Abstract
Background
Early prediction of acute pancreatitis severity remains a challenge. Circulating levels of histones are raised early in mouse models and correlate with disease severity. It was hypothesized that circulating histones predict persistent organ failure in patients with acute pancreatitis.
Methods
Consecutive patients with acute pancreatitis fulfilling inclusion criteria admitted to Royal Liverpool University Hospital were enrolled prospectively between June 2010 and March 2014. Blood samples were obtained within 48 h of abdominal pain onset and relevant clinical data during the hospital stay were collected. Healthy volunteers were enrolled as controls. The primary endpoint was occurrence of persistent organ failure. The predictive values of circulating histones, clinical scores and other biomarkers were determined.
Results
Among 236 patients with acute pancreatitis, there were 156 (66·1 per cent), 57 (24·2 per cent) and 23 (9·7 per cent) with mild, moderate and severe disease respectively, according to the revised Atlanta classification. Forty-seven healthy volunteers were included. The area under the receiver operating characteristic (ROC) curve (AUC) for circulating histones in predicting persistent organ failure and mortality was 0·92 (95 per cent c.i. 0·85 to 0·99) and 0·96 (0·92 to 1·00) respectively; histones were at least as accurate as clinical scores or biochemical markers. For infected pancreatic necrosis and/or sepsis, the AUC was 0·78 (0·62 to 0·94). Histones did not predict or correlate with local pancreatic complications, but correlated negatively with leucocyte cell viability (r = –0·511, P = 0·001).
Conclusion
Quantitative assessment of circulating histones in plasma within 48 h of abdominal pain onset can predict persistent organ failure and mortality in patients with acute pancreatitis. Early death of immune cells may contribute to raised circulating histone levels in acute pancreatitis.
Potential as early biomarker
Introduction
Acute pancreatitis is one of the leading gastrointestinal disorders that require urgent clinical care and is increasing in incidence1. The clinical course of acute pancreatitis is variable, ranging from mild (uneventful clinical course), through moderate (local complication or transient organ failure) to severe (occurrence of persistent organ failure) disease2,3. Infected pancreatic necrosis4 and/or sepsis5 are major complications contributing to death at any stage. However, the principal cause of early death is persistent organ failure5. Early recognition of patients at risk of persistent organ failure is critical to guide fluid resuscitation and initiate high-dependency or intensive care treatment, and reduce morbidity and mortality6. Indeed, early stratification of disease severity improves clinical outcomes and significantly reduces length of hospital stay7.
Improvements in imaging, such as CT, have not proven superior to clinical scoring systems in early prediction of acute pancreatitis severity8. A recent multicentre study9 has shown that existing clinical scores such as the Systemic Inflammatory Response Syndrome (SIRS) score, Bedside Index for Severity in Acute Pancreatitis (BISAP), Acute Physiology And Chronic Health Examination (APACHE) II and Sequential Organ Failure Assessment (SOFA), either alone or in combination, are of limited clinical use for predicting persistent organ failure. The best predictor had a sensitivity and specificity of only 75 per cent on or within 48 h of admission. The latest meta-analyses concluded that there is no adequate predictor of persistent organ failure within 48 h of hospital admission10, or justifiable prediction models for mortality11. There is thus a pressing need for the identification and development of more powerful predictive markers.
Recently, damage-associated molecular pattern molecules (DAMPs)12, such as high-mobility group box 1 (HMGB1)13, cell-free DNA14, nucleosomes15 and histones16, have been investigated in experimental acute pancreatitis models, and most have shown a correlation between circulating levels and acute pancreatitis severity12,17. Furthermore, levels of HMGB118, nucleosomes17 and cell-free DNA19 have been associated with organ failure in human acute pancreatitis. The proposed role of these DAMPs in acute pancreatitis is illustrated in Fig. 1. Histones are well conserved nuclear proteins that are essential for DNA packaging and gene regulation. During tissue damage and cell death, nuclear chromatin is cleaved and released outside the cell where it is degraded into individual histones20. Circulating histones, the most abundant nuclear proteins, are barely detectable in the blood unless there is extensive cell death, such as in severe sepsis21,22 and trauma23. A recent review12 has suggested that circulating histones act as DAMPs that cause sterile inflammation and contribute to SIRS and organ failure. Extracellular histones are also toxic to endothelial cells21,23, platelets24 and leucocytes25. Furthermore, they have been reported to activate coagulation and stimulate cytokine release23,26. In mouse models, histone infusion causes death by multiple organ failure, which can be rescued by antihistone antibodies21,23,27. Clinically, high levels of circulating histones have been found in patients with severe blunt trauma23 and sepsis22, and have been associated with the development of respiratory failure23, new-onset cardiac complications22 and thrombocytopenia28.
Fig. 1.
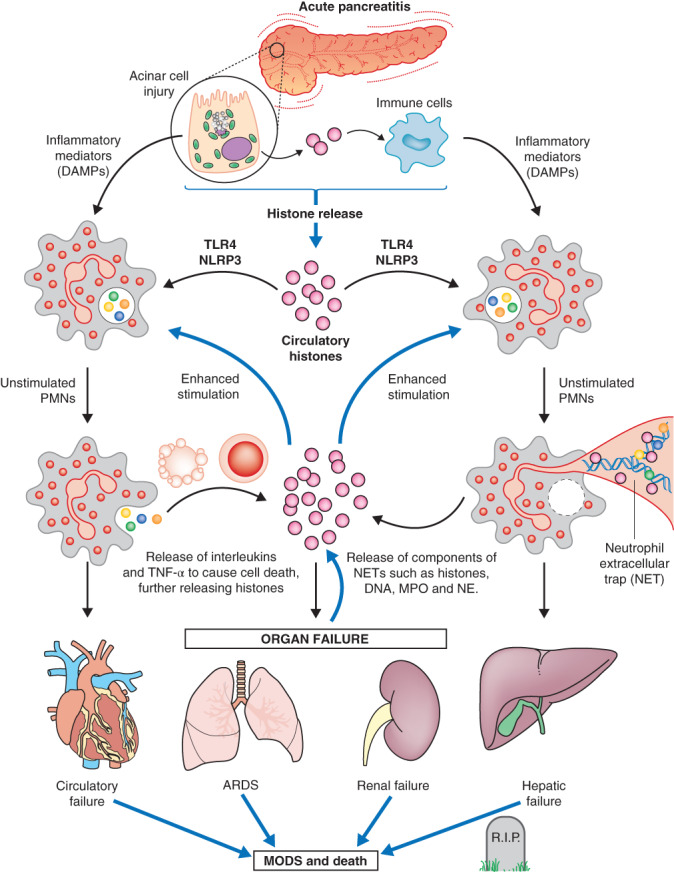
Proposed role of extracellular histones in acute pancreatitis. After injury caused by pancreatic toxins, the pancreas (primarily pancreatic acinar cells) releases damage-associated molecular pattern molecules including extracellular histones. The released extracellular histones and other inflammatory mediators stimulate resident immune cells to release more proinflammatory cytokines/chemokines. Once released into the circulation, the circulating histones activate polymorphonuclear neutrophils (PMNs) and facilitate neutrophil extracellular trap (NET) formation. Apoptotic and necrotic neutrophils and NETs release more histones and inflammatory mediators, which further stimulate PMN infiltration and NET formation. Stimulated PMNs, NET components, circulating histones and other inflammatory mediators cause distant organ dysfunction, such as acute respiratory distress syndrome or even multiple organ dysfunction syndrome (MODS). MODS in turn causes excessive release of circulating histones and other inflammatory mediators, triggering a vicious cycle of uncontrolled MODS, coagulation and death. TLR, Toll-like receptor; NLRP, NOD-like receptor family, pyrin domain-containing; IL, interleukin; TNF, tumour necrosis factor; MPO, myeloperoxidase; NE, neutrophil elastase
The authors16 have demonstrated previously that circulating histone levels rise very early in mouse acute pancreatitis models, and are strongly associated with disease severity and distant organ injury. Therefore, the hypothesis for the present study was that plasma levels of circulating histones may have early predictive value for persistent organ failure and other major clinical outcomes in patients with acute pancreatitis.
Methods
This study was designed, conducted and reported according to STROBE guidance29 for observational studies. A consecutive cohort of patients with acute pancreatitis admitted to the Royal Liverpool University Hospital between June 2010 and March 2014 were enrolled once written informed consent had been obtained. Inclusion criteria were: first episode of acute pancreatitis as defined by the revised Atlanta classification2; and availability of blood samples within 24 h of admission. Exclusion criteria were: age below 18 or over 85 years; advanced pulmonary, cardiac, renal diseases (chronic kidney disease stage 4–5), liver cirrhosis (modified Child–Pugh grade 2–3) or malignancy; pregnancy, chronic pancreatitis or trauma as the aetiology; and duration of abdominal pain before admission exceeding 24 h or referral from other hospitals. A group of healthy volunteers was also included.
The study protocols and acute pancreatitis biobank were approved by local research ethics committees (reference: 10/H1308/31).
Sample and data collection
Peripheral blood samples were collected within 24 h of admission (within 48 h of onset of abdominal pain). Serum (serum separator tube) and plasma (EDTA tube) were obtained after centrifugation at 1500 g for 10 min. Leucocytes were freshly isolated from whole blood and cell viability assessed using 0·1 per cent trypan blue (Life Technologies, Warrington, UK) and a Countess™ automated cell counter (Invitrogen, Glasgow, UK). Samples were stored at –80°C before use. Collection, processing, storage, monitoring and use of samples followed standard operating procedures (SOPs) and good clinical laboratory practice. Demographic and clinical data were recorded prospectively and maintained in an electronic database in accordance with SOPs. SIRS, BISAP, APACHE II and SOFA scores were calculated within 24 h of admission9. The first and the worst modified CT severity index (MCTSI)8 values were enumerated using contrast-enhanced CT images.
Outcomes of interest
The primary outcome of persistent organ failure was defined by a modified SOFA score of at least 2 for 48 h or more that manifested in failure of at least one of the respiratory, cardiovascular or renal systems3. In patients with pre-existing chronic kidney disease (stage 1–3), a 2-point worsening of kidney function, based on the estimated glomerular filtration rate30, was used to diagnose renal failure regardless of serum creatinine levels. Local complications were defined according to the revised Atlanta classfication2. Major infection was defined as the appearance of either infected pancreatic necrosis, sepsis or both, at least 3 days after admission. Mortality was recorded for the index hospital admission.
Clinical biomarker analysis
Plasma histone levels were determined by quantitative western blotting22,23,27,28, with intra-assay and inter-assay variability of 4·6 and 4·3 per cent respectively. Plasma interleukin (IL)-6 and IL-8 (R&D, Abingdon, UK) were measured by enzyme-linked immunosorbent assay, in accordance with the manufacturer's instructions. Haematocrit, urea, creatinine, C-reactive protein (CRP) and other routine clinical biomarkers were reported by the Department of Clinical Biochemistry of the hospital.
Statistical analysis
Continuous data are reported as median (i.q.r.). Continuous variables were compared by Mann–Whitney U test (2 groups) and Kruskal–Wallis test (3 or more groups). Categorical data were compared by means of χ2 or Fisher's exact tests. Spearman rank correlation was used for correlation analysis.
Receiver operating characteristic (ROC) curves were constructed for predictive variables, and the area under the curve (AUC) with 95 per cent confidence intervals (c.i.) calculated. Optimal cut-off values for sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR) and negative likelihood ratio (NLR) for each parameter were derived from the ROC curves. Post-test probability was obtained from the prevalence outcomes of interest and PLR. All tests were two-tailed and statistical significance was set at P < 0·050. The analyses were performed using SPSS® version 22.0 (IBM, Armonk, New York, USA).
Results
A total of 236 consecutive patients with pancreatitis (mild: 156, 66·1 per cent; moderate: 57, 24·2 per cent; severe: 23, 9·7 per cent) fulfilling the inclusion criteria were included in this study (Fig. 2). Baseline characteristics and clinical outcomes for each group are outlined in Table 1. Twenty-three patients (9·7 per cent) developed persistent organ failure; this occurred within 24 h of admission in 11 of these patients (Fig. S1, supporting information). Fifteen patients (6·4 per cent) had transient organ failure without local complications. Sixty patients (25·4 per cent) developed local complications; acute peripancreatic and acute necrotic collection each had an incidence of 12·7 per cent (30 patients). Major infection occurred in nine patients (3·8 per cent); there was one instance of infected pancreatic necrosis in the moderate group and eight in the severe group. Nine patients died (3·8 per cent), all of whom had severe pancreatitis.
Fig. 2.
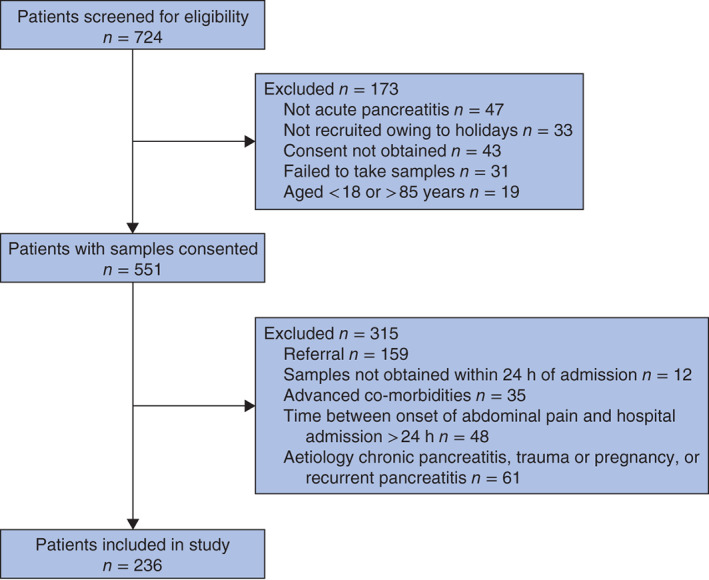
Flow chart showing patient selection
Table 1.
Demographic and clinical outcomes of the study population according to the severity of acute pancreatitis
| Mild | Moderate | Severe | P ‡ | |
|---|---|---|---|---|
| (n = 156) | (n = 57) | (n = 23) | ||
| Age (years)* | 56 (42–68) | 52 (40–67) | 62 (52–77) | 0·064 |
| Sex ratio (F : M) | 91 : 65 | 22 : 35 | 11 : 12 | 0·040§ |
| Updated Charlson co-morbidity index score* | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0·158 |
| Aetiology | ||||
| Biliary | 83 (53·2) | 29 (51) | 8 (35) | 0·256 |
| Alcohol | 30 (19·2) | 13 (23) | 5 (22) | 0·835 |
| Other | 43 (27·6) | 15 (26) | 10 (43) | 0·259 |
| Time to admission (h)* | 8 (4–14) | 6 (4–12) | 11 (6–15) | 0·111 |
| Time from admission to sampling (h)* | 14 (8–20) | 17 (10–20) | 16 (12–20) | 0·588 |
| Worst modified CT severity index*† | 2 (0–2) | 6 (4–8) | 6 (6–8) | < 0·001§ |
| Acute peripancreatic fluid collection | 0 (0) | 23 (40) | 7 (30) | < 0·001§ |
| Pancreatic necrosis | 0 (0) | 19 (33) | 11 (48) | < 0·001§ |
| Infected pancreatic necrosis and/or sepsis | 0 (0) | 1 (2) | 8 (35) | < 0·001¶ |
| Need for ICU admission | 0 (0) | 0 (0) | 19 (83) | < 0·001¶ |
| Need for antibiotics | 9 (5·8) | 9 (16) | 16 (70) | < 0·001# |
| Nutritional support | 0 (0) | 0 (0) | 13 (57) | < 0·001¶ |
| Necrosectomy and/or percutaneous drainage | 0 (0) | 3 (5) | 8 (35) | < 0·001# |
| Death | 0 (0) | 0 (0) | 9 (39) | < 0·001¶ |
| Length of hospital stay (days)* | 5·5 (3–9) | 14 (11–21) | 29 (13·5–65·5) | < 0·001# |
Values in parentheses are percentages unless indicated otherwise;
values are median (i.q.r.).
CT performed in 31 patients with mild, 48 with moderate and 20 with severe pancreatitis.
χ2 or Fisher's exact test for categorical data and Kruskal–Wallis test for continuous data.
P < 0·050, mild versus moderate or severe;
P < 0·050, severe versus mild or moderate;
P < 0·050 between any two groups (χ2 or Fisher's exact test for categorical data and Mann–Whitney U test for continuous data).
Prediction of disease severity
Levels of circulating histones in healthy volunteers and patients with acute pancreatitis are shown in Fig. 3a. Representative western blots for histone measurement are available in Fig. S2 (supporting information). Circulating histones were barely detectable in healthy volunteers, and comparable in patients with mild and moderate disease (median (i.q.r.) 1·1 (0·6–2·1) versus 1·3 (0·5–2·8) μg/ml; P = 0·633) (Table 2). Circulating histone levels were raised significantly only in patients with severe disease (18·8 (5·9–33·8) μg/ml; P < 0·001 versus all other groups), and were higher when persistent organ failure occurred within 24 h than more than 24 h after admission (P = 0·056) (Fig. 3b).
Fig. 3.
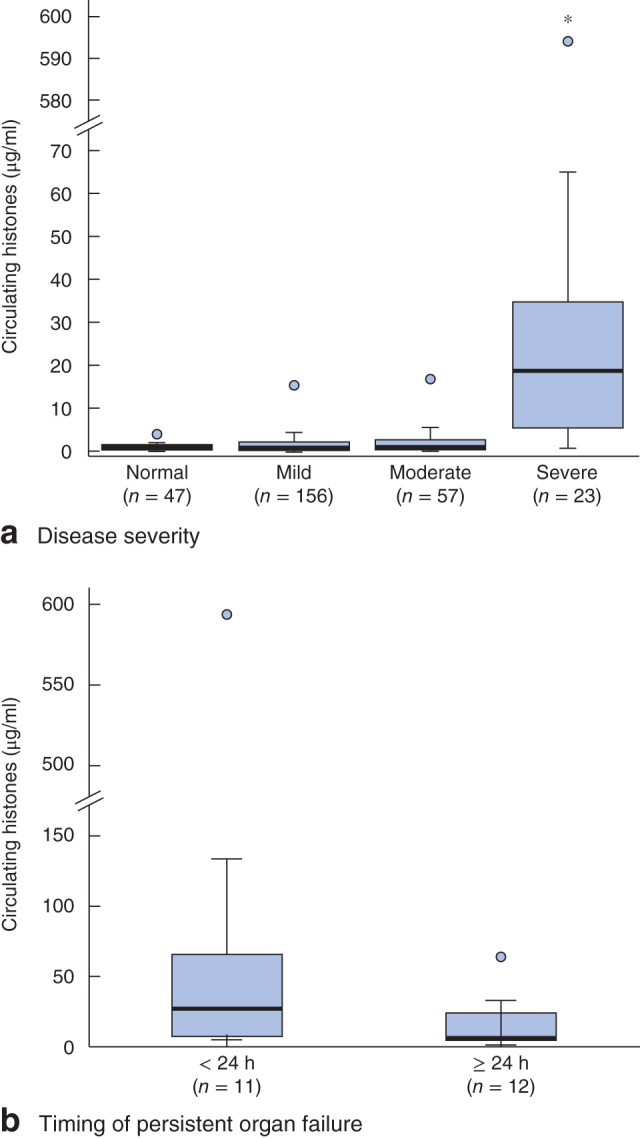
Comparison of circulating histone levels measured within 24 h of admission: a healthy volunteers and patients with acute pancreatitis on admission; b patients with persistent organ failure occurring in less than 24 h versus 24 h or more. Median values (bold line), i.q.r. (box), and range (error bars) including outliers (circles) are shown. *P < 0·050 versus each other group (Mann–Whitney U test)
Table 2.
Comparison of clinical scores and biomarkers in different severity groups
| Mild | Moderate | Severe | P * | |
|---|---|---|---|---|
| (n = 156) | (n = 57) | (n = 23) | ||
| Clinical scores within 24 h of admission | ||||
| SIRS | 1 (0–1) | 1 (1–2) | 2 (1–2) | 0·002† |
| BISAP | 1 (0–1) | 1 (0–1) | 2 (1–2) | < 0·001‡ |
| APACHE II | 5 (3–7) | 7 (5–9) | 10 (6–12·5) | < 0·001† |
| SOFA | 0 (0–1) | 1 (0–2) | 2 (1–4) | < 0·001† |
| Biomarkers within 24 h of admission | ||||
| White cell count (×109/l) | 12·4 (9·9–15·3) | 14·2 (11·5–17·4) | 14·4 (11·2–19·3) | 0·002‡ |
| Neutrophil to lymphocyte ratio | 6·9 (4·1–14·8) | 8 (4·3–14·7) | 7·7 (5·3–23·6) | 0·520 |
| Haematocrit (%) | 40·1 (38–42·9) | 43·5 (39·3–45·6) | 42·8 (37·6–45·4) | 0·003‡ |
| Urea (mmol/l) | 5 (3·7–6·1) | 4·8 (3·7–6·3) | 7·3 (5·2–8·9) | 0·001§ |
| Creatinine (μmol/l) | 71 (61–88) | 83 (65–99) | 104 (75–157·5) | < 0·001† |
| CRP (mg/l) | 8 (5–23) | 10 (5–34) | 10 (5–137) | 0·288 |
| IL-6 (pg/ml) | 13·8 (7–57·8) | 30·8 (8·6–96·2) | 65·1 (21·7–143·4) | 0·022¶ |
| IL-8 (pg/ml) | 9·9 (0·4–19·1) | 13·9 (5·4–28·9) | 44·5 (18·8–64·2) | < 0·001¶ |
| Circulating histones (μg/ml) | 1·1 (0·6–2·1) | 1·3 (0·5–2·8) | 18·8 (5·9–33·8) | < 0·001§ |
| Biomarkers at 48 h after admission | ||||
| Urea (mmol/l) | 3·5 (2·6–4·7) | 3·5 (2·7–5·5) | 8·7 (5·2–11·7) | < 0·001§ |
| Creatinine (μmol/l) | 66 (53·5–79) | 65 (54·5–86) | 71 (57–172) | 0·080 |
| CRP (mg/l) | 38 (11–116) | 234 (159–317) | 328 (250–368) | 0·001† |
Values are median (i.q.r.). SIRS, Systemic Inflammatory Response Syndrome; BISAP, Bedside Index for Severity in Acute Pancreatitis; APACHE, Acute Physiology And Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; CRP, C-reactive protein; IL, interleukin.
Kruskal–Wallis test.
P < 0·050 between any two groups;
P < 0·050, mild versus moderate or severe
P < 0·050, severe versus mild or moderate;
P < 0·050, mild versus severe (Mann–Whitney U test).
The levels of circulating histones and other clinical parameters used for assessing acute pancreatitis severity are summarized in Table 2. Like circulating histones, SIRS, BISAP, APACHE II and SOFA scores all increased with disease severity within 24 h of admission, and were significantly higher in those with severe disease compared with the mild or moderate groups. Levels of urea, creatinine, haematocrit, IL-6 and IL-8 were also significantly higher in patients with severe disease. In contrast, CRP levels showed no significant association with disease severity within 24 h of admission, but became significant after 48 h. These data indicate that, following the onset of acute pancreatitis, histones appear within the circulation more rapidly than CRP and synchronously with severe clinical manifestations, and may have potential value in the early prediction of severe acute pancreatitis.
Prediction of persistent organ failure
The AUC values for potential predictors of persistent organ failure are summarized in Table 3. The values for all the clinical scores were moderate (range 0·68–0·81) in predicting persistent organ failure, and both the sensitivity (51·6–68·4 per cent) and specificity (74·1–82·8 per cent) were poor. Levels of circulating histones within 24 h of admission outperformed clinical scores and had stronger predictive value (AUC 0·92, 95 per cent c.i. 0·85 to 0·99) than either CRP (AUC 0·54, 0·37 to 0·70) (Fig. 4a) or urea (AUC 0·75, 0·63 to 0·86) (Fig. 4b). CRP (AUC 0·89, 0·84 to 0·94) (Fig. 4a) and urea (AUC 0·82, 0·71 to 0·94) (Fig. 4b) only showed relatively strong predictive value for persistent organ failure at 48 h following admission. Using the optimal cut-off value of circulating histones (5·4 μg/ml), they surpassed all other parameters measured in this study, with a sensitivity, specificity, PPV and NPV of 82·6, 94·4, 61·3 and 98·1 per cent respectively, a PLR and NLR of 14·7 and 0·18, and post-test probability of 61·4 per cent (versus prevalence 9·7 per cent) (Table 4). However, combining circulating histone levels with either CRP or urea at 48 h did not increase the predictive values further.
Table 3.
Accuracy of potential predictors with time since admission
| Persistent organ failure | Mortality | |||
|---|---|---|---|---|
| AUC | P | AUC | P | |
| Clinical scores within 24 h of admission | ||||
| SIRS | 0·68 (0·55, 0·81) | 0·010 | 0·72 (0·53, 0·91) | 0·037 |
| BISAP | 0·81 (0·71, 0·91) | < 0·001 | 0·90 (0·80, 0·99) | < 0·001 |
| APACHE II | 0·74 (0·62, 0·87) | < 0·001 | 0·86 (0·70, 1·00) | 0·001 |
| SOFA | 0·79 (0·68, 0·90) | < 0·001 | 0·83 (0·66, 0·99) | 0·001 |
| Biomarkers within 24 h of admission | ||||
| White cell count (×109/l) | 0·62 (0·49, 0·74) | 0·066 | 0·67 (0·46, 0·87) | 0·085 |
| Haematocrit (%) | 0·58 (0·43, 0·73) | 0·253 | 0·52 (0·30, 0·74) | 0·848 |
| Urea (mmol/l) | 0·75 (0·63, 0·86) | < 0·001 | 0·83 (0·69, 0·98) | 0·001 |
| Creatinine (μmol/l) | 0·74 (0·62, 0·86) | < 0·001 | 0·91 (0·81, 1·00) | < 0·001 |
| CRP (mg/l) | 0·54 (0·37, 0·70) | 0·612 | 0·75 (0·54, 0·96) | 0·026 |
| IL-6 (pg/ml) | 0·67 (0·49, 0·74) | 0·018 | 0·73 (0·54, 0·91) | 0·045 |
| IL-8 (pg/ml) | 0·76 (0·64, 0·89) | 0·001 | 0·89 (0·78, 0·99) | 0·001 |
| Circulating histones (μg/ml) | 0·92 (0·85, 0·99) | < 0·001 | 0·96 (0·92, 1·00) | < 0·001 |
| Biomarkers at 48 h after admission | ||||
| Urea (mmol/l) | 0·82 (0·71, 0·94) | < 0·001 | 0·97 (0·95, 0·99) | < 0·001 |
| Creatinine (μmol/l) | 0·61 (0·44, 0·78) | 0·129 | 0·86 (0·65, 1·00) | 0·002 |
| CRP (mg/l) | 0·89 (0·84, 0·94) | < 0·001 | 0·86 (0·79, 0·93) | 0·003 |
Values in parentheses are 95 per cent confidence intervals. AUC, area under the receiver operating characteristic (ROC) curve; SIRS, Systemic Inflammatory Response Syndrome; BISAP, Bedside Index for Severity in Acute Pancreatitis; APACHE, Acute Physiology And Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; CRP, C-reactive protein; IL, interleukin.
Fig. 4.
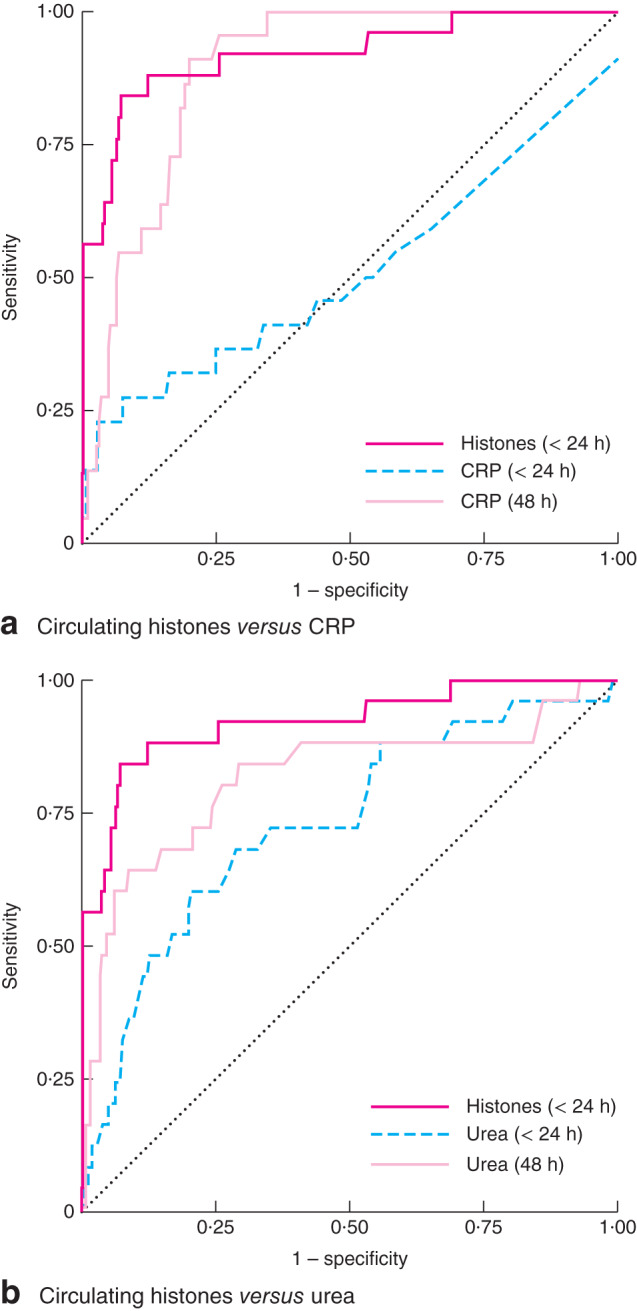
Comparison of receiver operating characteristic (ROC) curves for prediction of persistent organ failure since admission: a circulating histones within 24 h versus C-reactive protein (CRP) within 24 h or at 48 h; b circulating histones within 24 h versus urea within 24 h or at 48 h. Dotted line is the ROC reference line
Table 4.
Predictive values of the most effective predictors since admission
| Cut-off value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | PLR | NLR | PP (%) | |
|---|---|---|---|---|---|---|---|---|
| Persistent organ failure (prevalence 9·7%) | ||||||||
| BISAP (< 24 h) | ≥ 2 | 68·4 | 82·8 | 26·5 | 96·7 | 4·0 | 0·38 | 30·2 |
| Circulating histones (< 24 h) | ≥ 5·4 μg/ml | 82·6 | 94·4 | 61·3 | 98·1 | 14·7 | 0·18 | 61·4 |
| Urea (48 h) | ≥ 8 mmol/l | 60·9 | 94·7 | 56·0 | 95·6 | 11·5 | 0·41 | 55·4 |
| CRP (48 h) | ≥ 250 mg/l | 80·0 | 80·5 | 29·1 | 97·6 | 4·1 | 0·25 | 30·7 |
| Mortality (prevalence 3·8%) | ||||||||
| BISAP (< 24 h) | ≥ 2 | 87·5 | 80·9 | 14·3 | 99·4 | 4·6 | 0·15 | 15·4 |
| Circulating histones (< 24 h) | ≥ 5·4 μg/ml | 88·9 | 89·9 | 25·8 | 99·5 | 8·8 | 0·12 | 25·9 |
| Urea (48 h) | ≥ 8 mmol/l | 100 | 93·2 | 37·5 | 100 | 14·8 | 0 | 37·0 |
| CRP (48 h) | ≥ 250 mg/l | 100 | 77·1 | 10·9 | 100 | 4·4 | 0 | 14·9 |
PPV, positive predictive value; NPV, negative predictive value; PLR, positive likelihood ratio; NLR, negative likelihood ratio; PP, post-test probability based on the test result being above the cut-off value; BISAP, Bedside Index for Severity in Acute Pancreatitis; CRP, C-reactive protein.
Prediction of major infection
For predicting major infection, the AUC for circulating histones was 0·78 (0·62 to 0·94), which was similar to, or lower than the values for BISAP, APACHE II, SOFA, urea, creatinine and IL-6 (range 0·80 to 0·87) within 24 h (Table S1, supporting information). At 48 h, the AUC values for CRP (Fig. S3a, supporting information) and urea (Fig. S3b, supporting information) were 0·83 and 0·92 respectively, higher than that for circulating histones within 24 h. At a cut-off of 5·4 μg/ml, the sensitivity, specificity, PPV and NPV of circulating histones for the prediction of major infection were 44·4, 88·1, 12·9 and 97·6 per cent respectively, with a PLR and NLR of 3·7 and 0·63, and post-test probability of 12·8 per cent (versus prevalence 3·8 per cent) (Table S2, supporting information).
Prediction of mortality
Circulating histones had a higher predictive value for death (AUC 0·96, 95 per cent c.i. 0·92 to 1·00) than any other parameter within 24 h (Table 3), including both CRP (Fig. S4a, supporting information) and urea (Fig. S4b, supporting information), but was comparable to urea at 48 h (AUC 0·97, 0·95 to 0·99) (Table 3). At an optimal cut-off of 5·4 μg/ml, the sensitivity, specificity, PPV and NPV of circulating histones for predicting death were 88·9, 89·9, 25·8 and 99·5 respectively, with a PLR and NLR 8·8 and 0·12, and a post-test probability of 25·9 per cent (versus prevalence 3·8 per cent) (Table 4). For the urea level at 48 h with a cut-off of 8 mmol/l, the sensitivity, specificity, PPV and NPV were 100, 93·2, 37·5 and 100 per cent, with a PLR and NLR of 14·8 and 0, and a post-test probability of 37·0 per cent (Table 4). Both BISAP within 24 h (cut-off 2) and CRP (cut-off 250 mg/l) at 48 h had reasonable predictive values, but PLR values (4·6 and 4·4 respectively) were much lower than those of both circulating histones within 24 h and urea at 48 h. Combining circulating histones (cut-off 5·4 μg/ml) with either urea (cut-off 8 mmol/l, at 48 h) or CRP (cut-off 250 mg/l, at 48 h) did not increase specificity compared with the individual parameters alone.
Leucocyte viability, local complications and transient organ failure
Circulating levels of histones have been shown to correlate significantly with pancreatic necrosis scores in animal models16. However, the correlation between histone levels on admission and the first (n = 99; r = 0·17, P = 0·094) or the worst (n = 99; r = 0·195, P = 0·054) MCTSI value were not significant. In an analysis including only patients with mild and moderate disease, there was no correlation between circulating histone levels and local complications, acute peripancreatic fluid collection, pancreatic necrosis or transient organ failure with or without local complications (all P ≥ 0·237). During disease progression, pancreatic necrosis normally occurs 24 h after onset of symptoms and would therefore not directly affect histone levels within the first 24 h, but may be contributory after this time. Another source could be histones released from immune cells following cellular damage or death. The percentage of viable leucocytes was measured in the peripheral blood of 62 patients in this cohort, and a significant negative association was found between circulating histone levels and leucocyte viability within 24 h of admission (r = –0·511, P = 0·001). There was no significant impact of aetiology (biliary, alcohol or others) on circulating histone levels (r = –0·024, P = 0·712) or leucocyte viability (r = –0·101, P = 0·426).
Discussion
In this study of consecutive patients with acute pancreatitis, levels of circulating histones were an accurate index of disease severity, and capable of predicting persistent organ failure and mortality; they performed better than BISAP and urea, indices currently used in the clinical setting within 24 h of admission (48 h from disease onset to admission).
Both revised Atlanta2 and determinant-based3 classifications for severity stratification of acute pancreatitis recognize persistent organ failure as the predominant determinant of death. The present findings confirm this and mortality occurred only in the group with severe disease. Circulating histones had the best AUC value in predicting both persistent organ failure and mortality within 24 h of admission. On the other hand, patients with transient organ failure without local complications have a similar clinical outlook to those with mild disease31. Current clinical scores are imprecise at differentiating between organ failure that will be transient or persistent32. In the present cohort, only 11 of 23 patients with severe acute pancreatitis developed persistent organ failure within 24 h of admission, but circulating histone levels were still invaluable in differentiating this group from patients with transient organ failure. Only one patient with transient organ failure had circulating histone levels that exceeded the cut-off value for predicting persistent organ failure.
This study showed that circulating histones have moderate predictive value for major infection, underlining the challenges in fulfilling the clinical need in this area in the current absence of a good predictor10. Here, five of nine patients died within the first week of admission, precluding further assessment of the development of infected pancreatic necrosis. This introduces bias for circulating histones in predicting major infection. Patients with symptomatic sterile necrosis invariably develop infection after surgical intervention33,34. A previous study5 showed that baseline parameters and clinical outcomes were similar in patients with a primary diagnosis of infected pancreatic necrosis and those who presented initially with sterile necrosis but eventually required surgery despite maximal conservative treatment5. Infected pancreatic necrosis has been emphasized by the determinant-based classification as another determinant of mortality3,4. The modified determinant-based classification35 further stratifies patients with persistent organ failure into two groups: one with infected pancreatic necrosis and one without. It would be of interest to test whether circulating histone levels differ between these two groups in a large study.
Strong correlations have been observed between peak histone levels and pancreatic necrosis in animal models of acute pancreatitis16, but histone levels within 24 h of admission in this study did not correlate with MCTSI or predict pancreatic necrosis. This discrepancy may be due to the time point of blood collection or a fundamental difference in disease progression, as pancreatic necrosis occurs several days later in humans than in animal models. Therefore, pancreatic acinar cells are likely to make a limited contribution to levels of circulating histones in early-stage disease. Instead, immune cells such as neutrophils may be a major contributor to histone release, either via neutrophil extracellular trap formation (NETosis)19 or necrosis. The present observation that histone levels significantly and inversely correlated with the proportion of dead or dying peripheral leucocytes supports this argument. More data are needed to establish this point. The authors postulate that histone levels may reflect the intensity of systemic inflammation.
The most studied DAMP in the acute pancreatitis setting is HMGB112. Based on previous reports13, significant release of HMGB1 occurs beyond 24 h in experimental acute pancreatitis, which implies that it may not be useful in the early prediction of persistent organ failure. In contrast, the authors16 have previously demonstrated that levels of circulating histones rise within 2 h in experimental and early in human acute pancreatitis, as demonstrated here. Circulating nucleosomes have been tested in non-consecutive patients with acute pancreatitis based on the revised Atlanta classification17, but the predictive values were lower than those in the present study. This discrepancy may be explained by the different pathophysiological roles of circulating nucleosomes and histones. One of the fundamental differences is that nucleosomes are not toxic when released in an intact form36. Current assays cannot distinguish between intact and degraded nucleosomes, which feeds into the controversy surrounding their clinical value compared with the well established toxic effects of circulating histones37. As the present study included only 23 patients with severe acute pancreatitis, larger studies are needed to compare the predictive values of these nuclear DAMPs with current clinical indices in the setting of acute pancreatitis. Further studies are also needed to elucidate the temporal changes and source of circulating histones in acute pancreatitis, and how these correlate with systemic inflammation markers and individual organ failure. Such data could advance the consideration of monitoring histones routinely in clinical practice as well as the translational potential of targeting circulating histones in acute pancreatitis.
Supplementary Material
Fig. S1 Timing of persistent organ failure after admission. D, day
Fig. S2 Representative western blots for circulating histone measurement. Recombinant histone H3 was used as standard, and specific antihistone 3 antibody was used to measure histones. Normal, heathy volunteers; Se, severe pancreatitis; Mi, mild pancreatitis; Mo, moderate pancreatitis
Fig. S3 Comparison of receiver operating characteristic (ROC) curves for prediction of major infection since admission: a circulating histones within 24 h versus C-reactive protein (CRP) within 24 h or at 48 h; b circulating histones within 24 h versus urea within 24 h or at 48 h. Dashed line is the ROC reference line
Fig. S4 Comparison of receiver operating characteristic (ROC) curves for prediction of mortality since admission: a circulating histones within 24 h versus C-reactive protein (CRP) within 24 h or at 48 h; b circulating histones within 24 h versus urea within 24 h or at 48 h. Dashed line is the ROC reference line
Table S1 Accuracy of potential predictors of major infection with time since admission
Table S2 Comparison of predictive values of the most effective predictors for major infection since admission
Acknowledgements
G. Wang, R. Sutton and C. H. Toh are senior (correspondence) authors with equal contributions. [Correction added on 28 April, after first online publication: clarification of correspondence authors]. T.L. and W.H. contributed equally to this article as joint first authors. This work was supported by the National Institute for Health Research (NIHR) (NIHR-BRF-2011-026, II-FS-0110-14061), the British Heart Foundation (PG/14/19/30751), Newton International Fellowship, Royal College of Surgeons of England Fellowship (to P.S.), a NIHR Biomedical Research Unit Award and the Medical Research Council (G0501641).
Disclosure: The authors declare no conflict of interest.
References
- 1. Peery AF, Crockett SD, Barritt AS, Dellon ES, Eluri S, Gangarosa LMet al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology 2015; 149: 1731–1741. e1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MGet al. ; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis – 2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62: 102–111. [DOI] [PubMed] [Google Scholar]
- 3. Dellinger EP, Forsmark CE, Layer P, Lévy P, Maravi-Poma E, Petrov MSet al. ; Pancreatitis Across Nations Clinical Research and Education Alliance (PANCREA). Determinant-based classification of acute pancreatitis severity: an international multidisciplinary consultation. Ann Surg 2012; 256: 875–880. [DOI] [PubMed] [Google Scholar]
- 4. Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010; 139: 813–820. [DOI] [PubMed] [Google Scholar]
- 5. Guo Q, Li A, Xia Q, Liu X, Tian B, Mai Get al. The role of organ failure and infection in necrotizing pancreatitis: a prospective study. Ann Surg 2014; 259: 1201–1207. [DOI] [PubMed] [Google Scholar]
- 6. Wu BU, Banks PA. Clinical management of patients with acute pancreatitis. Gastroenterology 2013; 144: 1272–1281. [DOI] [PubMed] [Google Scholar]
- 7. Dimagno MJ, Wamsteker EJ, Rizk RS, Spaete JP, Gupta S, Sahay Tet al. A combined paging alert and web-based instrument alters clinician behavior and shortens hospital length of stay in acute pancreatitis. Am J Gastroenterol 2014; 109: 306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bollen TL, Singh VK, Maurer R, Repas K, van Es HW, Banks PAet al. A comparative evaluation of radiologic and clinical scoring systems in the early prediction of severity in acute pancreatitis. Am J Gastroenterol 2012; 107: 612–619. [DOI] [PubMed] [Google Scholar]
- 9. Mounzer R, Langmead CJ, Wu BU, Evans AC, Bishehsari F, Muddana Vet al. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology 2012; 142: 1476–1482. [DOI] [PubMed] [Google Scholar]
- 10. Yang CJ, Chen J, Phillips AR, Windsor JA, Petrov MS. Predictors of severe and critical acute pancreatitis: a systematic review. Dig Liver Dis 2014; 46: 446–451. [DOI] [PubMed] [Google Scholar]
- 11. Di MY, Liu H, Yang ZY, Bonis PA, Tang JL, Lau J. Prediction models of mortality in acute pancreatitis in adults: a systematic review. Ann Intern Med 2016; 165: 482–490. [DOI] [PubMed] [Google Scholar]
- 12. Kang R, Lotze MT, Zeh HJ, Billiar TR, Tang D. Cell death and DAMPs in acute pancreatitis. Mol Med 2014; 20: 466–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yasuda T, Ueda T, Shinzeki M, Sawa H, Nakajima T, Takeyama Yet al. Increase of high-mobility group box chromosomal protein 1 in blood and injured organs in experimental severe acute pancreatitis. Pancreas 2007; 34: 487–488. [DOI] [PubMed] [Google Scholar]
- 14. Hoque R, Sohail M, Malik A, Sarwar S, Luo Y, Shah Aet al. TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis. Gastroenterology 2011; 141: 358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kang R, Zhang Q, Hou W, Yan Z, Chen R, Bonaroti Jet al. Intracellular Hmgb1 inhibits inflammatory nucleosome release and limits acute pancreatitis in mice. Gastroenterology 2014; 146: 1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ou X, Cheng Z, Liu T, Tang Z, Huang W, Szatmary Pet al. Circulating histone levels reflect disease severity in animal models of acute pancreatitis. Pancreas 2015; 44: 1089–1095. [DOI] [PubMed] [Google Scholar]
- 17. Penttila AK, Rouhiainen A, Kylanpaa L, Mustonen H, Puolakkainen P, Rauvala Het al. Circulating nucleosomes as predictive markers of severe acute pancreatitis. J Intensive Care 2016; 4: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu GF, Guo M, Tian ZQ, Wu GZ, Zou XP, Zhang WJ. Increased of serum high-mobility group box chromosomal protein 1 correlated with intestinal mucosal barrier injury in patients with severe acute pancreatitis. World J Emerg Surg 2014; 9: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Merza M, Hartman H, Rahman M, Hwaiz R, Zhang E, Renström Eet al. Neutrophil extracellular traps induce trypsin activation, inflammation, and tissue damage in mice with severe acute pancreatitis. Gastroenterology 2015; 149: 1920–1931.e8. [DOI] [PubMed] [Google Scholar]
- 20. Holdenrieder S, Stieber P, Bodenmuller H, Fertig G, Furst H, Schmeller Net al. Nucleosomes in serum as a marker for cell death. Clin Chem Lab Med 2001; 39: 596–605. [DOI] [PubMed] [Google Scholar]
- 21. Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro Fet al. Extracellular histones are major mediators of death in sepsis. Nat Med 2009; 15: 1318–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alhamdi Y, Abrams ST, Cheng Z, Jing S, Su D, Liu Zet al. Circulating histones are major mediators of cardiac injury in patients with sepsis. Crit Care Med 2015; 43: 2094–2103. [DOI] [PubMed] [Google Scholar]
- 23. Abrams ST, Zhang N, Manson J, Liu T, Dart C, Baluwa Fet al. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med 2013; 187: 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood 2011; 118: 3708–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DDet al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A 2010; 107: 15 880–15 885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu J, Zhang X, Monestier M, Esmon NL, Esmon CT. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol 2011; 187: 2626–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alhamdi Y, Zi M, Abrams ST, Liu T, Su D, Welters Iet al. Circulating histone concentrations differentially affect the predominance of left or right ventricular dysfunction in critical illness. Crit Care Med 2016; 44: e278–e288. [DOI] [PubMed] [Google Scholar]
- 28. Alhamdi Y, Abrams ST, Lane S, Wang G, Toh CH. Histone-associated thrombocytopenia in patients who are critically ill. JAMA 2016; 315: 817–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JPet al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007; 335: 806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158: 825–830. [DOI] [PubMed] [Google Scholar]
- 31. Kwong WT, Ondrejkova A, Vege SS. Predictors and outcomes of moderately severe acute pancreatitis – evidence to reclassify. Pancreatology 2016; 16: 940–945. [DOI] [PubMed] [Google Scholar]
- 32. Johnson CD, Abu-Hilal M. Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut 2004; 53: 1340–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Raraty MG, Halloran CM, Dodd S, Ghaneh P, Connor S, Evans Jet al. Minimal access retroperitoneal pancreatic necrosectomy: improvement in morbidity and mortality with a less invasive approach. Ann Surg 2010; 251: 787–793. [DOI] [PubMed] [Google Scholar]
- 34. Gomatos IP, Halloran CM, Ghaneh P, Raraty MG, Polydoros F, Evans JCet al. Outcomes from minimal access retroperitoneal and open pancreatic necrosectomy in 394 patients with necrotizing pancreatitis. Ann Surg 2016; 263: 992–1001. [DOI] [PubMed] [Google Scholar]
- 35. Zubia-Olaskoaga F, Maravi-Poma E, Urreta-Barallobre I, Ramirez-Puerta MR, Mourelo-Farina M, Marcos-Neira MP; Epidemiology of Acute Pancreatitis in Intensive Care Medicine Study Group. Comparison between revised Atlanta classification and determinant-based classification for acute pancreatitis in intensive care medicine. Why do not use a modified determinant-based classification? Crit Care Med 2016; 44: 910–917. [DOI] [PubMed] [Google Scholar]
- 36. Abrams ST, Zhang N, Dart C, Wang SS, Thachil J, Guan Yet al. Human CRP defends against the toxicity of circulating histones. J Immunol 2013; 191: 2495–2502. [DOI] [PubMed] [Google Scholar]
- 37. Alhamdi Y, Toh CH. The role of extracellular histones in haematological disorders. Br J Haematol 2016; 173: 805–811. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Timing of persistent organ failure after admission. D, day
Fig. S2 Representative western blots for circulating histone measurement. Recombinant histone H3 was used as standard, and specific antihistone 3 antibody was used to measure histones. Normal, heathy volunteers; Se, severe pancreatitis; Mi, mild pancreatitis; Mo, moderate pancreatitis
Fig. S3 Comparison of receiver operating characteristic (ROC) curves for prediction of major infection since admission: a circulating histones within 24 h versus C-reactive protein (CRP) within 24 h or at 48 h; b circulating histones within 24 h versus urea within 24 h or at 48 h. Dashed line is the ROC reference line
Fig. S4 Comparison of receiver operating characteristic (ROC) curves for prediction of mortality since admission: a circulating histones within 24 h versus C-reactive protein (CRP) within 24 h or at 48 h; b circulating histones within 24 h versus urea within 24 h or at 48 h. Dashed line is the ROC reference line
Table S1 Accuracy of potential predictors of major infection with time since admission
Table S2 Comparison of predictive values of the most effective predictors for major infection since admission


