Abstract
Background
Patients undergoing surgery for soft tissue sarcoma have high morbidity rates, particularly after preoperative radiation therapy (RT). An enhanced recovery after surgery (ERAS) programme may improve perioperative outcomes in abdominal surgery. This study reported outcomes of an ERAS programme tailored to patients with soft tissue sarcoma.
Methods
A prospective ERAS protocol was implemented in 2015 at a high-volume sarcoma centre. Patients treated within the ERAS programme from 2015 to 2018 were case-matched retrospectively with patients treated between 2012 and 2018 without use of the protocol, matched by surgical site, surgeon, sarcoma histology and preoperative RT treatment. Postoperative outcomes, specifically wound complications and duration of hospital stay, were reported.
Results
In total, 234 patients treated within the ERAS programme were matched with 237 who were not. The ERAS group had lower wound dehiscence rates overall (2 of 234 (0·9 per cent) versus 31 of 237 (13·1 per cent); P < 0·001), after preoperative RT (0 of 41 versus 11 of 51; P = 0·004) and after extremity sarcoma surgery (0 of 54 versus 6 of 56; P = 0·040) compared with the non-ERAS group. Rates of postoperative ileus or obstruction were lower in the ERAS group (21 of 234 (9·9 per cent) versus 40 of 237 (16·9 per cent); P = 0·016) and in those with retroperitoneal sarcoma (4 of 36 versus 15 of 36; P = 0·007). Duration of hospital stay was shorter in the ERAS group (median 5 (range 0–36) versus 6 (0–67) days; P = 0·003).
Conclusion
Treatment within an ERAS protocol for patients with soft tissue sarcoma was associated with lower morbidity and shorter hospital stay.
Graphical Abstract
An enhanced recovery after surgery (ERAS) protocol may diminish the adverse effects of postoperative wound complications associated with preoperative radiotherapy. Further studies validating these findings may lead to a consensus on treatment algorithms for patients with trunk and extremity soft tissue sarcoma. The results support the use of ERAS to minimize acute postoperative complications and reduce duration of hospital stay.
Graphical Abstract.
Organized care better
Resumen
Antecedentes
Los pacientes sometidos a cirugía por sarcoma de tejido blando (soft tissue sarcoma, STS) tienen altas tasas de morbilidad, particularmente después de la radioterapia preoperatoria (RT). El programa de recuperación intensificada después de la cirugía (enhanced recovery after surgery, ERAS) puede mejorar los resultados perioperatorios en la cirugía abdominal. Este estudio analizó los resultados de un programa ERAS diseñado para pacientes con STS.
Métodos
Se implementó un protocolo prospectivo ERAS en el año 2015 en un centro de alto volumen de sarcomas. Los pacientes en ERAS desde 2015 hasta 2018 fueron emparejados retrospectivamente con pacientes sin ERAS desde 2012 hasta 2018, según la localización quirúrgica, el cirujano, la histología del sarcoma y el tratamiento con RT preoperatoria. Se analizaron los resultados postoperatorios, específicamente las complicaciones de la herida y la duración de la estancia hospitalaria (length of stay, LOS).
Resultados
En total, 234 pacientes tratados con ERAS se compararon con 237 pacientes no tratados con ERAS. Los pacientes con ERAS tuvieron tasas globales más bajas de dehiscencia de la herida (2 (0,9%) versus 31 (13,1%), P < 0,001)), después de la RT preoperatoria (0 versus 11 (21,6%), P = 0,004)), y después de la cirugía de STS de extremidades (0 versus 6 (0,7%), P = 0,04)) en comparación con los pacientes no ERAS. Las tasas de íleo u obstrucción postoperatorias fueron más bajas en el grupo ERAS (21 (9,9%) versus 40 (16,9%), P = 0,02)) y en aquellos pacientes con sarcoma retroperitoneal (4 (11,1%) versus 15 (41,7%), P = 0,007)). La mediana (rango) de la LOS fue más corta en los pacientes con ERAS que fue de 5 (0-36) días que en los pacientes sin ERAS que fue de 6 (0-67) días (P = 0,003).
Conclusión
ERAS para pacientes con STS se asoció con una menor morbilidad y una estancia hospitalaria más corta.
Introduction
Enhanced recovery after surgery (ERAS) protocols are multimodal perioperative care pathways that were designed in Europe in 2001 to improve postoperative recovery and outcomes1. Since their inception, ERAS protocols for 15 procedures have been published or are currently under production by the ERAS® Society, as of 20171. The principal goal of these protocols is to minimize patients' stress response by achieving the following elements: optimizing preoperative nutritional support, carbohydrate loading to minimize insulin resistance, emphasizing non-opioid analgesia and anti-inflammatory drugs, and initiating early postoperative enteral nutrition1–3. Another important component is the maintenance of euvolaemia by optimizing intraoperative and postoperative fluid administration1. ERAS protocols typically include over 20 individual elements that require participation and cooperation among several disciplines including surgery, anaesthesia and nursing1–6.
Multiple studies have demonstrated the safety and efficacy of ERAS protocols across various surgical disciplines7,8. A meta-analysis8 undertaken by the Department of Veterans Affairs showed overall reductions in duration of hospital stay and perioperative morbidity. Specifically, in colorectal surgery, adoption of ERAS protocols reduced mean hospital stay to 2·7 days, and a highly selected cohort of patients was discharged within 24 h9. Similar results have been reported for hepatobiliary, gastric, oesophageal, thoracic, urological, gynaecological, orthopaedic and emergency surgery1.
Studies of the implementation of ERAS protocols for patients with soft tissue sarcoma (STS) are limited, with published reports focused on extremity/trunk STS10. The aim of this study was to evaluate an ERAS protocol specifically tailored to patients undergoing surgery for STS. It was hypothesized that there could be an impact on wound complications and the timing of radiation therapy (RT), particularly for extremity STS.
Methods
An ERAS protocol initially designed for colorectal surgery was modified for patients with STS and implemented in the Division of Surgical Oncology at Brigham and Women's Hospital (BWH) in February 2015 (Table 1). All patients who had surgery for STS by one of three surgical oncologists from July 2015 to March 2018, with intention to treat according to this ERAS protocol, were included in the study. Patients on the STS ERAS programme before July 2015 were excluded to mitigate any non-compliance in the first 5 months of protocol implementation. There was variability in the rate of protocol adoption among the three surgeons. Patients undergoing surgery for indications other than STS, and those not placed on the ERAS protocol before operation were excluded. The ERAS cohort was case-matched retrospectively with patients who were not managed according the ERAS protocol, by several metrics in the following order: site of surgery, surgeon, sarcoma histology, and treatment with preoperative RT. Patients in the non-ERAS group underwent surgery between January 2012 and March 2018.
| Preoperative | Intraoperative | Postoperative |
|---|---|---|
| Patient/family education | Opioid-sparing, multimodal analgesia | Early nutrition |
| Medical optimization | Euvolaemia* | Early mobilization (out of bed) |
| Limiting fasting, made nil by mouth 6 h before operation | Normothermia | Minimize intravenous fluids, heplock fluids at 12 h |
| Carbohydrate-rich beverage (ClearFast®) given up to 2 h before operation | Normoglycaemia | Management of nausea and vomiting |
| Regional block versus epidural placement | Minimize tubes and drains | Minimize opioids |
As measured by adherence to institutional protocol for perioperative fluid administration, including but not restricted to limiting fluids in the preoperative setting, zero fluid balance or intraoperative oesophageal Doppler monitoring, and decreasing postoperative intravenous fluid administration to 75 ml/h for 6 h. ClearFast® (ClearFast, Atlanta, Georgia, USA).
The BWH Institutional Review Board (IRB) deemed evaluation of the STS ERAS protocol to be a quality improvement study and thus IRB-exempt. The present analysis of outcomes after institution of the sarcoma ERAS protocol was approved by the BWH IRB (protocol number: 2018P000183).
Data were collected on demographic (age, sex, BMI, smoking status and co-morbidities) and disease-specific (anatomical site, histology, history of chemotherapy and RT, use of neoadjuvant chemotherapy and RT, and whether surgery was for primary or recurrent disease) variables. Outcomes measured included: return to the emergency department, readmission, bleeding, surgical-site infection, wound dehiscence, ileus and/or obstruction, intra-abdominal fluid collection, initiation of total parenteral nutrition, discharge to a facility (skilled nursing facility or inpatient rehabilitation centre), and duration of hospital stay. Only postoperative outcomes occurring within 30 days after the initial operation were included. Bleeding was defined by the requirement for blood transfusion. Wound dehiscence was recorded in the following situations: reopening of the wound requiring daily dressing changes, vacuum-assisted wound closure, wound care consultation or a visiting nurse for home-based wound care, and reoperation for wound complications. Wound dehiscence was considered as a distinct category of wound complication separate from surgical-site infection, which was defined as culture-positive fluid collections or any wound complications treated with antibiotics. Bowel obstruction was confirmed by imaging that demonstrated a transition point and distinguished it from ileus. Ileus was defined by any documentation of extended time for return of bowel function requiring bowel rest and/or placement of a nasogastric tube for decompression.
Statistical analysis
Comparative univariable analyses were completed with Pearson's χ2 test for categorical variables and two-sample paired Student's t test for continuous variables. Numerical variables were compared using the non-parametric Wilcoxon rank-sum test or Student's t test. A multivariable logistic regression model was created to study the association between ERAS participation and several outcome metrics while controlling for potential confounders. Variables were chosen based on contextual plausibility. Subgroup analyses comparing postoperative outcomes such as wound complications and duration of hospital stay were undertaken for patients undergoing surgery for retroperitoneal and extremity sarcoma and those who had preoperative RT. Statistical significance was set at P < 0·050. Data were analysed using R software (R Development Core Team, Vienna, Austria).
Results
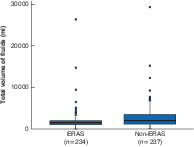
Between July 2015 and March 2018, 234 STS resections were performed within an ERAS protocol by three surgical oncologists. These were matched with 237 non-ERAS STS procedures undertaken by the same three surgical oncologists from January 2012 to March 2018. There were no significant differences between the ERAS and non-ERAS cohorts with respect to demographic variables, co-morbidities, oncological treatment details, tumour site or histology (Tables 2 and 3). There was a significant difference in the amount of intravenous fluid administered during surgery, as expected considering the institution's ERAS protocol; patients in the ERAS group received a median of 1500 ml compared with 2000 ml for those in the non-ERAS group (P < 0·001) (Fig. 1).
| ERAS (n = 234) | Non-ERAS (n = 237) | P * | |
|---|---|---|---|
| Mean age (years) | 58·6 | 58·5 | 0·614† |
| Sex ratio (M : F) | 102 : 132 | 101 : 136 | 0·904 |
| Mean BMI (kg/m2) | 27·8 | 28·6 | 0·437† |
| Co-morbidities | |||
| Diabetes mellitus | 24 (10·3) | 29 (12·2) | 0·582 |
| Coronary artery disease | 9 (3·8) | 19 (8·0) | 0·086 |
| COPD | 5 (2·1) | 4 (1·7) | 0·985 |
| Chronic kidney disease | 15 (6·4) | 12 (5·1) | 0·667 |
| Transient ischaemic attack/stroke | 3 (1·3) | 6 (2·5) | 0·517 |
| Current smoker | 14 (6·0) | 18 (7·6) | 0·517 |
| History of radiation | 37 (15·8) | 40 (16·9) | 0·835 |
| Preoperative radiation | 41 (17·5) | 51 (21·5) | 0·328 |
| History of chemotherapy | 51 (21·8) | 60 (25·3) | 0·429 |
| Preoperative chemotherapy | 69 (29·5) | 66 (27·8) | 0·771 |
| Surgery for initial presentation | 176 (75·2) | 160 (67·5) | 0·068 |
| Surgery for recurrence | 79 (33·8) | 85 (35·9) | 0·702 |
Values in parentheses are percentages unless indicated otherwise. ERAS, enhanced recovery after surgery; COPD, chronic obstructive pulmonary disease.
Pearson χ2 test, except
Student's t test.
| ERAS (n = 234) | Non-ERAS (n = 237) | P * | |
|---|---|---|---|
| Tumour anatomical site | 0·999 | ||
| Stomach | 23 (9·8) | 23 (9·7) | |
| Abdomen (metastatic GIST) | 25 (10·7) | 20 (8·4) | |
| Abdomen/pelvis | 47 (20·1) | 47 (19·8) | |
| Retroperitoneum | 36 (15·4) | 36 (15·2) | |
| Lower extremity (above knee) | 38 (16·2) | 40 (16·9) | |
| Lower extremity (below knee) | 11 (4·7) | 11 (4·6) | |
| Upper extremity | 5 (2·1) | 5 (2·1) | |
| Trunk/flank | 25 (10·7) | 25 (10·5) | |
| Breast | 18 (7·7) | 23 (9·7) | |
| Head and neck | 6 (2·6) | 7 (3·0) | |
| Tumour histology | 0·938 | ||
| GIST | 48 (20·5) | 42 (17·7) | |
| Liposarcoma/atypical lipomatous tumour | 63 (26·9) | 61 (25·7) | |
| Leiomyosarcoma | 33 (14·1) | 39 (16·5) | |
| Angiosarcoma | 16 (6·8) | 19 (8·0) | |
| Dermatofibrosarcoma protuberans | 12 (5·1) | 17 (7·2) | |
| Malignant peripheral nerve sheath tumour | 11 (4·7) | 8 (3·4) | |
| Other | 51 (21·8) | 51 (21·5) |
Values in parentheses are percentages. ERAS, enhanced recovery after surgery; GIST, gastrointestinal stromal tumour.
Pearson χ2 test.
Fig. 1.
Intraoperative fluid volume stratified by enhanced recovery after surgery protocol implementation
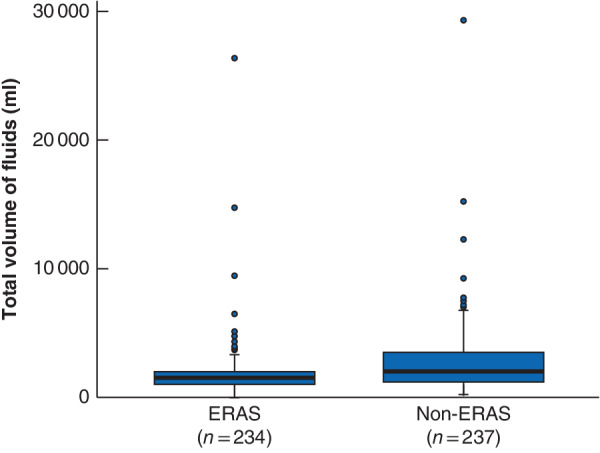
Median value (bold line), i.q.r. (box), and range (error bar) excluding outliers (circles) are shown. ERAS, enhanced recovery after surgery. P < 0·001 (Wilcoxon rank-sum test).
Postoperative outcomes
Wound dehiscence rates (in the absence of infection) were significantly lower in the ERAS group than the non-ERAS group (0·9 versus 13·1 per cent; P < 0·001); however, surgical-site infection rates were similar (9·0 versus 11·8 per cent; P = 0·484) (Table 4). Rates of postoperative ileus and/or bowel obstruction were significantly lower in the ERAS cohort (9·0 versus 16·9 per cent; P = 0·016). Patients in the ERAS cohort were less likely to be discharged to a facility (5·6 versus 13·1 per cent; P = 0·008). Overall hospital stay was shorter by 1 day for the ERAS cohort (median 5 versus 6 days; P = 0·003).
| ERAS (n = 234) | Non-ERAS (n = 237) | P † | |
|---|---|---|---|
| Return to emergency room | 8 (3·4) | 8 (3·4) | 1·000 |
| Readmission | 18 (7·7) | 23 (9·7) | 0·532 |
| Bleeding | 16 (6·8) | 30 (12·7) | 0·050 |
| Surgical-site infection | 21 (9·0) | 28 (11·8) | 0·484 |
| Wound dehiscence | 2 (0·9) | 31 (13·1) | < 0·001 |
| Seroma formation | 10 (4·3) | 13 (5·5) | 0·684 |
| Ileus and/or obstruction | 21 (9·0) | 40 (16·9) | 0·016 |
| Intra-abdominal fluid collection | 6 (2·6) | 3 (1·3) | 0·468 |
| Discharge to facility | 13 (5·6) | 31 (13·1) | 0·008 |
| Duration of hospital stay (days)* | 5 (0–36) | 6 (0–67) | 0·003‡ |
Values in parentheses are percentages unless indicated otherwise;
values are median (range). ERAS, enhanced recovery after surgery.
Pearson χ2 test, except
Wilcoxon rank-sum test.
Participation in the ERAS programme was associated with a lower rate of wound complications and discharge to a facility, and a shorter hospital stay, after adjustment for multiple confounders including age, BMI, sex, smoking history, preoperative chemotherapy and RT, and medical co-morbidities including diabetes, coronary artery disease, chronic kidney disease and chronic obstructive pulmonary disease (P < 0·001, P = 0·004 and P < 0·001 respectively).
Subgroup analysis
Subgroup analyses were undertaken to determine whether differences in rates of specific postoperative outcomes remained statistically significant in certain populations (Table 5). Among 92 patients who received preoperative RT, none of 41 in the ERAS cohort had postoperative wound dehiscence, compared with 11 of 51 in the non-ERAS cohort (P = 0·004). Similarly, of 110 patients who underwent surgery for extremity STS, none of 54 in the ERAS group and six of 56 in the non-ERAS group developed wound dehiscence (P = 0·040). Participation in the ERAS programme was associated with fewer wound complications after adjusting for preoperative RT (P = 0·008). The difference in duration of hospital stay remained significant in the subgroup of patients who had extremity STS (median 4 versus 5 days for ERAS and non-ERAS groups respectively; P < 0·001).
| Retroperitoneal | Extremity | |||||
|---|---|---|---|---|---|---|
| ERAS (n = 36) | Non-ERAS (n = 36) | P † | ERAS (n = 54) | Non-ERAS (n = 56) | P † | |
| Return to emergency room | 1 (3) | 0 (0) | 1·000 | 2 (4) | 5 (9) | 0·464 |
| Readmission | 1 (3) | 10 (28) | 0·009 | 7 (13) | 3 (5) | 0·305 |
| Bleeding | 3 (8) | 10 (28) | 0·066 | 3 (6) | 1 (2) | 0·585 |
| Surgical-site infection | 1 (3) | 6 (17) | 0·167 | 11 (20) | 10 (18) | 0·926 |
| Wound dehiscence | 0 (0) | 8 (22) | 0·009 | 0 (0) | 6 (11) | 0·040 |
| Seroma formation | 1 (3) | 0 (0) | 1·000 | 5 (9) | 7 (13) | 0·785 |
| Ileus and/or obstruction | 4 (11) | 15 (42) | 0·007 | 1 (2) | 0 (0) | 0·985 |
| Intra-abdominal fluid collection | 0 (0) | 2 (6) | 0·473 | 0 (0) | 0 (0) | 0·774 |
| Discharge to facility | 2 (6) | 10 (28) | 0·027 | 6 (11) | 10 (18) | 0·464 |
| Duration of hospital stay (days)* | 8 (0–36) | 14 (4–67) | < 0·001 | 4 (0–15) | 5 (0–26) | < 0·001 |
Values in parentheses are percentages unless indicated otherwise;
values are median (range). ERAS, enhanced recovery after surgery.
Pearson χ2 test, except
Wilcoxon rank-sum test.
Among 72 patients who underwent resection of retroperitoneal sarcoma, four of 36 in the ERAS group developed postoperative ileus and/or obstruction compared with 15 of 36 in the non-ERAS group (P = 0·007). The difference in duration of hospital stay remained statistically significant in this subgroup (8 versus 14 days; P < 0·001).
Discussion
Patients treated according to the ERAS protocol had lower rates of wound dehiscence and ileus and/or bowel obstruction, were less likely to be discharged to a facility, and had a shorter median hospital stay by 1 day. A previous study10,11 of ERAS in the setting of STS showed that implementation of the protocol reduced hospital stay but with no significant reduction in major morbidity11. That study was limited to patients with extremity and trunk STS. The impact of the ERAS protocol on duration of hospital stay was less dramatic in the present study because it included patients who underwent abdominal surgery, and those from outside the state for whom discharge was delayed by logistical barriers.
Rates of ileus and bowel obstruction were lower in the ERAS group, and this difference remained meaningful for patients undergoing surgery for retroperitoneal sarcoma. ERAS studies in other malignancies have shown significant differences in time to flatulence and time to bowel movement after surgery, but obstruction rates specifically have not been studied widely12. Principal components of the ERAS protocol likely contributing to improved bowel function include preoperative carbohydrate loading, goal-directed fluid therapy, early mobilization, multimodal pain regimens minimizing use of opioid pain medications, and early oral diet.
Wound dehiscence rates were also significantly lower in the ERAS cohort compared with the non-ERAS cohort, and this finding was preserved in subgroups of patients who had preoperative RT and those with extremity STS. It is difficult to know which components of the ERAS protocol specifically contributed to improving wound dehiscence rates. Excessive perioperative fluid administration has been identified previously as a risk factor for increased complication rates13. Reduction in overall volume, including intraoperative fluid administered as demonstrated here, may be a significant contributor to the decreased rates of wound dehiscence. Other instrumental elements of the protocol that are intended to reduce the overall stress response, such as the elimination of unnecessary drainage catheters, preservation of normothermia, methods to reduce insulin resistance, and early postoperative nutrition, may have contributed to the present findings14.
Although reducing wound complication rates is associated with administrative and cost benefits, there are potentially more significant clinical implications, particularly for patients with extremity STS. Better functional recovery is independently associated with implementation of ERAS protocols11. Currently, the standard of care for extremity STS is surgery combined with RT, which can be administered either before or after surgery15. Preoperative and postoperative RT have similar rates of local control and overall survival rates15. The preferred approach varies between institutions, balancing the risks and benefits of preoperative versus postoperative RT. Patients treated with postoperative RT often receive higher doses and larger treatment volumes than those who undergo preoperative RT; these, in turn, are associated with more long-term fibrosis, oedema, and joint stiffness affecting functional outcomes16–19. Preoperative RT enables the use of smaller field sizes and lower doses, often associated with better functional outcomes20,21. On the other hand, preoperative RT is associated with higher rates of major wound complications than postoperative radiation (35 versus 17 per cent)16. Although the use of preoperative RT has been increasing since 2000, a study22 in 2017 showed that approximately 76 per cent of patients are still being treated with postoperative RT. The ERAS-associated reduction in wound dehiscence offset the most notable morbidity of preoperative RT in patients with extremity STS.
A correlation between the use of ERAS protocols and oncological survival has been reported23,24. Gustafsson and colleagues24 showed that patients with colorectal cancer with 70 per cent or more adherence to ERAS interventions had a 5 per cent decrease in 5-year cancer-specific mortality23,24. It is difficult to establish a true cause and effect relationship between differences in cancer-specific mortality rates and implementation of an ERAS protocol. However, compliance with such a protocol may lead to a perioperative reduction in surgically induced stress for patients with cancer, which may indirectly influence tumour recurrence or metastases24. A study of the effect of ERAS on long-term, cancer-specific outcomes is warranted for patients undergoing surgery for STS.
This study has some limitations, including that the data are from a single institution. The results should be validated using data from multiple high-volume sarcoma centres; however, this is not possible at the moment because few centres have implemented ERAS protocols adapted specifically for patients with STS. Given these initial findings, the development of an international and standardized ERAS protocol for those with STS is currently under way. This was an intention-to-treat analysis, so compliance with the various components of the ERAS protocol was not tracked. It is therefore difficult to isolate the specific elements of the ERAS protocol that contributed to the findings. Although this was a comprehensive study of all patients with STS undergoing surgery, it included a retrospective comparator group of patients treated before implementation of the ERAS protocol. This study design cannot fully account for changes in clinical care and patient selection that occurred over time but were unrelated to ERAS protocol implementation. Nevertheless, it seems that treatment according to an ERAS protocol can improve aspects of patient care with no apparent disadvantages.
Acknowledgements
H.G.L. was supported by a National Library of Medicine institutional training grant for research training in biomedical informatics and data science (T15LM007092).
Disclosure: The authors declare no conflict of interest.
References
- 1. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg 2017; 152: 292–298. [DOI] [PubMed] [Google Scholar]
- 2. Martin L, Gillis C, Atkins M, Gilliam M, Sheppard C, Buhler Set al. Implementation of an enhanced recovery after surgery program can change nutrition care practice: a multicenter experience in elective colorectal surgery. J Parenter Enteral Nutr 2019; 43: 206–219. [DOI] [PubMed] [Google Scholar]
- 3. D'Souza K, Choi J, Wootton J, Wallace T. Impact of sequential implementation of multimodal perioperative care pathways on colorectal surgical outcomes. Can J Surg 2019; 62: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Steenhagen E. Enhanced recovery after surgery: it's time to change practice! Nutr Clin Pract 2016; 31: 18–29. [DOI] [PubMed] [Google Scholar]
- 5. Greco M, Capretti G, Beretta L, Gemma M, Pecorelli N, Braga M. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg 2014; 38: 1531–1541. [DOI] [PubMed] [Google Scholar]
- 6. Bugada D, Bellini V, Fanelli A, Marchesini M, Compagnone C, Baciarello Met al. Future perspectives of ERAS: a narrative review on the new applications of an established approach. Surg Res Pract 2016; 2016: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Currie A, Soop M, Demartines N, Fearon K, Kennedy R, Ljungqvist O. Enhanced recovery after surgery interactive audit system: 10 years' experience with an international web-based clinical and research perioperative care database. Clin Colon Rectal Surg 2019; 32: 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Greer N, Sultan S, Shaukat A, Dahm P, Lee A, MacDonald Ret al. Enhanced Recovery After Surgery (ERAS) Programs for Patients Undergoing Colorectal Surgery. Department of Veterans Affairs (US): Washington, DC, 2017. [PubMed] [Google Scholar]
- 9. Levy BF, Scott MJ, Fawcett WJ, Rockall TA. 23-Hour-stay laparoscopic colectomy. Dis Colon Rectum 2009; 52: 1239–1243. [DOI] [PubMed] [Google Scholar]
- 10. Michot A, Stoeckle E, Bannel J, Colombani S, Sargos P, Brouste Vet al. The introduction of early patient rehabilitation in surgery of soft tissue sarcoma and its impact on post-operative outcome. Eur J Surg Oncol 2015; 41: 1678–1684. [DOI] [PubMed] [Google Scholar]
- 11. Stoeckle E, Michot A, Rigal L, Babre F, Sargos P, Henriques de Figueiredo Bet al. The risk of postoperative complications and functional impairment after multimodality treatment for limb and trunk wall soft-tissue sarcoma: long term results from a monocentric series. Eur J Surg Oncol 2017; 43: 1117–1125. [DOI] [PubMed] [Google Scholar]
- 12. Frees SK, Anning J, Black P, Struss W, Bell R, Chavez-Munoz Cet al. A prospective randomized pilot study evaluating an ERAS protocol versus a standard protocol for patients treated with radical cystectomy and urinary diversion for bladder cancer. World J Urol 2018; 36: 215–220. [DOI] [PubMed] [Google Scholar]
- 13. Teeuwen P, Bleichrodt R, Strik C, Groenewoud JJ, Brinkert W, van Laarhoven CJet al. Enhanced recovery after surgery (ERAS) versus conventional postoperative care in colorectal surgery. J Gastrointet Surg 2010; 14: 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Melnyk M, Casey R, Black P, Koupparis A. Enhanced recovery after surgery (ERAS) protocols: time to change practice? Can Urol Assoc J 2011; 5: 342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baldini EH, Lapidus MR, Wang Q, Manola J, Orgill DP, Pomahac Bet al. Predictors for major wound complications following preoperative radiotherapy and surgery for soft-tissue sarcoma of the extremities and trunk: importance of tumor proximity to skin surface. Ann Surg Oncol 2013; 20: 1494–1499. [DOI] [PubMed] [Google Scholar]
- 16. Davis AM, O'Sullivan B, Turcotte R, Bell R, Catton C, Chabot Pet al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 2005; 75: 48–53. [DOI] [PubMed] [Google Scholar]
- 17. Stinson SF, DeLaney TF, Greenberg J, Yang JC, Lampert MH, Hicks JEet al. Acute and long-term effects on limb function of combined modality limb sparing therapy for extremity soft tissue sarcoma. Int J Radiat Oncol Biol Phys 1991; 21: 1493–1499. [DOI] [PubMed] [Google Scholar]
- 18. Mundt AJ, Awan A, Sibley GS, Simon M, Rubin SJ, Samuels Bet al. Conservative surgery and adjuvant radiation therapy in the management of adult soft tissue sarcoma of the extremities: clinical and radiobiological results. Int J Radiat Oncol Biol Phys 1995; 32: 977–985. [DOI] [PubMed] [Google Scholar]
- 19. Robinson MH, Spruce L, Eeles R, Fryatt I, Harmer CL, Thomas JMet al. Limb function following conservation treatment of adult soft tissue sarcoma. Eur J Cancer 1991; 27: 1567–1574. [DOI] [PubMed] [Google Scholar]
- 20. O'Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot Pet al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 2002; 359: 2235–2241. [DOI] [PubMed] [Google Scholar]
- 21. Delaney TF. Radiation therapy: neoadjuvant, adjuvant, or not at all. Surg Oncol Clin N Am 2012; 21: 215–241. [DOI] [PubMed] [Google Scholar]
- 22. Lazarev S, McGee H, Moshier E, Ru M, Demicco EG, Gupta V. Preoperative vs postoperative radiation therapy in localized soft tissue sarcoma: nationwide patterns of care and trends in utilization. Pract Radiat Oncol 2017; 7: e507–e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kang SH, Lee Y, Min SH, Park YS, Ahn SH, Park DJet al. Multimodal enhanced recovery after surgery (ERAS) program is the optimal perioperative care in patients undergoing totally laparoscopic distal gastrectomy for gastric cancer: a prospective, randomized, clinical trial. Ann Surg Oncol 2018; 25: 3231–3238. [DOI] [PubMed] [Google Scholar]
- 24. Gustafsson UO, Oppelstrup H, Thorell A, Nygren J, Ljungqvist O. Adherence to the ERAS protocol is associated with 5-year survival after colorectal cancer surgery: a retrospective cohort study. World J Surg 2016; 40: 1741–1747. [DOI] [PubMed] [Google Scholar]


