Abstract
Background
Numerous factors affect the prognosis of colorectal cancer (CRC), many of which have long been identified, such as patient demographics and the multidisciplinary team. In more recent years, molecular and immunological biomarkers have been shown to have a significant influence on patient outcomes. Whilst some of these biomarkers still require ongoing validation, if proven to be worthwhile they may change our understanding and future management of CRC. The aim of this review was to identify the key prognosticators of CRC, including new molecular and immunological biomarkers, and outline how these might fit into the whole wider context for patients.
Methods
Relevant references were identified through keyword searches of PubMed and Embase Ovid SP databases.
Results
In recent years there have been numerous studies outlining molecular markers of prognosis in CRC. In particular, the Immunoscore® has been shown to hold strong prognostic value. Other molecular biomarkers are useful in guiding treatment decisions, such as mutation testing of genes in the epidermal growth factor receptor pathway. However, epidemiological studies continue to show that patient demographics are fundamental in predicting outcomes.
Conclusion
Current strategies for managing CRC are strongly dependent on clinicopathological staging, although molecular testing is increasingly being implemented into routine clinical practice. As immunological biomarkers are further validated, their testing may also become routine. To obtain clinically useful information from new biomarkers, it is important to implement them into a model that includes all underlying fundamental factors, as this will enable the best possible outcomes and deliver true precision medicine.
Defines modern practice
Introduction
Colorectal cancer (CRC) is one of the most common cancers worldwide1, yet accurate prognostics evade us, often due to the heterogeneous nature of the disease. Current excitement about single fashionable factors, such as Oncotype DX® (Genomic Health, Redwood, California, USA), consensus molecular subtypes, the Immunoscore® (Integrative Cancer Immunology Laboratory, INSERM, Paris, France), tumour budding and stromal content, belies the complexity of the disease2–5. These factors may be insufficiently validated by limited studies of inadequate patient numbers, lack of robust comparison against standard prognosticators, or being tested only in trial populations. Most importantly, they rarely consider the individual characteristics of the patient, the tumour, the treating team or the expected site of recurrence (Fig. 1). Prognosis is improving with more access to screening and targeted oncology treatments that have reduced metastatic disease burdens. Delivery of precision cancer care requires sophisticated prognostic modelling that accounts for all these levels of complexity in differing socioeconomic populations and healthcare settings.
Fig. 1.
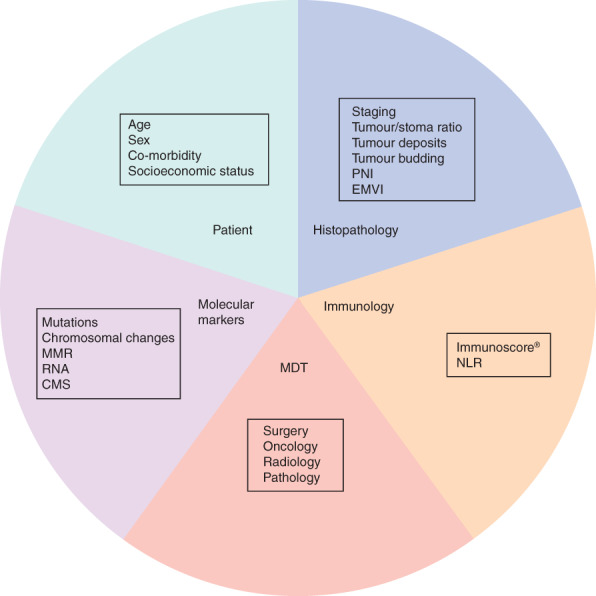
Prognostic model for colorectal cancer, showing the many factors that influence outcome. PNI, perineural invasion; EMVI, extramural vascular invasion; MMR, mismatch repair status; MDT, multidisciplinary team; NLR, neutrophil/lymphocyte ratio; CMS, consensus molecular subtypes
The anatomical extent of the disease, as assessed by clinicopathological staging, remains the most informing aspect of prognostic estimation. However, there are many patients whose outcomes do not match those typical for their tumour stage. Understanding the mechanisms behind these discrepancies will allow for a more precise, personalized approach, better informed treatment options, and ultimately improved outcomes. Over recent years, new evidence has impacted positively on the understanding of individual prognosis. This includes the importance of specific patient characteristics, the effectiveness of the multidisciplinary team, refinement of histopathological morphological factors, new molecular markers, and immunological indicators. Much of the immunological and molecular information provides promising new ways to classify and understand the prognosis of CRC, but the knowledge base of their relative importance remains incomplete. This review details key prognosticators of CRC, including new molecular and immunological biomarkers, and outlines how these might fit into the wider context for patients.
Methods
Searches of the PubMed and Embase Ovid SP databases were conducted for keywords ‘colon’ or ‘rectum’ or ‘colorectal’ and ‘cancer’ combined with ‘prognosis’ or ‘outcomes’, for papers published between July 2007 and July 2017. English-language references from these searches were then uploaded into a database, which was further interrogated for keywords in specific key areas such as ‘biomarkers, immunology, molecular markers’. Additional relevant papers published before 2007 were identified through review articles.
Patient factors
It is a fundamental principle that prognosis and appropriate management in CRC is strongly related to individual patient characteristics. These include age, sex, co-morbidity and socioeconomic status. CRC is often a disease of the elderly, with the incidence increasing with age. In the UK, more than 44 per cent of new cases are diagnosed in those aged 75 years or over, and the peak incidence is seen in the 85–89 years age group6. It is thought that this is due to an accumulation of aberrant genetic changes and a loss of the body's tumour defence systems with time. Co-morbidity and the patient's overall health should have a greater influence than absolute age, but there tends to be negative bias in the clinical decision regarding treatment (chemotherapy) regardless of overall state7–9. Elderly patients are poorly represented in clinical oncology trials and the benefit to elderly patients may be underappreciated7,10,11.
It is also observed that women more frequently have right-sided cancers with microsatellite instability (MSI) and are less likely to have a rectal cancer than men. They have a survival advantage that may be due in part to oestrogen exposure, although the mechanisms are still unclear. It is known that women have a lower neutrophil/lymphocyte ratio than men, which may contribute to better long-term survival12. A mutation in the p16 gene is found more often in right-sided tumours, and this mutation is nine times more frequent in all CRC in women than in men13–15. However, the mechanism behind this finding has yet to be fully explored. Women are more likely to present in an emergency situation with CRC, an independently poor prognostic factor. However, overall, women still have a better long-term survival, even when accounting for these and other differences in disease extent and treatment16,17.
Levels of accompanying co-morbid disease represent an important influence on CRC prognosis. This is due not only to the increased risk of death from non-cancer causes but also to the influence on disease-specific mortality9,18,19. To account for the influence that co-morbidity may have on outcome, a number of classification systems are used in clinical practice. One of more commonly used methods in epidemiological studies is the Charlson co-morbidity index (CCI)20. This scoring system classifies co-morbidities according to the patient's risk of 1-year mortality. The CCI score has been used in numerous clinical trials and studies to enable multivariable analyses to account for co-morbidities influencing the effect of the studied intervention. Another commonly employed method to quantify the effect of co-morbid disease on a patient's frailty is to classify performance status (PS). The most commonly used PS systems are the WHO PS (also known as the Zubrod or Eastern Cooperative Oncology Group scale) and the Karnofsky score21,22. These scoring systems differ from methods such as the CCI in that they focus on the effect of co-morbidities on the ability to perform activities of daily living rather than the diagnosed condition itself. Scoring systems are blunt measures of quantifying co-morbidity. However, by using these systems, trials can provide more clinically useful information and prognosis can be assessed more accurately.
Another highly important patient characteristic is socioeconomic status, which is independently associated with prognosis23–26. Similar to co-morbid state, socioeconomic status is categorized by a number of different classification and scoring systems. In most epidemiological studies, socioeconomic groups are determined by the location of the patient's home and/or educational level. This means that they may not reflect the patient's true socioeconomic status, and this has to be considered in the interpretation of studies that use this method. Socioeconomic status can be viewed as a surrogate for prognosis rather than as a prognostic factor. For example, patients with a poorer socioeconomic status may have higher levels of co-morbidity, poorer diet, present with late disease, be more likely to present as an emergency, have high levels of smoking, and so on. These factors may be the influence on poor prognosis rather than socioeconomic status itself27.
Surgery and the multidisciplinary team
The multidisciplinary team delivering care and treatment to the patient has a significant influence on prognosis, regardless of the molecular make-up of the tumour and patient factors. Surgery is often the main curative component of treatment, especially for earlier stage tumours. The plane of surgery has been demonstrated as an important prognostic factor for recurrence following both rectal and colonic cancer resections28,29. In rectal cancer, it has been demonstrated30 that the presence of tumour cells at or within 1 mm of the surgical circumferential resection margin is an independent poor prognostic factor. Involvement of the circumferential resection margin is dependent on the extent of the disease, the effectiveness of radiological techniques in its prediction, and the quality of surgery. Total mesorectal excision involves full excision of the mesorectum in an intact fascia-lined package, thereby achieving the best plane of surgery, and the lowest incidence of involved margins and local recurrence31,32. High-quality, standardized surgery for low rectal cancer and colonic cancer can have an effect on outcomes33–36.
From a radiological perspective, staging accuracy has a significant impact on subsequent surgical outcome. Improved imaging technologies have allowed for more accurate staging and therefore more accuracy in predicting prognosis. In addition, optimal planning of surgical approaches relies on robust imaging, which leads to oncologically superior outcomes whilst limiting morbidity (where possible). MRI, combined with CT, is a robust method for accurate prediction of surgical margin involvement37,38.
For rectal cancer, radiotherapy is now a well established treatment modality in addition to surgery. It reduces the risk of local recurrence, although short-course radiotherapy may not influence overall survival. There are therefore differences in its application across the world. In the USA and Europe, the most common approach is preoperative short-course radiotherapy; however, in Japan this is not standard practice39,40. In England, wide variation has been demonstrated41 in the use of radiotherapy, with men and patients having an abdominoperineal excision receiving it more often, and elderly patients and those with co-morbidities less so. This will have an impact on potential side-effects and local recurrence rates, and therefore may influence prognosis.
There is a strong association between the total number of lymph nodes examined after resection and overall survival. A large clinical trial42 showed that for node-negative patients, median 5-year survival was significantly improved when over 20 lymph nodes were identified. This is dependent on many factors, including patient immunology, tumour biology, anatomical location in the bowel, age, sex, preoperative treatment, and the quality of the surgery and the pathological assessment.
Histopathologists may face challenges when assessing the morphology of CRC specimens; the histological features are often subjective and opinion may vary between assessors. An example of this is when establishing the tumour grade. Grading tumours involves classifying the degree of cellular differentiation. The WHO grading system43 consists of four subcategories based on the percentage of glandular differentiation, whereby grade 1 denotes well differentiated tumours, grade 2 is moderate differentiation, grade 3 is poor differentiation, and grade 4 is undifferentiated. It is well established44,45 that tumour grade is significantly associated with survival. However, for day-to-day use a two-grade system of poor or other is more helpful as an aid to decision-making, as this improves interobserver agreement. Algorithms that automatically and consistently grade tumours need development, potentially by machine learning to improve the benefit of this important feature.
Another morphological feature that can be difficult to assess is the depth of tumour invasion, which has long been established as a prognostic marker. For a depth of submucosal invasion greater than 1 mm in pT1 cancers, there is a significantly higher risk of lymph node metastasis46,47. As tumours grow and invade through subsequent layers of the bowel, there may eventually be peritoneal involvement. This has independent adverse prognostic significance and it is therefore important to classify these tumours accurately. However, on histopathological examination it can be difficult to identify peritoneal involvement, which is therefore subjective with the potential to be missed. A study48 that involved cytological examination of serosal scrapings identified that 26 per cent of tumours classified as pT3 contained malignant cells. Newer methods of assessing peritoneal disease might be valuable.
Tumour pathology
The tumour itself provides a large amount of information that holds important value for prognosis. This includes studying macroscopic and microscopic histopathological features, as well as the molecular signature. The stage at which a tumour is diagnosed and the grade of differentiation provide fundamental information regarding likely prognosis to guide management. The AJCC and the UICC TNM staging system informs clinicians about the extent of tumour growth and spread into lymph nodes and/or distant metastatic deposits. It is continually reviewed and updated, as the role and impact that diagnostic features have on outcomes are further understood. Important histopathological features that are independent prognostic markers include depth of invasion in the bowel wall, peritoneal involvement, proportion of tumour cells/stroma in a given area, perineural and lymphovascular invasion, extramural venous invasion, tumour deposits and tumour budding49. One notable criticism of the TNM system is that it is frequently based on retrospective data from limited patient populations and from mainly within the USA, which may or may not be representative of healthcare systems around the world.
CRC consists of malignant epithelial cells mixed with benign stroma comprising fibroblasts, lymphatic and vascular structures, and inflammatory cells. Studies50,51 have shown that the proportion of tumour cells within a tumour area is an independent prognostic marker, and increases with stage. This is thought to be due to the cross-talk between stroma and carcinoma cells producing a greater level and number of growth factors, possible protection of tumour cells from immune attack, and the inverse association with deficient mismatch repair51,52.
The presence of vascular or perineural invasion has long been recognized as a prognostic factor45,53. Given its clinical impact, perineural invasion was added to the seventh edition of the AJCC TNM staging manual49. Another factor that has prognostic value but that may be difficult to determine on pathological assessment is direct invasion of the tumour into blood vessels. This is determined as intramural invasion when it occurs in the submucosa and/or muscular layer, and as extramural venous invasion when it invades beyond the muscularis propria54.
An important component of TNM staging is the detection of tumour cells within lymph nodes. Occult tumour cells within lymph nodes have previously been categorized as micrometastasis when 0·2–2 mm in size or as isolated tumour cells when less than 0·2 mm. Meta-analyses55,56 have shown that, for stage II and III tumours, micrometastases are associated with worse prognosis but isolated tumour cells are not. This is reflected in TNM staging, whereby micrometastases are counted as an involved node, whereas isolated tumour cells are included in the pN0 category49.
Tumour deposits are an important prognostic factor. They refer to a focus of tumour in the pericolic/perirectal fat within the lymph drainage area of the primary tumour but without identifiable lymph node, neural or vascular structure. Their presence as an adverse prognostic marker has been recognized since the fifth version of the AJCC TNM staging manual, but their definition has been refined over time. Although still suboptimal with the potential for subjectivity, there is no doubt their description adds value to TNM57,58. A recent meta-analysis59 suggested that the presence of both tumour deposits and lymph node metastases is additive in indicating a poor prognosis, possibly suggesting the presence of more than one metastatic pathway.
A histopathological feature that is not currently part of the TNM staging system is tumour budding. This refers to individual or small discrete clusters of tumour cells (fewer than 5) present at the invasive edge of the tumour. Its underlying mechanism is not fully understood, but is thought to represent an epithelial–mesenchymal transition whereby cell adhesion is lost, there is resistance to apoptosis, and cells gain an invasive phenotype60. Tumour budding is strongly associated with a number of poor prognostic histopathological factors, including higher tumour grade, lymphovascular invasion, lymph node metastasis and distant metastasis. It has been shown to be a strongly independent prognostic factor in numerous studies5,60–62. Despite extensive evidence for its usefulness as a marker of prognosis, until recently there has been a lack of consensus in the practical assessment of tumour budding, leading to subjectivity in the interpretation. Factors to be considered include the best topographical area for assessment, the microscopic field number and size that should be used, and whether staining should be with haematoxylin and eosin or by immunohistochemistry. However, work is ongoing to address this, including standards set by the International Tumor Budding Working Group so that this marker can be incorporated into staging in the future62.
Molecular markers
There are numerous prognostic molecular markers for CRC at all levels, including DNA, RNA and protein (Table 1)63. DNA can be affected in multiple ways in cancer pathways; this includes small-scale changes such as mutations, deletions and insertions, and larger changes such as methylation, MSI and chromosomal rearrangements.
Table 1.
Summary of recommended clinical molecular biomarkers in colorectal cancer63
| Biomarker | Mechanism | Use |
|---|---|---|
| KRAS | EGFR signalling pathway | Gene mutation status. For patients to be considered for anti-EGFR monoclonal antibodies |
| NRAS | EGFR signalling pathway | Gene mutation status. For patients to be considered for anti-EGFR monoclonal antibodies |
| BRAF | EGFR signalling pathway | Gene mutation status. For prognostic stratification and for dMMR tumours with loss of MLH1 to evaluate risk of Lynch syndrome |
| Mismatch repair status | DNA mismatch repair | Immunohistochemistry or MSI testing. For prognostic stratification and screening of Lynch syndrome |
EGFR, epidermal growth factor receptor; dMMR, DNA mismatch repair; MSI, microsatellite instability.
The ‘classical’ model of CRC tumour development is from normal mucosa, through to adenoma and then carcinoma, and it is thought that the majority of cancers develop in this way. At the molecular level, the model consists of early loss of regulation of the Wnt signalling pathway, followed by accumulation of activating mutations in oncogenes such as KRAS, PIK3CA and BRAF. Malignant transformation is then thought to occur through mutations in genes such as TP53 and SMAD4, and via chromosomal instability. Although, at present, none of these individual molecular events is used as a clinical prognostic marker, the overall chromosomal instability phenotype is associated with worse survival in comparison with that in patients with MSI tumours, as also found in studies looking at DNA aneuploidy, a more crude assessment of DNA content64–66.
Within CRC a number of biological classifications have been proposed: those cancers with MSI, CpG island methylator phenotype (CIMP) or chromosomal instability, and more recently based on RNA profiles into consensus molecular subtypes I–IV3,67,68. Although these biological subtypes also correlate with prognosis, there is not yet sufficient detailed information to identify their true place in clinical practice compared with other important parameters. There is also considerable overlap of these with other prognostic markers. Large-scale international collaborations on populations are needed to truly define the role of what can be commercially expensive tests.
Although they may have limited use as prognostic markers, mutations are valuable as predictive markers for guiding treatment. In metastatic CRC there are drugs that target the epidermal growth factor receptor (EGFR) such as panitumumab and cetuximab, but for patients with tumours that have activating mutations of the downstream RAS proteins, targeting EGFR does not improve outcomes. Studies69–71 have shown consistently that in tumours with KRAS mutations, there are poorer progression-free and overall survival rates when treatment includes anti-EGFR therapy compared with those in patients with tumours that are wild-type for KRAS. More controversy exists over mutations in BRAF and PIK3CA, EGFR amplification, amphiregulin and epiregulin RNA levels, and markers such as PTEN (phosphatase and tensin homologue) protein expression70,72,73.
Although it is considered that the majority of tumours develop via chromosomal instability, approximately 12–15 per cent are deficient in DNA mismatch repair (dMMR) and therefore have high levels of MSI. This loss of MMR can occur through germline mutations, known as Lynch syndrome, or sporadic epigenetic silencing and CIMP of the MMR genes. Lynch syndrome is associated with a high risk of developing metachronous tumours, and a more extensive surgical approach such as extended colectomy is therefore often recommended74,75.
Approximately half of sporadic CRCs with dMMR also carry a mutation in the BRAF gene. There are also phenotypic associations seen with dMMR tumours such as a proximal location within the colon, female sex, poor differentiation, mucinous histological phenotype and higher levels of lymphocytic infiltration. Tumours with dMMR are associated with better stage-adjusted survival compared with that in proficient MMR tumours76–78. They also have a decreased risk of metastasizing and are therefore associated with earlier stage79. The increased antigen-driven immune response caused by dMMR is thought to be part of the reason behind this. It has also been suggested80 that in dMMR the β2-microglobulin gene is often mutated in its microsatellite coding regions. This results in an inability to present antigens at the cell surface, which, in turn, stimulates natural killer cell-mediated tumour cell death80.
MMR status is not only a useful prognostic tool, but also may have a role in predicating response to therapy. For stage II dMMR tumours, some clinical trials have shown that 5-fluorouracil (5FU)-based adjuvant chemotherapy may not provide clinical benefit; however, this has not been found in all clinical trials and its clinical significance is therefore unclear81. In stage III disease, treatment with adjuvant FOLFOX (folinic acid, 5FU and oxaliplatin) is recommended regardless of MMR status. As well as traditional chemotherapy, more recently there have been advances in the treatment of dMMR tumours with immunotherapy. These tumours often have increased expression of immune checkpoints including programmed death (PD) 1. A phase 2 clinical trial of the monoclonal antibody to PD-1, pembrolizumab, has shown high rates of response in MSI tumours, and the 20-week progression-free survival rate was up to 78 per cent, compared with no response in proficient MMR tumours82. There are ongoing trials to explore further other immune checkpoint targets for dMMR tumours.
Assessment of MMR status has a number of clinical implications including understanding prognosis, an increased incidence of metachronous cancers, differential response to treatment (especially immunotherapy), and the identification of patients at higher risk of Lynch syndrome. It is recommended by the National Institute for Health and Care Excellence83 and in the eighth version of the AJCC TNM staging manual49, but is not currently widely implemented in routine testing in the UK. This needs to change rapidly.
RNA includes both coding and non-coding RNAs (ncRNAs) such as microRNAs and long non-coding RNAs amongst others. MicroRNAs regulate gene expression, and their dysregulation is associated with a number of malignancies84. There has been extensive work to understand and classify the functions of ncRNAs within CRC. Many ncRNAs have been shown to be independent prognostic factors and are associated with later stages of CRC, and therefore a worse prognosis85,86. However, the true prognostic potential of ncRNAs has yet to be fully explored and their relative value translated through to clinical practice.
Immunological factors
The immunological status of a patient with CRC can have a significant impact on their prognosis. There may be systemic inflammation, and host response factors also play a key role locally within the tumour. On a systemic level, a marker of inflammation that is increasingly used is the neutrophil/lymphocyte ratio (NLR), calculated by dividing the neutrophil count by the lymphocyte count. An increased NLR is seen with lymphocytopenia and neutrophilia. Lymphocytopenia indicates impaired cell-mediated immunity, whereas neutrophilia is seen as an acute inflammatory response. A raised NLR, and therefore higher levels of systemic inflammation, have been associated with worse prognosis in numerous studies, and a recent meta-analysis87 showed that an increased NLR was associated with significantly shorter overall and progression-free survival rates. NLR is an easily measured, cost-effective marker that has a significant association with outcome in a number of solid tumours, and it may hold greater clinical impact in the future. The additional value of other immune markers needs to be understood in the context of this very cheap routine test.
The immune system also plays an important role locally within CRC. Numerous immune cell types are found in tumours; in particular, higher levels of lymphocytic infiltrate are associated with improved outcomes. The lymphocytic infiltrate can be profiled using immunohistochemistry for T cell markers, such as cluster of differentiation (CD) 3, CD4, CD8 and FOXP3. Studies have shown that the presence of CD3 cytotoxic T cells and CD45RO memory T cells is a strong marker of prognosis. For patients with low densities of CD3 and CD45RO cells, both in the tumour core and in the invasive margin, it has been demonstrated88 that, regardless of stage, their overall survival is similar to that in patients with stage IV tumours. Standardized scoring of the immune infiltrate in this way, denoted as the Immunoscore® by Fridman and colleagues89, has been suggested as a clinically useful prognostic marker owing to its strong association with outcome in numerous studies. However, its implementation requires ongoing independent validation with retrospective case series and further work to identify how it relates to the MSI subset of tumours. As this method continues to be standardized it has the potential to be included in TNM staging4. Interestingly, assessment of the total number of lymphocytes present may also be an effective method of predicting prognosis, as shown for other cancer types, although further work is needed to explore its role in CRC90.
Conclusion
Current strategies for managing CRC are strongly dependent on clinopathological staging. Staging systems are continually reviewed and updated to include new validated markers of prognosis. A large number of new markers and further markers are emerging – not only histopathological features, but also molecular and immunological data. Some of these hold a strong association with prognosis, but it is important not to forget the greater context for the individual patient. A patient's tumour may have excellent biological markers, but if the person is elderly, has many co-morbidities, is socioeconomically deprived or is managed outside a high-quality multidisciplinary team by a non-specialist surgeon then the isolated biological profile may not hold as much relevance for overall prognosis. In the future, it is vital to understand how we can use this new biological information in combination with current established markers, patient demographics and data on the effectiveness of treatment. This can be undertaken only by the creation of new international networks that can generate high-resolution, high-quality, anonymized, large public data sets from different healthcare systems. These can then be used to model CRC more accurately and deliver truly precision medicine for the individual patient.
Acknowledgements
K.M.M. is a recipient of the National Institute of Health Research Academic Clinical Fellowship. N.P.W., E.M. and P.Q. are funded by Yorkshire Cancer Research. E.M. and P.Q. are also funded by a Cancer Research UK programme grant and research support from Roche. P.Q. is the recipient of a National Institute of Health Research Senior Investigator Award.
Disclosure: The authors declare no conflict of interest.
References
- 1. GLOBOCAN, International Agency for Research on Cancer, WHO . Cancer Today: Estimated Number of Incident Cases, Both Sexes, Worldwide (Top 10 Cancer Sites) in 2012; 2017. http://gco.iarc.fr/today/online-analysis-multi-bars?mode=cancer&mode_population=continents&population=900&sex=0&cancer=29&type=0&statistic=0&prevalence=0&color_palette=default [accessed 31 July 2017]. [Google Scholar]
- 2. Srivastava G, Renfro LA, Behrens RJ, Lopatin M, Chao C, Soori GSet al. Prospective multicenter study of the impact of oncotype DX colon cancer assay results on treatment recommendations in stage II colon cancer patients. Oncologist 2014; 19: 492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson Cet al. The consensus molecular subtypes of colorectal cancer. Nat Med 2015; 21: 1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Galon J, Pagès F, Marincola FM, Thurin M, Trinchieri G, Fox BAet al. The immune score as a new possible approach for the classification of cancer. J Transl Med 2012; 10: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rogers AC, Winter DC, Heeney A, Gibbons D, Lugli A, Puppa Get al. Systematic review and meta-analysis of the impact of tumour budding in colorectal cancer. Br J Cancer 2016; 115: 831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cancer Research UK . Bowel Cancer Incidence Statistics. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer/incidence [accessed 15 July 2017]. [Google Scholar]
- 7. Papamichael D, Audisio R, Horiot J-C, Glimelius B, Sastre J, Mitry Eet al. ; SIOG. Treatment of the elderly colorectal cancer patient: SIOG expert recommendations. Ann Oncol 2009; 20: 5–16. [DOI] [PubMed] [Google Scholar]
- 8. Glimelius B, Gunnarsdóttir KÁ, Derwinger K, Wettergren Y, Glimelius B, Kodeda Ket al. Optimal time intervals between pre-operative radiotherapy or chemoradiotherapy and surgery in rectal cancer? Front Oncol 2014; 4(Suppl 6): 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Eeghen EE, Bakker SD, van Bochove A, Loffeld RJLF. Impact of age and comorbidity on survival in colorectal cancer. J Gastrointest Oncol 2015; 6: 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bojer AS, Roikjœr O. Elderly patients with colorectal cancer are oncologically undertreated. Eur J Surg Oncol 2015; 41: 421–425. [DOI] [PubMed] [Google Scholar]
- 11. Nitsche U, Späth C, Müller TC, Maak M, Janssen K-P, Wilhelm Det al. Colorectal cancer surgery remains effective with rising patient age. Int J Colorectal Dis 2014; 29: 971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chua W, Charles KA, Baracos VE, Clarke SJ. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer 2011; 104: 1288–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Elsaleh H, Joseph D, Grieu F, Zeps N, Spry N, Iacopetta B. Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet 2000; 355: 1745–1750. [DOI] [PubMed] [Google Scholar]
- 14. Majek O, Gondos A, Jansen L, Emrich K, Holleczek B, Katalinic Aet al. ; GEKID Cancer Survival Working Group. Sex differences in colorectal cancer survival: population-based analysis of 164 996 colorectal cancer patients in Germany. PLoS One 2013; 8: e68077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ait Ouakrim D, Pizot C, Boniol M, Malvezzi M, Boniol M, Negri Eet al. Trends in colorectal cancer mortality in Europe: retrospective analysis of the WHO mortality database. BMJ 2015; 351: h4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Renzi C, Lyratzopoulos G, Card T, Chu TPC, Macleod U, Rachet B. Do colorectal cancer patients diagnosed as an emergency differ from non-emergency patients in their consultation patterns and symptoms? A longitudinal data-linkage study in England. Br J Cancer 2016; 115: 866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Quirt JS, Nanji S, Wei X, Flemming JA, Booth CM. Is there a sex effect in colon cancer? Disease characteristics, management, and outcomes in routine clinical practice. Curr Oncol 2017; 24: e15–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Edwards BK, Noone A-M, Mariotto AB, Simard EP, Boscoe FP, Henley SJet al. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 2014; 120: 1290–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Erichsen R, Horváth-Puhó E, Iversen LH, Lash TL, Sørensen HT. Does comorbidity interact with colorectal cancer to increase mortality? A nationwide population-based cohort study. Br J Cancer 2013; 109: 2005–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 21. West HJ, Jin JO. Performance status in patients with cancer. JAMA Oncol 2015; 1: 998. [DOI] [PubMed] [Google Scholar]
- 22. Mor V, Laliberte L, Morris JN, Wiemann M. The Karnofsky performance status scale: an examination of its reliability and validity in a research setting. Cancer 1984; 53: 2002–2007. [DOI] [PubMed] [Google Scholar]
- 23. Morris EJA, Taylor EF, Thomas JD, Quirke P, Finan PJ, Coleman MPet al. Thirty-day postoperative mortality after colorectal cancer surgery in England. Gut 2011; 60: 806–813. [DOI] [PubMed] [Google Scholar]
- 24. Hole DJ, McArdle CS. Impact of socioeconomic deprivation on outcome after surgery for colorectal cancer. Br J Surg 2002; 89: 586–590. [DOI] [PubMed] [Google Scholar]
- 25. Smith JJ, Tilney HS, Heriot AG, Darzi AW, Forbes H, Thompson MRet al. ; Association of Coloproctology of Great Britain and Ireland. Social deprivation and outcomes in colorectal cancer. Br J Surg 2006; 93: 1123–1131. [DOI] [PubMed] [Google Scholar]
- 26. Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi Aet al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017; 67: 177–193. [DOI] [PubMed] [Google Scholar]
- 27. ELHadi A, Ashford-Wilson S, Brown S, Pal A, Lal R, Aryal K. Effect of social deprivation on the stage and mode of presentation of colorectal cancer. Ann Coloproctol 2016; 32: 128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. West NP, Morris EJ, Rotimi O, Cairns A, Finan PJ, Quirke P. Pathology grading of colon cancer surgical resection and its association with survival: a retrospective observational study. Lancet Oncol 2008; 9: 857–865. [DOI] [PubMed] [Google Scholar]
- 29. Quirke P, Steele R, Monson J, Grieve R, Khanna S, Couture Jet al.; MRC CR07/NCIC-CTG CO16 Trial Investigators; NCRI Colorectal Cancer Study Group . Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet 2009; 373: 821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Birbeck KF, Macklin CP, Tiffin NJ, Parsons W, Dixon MF, Mapstone NPet al. Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg 2002; 235: 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986; 1: 1479–1482. [DOI] [PubMed] [Google Scholar]
- 32. Wibe A, Møller B, Norstein J, Carlsen E, Wiig JN, Heald RJet al.; Norwegian Rectal Cancer Group . A national strategic change in treatment policy for rectal cancer – implementation of total mesorectal excision as routine treatment in Norway. A national audit. Dis Colon Rectum 2002; 45: 857–866. [DOI] [PubMed] [Google Scholar]
- 33. Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation – technical notes and outcome. Colorectal Dis 2009; 11: 354–364. [DOI] [PubMed] [Google Scholar]
- 34. West NP, Hohenberger W, Weber K, Perrakis A, Finan PJ, Quirke P. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol 2010; 28: 272–278. [DOI] [PubMed] [Google Scholar]
- 35. Søndenaa K, Quirke P, Hohenberger W, Sugihara K, Kobayashi H, Kessler Het al. The rationale behind complete mesocolic excision (CME) and a central vascular ligation for colon cancer in open and laparoscopic surgery. Int J Colorectal Dis 2014; 29: 419–428. [DOI] [PubMed] [Google Scholar]
- 36. Bertelsen CA, Neuenschwander AU, Jansen JE, Kirkegaard-Klitbo A, Tenma JR, Wilhelmsen Met al. ; Copenhagen Complete Mesocolic Excision Study (COMES); Danish Colorectal Cancer Group (DCCG). Short-term outcomes after complete mesocolic excision compared with ‘conventional’ colonic cancer surgery. Br J Surg 2016; 103: 581–589. [DOI] [PubMed] [Google Scholar]
- 37. MERCURY Study Group . Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ 2006; 333: 779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Battersby NJ, How P, Moran B, Stelzner S, West NP, Branagan Get al.; MERCURY II Study Group . Prospective validation of a low rectal cancer magnetic resonance imaging staging system and development of a local recurrence risk stratification model. Ann Surg 2016; 263: 751–760. [DOI] [PubMed] [Google Scholar]
- 39. Boland PM, Fakih M. The emerging role of neoadjuvant chemotherapy for rectal cancer. J Gastrointest Oncol 2014; 5: 362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tokuhara K, Ueyama Y, Nakatani K, Yoshioka K, Kon M. Outcomes of neoadjuvant chemoradiotherapy in Japanese locally advanced rectal carcinoma patients. World J Surg Oncol 2016; 14: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Morris EJA, Finan PJ, Spencer K, Geh I, Crellin A, Quirke Pet al. Wide variation in the use of radiotherapy in the management of surgically treated rectal cancer across the English National Health Service. Clin Oncol (R Coll Radiol) 2016; 28: 522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Le Voyer TE, Sigurdson ER, Hanlon AL, Mayer RJ, Macdonald JS, Catalano PJet al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol 2003; 21: 2912–2919. [DOI] [PubMed] [Google Scholar]
- 43. Bosman FT, Carneiro F, Hruban RH, Theise ND (eds). WHO Classification of Tumours of the Digestive System. International Agency for Research on Cancer, WHO: Lyon, 2010. [Google Scholar]
- 44. Halvorsen TB, Seim E. Degree of differentiation in colorectal adenocarcinomas: a multivariate analysis of the influence on survival. J Clin Pathol 1988; 41: 532–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Compton CC. Colorectal carcinoma: diagnostic, prognostic, and molecular features. Mod Pathol 2003; 16: 376–388. [DOI] [PubMed] [Google Scholar]
- 46. Ueno H, Mochizuki H, Hashiguchi Y, Shimazaki H, Aida S, Hase Ket al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology 2004; 127: 385–394. [DOI] [PubMed] [Google Scholar]
- 47. Beaton C, Twine CP, Williams GL, Radcliffe AG. Systematic review and meta-analysis of histopathological factors influencing the risk of lymph node metastasis in early colorectal cancer. Colorectal Dis 2013; 15: 788–797. [DOI] [PubMed] [Google Scholar]
- 48. Panarelli NC, Schreiner AM, Brandt SM, Shepherd NA, Yantiss RK. Histologic features and cytologic techniques that aid pathologic stage assessment of colonic adenocarcinoma. Am J Surg Pathol 2013; 37: 1252–1258. [DOI] [PubMed] [Google Scholar]
- 49. Amin MB, Edge SB, Greene F, Byrd DR, Brookland RK, Washington MKet al. (eds). AJCC Cancer Staging Manual. American Joint Committee on Cancer: Chicago, 2017. [Google Scholar]
- 50. West NP, Dattani M, McShane P, Hutchins G, Grabsch J, Mueller Wet al. The proportion of tumour cells is an independent predictor for survival in colorectal cancer patients. Br J Cancer 2010; 102: 1519–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hutchins GGA, Treanor D, Wright A, Handley K, Magill L, Tinkler-Hundal Eet al. ; QUASAR trial collaborators and the UK National Cancer Research Institute Colorectal Cancer Clinical Studies Group. Intra-tumoural stromal morphometry predicts disease recurrence but not response to 5-fluorouracil - results from the QUASAR trial of colorectal cancer. Histopathology 2017; [Epub ahead of print]. [DOI] [PubMed]
- 52. Tsujino T, Seshimo I, Yamamoto H, Ngan CY, Ezumi K, Takemasa Iet al. Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin Cancer Res 2007; 13: 2082–2090. [DOI] [PubMed] [Google Scholar]
- 53. Liebig C, Ayala G, Wilks J, Verstovsek G, Liu H, Agarwal Net al. Perineural invasion is an independent predictor of outcome in colorectal cancer. J Clin Oncol 2009; 27: 5131–5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Betge J, Pollheimer MJ, Lindtner RA, Kornprat P, Schlemmer A, Rehak Pet al. Intramural and extramural vascular invasion in colorectal cancer. Cancer 2012; 118: 628–638. [DOI] [PubMed] [Google Scholar]
- 55. Rahbari NN, Bork U, Motschall E, Thorlund K, Büchler MW, Koch Met al. Molecular detection of tumor cells in regional lymph nodes is associated with disease recurrence and poor survival in node-negative colorectal cancer: a systematic review and meta-analysis. J Clin Oncol 2012; 30: 60–70. [DOI] [PubMed] [Google Scholar]
- 56. Sloothaak DAM, Sahami S, van der Zaag-Loonen HJ, van der Zaag ES, Tanis PJ, Bemelman WAet al. The prognostic value of micrometastases and isolated tumour cells in histologically negative lymph nodes of patients with colorectal cancer: a systematic review and meta-analysis. Eur J Surg Oncol 2014; 40: 263–269. [DOI] [PubMed] [Google Scholar]
- 57. Nagtegaal ID, Quirke P. Colorectal tumour deposits in the mesorectum and pericolon; a critical review. Histopathology 2007; 51: 141–149. [DOI] [PubMed] [Google Scholar]
- 58. Nagtegaal ID, Tot T, Jayne DG, McShane P, Nihlberg A, Marshall HCet al. Lymph nodes, tumor deposits, and TNM: are we getting better? J Clin Oncol 2011; 29: 2487–2492. [DOI] [PubMed] [Google Scholar]
- 59. Nagtegaal ID, Knijn N, Hugen N, Marshall HC, Sugihara K, Tot Tet al. Tumor deposits in colorectal cancer: improving the value of modern staging – a systematic review and meta-analysis. J Clin Oncol 2017; 35: 1119–1127. [DOI] [PubMed] [Google Scholar]
- 60. Mitrovic B, Schaeffer DF, Riddell RH, Kirsch R. Tumor budding in colorectal carcinoma: time to take notice. Mod Pathol 2012; 25: 1315–1325. [DOI] [PubMed] [Google Scholar]
- 61. Schneider NI, Langner C. Prognostic stratification of colorectal cancer patients: current perspectives. Cancer Manag Res 2014; 6: 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lugli A, Kirsch R, Ajioka Y, Bosman F, Cathomas G, Dawson Het al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol 2017; 30: 1299–1311. [DOI] [PubMed] [Google Scholar]
- 63. Sepulveda AR, Hamilton SR, Allegra CJ, Grody W, Cushman-Vokoun AM, Funkhouser WKet al. Molecular biomarkers for the evaluation of colorectal cancer: guideline from the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. J Clin Oncol 2017; 35: 1453–1486. [DOI] [PubMed] [Google Scholar]
- 64. Sinicrope FA, Rego RL, Halling KC, Foster N, Sargent DJ, La Plant Bet al. Prognostic impact of microsatellite instability and DNA ploidy in human colon carcinoma patients. Gastroenterology 2006; 131: 729–737. [DOI] [PubMed] [Google Scholar]
- 65. Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology 2010; 138: 2059–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hveem TS, Merok MA, Pretorius ME, Novelli M, Bævre MS, Sjo OHet al. Prognostic impact of genomic instability in colorectal cancer. Br J Cancer 2014; 110: 2159–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Walther A, Johnstone E, Swanton C, Midgley R, Tomlinson I, Kerr D. Genetic prognostic and predictive markers in colorectal cancer. Nat Rev Cancer 2009; 9: 489–499. [DOI] [PubMed] [Google Scholar]
- 68. Phipps AI, Limburg PJ, Baron JA, Burnett-Hartman AN, Weisenberger DJ, Laird PWet al. Association between molecular subtypes of colorectal cancer and patient survival. Gastroenterology 2015; 148: 77–87.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lièvre A, Bachet J-B, Le Corre D, Boige V, Landi B, Emile J-Fet al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 2006; 66: 3992–3995. [DOI] [PubMed] [Google Scholar]
- 70. Sartore-Bianchi A, Martini M, Molinari F, Veronese S, Nichelatti M, Artale Set al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res 2009; 69: 1851–1857. [DOI] [PubMed] [Google Scholar]
- 71. Maughan TS, Meade AM, Adams RA, Richman SD, Butler R, Fisher Det al. A feasibility study testing four hypotheses with phase II outcomes in advanced colorectal cancer (MRC FOCUS3): a model for randomised controlled trials in the era of personalised medicine? Br J Cancer 2014; 110: 2178–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jacobs B, De Roock W, Piessevaux H, Van Oirbeek R, Biesmans B, De Schutter Jet al. Amphiregulin and epiregulin mRNA expression in primary tumors predicts outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol 2009; 27: 5068–5074. [DOI] [PubMed] [Google Scholar]
- 73. De Roock W, De Vriendt V, Normanno N, Ciardiello F, Tejpar S. KRAS, BRAF, PIK3CA, and PTEN mutations: implications for targeted therapies in metastatic colorectal cancer. Lancet Oncol 2011; 12: 594–603. [DOI] [PubMed] [Google Scholar]
- 74. Ryan E, Sheahan K, Creavin B, Mohan HM, Winter DC. The current value of determining the mismatch repair status of colorectal cancer: a rationale for routine testing. Crit Rev Oncol Hematol 2017; 116: 38–57. [DOI] [PubMed] [Google Scholar]
- 75. Anele CC, Adegbola SO, Askari A, Rajendran A, Clark SK, Latchford Aet al. Risk of metachronous colorectal cancer following colectomy in Lynch syndrome: a systematic review and meta-analysis. Colorectal Dis 2017; 19: 528–536. [DOI] [PubMed] [Google Scholar]
- 76. Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology 2010; 138: 2073–2087.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Webber EM, Kauffman TL, O'Connor E, Goddard KA. Systematic review of the predictive effect of MSI status in colorectal cancer patients undergoing 5FU-based chemotherapy. BMC Cancer 2015; 15: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kawakami H, Zaanan A, Sinicrope FA. Microsatellite instability testing and its role in the management of colorectal cancer. Curr Treat Options Oncol 2015; 16: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Malesci A, Laghi L, Bianchi P, Delconte G, Randolph A, Torri Vet al. Reduced likelihood of metastases in patients with microsatellite-unstable colorectal cancer. Clin Cancer Res 2007; 13: 3831–3839. [DOI] [PubMed] [Google Scholar]
- 80. Tikidzhieva A, Benner A, Michel S, Formentini A, Link K-H, Dippold Wet al. Microsatellite instability and beta2-microglobulin mutations as prognostic markers in colon cancer: results of the FOGT-4 trial. Br J Cancer 2012; 106: 1239–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Quasar Collaborative Group, Gray R, Barnwell J, McConkey C, Hills RK, Williams NSet al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007; 370: 2020–2029. [DOI] [PubMed] [Google Scholar]
- 82. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring ADet al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015; 372: 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. National Institute for Health and Care Excellence . Molecular Testing Strategies for Lynch Syndrome in People with Colorectal Cancer. Diagnostics Guidance [DG27]; 2017. https://www.nice.org.uk/guidance/dg27 [accessed 12 September 2017]. [Google Scholar]
- 84. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009; 136: 215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Weng M, Wu D, Yang C, Peng H, Wang G, Wang Tet al. Noncoding RNAs in the development, diagnosis, and prognosis of colorectal cancer. Transl Res 2017; 181: 108–120. [DOI] [PubMed] [Google Scholar]
- 86. Kita Y, Yonemori K, Osako Y, Baba K, Mori S, Maemura Ket al. Noncoding RNA and colorectal cancer: its epigenetic role. J Hum Genet 2017; 62: 41–47. [DOI] [PubMed] [Google Scholar]
- 87. Li M-X, Liu X-M, Zhang X-F, Zhang J-F, Wang W-L, Zhu Yet al. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer 2014; 134: 2403–2413. [DOI] [PubMed] [Google Scholar]
- 88. Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès Cet al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313: 1960–1964. [DOI] [PubMed] [Google Scholar]
- 89. Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12: 298–306. [DOI] [PubMed] [Google Scholar]
- 90. Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LNet al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 2014; 32: 2959–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]


