogens14. However, this conceptual framework has shifted from the simple notion that the normal intestinal microbiota provide a mass effect to exclude transient pathogens competitively, to one in which the intestinal microbiome is engaged actively with the host through receptors on the lining of the intestinal epithelia that communicate via dendritic cells with elements of the systemic immune system and provide health-promoting influences on overall host health maintenance14. Data are now emerging to suggest that tissue healing remote from the gut microbiome, such as liver, lung and surgical incision, are influenced positively when a patient has a diverse and functionally stable gut microbiome.
Recently, the neuropeptide oxytocin has been shown to be involved in surgical wound healing, and there is compelling evidence that the intestinal microbiota may play a significant role in this interaction15. In this study, the authors demonstrated that supplementing food with bacteria that ferment lactic acid accelerated wound healing in the skin via vagus nerve-mediated oxytocin stimulation, the effect of which recruited T cells to the wound, resulting in accelerated healing. In another study16, mice raised in a germ-free environment produced scarless wounds characterized by less neutrophil recruitment to the wound site and a greater degree of vascularization as judged by the accumulation of vascular endothelial growth factor within wounds. Taken together these studies indicate the intestinal microbial environment may have an unappreciated regulatory role in wound healing throughout the body. Furthermore, the techniques employed by these studies will finally afford surgeon-investigators the opportunity to elucidate the mechanisms by which a given management (nutritional support, antibiotics, use of opioids, bowel preparation) either enhances or hinders recovery from surgery.
The gut microbiome can influence the host immune system and metabolome without bacterial translocation or dissemination
To understand how the gut microbiome influences the occurrence, course and outcome of surgical infection, it is important to be aware of the various emerging mechanisms that indicate a diverse and abundant intestinal microbiome provides stimulation to the immune system at the microbial–epithelial interface. Work by Donaldson and colleagues14 has demonstrated that highly abundant commensals such as Bacteroides fragilis and Bifidobacterium secrete key metabolites that provide tonic stimulation to epithelial receptors, leading to profound effects on immune function throughout the body. Intestinal bacteria need not translocate to activate these signals as their simple engagement with epithelial receptors, or the long-reaching arms of dendritic cells (antigen-presenting cells) insinuated between the epithelia, is sufficient to transduce the information. Alternatively, many of the metabolites of intestinal bacteria can be taken up by epithelial cells and undergo modification to active compounds, for example when bacteria metabolize tryptophan to 5-hydroxytryptophan, which is absorbed and used as a precursor to produce serotonin17. Yet the fate of bacterial metabolites is difficult to predict given that the gut lumen is a challenging environment where bacterial metabolites can be either degraded or absorbed depending on competing microbes and the particulars of the local physicochemical environment. Therefore, attempts to characterize the diversity of the intestinal microbiome by extracting DNA from stool and applying 16S rRNA amplicon sequencing, as well as by classifying the metabolites and proteins in that stool, cannot necessarily be contextualized to a specific disease phenotype. Experimentally, the use of germ-free mice, with transfer of the microbiota from one mouse to the next displaying a particular clinical phenotype via faecal transplant, can provide some causal inference as to the relationship between these factors and the disease of interest. Today, what can be inferred from microbiome data generated from human faecal samples among injured and infected patients is that a loss of microbial diversity and abundance in the gut is associated with worse outcomes18,19. However, the mechanisms by which these observations are linked still need to be clarified. It is possible that the adverse consequences of loss of the intestinal microbial consortia and their exoproducts following surgical injury results in loss of host resilience mechanisms that are driven by loss of tonic stimulation of the immune system by the colonizing microbiota (Figs 1,2).
Fig. 1.
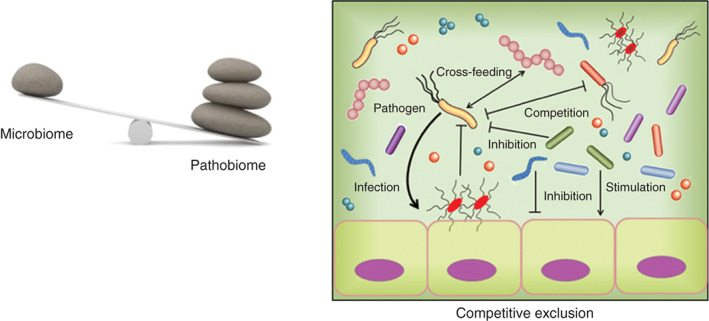
Host–microbiome dynamics in the gut during surgery. The normal microbiota constitute a major defence mechanism against the colonization and persistence of pathogenic bacteria (pathobiota), which are transiently encountered during hospitalization. Pathobiota are excluded by various mechanisms including competition for resources as they are outnumbered by the microbiota, secretion of antibiotics by the microbiota, and induction of antimicrobial peptides and immune elements induced by the microbiota.
Fig. 2.
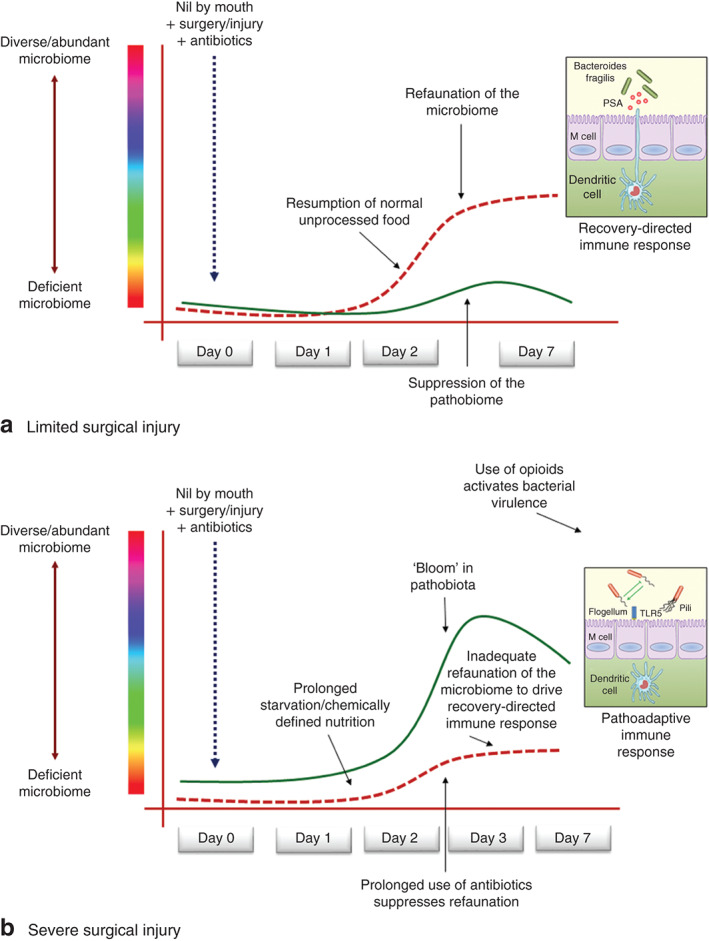
Host-microbiome dynamics in the gut during surgery. a When surgical injury is limited by minimally invasive techniques and attenuated physiological stress, the impact of antibiotic use on the microbiota is also limited, allowing the normal microbiota to refaunate and provide competitive exclusion to any transient pathobiota. b When surgical injury is severe and prolonged, causing a delay in resumption of normal foodstuff, refaunation of the microbiome can become impaired. This may result in a period of vulnerability to colonizing pathobiota, the consequences of which can be a loss of systemic immune function from lack of tonic immune stimulation by the microbiota. PSA, polysaccharide A; DC, dendritic cell, TLR, toll-like receptor
Several studies have now documented that loss of the intestinal microbial consortia and their exoproducts can occur within as little as 6 h following a sudden insult20,21. There is a near 90 per cent loss of microbial abundance and a significant decrease in important cytoprotective metabolites such as short-chain fatty acids following a sudden insult such as a myocardial infarction, stroke or burn injury22. Although data are not yet available, as the underlying insult is treated by modern medical and surgical care, it can be assumed that intestinal refaunation occurs and then drives a recovery-directed immune response. It may be for this reason, among others, that fast, technically meticulous and bloodless operations tend to be associated with excellent outcomes, whereas longer more complex operations requiring increased antibiotic exposure and prolonged admission to hospital are associated with worse outcomes. However, the precision to predict which patients will do worse may lie in the degree to which the microbiome can refaunate and drive a recovery-directed immune response. Although the science behind this notion is in its early descriptive phase, there is a growing body of literature to substantiate that patients who are critically ill8,23, and those undergoing bone marrow and stem cell transplantation24, recover in association with re-establishment of the intestinal microbiome.
Microbiota are key participants in the gut–lung, gut–liver, gut–brain and gut–wound axis
As discussed, the intestinal microbiome may have positive influences on the healing of remote tissues via its interaction with the intestinal epithelium, which can engage and activate downstream signals of the immune system14. Yet it is also important to recognize that the intestinal microbiome may exert similar influences via the enteric nervous system and via absorption of as yet unidentified metabolites that reach remote tissues. With the availability of germ-free mice and transgenic mice harbouring various reporter genes, it is now possible to invoke alterations in the gut microbiome to disorders within the brain, lung, liver and other tissues. For example, the lung is protected against pneumonia when the intestinal microbiome maintains a diverse and abundant community25,26. Antibiotics impair this process directly by eliminating the microbiome's probiotic effect on the immune system, which has far-reaching implications for the local inflammatory response in the lung. Similarly, liver regeneration, a critical response following major hepatic resection, is highly influenced by the normal structure and function of the intestinal microbiome. Recent studies suggest that commensal bacteria maintain Kupffer cells in a tolerant state, thus preventing subsequent natural killer T cell overactivation during liver regeneration27. Use of antibiotics (ampicillin) has a potent inhibitory effect on liver regeneration in mice28. The use of faecal transplantation in these models is another mechanism by which the observed host phenotype can be traced back to the membership of the intestinal microbiota. Yet, in many of these cases involving a positive effect of faecal transplantation on the host phenotype, the conclusion that the whole is greater than the sum of the parts makes it challenging to identify a single microbe and/or secreted exoproduct that is responsible for the observed effect. To date, many of these experimental models remain descriptive and much remains to be learned about the mechanisms underlying the striking observations. Particularly exciting is the prospect that the gut microbiome may have a major influence on brain function and autoimmune disease through common mechanisms outlined above.
The information thus far provided should encourage surgeons to consider the indications, doses and duration of antibiotics they use for surgical patients. This has been debated, for example in the treatment of acute pancreatitis29. Although, traditionally, surgeons have been cautious about their antibiotic use to avoid superinfections such as candidiasis or Clostridium difficile colitis, emerging information on the important role of the intestinal microbiome in maintaining a recovery-directed immune response provides yet another incentive not to start antibiotics when they are not needed. Recent studies from the STOP-IT trial suggest that 5 days of antibiotics are equivalent to 10 days in eradicating intra-abdominal infection30. The longstanding controversy surrounding the use of antibiotics in pancreatitis has not usually considered that preservation of the normal microbiota by withholding antibiotics may actually provide an immune advantage. Trials in which patients with severe acute pancreatitis were randomized to a probiotic or placebo, but received prolonged broad-spectrum antibiotics, showed no difference in infections or mortality31. This is not surprising given that antibiotics alone could have negated any effect of the probiotic regimen in both groups. In light of emerging knowledge demonstrating the role of the microbiome in driving a recovery-directed immune response, clinical trials on the role of the microbiota on the course and outcome of severe acute pancreatitis will need to be redesigned. These studies might include a high-resolution analysis of the microbiome in each patient, and an assessment of the community structure, membership and diversity of each patient. Patients whose microbiome appears to have collapsed during the course of their pancreatitis could then be randomized to repletion, with either a probiotic regimen or a faecal transplant, or placebo. In the current era of precision medicine, it is no longer tenable to randomize patients to one treatment versus another and examine only gross clinical outcomes. In this regard, the microbiome can be considered yet another variable to be accounted for in the host response to surgical intervention, infection and injury.
Adhesions, ileus, and anastomotic leak: role of the microbiome beyond surgical-site infections
Enhanced recovery after surgery (ERAS) programmes have emerged as a method to develop practical approaches to minimize the stress of surgery and limit the time of hospital admission, with the overall goal of improving patient outcome32. Among the various goals of ERAS is to prevent the development of ileus and infection, two of the most common reasons for prolonged hospital stay and readmission after gastrointestinal surgery. If each of the elements of ERAS were examined for the effect on the intestinal microbiome, it would be significant and substantial. For example, lack of enteral nutrition has been shown have a profound effect on the community structure, membership and function of the microbiome, with consequences for the incidence of surgical-site infections33. Similarly, the use of opioids after surgery is an independent risk factor for the development of surgical-site infections, sepsis and ileus34. Not only do opioids have a suppressive effect on the immune system but, more importantly, they have been shown to affect the community structure and function of the intestinal microbiome35,36. Opioids can directly shift the virulence state of intestinal pathogens by activating their quorum-sensing system, a molecular pathway in which bacteria sense ‘cues’ in the local microenvironment and accordingly express virulence genes37,38. The consequences of this direct action of opioids on bacterial virulence expression are an increase in sepsis and sepsis-related mortality in mice. Blockage of the peripheral action of opioids with specific competitive antagonists such as alvimopan has been shown to decrease surgical complications following intestinal surgery39. Although the precise mechanism of action of this effect remains to be clarified, there is likely a significant contribution of the intestinal microbiome.
Ileus and adhesion formation remain important concerns for surgeons operating within the abdominal cavity. The extent to which the intestinal microbiome contributes to both of these complications is unknown. However, there is compelling evidence to suggest that the intestinal microbiome plays a key and contributory role in the pathogenesis of both ileus and adhesion formation. This conclusion is based on experimental and clinical observations in which germ-free conditions or antibiotic use, such as oral non-absorbable antibiotics, reduce or eliminate the incidence of these complications40–42. Yet, despite these observations, because of the empiricism of the clinical studies and their lack of mechanistic detail, applying universal guidelines for patient management cannot move forward. Today it is still not known which of the intestinal microbes should be preserved and which should be eliminated. Furthermore, the pathogens that drive surgical complications within the microbiome cannot be eliminated selectively while at the same time preserving the health-promoting microbiota. It is important to realize that many of the identified causative pathogens, such as Enterococcus faecalis, Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus, exist within the intestinal microbiome as low-abundance pathogens (less than 1 per cent of the total). They bloom only when the normal microbiota are eliminated by either broad-spectrum antibiotics, surgical injury or other factors related to physiological stress. As such, the broad kill strategy employed by surgeons to suppress the harmful effects of intestinal pathogens remains not only empirical, but based on the limited knowledge provided by culture alone. The activity of many of the antibiotics used today is known only from 60–70-year-old cultured isolate trials, which do not consider how the antibiotic may influence the commensal microbiome in today's patients, who have been exposed to newer antibiotics, new chemotherapy agents, processed foods and global travel.
Anastomotic leak: a modern example of failure to incorporate microbiome sciences into surgical thinking and practice
More than 60 years ago, experimental studies were performed in dogs that unambiguously and uncontestably demonstrated that intestinal microbes play a key and causative role in the pathogenesis of anastomotic leak. One study created a transverse colonic anastomosis, with gross ischaemia created by dividing the feeding blood vessels to the anastomotic segment, and then administered either saline or antibiotics into the colonic lumen via a feeding tube placed just proximal to the anastomosis43,44. Saline-treated colonic segments developed gross leaks and peritonitis, whereas the antibiotic-treated dogs demonstrated complete reversal of the ischaemia and completely healed and intact anastomoses. Similar studies were performed later in rats, showing again that microbes were the cause of anastomotic leak45. Several randomized prospective placebo-blinded trials, and now retrospective large database analyses, have come to the same conclusion46,47. Finally, the mechanisms were elucidated, in high-resolution molecular detail, by which high collagenase-producing intestinal microbes (E. faecalis, P. aeruginosa) cause anastomotic leak in rats and mice, with causative inference in humans, by demonstrating the presence of these pathogens in patients with an anastomotic leak12. Yet to surgeons this hypothesis still remains untenable because bacteria are there all the time, and because variations in clinical practice, that is bowel preparation and antibiotic choice, do not seem to correlate with rates of anastomotic leak. For the purposes of elucidating how microbes might play a key and causative role in anastomotic leak and other processes of seemingly non-microbe-related complications (ileus, adhesions, etc.), it might be useful to unpick this line of reasoning.
The comment that bacteria are there all the time needs to be qualified with a more in-depth understanding of the molecular Koch's postulates in the context of advances in microbiome science. For the past 50 years, clinicians have been taught that most infectious-related diseases are monomicrobial. The isolation of a single pathogen can be implicated in the disease process by demonstrating that: it is present in patients with the disease and absent in those without the disease; it can reproduce the disease when introduced in animals; and the disease is cured when eradicated with antibiotics (which also kill many other adjacent microbes). Yet the molecular Koch's postulates state that microbial phenotype (for example ability to produce collagenase which varies by isolate not species), the presence of the local microbiome (microbial community structure, function and membership) and the local environmental cues that activate virulence genes in a given pathogenic isolate are the elements that govern whether a particular microbial species will behave commensally or pathogenically48. It is for this reason that isolation of a single causative pathogen in surgical disorders, such as neonatal necrotizing enterocolitis, anastomotic leak, ileus or adhesion formation, has eluded surgeon-investigators. Even in disease states in which a single pathogen is considered to be the causative agent, such as with C. difficile colitis or H. pylori ulcer disease, the mere presence and abundance of the pathogen does not predict the development of the clinical disorder49,50. Based on the emerging theories of the molecular Koch's postulates, this observation may be explained as a function of the pathogen's dominance in the microenvironment, the loss of the protective microbiome, its expression of virulence genes (adhesins, toxins, etc.), alignment of the pathogen's adhesins and toxins with the host receptor genetics (glycosylation of receptors, receptor homology to the adhesin, activation or damping of host inflammation), and probably many more factors. For this reason, as highlighted above, scientists who perform reductionist modelling of these events use the terms necessary and sufficient to invoke the role of a causative agent in a particular host phenotype (or disease). For example, work on anastomotic leak demonstrates that collagenase-producing microbes are necessary to cause anastomotic leak, but alone are not sufficient to cause leak. The microbial community must be degraded so that the causative pathogen can predominate and adhere to anastomotic tissues, and compensatory host factors from surgical injury (the release of host stress factors known to activate bacterial virulence genes, opioids, end-products of ischaemia, cytokines) must be present locally to shift the pathogen's phenotype from innocuous colonizer to invasive and virulent pathogen12,51–52. Analysing such a complex molecular dialogue within the regional and spatial context of anastomotic tissues is challenging and cannot be determined simply by assessment of expelled stool or a blood test14. As such, this is just the tip of the iceberg in the molecular pathogenesis of these disorders, and many technological advances are forthcoming that will enable more predictive models and biomarkers.
Along this line of reasoning and with the new information, perhaps Schwartz's dictum ‘no acid, no ulcer’ could better be stated as: acid is necessary but not sufficient to cause peptic ulcer disease. In the case of H. pylori, experimentally, it alone may be insufficient to cause peptic ulcer disease, even if an abundance is ingested, unless it occurs in a susceptible host53. Alternatively, it could be stated that in some cases of bacteria-mediated peptic ulcer disease, H. pylori may be necessary, but alone not sufficient, requiring host stress factors to induce the bacteria to express their adhesins in vivo, loss of the mucous barrier, and loss of the resilience of the gastric and duodenal microbiome. Perhaps this is what Dr Barry Marshall indicated when he stated ‘in a susceptible host’53. Most investigators in the field believe that if 100 human volunteers ingested H. pylori in the manner that Marshall did, most would not develop an ulcer. Perhaps Marshall was nervous the night before the experiment and did not sleep, activating stress-induced cytokines and hormones that have now been shown to change the microbiome and local mucous barrier54. Perhaps the short period of starvation before the experiments affected these same parameters. Variation in disease presentation and the invoking of pathogens as causative agents of disease must now march along a very complex and challenging experimental platform to enable an understanding of diseases whose protean manifestations have eluded traditional concepts of disease pathogenesis. In this regard, the complex bioreactor of microbial cells within the gut is likely to play a significant role.
Role of nutrition to modify recovery after surgery
In the absence of significant knowledge and consideration of the role of the intestinal microbiome to modify, and be modified by, foodstuffs as they pass through the intestinal bioreactor, surgeons have over the years attempted to modify the metabolic response to injury with nutrients. Failure to enhance recovery after surgery was witnessed, including trials of glutamine55, branched-chain amino acids56,57, arginine58 and various fat emulsions. Viewing the gut as a mere conduit for nutrient absorption led to missteps and miscalculations that nutrients in their most elemental form could be delivered parenterally and would be sufficient to reprogramme the immune system to enhance recovery. The greatest pay-off for such studies was clearly predicted to be in high-risk patients, many of whom would harbour altered microbiomes as a result of chronic illness, the presence of cancer, and multiple and concurrent exposure to antibiotics. Enterally presented, chemically defined nutrients do not normally reach the distal intestine, where much of the probiotic microbiome activity affects the immune system. This was not accounted for in the design of clinical trials or their anticipated results. Alongside these studies emerged rapid advances in surgery and anaesthesia, pain management, adoption of ERAS protocols, and restraint and governance on the use of antibiotics. Many, if not most, of these studies suffered from a tradition of therapeutic empiricism. The limitation of such studies with only crude outcome measures is that they do not inform mechanism, and therefore the path forward remains undefined.
Today, next-generation technology offers the promise of unprecedented insight into disease pathogenesis through the use of genomic, metabolomic and proteomic analyses. However, as information is gathered and large data sets are assembled, analysis and interpretation require the expertise of bioinformatic specialists. Although there is much excitement in this approach, in many instances the costs can outstrip the available resources. However, without such analysis, single identified biomarkers will continue to fall short of their predictive capacity, and will not be sufficient to inform preventive therapies.
Practical application of microbiome sciences to surgery
In many countries today, the quality of surgical care is being measured by readmission rates to hospital. As most elective surgery today requires 3 days or fewer in hospital, using readmission rates as a proxy for quality is rapidly gaining acceptance, as it is both practical and trackable. Today, the most common reason for readmission to a US hospital is infection59. This statistic has forced surgeons to be watchful for the possibility of an infection becoming manifest clinically outside the hospital setting. Yet, despite multiple measures to prevent readmissions, many infections cannot be anticipated and the mechanisms underlying their occurrence often remain obscure.
Although technology can now generate an overwhelming amount of genetic and metabolomic information on both host tissues and the microbiota that surround them, the costs and discriminative value of this type of megadata medicine remain to be determined. Alongside this, there is a practical lesson: when surgery is done in a manner in which tissue trauma and blood loss are minimized, when drugs such as opioids and antibiotics are used judiciously, and when non-processed foods are resumed at the earliest time point, a previously unappreciated resilience factor driving a recovery-directed immune response, the microbiome, may be operative. Perhaps surgeons have known this all along, as they were the first to recognize the importance of the intestinal microbiota to postoperative infection and sepsis60,61, the first to administer nutrient enemas62 and the first to deliver faecal transplants to treat life-threatening antibiotic-induced fulminant colitis63. Although these early empirical approaches have turned out to have a scientific basis to their efficacy, the quest to elucidate the molecular details behind them must continue.
Acknowledgements
This work was supported by a grant from the US National Institutes of Health to J.C.A. (NIH RO1 5R01GMO62344-15).
Disclosure: The authors declare no conflict of interest.
References
- 1. Kronsteiner B, Bassaganya-Riera J, Philipson C, Viladomiu M, Carbo A, Abedi Vet al. Systems-wide analyses of mucosal immune responses to Helicobacter pylori at the interface between pathogenicity and symbiosis. Gut Microbes 2016; 7: 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Naughton J, Duggan G, Bourke B, Clyne M. Interaction of microbes with mucus and mucins: recent developments. Gut Microbes 2014; 5: 48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robert A, Asano T. Resistance of germfree rats to indomethacin-induced intestinal lesions. Prostaglandins 1977; 14: 333–341. [DOI] [PubMed] [Google Scholar]
- 4. Mayo SA, Song YK, Cruz MR, Phan TM, Singh KV, Garsin DAet al. Indomethacin injury to the rat small intestine is dependent upon biliary secretion and is associated with overgrowth of enterococci. Physiol Rep 2016; 4: e12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zwolinska-Wcislo M, Krzysiek-Maczka G, Ptak-Belowska A, Karczewska E, Pajdo R, Sliwowski Zet al. Antibiotic treatment with ampicillin accelerates the healing of colonic damage impaired by aspirin and coxib in the experimental colitis. Importance of intestinal bacteria, colonic microcirculation and proinflammatory cytokines. J Physiol Pharmacol 2011; 62: 357. [PubMed] [Google Scholar]
- 6. Satoh HI, Guth PH, Grossman MI. Role of bacteria in gastric ulceration produced by indomethacin in the rat: cytoprotective action of antibiotics. Gastroenterology 1983; 84: 483–489. [PubMed] [Google Scholar]
- 7. Rogers MA, Aronoff DM. The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Clin Microbiol Infect 2016; 22: 178.e1–178.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krezalek MA, DeFazio J, Zaborina O, Zaborin A, Alverdy JC. The shift of an intestinal ‘microbiome’ to a ‘pathobiome’ governs the course and outcome of sepsis following surgical injury. Shock 2016; 45: 475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gilbert JA, Quinn RA, Debelius J, Xu ZZ, Morton J, Garg Net al. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature 2016; 535: 94–103. [DOI] [PubMed] [Google Scholar]
- 10. Gilbert JA, Dupont CL. Microbial metagenomics: beyond the genome. Annu Rev Mar Sci 2011; 3: 347–371. [DOI] [PubMed] [Google Scholar]
- 11. Lax S, Smith DP, Hampton-Marcell J, Owens SM, Handley KM, Scott NMet al. Longitudinal analysis of microbial interaction between humans and the indoor environment. Science 2014; 345: 1048–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shogan BD, Belogortseva N, Luong PM, Zaborin A, Lax S, Bethel Cet al. Collagen degradation and MMP9 activation by Enterococcus faecalis contribute to intestinal anastomotic leak. Sci Transl Med 2015; 7: 286ra68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nekrutenko A, Taylor J. Next-generation sequencing data interpretation: enhancing reproducibility and accessibility. Nat Rev Genet 2012; 13: 667–672. [DOI] [PubMed] [Google Scholar]
- 14. Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol 2016; 14: 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poutahidis T, Kearney SM, Levkovich T, Qi P, Varian BJ, Lakritz JRet al. Microbial symbionts accelerate wound healing via the neuropeptide hormone oxytocin. PLoS One 2013; 8: e78898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Canesso MC, Vieira AT, Castro TB, Schirmer BG, Cisalpino D, Martins FSet al. Skin wound healing is accelerated and scarless in the absence of commensal microbiota. J Immunol 2014; 193: 5171–5180. [DOI] [PubMed] [Google Scholar]
- 17. Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma Let al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015; 161: 264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shimizu K, Ogura H, Goto M, Asahara T, Nomoto K, Morotomi Met al. Altered gut flora and environment in patients with severe SIRS. J Trauma Acute Care Surg 2006; 60: 126–133. [DOI] [PubMed] [Google Scholar]
- 19. Osuka A, Shimizu K, Ogura H, Tasaki O, Hamasaki T, Asahara Tet al. Prognostic impact of fecal pH in critically ill patients. Crit Care 2012; 16: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hayakawa M, Asahara T, Henzan N, Murakami H, Yamamoto H, Mukai Net al. Dramatic changes of the gut flora immediately after severe and sudden insults. Dig Dis Sci 2011; 56: 2361–2365. [DOI] [PubMed] [Google Scholar]
- 21. Houlden A, Goldrick M, Brough D, Vizi ES, Lénárt N, Martinecz Bet al. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav Immun 2016; 57: 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Earley ZM, Akhtar S, Green SJ, Naqib A, Khan O, Cannon ARet al. Burn injury alters the intestinal microbiome and increases gut permeability and bacterial translocation. PLoS One 2015; 10: e0129996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dickson RP. The microbiome and critical illness. Lancet Respir Med 2016; 4: 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taur Y, Jenq RR, Ubeda C, van den Brink M, Pamer EG. Role of intestinal microbiota in transplantation outcomes. Best Pract Res Clin Haematol 2015; 28: 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fox AC, McConnell KW, Yoseph BP, Breed E, Liang Z, Clark ATet al. The endogenous bacteria alter gut epithelial apoptosis and decrease mortality following Pseudomonas aeruginosa pneumonia. Shock 2012; 38: 508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schuijt TJ, Lankelma JM, Scicluna BP, de Sousa e Melo F, Roelofs JJ, de Boer JDet al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 2016; 65: 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu HX, Hu Y, Wan YJ. Microbiota and bile acid profiles in retinoic acid-primed mice that exhibit accelerated liver regeneration. Oncotarget 2016; 7: 1096–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu X, Sun R, Chen Y, Zheng X, Bai L, Lian Zet al. Oral ampicillin inhibits liver regeneration by breaking hepatic innate immune tolerance normally maintained by gut commensal bacteria. Hepatology 2015; 62: 253–264. [DOI] [PubMed] [Google Scholar]
- 29. Wittau M, Mayer B, Scheele J, Henne-Bruns D, Dellinger EP, Isenmann R. Systematic review and meta-analysis of antibiotic prophylaxis in severe acute pancreatitis. Scand J Gastroenterol 2011; 46: 261–270. [DOI] [PubMed] [Google Scholar]
- 30. Sawyer RG, Claridge JA, Nathens AB, Rotstein OD, Duane TM, Evans HLet al. Trial of short-course antimicrobial therapy for intraabdominal infection. N Engl J Med 2015; 372: 1996–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HMet al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 371: 651–659. [DOI] [PubMed] [Google Scholar]
- 32. Nicholson A, Lowe MC, Parker J, Lewis SR, Alderson P, Smith AF. Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. Br J Surg 2014; 101: 172–188. [DOI] [PubMed] [Google Scholar]
- 33. Alverdy JO, Chi HS, Sheldon GF. The effect of parenteral nutrition on gastrointestinal immunity. The importance of enteral stimulation. Ann Surg 1985; 202: 681–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cron DC, Englesbe MJ, Bolton CJ, Joseph MT, Carrier KL, Moser SEet al. Preoperative opioid use is independently associated with increased costs and worse outcomes after major abdominal surgery. Ann Surg 2016; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 35. Shakhsheer BA, Versten LA, Luo JN, Defazio JR, Klabbers R, Christley Set al. Morphine promotes colonization of anastomotic tissues with collagenase-producing Enterococcus faecalis and causes leak. J Gastrointest Surg 2016; 20: 1744–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Banerjee S, Sindberg G, Wang F, Meng J, Sharma U, Zhang Let al. Opioid-induced gut microbial disruption and bile dysregulation leads to gut barrier compromise and sustained systemic inflammation. Mucosal Immunol 2016; 9:1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Babrowski T, Holbrook C, Moss J, Gottlieb L, Valuckaite V, Zaborin Aet al. Pseudomonas aeruginosa virulence expression is directly activated by morphine and is capable of causing lethal gut derived sepsis in mice during chronic morphine administration. Ann Surg 2012; 255: 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zaborina O, Lepine F, Xiao G, Valuckaite V, Chen Y, Li Tet al. Dynorphin activates quorum sensing quinolone signaling in Pseudomonas aeruginosa. PLoS Pathog 2007; 3: e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Adam MA, Lee LM, Kim J, Shenoi M, Mallipeddi M, Aziz Het al. Alvimopan provides additional improvement in outcomes and cost savings in enhanced recovery colorectal surgery. Ann Surg 2016; 264: 141–146. [DOI] [PubMed] [Google Scholar]
- 40. Oncel M, Kurt N, Remzi FH, Sensu SS, Vural S, Gezen CFet al. The effectiveness of systemic antibiotics in preventing postoperative, intraabdominal adhesions in an animal model. J Surg Res 2001; 101: 52–55. [DOI] [PubMed] [Google Scholar]
- 41. Bothin C, Midtvedt T. The role of the gastrointestinal microflora in postsurgical adhesion formation – a study in germfree rats. Eur Surg Res 1992; 24: 309–312. [DOI] [PubMed] [Google Scholar]
- 42. Barbara G, Stanghellini V, Brandi G, Cremon C, Di Nardo G, De Giorgio Ret al. Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am J Gastroenterol 2005; 100: 2560–2568. [DOI] [PubMed] [Google Scholar]
- 43. Cohn I, Rives JD. Antibiotic protection of colon anastomoses. Ann Surg 1955; 141: 707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fry DE. Colon preparation and surgical site infection. Am J Surg 2011; 202: 225–232. [DOI] [PubMed] [Google Scholar]
- 45. Cohen SR, Cornell CN, Collins MH, Sell JE, Blanc WA, Altman RP. Healing of ischemic colonic anastomoses in the rat: role of antibiotic preparation. Surgery 1985; 97: 443–446. [PubMed] [Google Scholar]
- 46. Schardey HM, Kamps T, Rau HG, Gatermann S, Baretton G, Schildberg FW. Bacteria: a major pathogenic factor for anastomotic insufficiency. Antimicrob Agents Chemother 1994; 38: 2564–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schardey HM, Joosten U, Finke U, Staubach KH, Schauer R, Heiss Aet al. The prevention of anastomotic leakage after total gastrectomy with local decontamination. A prospective, randomized, double-blind, placebo-controlled multicenter trial. Ann Surg 1997; 225: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Seal JB, Morowitz M, Zaborina O, An G, Alverdy JC. The molecular Koch's postulates and surgical infection: a view forward. Surgery 2010; 147: 757–765. [DOI] [PubMed] [Google Scholar]
- 49. Pakodi F, Abdel-Salam OM, Debreceni A, Mózsik G. Helicobacter pylori. One bacterium and a broad spectrum of human disease! An overview. J Physiol Paris 2000; 94: 139–152. [DOI] [PubMed] [Google Scholar]
- 50. Monaghan TM. New perspectives in Clostridium difficile disease pathogenesis. Infect Dis Clin North Am 2015; 29: 1–11. [DOI] [PubMed] [Google Scholar]
- 51. Olivas AD, Shogan BD, Valuckaite V, Zaborin A, Belogortseva N, Musch Met al. Intestinal tissues induce an SNP mutation in Pseudomonas aeruginosa that enhances its virulence: possible role in anastomotic leak. PLoS One 2012; 7: e44326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Luong PM, Shogan BD, Zaborin A, Belogortseva N, Shrout JD, Zaborina Oet al. Emergence of the P2 phenotype in Pseudomonas aeruginosa PAO1 strains involves various mutations in mexT or mexF. J Bacteriol 2014; 196: 504–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Marshall BJ. Helicobacter pylori in peptic ulcer: have Koch's postulates been fulfilled? Ann Med 1995; 27: 565–568. [DOI] [PubMed] [Google Scholar]
- 54. Asher G, Sassone-Corsi P. Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell 2015; 161: 84–92. [DOI] [PubMed] [Google Scholar]
- 55. Ziegler TR, May AK, Hebbar G, Easley KA, Griffith DP, Dave Net al. Efficacy and safety of glutamine-supplemented parenteral nutrition in surgical ICU patients: an American multicenter randomized controlled trial. Ann Surg 2016; 263: 646–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Les I, Doval E, García-Martínez R, Planas M, Cárdenas G, Gómez Pet al. Effects of branched-chain amino acids supplementation in patients with cirrhosis and a previous episode of hepatic encephalopathy: a randomized study. Am J Gastroenterol 2011; 106: 1081–1088. [DOI] [PubMed] [Google Scholar]
- 57. Kephart WC, Mumford PW, McCloskey AE, Holland AM, Shake JJ, Mobley CBet al. Post-exercise branched chain amino acid supplementation does not affect recovery markers following three consecutive high intensity resistance training bouts compared to carbohydrate supplementation. J Int Soc Sports Nutr 2016; 13: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Luiking YC, Deutz NE. Exogenous arginine in sepsis. Crit Care Med 2007; 35: S557–S563. [DOI] [PubMed] [Google Scholar]
- 59. Merkow RP, Ju MH, Chung JW, Hall BL, Cohen ME, Williams MVet al. Underlying reasons associated with hospital readmission following surgery in the United States. JAMA 2015; 313: 483–495. [DOI] [PubMed] [Google Scholar]
- 60. Dragstedt LR, Moorhead JJ, Burcky FW. Intestinal obstruction: an experimental study of the intoxication in closed intestinal loops. J Exp Med 1917; 25: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yeo IB, Harley V, Goodbody F, Pope FM, Herschell G, Wild RBet al. A discussion on intestinal antiseptics. Br Med J 1899; 1250–1257. [Google Scholar]
- 62. Spriggs EI. The treatment of gastric ulcer by immediate feeding: based on a comparison of cases on the Lenhartz dietary and cases treated by saline or nutrient enemas and a graduated milk diet. Proc R Soc Med 1909; 2(Ther Pharmacol Sect): 81. [PMC free article] [PubMed] [Google Scholar]
- 63. Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958; 44: 854–859. [PubMed] [Google Scholar]


