Abstract
Background
There are few prospective studies of outcomes following surgery in rural district hospitals in sub-Saharan Africa. This study aimed to estimate the prevalence and predictors of surgical-site infection (SSI) following caesarean section at Kirehe District Hospital in rural Rwanda.
Methods
Adult women who underwent caesarean section between March and October 2017 were given a voucher to return to the hospital on postoperative day (POD) 10 (±3 days). At the visit, a physician evaluated the patient for an SSI. A multivariable logistic regression model was used to identify risk factors for SSI, built using backward stepwise selection.
Results
Of 729 women who had a caesarean section, 620 were eligible for follow-up, of whom 550 (88·7 per cent) returned for assessment. The prevalence of SSI on POD 10 was 10·9 per cent (60 women). In the multivariable analysis, the following factors were significantly associated with SSI: bodyweight more than 75 kg (odds ratio (OR) 5·98, 1·56 to 22·96; P = 0·009); spending more than €1·1 on travel to the health centre (OR 2·42, 1·31 to 4·49; P = 0·005); being a housewife compared with a farmer (OR 2·93, 1·08 to 7·97; P = 0·035); and skin preparation with a single antiseptic compared with a combination of two antiseptics (OR 4·42, 1·05 to 18·57; P = 0·043). Receiving either preoperative or postoperative antibiotics was not associated with SSI.
Conclusion
The prevalence of SSI after caesarean section is consistent with rates reported at tertiary facilities in sub-Saharan Africa. Combining antiseptic solutions for skin preparation could reduce the risk of SSI.
Graphical Abstract
This study included adult women who underwent caesarean section at a rural district hospital in Rwanda between March and October 2017. Weighing more than 75 kg, spending more than €1·1 on transport to the nearest health centre, being a housewife, and skin preparation with a single solution were identified as predictors of surgical-site infection (SSI) in the multivariable analysis. The relationship between travel costs and SSI risk may be related to access to postoperative care and needs further exploration.
Graphical Abstract.
Surgical-site infection associated with markers of poverty
Introduction
Surgical-site infections (SSIs) are an important global public health problem, disproportionately affecting low- and middle- income countries (LMICs), where the burden is 75 per cent higher than in developed countries1. Globally, SSIs lead to longer hospital stays and more health complications, increasing mortality risks and costs for patients, families and healthcare facilities1–5.
Lower (uterine) segment caesarean section (LSCS) is the most commonly performed surgical procedure in the world6. The rates of LSCS have increased over the past three decades, up to an average of 19·1 per cent of deliveries worldwide7. Although sub-Saharan Africa has the lowest rates of LSCS at 7·3 per cent of all deliveries, increased access to LSCS has contributed to a decline in maternal mortality in the region7,8.
However, the increased access to LSCS has also led to an increased number of SSIs9. In sub-Saharan Africa, the prevalence of SSI after LSCS ranges from 7 to 48 per cent10–14, and is associated with younger age, obesity, hyperthermia on admission, difficult delivery, premature rupture of the membranes, neonatal death, prolonged labour and long duration of LSCS13–17. The majority of these studies were from urban and/or tertiary facilities, and there is limited information on SSI prevalence and risks for women delivering in district hospitals serving the rural areas, where 72·4 per cent of the sub-Saharan African population resides18.
In Rwanda, 13 per cent of all women and 11 per cent of women residing in rural areas deliver their baby via LSCS19; LSCS is the most commonly performed surgical procedure in Rwandan district hospitals20. This study estimated the prevalence of SSI on postoperative day (POD) 10 and identified risk factors for these infections for women who underwent LSCS at Kirehe District Hospital (KDH) in rural Rwanda. The aim was to characterize the burden of SSI to patients and district hospitals, and to identify which patients are most at risk, in order to target future interventions.
Methods
All women provided informed consent before study enrolment. Study data were entered directly into a research electronic data capture database (REDCap; https://www.project-redcap.org/)21 using password-protected, encrypted study tablets. To link data and clinical files, a separate password-protected file with patient and study identifiers was created and destroyed at the end of data validation. The study received approvals from Partners In Health/Inshuti Mu Buzima (PIH/IMB) Research Committee and the Rwandan National Health Research Committee. It received ethics review and approvals from the Rwanda National Ethics Committee (Kigali, Rwanda; no. 848/RNEC/2016) and Partners Human Research Committee (Boston, Massachusetts, USA; no. 2016P001943/MGH). The study was approved by the Rwandan Ministry of Health before the start of data collection.
This prospective cohort study included women who underwent LSCS between 22 March and 18 October 2017 at KDH. This hospital, located in the rural Eastern Province of Rwanda, is managed by the Rwandan Ministry of Health with technical and financial support from PIH/IMB, a US-based non-governmental organization. The KDH catchment area includes 16 health centres located in the district and two located in the Mahama Refugee Camp, and the hospital serves a population of 360 56522. The hospital has 233 beds and is staffed by 136 employees: 15 general practitioners (GPs), 77 nurses, 11 midwives, 18 paramedical staff, four administrators and 11 support staff. During a portion of the study period (March to July 2017), there was also a visiting PIH/IMB-sponsored obstetrician/gynaecologist working part-time at the hospital, performing complex obstetric and gynaecological procedures and providing professional development training to hospital GPs. An estimated 7·8 per cent of deliveries in Kirehe District were by LSCS19, with a mean of 136 LSCS done at KDH each month. About 80 per cent of Rwandans have medical insurance; 97 per cent are covered by the community-based health insurance, which pays for 90 per cent of total medical costs19.
In Kirehe District, and in other parts of rural Rwanda, a woman presents first to her nearest health centre for assessment and management. If a nurse or midwife there identifies an urgent problem, the woman is referred to the district hospital. For a limited number of women who are identified as having a high-risk pregnancy, they present directly to the district hospital to be attended by a GP. Once at the district hospital, a midwife monitors the woman's progress, and if needed, calls a GP who may recommend an LSCS for delivery.
Surgical technique
In accordance with hospital protocols, the woman's skin is prepared with aqueous-based 10 per cent chlorhexidine gluconate followed by 10 per cent povidone–iodine solution before incision. The 2016 WHO guidelines23 for the prevention of SSIs recommend administration of preoperative antibiotics within 120 min before skin incision and no postoperative antibiotics, except in the case of an infection. The previous 2015 WHO recommendations24 were to administer preoperative antibiotics 30–60 min before incision with no postoperative antibiotics, which were still the guidelines of the Rwandan gynaecology and obstetrics clinic. Although women undergoing LSCS at KDH receive preoperative antibiotic prophylaxis, typically a single dose of 1 g ceftriaxone within 1 h before incision, almost all receive postoperative antibiotics25. Braided absorbable sutures, usually Vicryl® (Ethicon, Somerville, New Jersey, USA), are used to suture subcutaneous tissue and for skin closure. A gauze soaked with povidone–iodine is used for dressing and is replaced on POD 3.
After LSCS, the woman is admitted to the postpartum ward for at least 3 days for monitoring and postoperative care. A GP attends daily to assess the woman's healing and decides when she is fit to be discharged. A GP then fills out a discharge form with a brief note on the patient's follow-up plan and, in some instances, prescribes medications (mostly pain medication and/or antibiotics). A midwife gives additional instructions about wound care, neonatal care, medications and follow-up. A follow-up date is then scheduled for the patient's wound dressing change at her nearest health centre.
Study population and data collection
All women 18 years and older who underwent LSCS at KDH between 22 March and 18 October 2017 were eligible for the study. Patients from Mahama Refugee Camp were excluded because guidelines regulating refugees' movements hinder their ability to be followed after discharge. After LSCS and during hospitalization, study team members consented and enrolled eligible women to participate in the study. At the time of enrolment, a trained study data collector interviewed patients, collecting basic information on demographics and clinical history. After discharge, data collectors extracted clinical details from patients' files.
Patients were screened on POD 10 (± 3 days) by a GP for the presence of an SSI. This window was selected because the majority of SSIs develop between POD 5 and 1026. Furthermore, timely identification of SSI is crucial for minimizing morbidity and mortality. Two study clinics were held each week. Patients were assigned to the first study clinic that fell within the POD-7–13 screening window. Patients still in hospital on the scheduled clinic date were assessed for SSI at the bedside. A woman discharged before her scheduled clinic date was given a transport voucher to be redeemed when she returned for screening. She was called a day before her clinic day as a reminder. If she missed her scheduled clinic day, a study team member attempted to call her and reschedule an appointment for the next study clinic, also within the POD-7–13 screening window. Women who missed two appointments were considered lost to follow-up, and excluded from the analysis.
The GP first administered a ten-question screening protocol assessing: increased pain since discharge, fever since discharge, erythema, oedema, induration, dehiscence, drainage from the wound, drainage with discolouration, drainage with a foul odour and drainage of pus (purulent discharge). The GP then conducted a physical examination. The diagnosis of SSI was based on the physical examination.
Evaluation of risk factors
Based on previous literature and the researchers' knowledge of the Rwandan healthcare system, demographic and clinical variables were identified that could be potential predictors of SSI. One set of variables of interest, both for predelivery and postdischarge risks, were related to the costs and time required for travel: travel time from home to health centre, travel time from health centre to hospital, cost of transport, and total time spent getting to the hospital. For women with missing travel data, the researchers imputed values based on data from participants from the same village. If there were no other participants from the same village, the imputed value was the mean of values from participants from the same cell, that is the next administrative level, typically a cluster of between four and 19 villages. Cost of transport and monthly income were analysed based on a value of up to or greater than €1·1 per day (US $1·3), which is the poverty line cut-off in purchasing power parity per day27. The time taken and cost of transport were self-reported by the patient. The cost of transport was calculated using an exchange rate of 1 euro to 887·7 Rwandan francs, the mean exchange rate during the study interval according to the Rwandan national central bank.
Statistical analysis
Fisher's exact test (categorical variables) or Wilcoxon rank-sum test (continuous variables) was used to assess the relationship between co-variables and the presence of an SSI. Variables that were significant at α = 0·2 in the bivariable analyses were considered for the multivariable logistic regression model. For variables with more than 10 per cent missing data, an explicit missing category was created for the multivariable modelling. A reduced multivariable logistic regression model was built using backward stepwise selection, stopping when all remaining co-variables were significant at the α = 0·05 level. Odds ratios, 95 per cent confidence intervals and P values were reported for the multivariable analysis. All analyses were completed in Stata® version 13 (StataCorp, College Station, Texas, USA).
Results
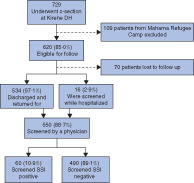
Of the 729 women who had an LSCS at KDH during the study, 620 (85·0 per cent) were eligible for follow-up. Of these, 550 (88·7 per cent) were screened by a GP for SSI, 16 (2·9 per cent) were still in hospital on POD 10 and 534 (97·1 per cent) returned for follow-up. The majority (316, 57·5 per cent) were between 22 and 30 years old, were married (237, 43·1 per cent), had primary education (382, 69·5 per cent) and were farmers (478, 86·9) (Table 1). Most women had community-based health insurance (523, 95·1 per cent) and a monthly household income of less than €33·8 (508, 92·4 per cent). Of the 390 women (70·9 per cent) with bodyweight documented, 359 (92·1 per cent) weighed between 50 and 75 kg.
Table 1.
Demographic characteristics of study participants
| No. of women* (n = 550) | |
|---|---|
| Age (years) | |
| 18–21 | 102 (18·6) |
| 22–30 | 316 (57·5) |
| ≥ 31 | 132 (24·0) |
| Marital status | |
| Single | 195 (35·5) |
| Married | 237 (43·1) |
| Living with a partner | 111 (20·2) |
| Separated (divorced or widowed) | 7 (1·3) |
| Education level | |
| No education | 41 (7·5) |
| Primary education | 382 (69·5) |
| Secondary education or higher | 127 (23·1) |
| Occupation | |
| Farmer | 478 (86·9) |
| Employed, trader | 40 (7·3) |
| Housewife | 32 (5·8) |
| Type of insurance | |
| No insurance | 6 (1·1) |
| Community-based health insurance | 523 (95·1) |
| Private insurance | 21 (3·8) |
| Monthly household income (€) | |
| < 33·8 | 508 (92·4) |
| ≥ 33·8 | 42 (7·6) |
| Weight (kg) (n = 390) | |
| < 50 | 18 (4·6) |
| 50–75 | 359 (92·1) |
| > 75 | 13 (3·3) |
| Mode of transport from home to health centre (n = 367)‡ | |
| Walked | 85 (23·2) |
| Public transport | 282 (76·8) |
| Private transportation | 8 (2·2) |
| Ambulance | 4 (1·1) |
| Rainfall status > 1 within 1 week after discharge (n = 534)§ | 36 (6·7) |
| Travel time (min) (n = 517)† | |
| Home to health centre | 30 (20–50) |
| Health centre to hospital | 45 (20–60) |
| Total time from home to hospital (h) (n = 355)† | 6·3 (2·5, 18·0) |
| Cost of transport (€) (n = 517 patients)† | |
| Home to health centre | 1·3 (0·6, 2·3) |
| Health centre to hospital | 1·9 (0·6, 2·7) |
| Total from home to hospital | 3·4 (1·9, 4·6) |
With percentages in parentheses unless indicated otherwise;
values are median (i.q.r.).
Patients could use more than one form of transport.
Patients for whom interval between discharge from hospital and surgical-site infection screening clinic day was rainy period.
Most women (282, 76·8 per cent) used public transport to reach a health centre and an ambulance (256, 69·8 per cent) to move from there to the hospital. The median time taken to travel from home to the health centre was 30 (i.q.r. 20–50) min and that from the health centre to the hospital was 45 (20–60) min. The median total time from home to hospital, including time receiving care at the health centre, waiting for transport to the hospital and waiting to be admitted to the hospital, was 6·3 (2·5–18·0) h. The median total amount spent to reach KDH was €3·4 (i.q.r. 1·9–4·6), 10 per cent of the mean monthly household income. This included a median of €1·3 (0·6–2·3) spent to reach the health centre and €1·9 (0·6–2·7) to travel from there to the hospital.
Co-morbidities, including cardiovascular disease, diabetes mellitus and human immunodeficiency virus/acquired immune deficiency syndrome, were rare (13, 2·4 per cent) (Table S1, supporting information). Nearly half of the LSCSs (270, 49·1 per cent) had an urgent indication and 260 (47·3 per cent) were emergencies, with fetal distress accounting for 31·3 per cent. Two antiseptic solutions were used for skin preparation in 97·6 per cent of procedures (536 of 549). The majority of LSCSs (388 of 539, 72·0 per cent) took 45 min or less. Two-thirds of patients (367, 66·7 per cent) received preoperative antibiotics and 532 (96·7 per cent) received at least one dose of postoperative antibiotic.
The prevalence of SSI on POD 10 was 10·9 per cent (60 women) (Table 2; Table S2, supporting information); of these, only two (3 per cent) were identified before discharge from hospital, and 75 per cent were superficial SSIs. In the bivariable analysis, the following factors were associated with SSI, significant at the α = 0·2 level: women who were living with a partner or separated/widowed (P = 0·177), occupation housewife rather than farmer (P = 0·127), monthly income less than €33·8 per month (P = 0·184), weighing more than 75 kg (P = 0·011), long travel time to reach the nearest health centre (P = 0·156), transport cost to health centre greater than €1·1 (P = 0·039), cord and membrane complication (P = 0·188), skin preparation with a single solution (P = 0·161), and duration of surgery greater than 45 min (P = 0·157). Neither preoperative nor postoperative antibiotic therapies were associated with the development of SSI.
Table 2.
Comparison of characteristics in women with versus those without surgical-site infection
| No surgical-site infection | Surgical-site infection | P † | |
|---|---|---|---|
| Overall | 490 (89·1) | 60 (10·9) | – |
| Age (years) | 0·590 | ||
| 18–21 | 88 (86·3) | 14 (13·7) | |
| 22–30 | 283 (89·6) | 33 (10·4) | |
| ≥ 31 | 119 (90·2) | 13 (9·8) | |
| Marital status | 0·177 | ||
| Single | 178 (91·3) | 17 (8·7) | |
| Married | 213 (89·9) | 24 (10·1) | |
| Living with a partner | 93 (83·8) | 18 (16·2) | |
| Separated (divorced or widowed) | 6 (86) | 1 (14) | |
| Education level | 0·973 | ||
| No education | 37 (90) | 4 (10) | |
| Primary education | 339 (88·7) | 43 (11·3) | |
| Secondary education or higher | 114 (89·8) | 13 (10·2) | |
| Occupation | 0·127 | ||
| Farmer | 429 (89·7) | 49 (10·3) | |
| Employed, trader | 36 (90) | 4 (10) | |
| Housewife | 25 (78) | 7 (22) | |
| Type of insurance | 0·533 | ||
| No insurance | 5 (83) | 1 (17) | |
| Community-based health insurance | 465 (88·9) | 58 (11·1) | |
| Private insurance | 20 (95) | 1 (5) | |
| Monthly household income (€) | 0·184 | ||
| < 33·8 | 450 (88·6) | 58 (11·4) | |
| ≥ 33·8 | 40 (95) | 2 (5) | |
| Weight (kg) | 0·011 | ||
| < 50 | 17 (94) | 1 (6) | |
| 50–75 | 326 (90·8) | 33 (9·2) | |
| > 75 kg | 8 (62) | 5 (38) | |
| Travel time (min) (n = 517)* | n = 460 | n = 57 | |
| Home to health centre | 30 (20–50) | 30 (25–60) | 0·156‡ |
| Health centre to hospital | 45 (20–97) | 50 (30–60) | 0·646‡ |
| Total time from home to hospital (h) (n = 355)* | 6·8 (2·5–18·1) (n = 325) | 5·1 (1·7–37·6) (n = 30) | 0·233‡ |
| Cost of transport (€) (n = 517)* | n = 460 | n = 57 | |
| Home to health centre | 1·1 (0·6–2·2) | 1·7 (0·8–2·3) | 0·039‡ |
| Health centre to hospital | 1·9 (0·8–2·7) | 2·1 (0·3–2·6) | 0·708‡ |
| Total from home to hospital | 3·4 (1·8–4·5) | 3·6 (2·0–5·0) | 0·310‡ |
| Preoperative antibiotic | 326 (88·8) | 41 (11·2) | 0·780 |
| Duration of postoperative antibiotic therapy (days) | 0·489 | ||
| No antibiotic | 17 (94) | 1 (6) | |
| 1–3 | 370 (88·1) | 50 (11·9) | |
| > 3 | 103 (92·0) | 9 (8·0) | |
| Duration of hospital stay (days) | 0·583 | ||
| ≤ 3 | 279 (89·7) | 32 (10·3) | |
| > 3 | 209 (88·2) | 28 (11·8) |
Values in parentheses are percentages unless indicated otherwise;
values are median (i.q.r.).
Fisher's exact test except
Wilcoxon rank sum test.
In the multivariable regression analysis, weighing more than 75 kg, spending more than €1·1 travelling to the health centre, occupation housewife rather than farmer, and use of a single antiseptic were independently associated with SSI (Table 3).
Table 3.
Results of multivariable logistic regression analysis to identify predictors of surgical-site infection after caesarean section
| Odds ratio | P | |
|---|---|---|
| Weight (kg) | ||
| 50–75 | 1·00 (reference) | |
| < 50 | 0·60 (0·08, 4·72) | 0·625 |
| > 75 | 5·98 (1·56, 22·96) | 0·009 |
| Missing | 1·28 (0·69, 2·35) | 0·436 |
| Cost of transport from home to health centre (€) | ||
| ≤ 1·1 | 1·00 (reference) | |
| > 1·1 | 2·42 (1·31, 4·49) | 0·005 |
| Occupation | ||
| Farmer | 1·00 (reference) | |
| Employed, trader | 0·91 (0·27, 3·02) | 0·875 |
| Housewife | 2·93 (1·08, 7·97) | 0·035 |
| Skin preparation | ||
| With two solutions | 1·00 (reference) | |
| With one solution | 4·42 (1·05, 18·57) | 0·043 |
Values in parentheses are 95 per cent confidence intervals. The logistic multivariable regression model was built using backward stepwise selection, stopping when all remaining co-variables were significant at the α = 0·05 level.
Discussion
This study estimated the prevalence and risk factors for SSI among women who had LSCS in a rural district hospital in the region. This study population's characteristics of being poor, with low levels of education, and long travel distances to reach a health centre or hospital reflect the characteristics of Kirehe District and much of rural East Africa. The SSI rate was 10·9 per cent, which is consistent with reports from other countries in sub-Saharan Africa, although notably these earlier estimates were based largely on studies in tertiary and/or urban facilities10–13,16–17,28. The prevalence was higher than the average of 7·1 per cent in developed countries16,29–30.
Some individual risks for SSIs were identified. First, consistent with the literature15,31, women who weighed more were more likely to develop an SSI. Second, it was found that women whose skin was prepared with two antiseptic solutions (10 per cent chlorhexidine followed by 10 per cent povidone–iodine in accordance with hospital protocol) were less likely to develop an SSI. The rare instances (2·4 per cent) where a single antiseptic solution was used were likely due to lack of stock or variation in GP practice.
Two interesting findings of this study were the increased risk of SSI in women who spent more money to access the health centre, and for housewives compared with farmers. Postoperative follow-up, including wound dressing changes, occurs mostly at health centres. This care is a burden for the majority of this study population as the median time to travel to the health centre is 30 min and the median cost of transport to the HC is 4–10 per cent of monthly income. Women with high transport costs and housewives may have the least disposable income, and may not be able to return to the health centre for regular dressing changes. Other studies19,32–33 support this hypothesis and have shown that transportation costs are common barriers to surgical care in LMICs; vouchers covering transport costs increased access to maternal health services34. It is possible that financial barriers may also be associated with loss to follow-up, and so there may be a higher SSI rate among women who did not attend follow-up in this study.
This study did not show any association between SSI development and administration of preoperative or postoperative antibiotic therapy. The recent WHO guidelines (2016)35 recommend administration of antibiotic prophylaxis at least 120 min before surgical incision and no administration of postoperative antibiotics. In the present study, 66·7 per cent of women received preoperative prophylaxis, and nearly all had an extended course of postoperative antibiotics. Overuse of antibiotics risks antimicrobial resistance in this population, particularly of Gram-negative strains36. A systematic review37 showed high levels of antimicrobial resistance across a variety of populations in sub-Saharan Africa.
Previous studies16,29–30,38–39 have discussed other factors that could be linked to an increased risk of SSI development, such as poor operating room infrastructure, poor adherence to operating room guidelines including hand-washing techniques, inadequate hygiene and sanitation at the hospital and at home, and inadequate quality and quantity of staffing. The present study did not have data to compare from this population. In addition, the impact of water quality, sanitation and hygiene conditions both in the patient's home and at health facilities could affect infection control. Another limitation of this study was missing data, particularly those extracted from clinical charts. Because height was often not recorded, the researchers were unable to calculate BMI. Instead, they used weight categories as a proxy estimation of overweight versus normal weight. The study also missed patients who returned to health facilities before their scheduled follow-up. Finally, KDH receives significant support from PIH/IMB, which may limit the generalizability of these findings. This included the presence of an obstetrician/gynaecologist for 5 months of the study; however, secondary analysis found no significant difference in SSIs or other complications during and after the time the surgeon was present in the hospital, indicating minimal confounding.
Supplementary Material
Table S1 Clinical characteristics of study participants (N = 550)
Table S2 Comparison of characteristics for women with SSI versus those with no SSSI (n = 550)
Acknowledgements
The researchers acknowledge the support of the KDH leadership and staff during data collection and thank the study participants for their voluntary participation. They also acknowledge N. Rudolfson for his support with variable coding. This study was supported financially by the US National Institutes of Health grant number R21EB022368.
Disclosure: The authors declare no conflict of interest.
References
- 1. Abbas M, Pittet D. Surgical site infection prevention: a global priority. J Hosp Infect 2016; 93: 319–322. [DOI] [PubMed] [Google Scholar]
- 2. Saeed KBM, Greene RA, Corcoran P, O'Neill SM. Incidence of surgical site infection following caesarean section: a systematic review and meta-analysis protocol. BMJ Open 2017; 7: e013037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bahufite A, Ndagijimana A, Adomako E, Zerihun A, Simba CA, Ntakirutimana Cet al. Implementing wound dressing protocol to reduce post cesarean section surgical site infections in Mibilizi District Hospital, Rwanda. On the Horizon 2016; 24: 369–376. [Google Scholar]
- 4. WHO . WHO Recommends 29 Ways to Stop Surgical Infections and Avoid Superbugs; 2016. http://www.who.int/mediacentre/news/releases/2016/recommendations-surgical-infections/en/ [accessed 10 August 2017]. [Google Scholar]
- 5. Alfonso JL, Pereperez SB, Canoves JM, Martinez MM, Martinez IM, Martin-Moreno JM. Are we really seeing the total costs of surgical site infections? A Spanish study. Wound Repair Regen 2007; 15: 474–481. [DOI] [PubMed] [Google Scholar]
- 6. Harrison MS, Goldenberg RL. Cesarean section in sub-Saharan Africa. Matern Health Neonatol Perinatol 2016; 2: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Betrán AP, Ye J, Moller AB, Zhang J, Gülmezoglu AM, Torloni MR. The increasing trend in caesarean section rates: global, regional and national estimates: 1990–2014. PLoS One 2016; 11: e0148343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nyamtema AS, Mwakatundu N, Dominico S, Mohamed H, Pemba S, Rumanyika Ret al. Enhancing maternal and perinatal health in under-served remote areas in Sub-Saharan Africa: a Tanzanian model. PLoS One 2016; 11: e0151419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ye J, Zhang J, Mikolajczyk R, Torloni MR, Gülmezoglu AM, Betran AP. Association between rates of caesarean section and maternal and neonatal mortality in the 21st century: a worldwide population-based ecological study with longitudinal data. BJOG 2016; 123: 745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mivumbi VN, Little SE, Rulisa S, Greenberg JA. Prophylactic ampicillin versus cefazolin for the prevention of post-cesarean infectious morbidity in Rwanda. Int J Gynaecol Obstet 2014; 124: 244–247. [DOI] [PubMed] [Google Scholar]
- 11. De Nardo P, Gentilotti E, Nguhuni B, Vairo F, Chaula Z, Nicastri Eet al. Post-caesarean section surgical site infections at a Tanzanian tertiary hospital: a prospective observational study. J Hosp Infect 2016; 93: 355–359. [DOI] [PubMed] [Google Scholar]
- 12. Mpogoro FJ, Mshana SE, Mirambo MM, Kidenya BR, Gumodoka B, Imirzalioglu C. Incidence and predictors of surgical site infections following caesarean sections at Bugando Medical Centre, Mwanza, Tanzania. Antimicrob Resist Infect Control 2014; 3: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chu K, Maine R, Trelles M. Cesarean section surgical site infections in sub-Saharan Africa: a multi-country study from Medecins sans Frontieres. World J Surg 2015; 39: 350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaboré B, Soudouem G, Seck I, Millogo T, Evariste Yaméogo WM, Kouanda S. A case–control study of risk factors for surgical site infection after cesarean delivery in eastern Burkina Faso. Int J Gynaecol Obstet 2016; 135(Suppl 1): S107–S110. [DOI] [PubMed] [Google Scholar]
- 15. Johnson A, Young D, Reilly J. Caesarean section surgical site infection surveillance. J Hosp Infect 2006; 64: 30–35. [DOI] [PubMed] [Google Scholar]
- 16. Ngaroua NJE, Bénet T, Djibrilla Y. Incidence of surgical site infections in sub-Saharan Africa: systematic review and meta-analysis. Pan Afr Med J 2016; 24: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bizimana JK, Ndoli J, Bayingana C, Baluhe I, Gilson GJ, Habimana E. Prevalence and risk factors for post cesarean delivery surgical site infection in a teaching hospital setting in rural Rwanda: a prospective cross sectional study. Int J Curr Microbiol App Sci 2016; 5: 631–641. [Google Scholar]
- 18. Corker J. Urbanization and Demographic Change in Sub-Saharan Africa: Three Essays on Fertility and Child Mortality Differentials in a Rapidly Urbanizing Context (Doctoral Thesis). University of Pennsylvania: Philadelphia, 2014. [Google Scholar]
- 19. National Institute of Statistics of Rwanda (NISR), Ministry of Health Rwanda (MOH) and ICF International . Rwanda Demographic and Health Survey 2014–15. NISR, MOH and ICF International: Rockville, 2015. [Google Scholar]
- 20. Petroze RT, Nzayisenga A, Rusanganwa V, Ntakiyiruta G, Calland JF. Comprehensive national analysis of emergency and essential surgical capacity in Rwanda. Br J Surg 2012; 99: 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sonderman KA, Nkurunziza T, Kateera F, Gruendl M, Koch R, Gaju Eet al. Using mobile health technology and community health workers to identify and refer caesarean-related surgical site infections in rural Rwanda: a randomised controlled trial protocol. BMJ Open 2018; 8: e022214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. WHO . Global Guidelines on the Prevention of Surgical Site Infection; 2016. http://www.who.int/gpsc/ssi-guidelines/en/ [accessed 14 September 2018]. [Google Scholar]
- 24. WHO . WHO Recommendations for Prevention and Treatment of Maternal Peripartum Infections; 2015. http://apps.who.int/iris/bitstream/handle/10665/186684/?sequence=1 [accessesed 14 September 2018]. [PubMed] [Google Scholar]
- 25. Ministry of Health . Gynecology and Obstetric: Clinical Protocols & Treatment Guidelines. Ministry of Health Rwanda: Kigali, Rwanda, 2012. [Google Scholar]
- 26. National Collaborating Centre for Women's and Children's Health (UK) . Surgical Site Infection: Prevention and Treatment of Surgical Site Infection. NICE Clinical Guidelines, No. 74; 2008. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0010039/ [accessed 14 September 2018] [Google Scholar]
- 27. United Nations Development Program . Population Living Below $1.25 PPP per day (%). http://hdr.undp.org/en/content/population-living-below-125-ppp-day [accessed 29 September 2018].
- 28. Amenu D, Belachew T, Araya F. Surgical site infection rate and risk factors among obstetric cases of Jimma University Specialized Hospital, Southwest Ethiopia. Ethiop J Health Sci 2011; 21: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Allegranzi B The Burden of Surgical Site Infections Worldwide; 2014. http://theific.org/wp-content/uploads/2014/10/025.pdf [accessed 13 June 2018]. [Google Scholar]
- 30. van Paridon BV. Incidence of Surgical Site Infections Highest in Lower-Income Countries; 2018. https://www.infectiousdiseaseadvisor.com/nosocomial-infections/surgical-site-infections-highest-in-lower-income-countries/article/751241/ [accessed 15 June 2018]. [Google Scholar]
- 31. Corcoran S, Jackson V, Coulter-Smith S, Loughrey J, McKenna P, Cafferkey M. Surgical site infection after cesarean section: implementing 3 changes to improve the quality of patient care. Am J Infect Control 2013; 41: 1258–1263. [DOI] [PubMed] [Google Scholar]
- 32. Shrime MG, Dare A, Alkire BC, Meara JG. A global country-level comparison of the financial burden of surgery. Br J Surg 2016; 103: 1453–1461. [DOI] [PubMed] [Google Scholar]
- 33. United States Agency for International Development . Ending Preventable Maternal Mortality: USAID Maternal Health Vision for Action; 2014. https://www.usaid.gov/sites/default/files/documents/1864/MCHVision.pdf [accessed 18 June 2018]. [Google Scholar]
- 34. Ensor T, Cooper S. Overcoming barriers to health service access: influencing the demand side. Health Policy Plan 2004; 19: 69–79. [DOI] [PubMed] [Google Scholar]
- 35. Allegranzi B, Bischoff P, de Jonge S, Kubilay NZ, Zayed B, Gomes SMet al. New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis 2016; 16: e276–e287. [DOI] [PubMed] [Google Scholar]
- 36. Bebell LM, Ngonzi J, Bazira J, Fajardo Y, Boatin AA, Siedner MJet al. Antimicrobial-resistant infections among postpartum women at a Ugandan referral hospital. PLoS One 2017; 12: e0175456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Workneh M, Katz MJ, Lamorde M, Cosgrove SE, Manabe YC. Antimicrobial resistance of sterile site infections in Sub-Saharan Africa: a systematic review. Open Forum Infect Dis 2017; 4: ofx209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Allegranzi B, Aiken AM, Zeynep Kubilay N, Nthumba P, Barasa J, Okumu Get al. A multimodal infection control and patient safety intervention to reduce surgical site infections in Africa: a multicentre, before–after, cohort study. Lancet Infect Dis 2018; 18: 507–515. [DOI] [PubMed] [Google Scholar]
- 39. Henry JA, Bem C, Grimes C, Borgstein E, Mkandawire N, Thomas WEet al. Essential surgery: the way forward. World J Surg 2015; 39: 822–832. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Clinical characteristics of study participants (N = 550)
Table S2 Comparison of characteristics for women with SSI versus those with no SSSI (n = 550)


