Abstract
Background
Minimally invasive surgery (MIS) and enhanced recovery protocols (ERPs) have improved postoperative recovery and shortened length of hospital stay (LOS). Telemedicine technology has potential to improve outcomes and patient experience further. This study was designed to determine whether the combination of MIS, ERP and a structured telemedicine programme (TeleRecovery) could shorten total 30-day LOS by 50 per cent.
Methods
This was a phase II prospective RCT at a large academic medical centre. Eligible patients aged 18–80 years undergoing minimally invasive colorectal resection using an ERP were randomized after surgery. The experimental arm (RecoverMI) included accelerated discharge on postoperative day (POD) 1 with or without evidence of bowel function and a televideoconference on POD 2. The control arm was standard postoperative care. The primary endpoint was total 30-day LOS (postoperative stay plus readmission/emergency department/observation days). Secondary endpoints included patient-reported outcomes measured by EQ-5D-5L™, Brief Pain Inventory (BPI) and a satisfaction questionnaire.
Results
Thirty patients were randomized after robotic (21 patients) or laparoscopic (9) colectomy, including 14 patients in the RecoverMI arm. Median 30-day total LOS was 28·3 (i.q.r. 23·7–43·6) h in the RecoverMI arm and 51·5 (43·8–67·0) h in the control arm (P = 0·041). There were no differences in severe adverse events or EQ-5D-5L™ score between the study arms. The BPI revealed low pain scores regardless of treatment arm. Satisfaction was high in both arms.
Conclusion
In patients having surgery for colorectal neoplasms, the trimodal combination of MIS, ERP and TeleRecovery can reduce 30-day LOS while preserving patients' quality of life and satisfaction. Registration number: NCT02613728 (https://clinicaltrials.gov).
Graphical Abstract
In this phase II randomized trial, 30 patients undergoing minimally invasive colorectal resection were randomized to standard care or an accelerated enhanced recovery after surgery programme with TeleRecovery (RecoverMI). Patients in the RecoverMI arm achieved a median 30-day total length of hospital stay of 28·3 (i.q.r. 23·7–43·6) h, compared with 51·5 (43·8–67·0) h in the control arm (P = 0·041) while maintaining EQ-5D-5L™ scores and patient satisfaction. The trimodal combination of minimally invasive surgery, enhanced recovery and TeleRecovery can achieve a reduced 30-day total length of stay while preserving patient quality of life and satisfaction.
Graphical Abstract.
24-hour stay possible
Resumen
Antecedentes
La cirugía mínimamente invasiva (minimally invasive surgery, MIS) y los protocolos de recuperación intensificada (enhanced recovery protocols, ERP) han mejorado la recuperación postoperatoria y acortan la duración de la estancia (length of stay, LOS). La tecnología de la telemedicina tiene potencial para mejorar aún más los resultados y la experiencia del paciente. Este estudio se diseñó para determinar si la combinación de MIS, ERP y un programa estructurado de telemedicina (TeleRecovery) podría acortar la LOS total a los 30 días en un 50%.
Métodos
Se efectuó un ensayo controlado aleatorizado, prospectivo, de fase II en un gran centro médico académico. Los pacientes elegibles de 18-80 años de edad que se sometieron a resección colorrectal MIS mediante ERP se asignaron al azar después de la resección quirúrgica. El brazo experimental (RecoverMI) incluyó el alta acelerada en el día 1 del postoperatorio (postoperative day, POD) con o sin evidencia de recuperación del tránsito intestinal y una televideoconferencia en el día 2 POD. Los pacientes en el grupo control recibieron los cuidados postoperatorios habituales. El criterio de valoración principal fue la LOS total (estancia postoperatoria más reingreso/estancia en urgencias/días de observación) a los 30 días. Los criterios de valoración secundarios incluyeron los resultados referidos por los pacientes medidos por los cuestionarios EQ-5D-5L, el Cuestionario Breve del Dolor (Brief Pain Inventory, BPI) y un cuestionario de satisfacción.
Resultados
Treinta pacientes fueron aleatorizados después de una colectomía robótica (21) o laparoscópica (9), incluidos 14 pacientes en el grupo de RecoverMI. La mediana de la LOS total a los 30 días fue de 28,3 horas (rango intercuartílico, RIQ 23,7-43,6) en el grupo de RecoverMI y de 51,5 horas (RIQ 43,8-67,0) en el grupo control (P = 0,04). No hubo diferencias entre los grupos de estudio en los eventos adversos graves o en las puntuaciones del EQ-5D-5L. El BPI mostró puntuaciones bajas de dolor independientemente del grupo de tratamiento. La satisfacción fue alta en ambos grupos.
Conclusión
Entre los pacientes que se someten a cirugía por cáncer colorrectal, la combinación trimodal de MIS, ERP y TeleRecovery puede reducir la LOS a los 30 días, preservando la calidad de vida y la satisfacción del paciente.
Introduction
Colorectal cancer is the third commonest cancer in the USA, with 135 000 new cases diagnosed during 20171. The majority of patients are treated by radical surgical resection2,3. Surgery for colorectal cancer has improved as a result of minimally invasive procedures (MIS) and the implementation of enhanced recovery after surgery (ERAS) programmes. Their use has reduced perioperative morbidity and reduced length of hospital stay (LOS)4–6. The combination of ERAS principles and advances in MIS have inspired the concept of accelerated postoperative pathways for discharge7–9.
The potential for short-stay surgery with next-day discharge after colorectal resection has been shown in highly selected, small, retrospective single-surgeon or single-institution studies7,8. Implementation of short-stay colorectal resection could further improve outcomes and reduce healthcare resource use. However, concerns about lack of clinical supervision and the perceived need for return of bowel function before discharge may impair its adoption. To date, short-stay accelerated recovery has not been evaluated in a randomized study.
The use of telemedicine technology after surgery has been explored for a variety of surgical procedures and may support the implementation of short-stay colorectal resection10–13. Telemedicine allows remote monitoring of patients after discharge, to allow prompt intervention for complications, and provides an additional means of communication between provider and patient.
The aim of this study was to examine the safety and efficacy of an accelerated recovery pathway using the trimodality combination of MIS, an ERAS programme and a telemedicine technology in patients undergoing resection of colorectal cancer in a single-centre phase II RCT.
Methods
RecoverMI is an investigator initiated, single-institution, phase II randomized trial of short-stay colorectal resection comparing the use of MIS, an ERAS programme and a structured telemedicine programme (TeleRecovery) with early discharge versus standard postoperative care. This study was approved by the Institutional Review Board at the University of Texas MD Anderson Cancer Center (MDACC), and registered with clinicaltrials.gov (NCT02613728). Details of the trial design have been published previously14.
Participants
English-speaking patients aged 18–80 years undergoing surgery with curative intent for colonic or rectal cancer at MDACC were eligible for inclusion in the study. Considering the personnel support for the study and the timing of follow-up, eligible patients underwent surgery on Monday, Tuesday or Wednesday. Patients with endoscopically unresectable polyps requiring surgical management were also eligible. The planned surgical resection had to be performed by laparoscopic or robotic surgery, and the surgeon had to plan for primary anastomosis without creation of an ostomy14. Patients were approached at their preoperative clinic visit regarding participation in the study.
Exclusion criteria included patient-reported history of severe postoperative nausea/vomiting. Patients with a serum creatinine level above 1·5 ng/ml, measured within 30 days of surgery, or a history of congestive heart failure, defined as ejection fraction of 40 per cent or less, or of more than 40 per cent with associated systemic signs of heart failure, were also excluded. Finally, any patients requiring conversion to open surgery or in whom an ostomy was necessary at completion of surgery were removed from the study and not randomized14.
Interventions
Details of the interventions used in the trial have been described previously14. Briefly, all patients enrolled in the study had standardized preadmission, preoperative and intraoperative care, including mechanical bowel preparation with oral antibiotics, narcotic-sparing anaesthesia, goal-directed fluid management and MIS (laparoscopic or robotic). Full details of perioperative care are shown in Appendix S1 (supporting information). The MDACC ERAS programme is compliant with standard guidelines, except for the omission of preoperative carbohydrate loading, the allowance of pelvic drains following proctectomy, and the restriction of diet to liquids on POD 0 with advancement if tolerated on POD 115 (Table S1, supporting information). All patients were discharged from hospital to either their own home or hotel lodging in the immediate surrounding area. Patients were required to reside within travelling distance (2–3 h) of the hospital after discharge.
Patients randomized to the RecoverMI arm were discharged on POD 1 if they were afebrile, had satisfactory pain control with oral analgesics and were able to maintain hydration with oral intake of fluids. At discharge, a TeleRecovery appointment was scheduled with the surgical team on POD 2. Patients were given instructions on how to perform videoconferencing and instant messaging, and were provided with an iPad® (Apple, Cupertino, California, USA) with WiFi and cellular data capability for communication. The trial coordinator monitored the instant messaging and communicated with the healthcare team. Physician assistants within the colonic and rectal surgery section at MDACC conducted the televideoconferences. The study protocol allowed for outpatient intravenous fluid hydration at an ambulatory infusion centre on POD 2 and/or POD 3 when the patient was identified to have inadequate oral intake or thought to be at high risk of dehydration during the scheduled televideoconference or other unscheduled telemedicine communications with the surgical team. Inadequate oral intake was defined as less than 1 litre of oral fluid intake per day and urine output of less than 0·3 ml per kg per h for 8 h.
Patients randomized to the control arm had routine postoperative management and were eligible for discharge once they were able to maintain oral liquid intake, had satisfactory pain control with oral analgesics, and had either passed flatus or had a bowel movement.
Patients had office-based follow-up within 14 days of surgery and telephone follow-up by study personnel on POD 30. Adverse events and complications including readmissions and emergency department (ED) visits within 30 days of surgery were monitored according to the Clavien–Dindo classification16. Postoperative quality of life (QoL) was assessed using the EQ-5D-5L™ (EuroQol Group, Rotterdam, the Netherlands) and the Brief Pain Inventory (BPI) at enrolment, discharge, first postoperative clinic visit, and on POD 3017,18. Patient satisfaction was evaluated at POD 30 with a 20-item questionnaire.
Outcomes
The primary outcome was total 30-day LOS in hours. Total LOS was defined as the initial postoperative stay plus any subsequent time spent in the ED, under observation or readmitted to the hospital. The initial postoperative stay was calculated from the time the patient left the operating theatre to the time the discharge order was written. Any time in the ED or during readmission was calculated from arrival in the unit to the time the discharge order was written.
Secondary outcomes included perioperative complications, QoL scores, pain scores and patient satisfaction.
Safety was ensured using a Bayesian stopping rule. During the study, the failure rate in the RecoverMI arm was monitored such that the trial would be stopped early if it was unacceptably high. Full details of safety monitoring are shown in Appendix S2 (supporting information).
Sample size
Historical review before a formalized, comprehensive ERAS programme demonstrated a mean length of stay of 96 h. Based on the hypothesis that the RecoverMI arm would result in a 50 per cent reduction in total 30-day LOS, it was calculated that 28 patients (14 per treatment arm) would have 80 per cent power to detect a mean difference of 48 h (assuming a common standard deviation of 42) in LOS between the two groups using a two-sample t test and with a two-sided type I error rate of 0·05 (nQuery Advisor® software version 7.0; Statistical Solutions, Boston, Massachusetts, USA)14.
Randomization
Eligible patients were randomized after undergoing minimally invasive colorectal resection and intraoperative anaesthesia using ERAS principles as detailed above, with a 1 : 1 ratio to either RecoverMI or routine care. Permuted-block randomization with variable block size was utilized through the MDACC Clinical Trial Conduct website (https://biostatistics.mdanderson.org/ClinicalTrialConduct).
Statistical analysis
Continuous variables were compared between treatment groups using the Wilcoxon rank sum test, and categorical data using Fisher's exact test. Summary scores were calculated based on standardized manuals associated with each survey instrument. Mean EQ-5D-5L™ QoL scores along with corresponding 95 per cent c.i. were plotted over time, including baseline, discharge, postoperative follow-up and POD 30, where a higher score represents a better health condition. Similar plots were generated for the BPI scores at the same time points; higher scores indicate more severe pain. All statistical evaluations were two-sided. P ≤ 0·050 was considered statistically significant. All statistical analyses were carried out in SAS® version 9.3 (SAS Institute, Cary, North Carolina, USA).
Results
Between 1 July 2016 and 22 August 2017, 63 eligible patients under the care of five surgeons were identified, of whom 32 were consented for participation. The reasons why the remaining 31 patients did not participate are shown in Fig. 1. One patient withdrew consent before surgery and another after randomization. As a result, 16 patients were randomized to the control arm and 14 to the RecoverMI arm. All patients completed the study and none was lost to follow-up. Results were analysed according to treatment arm.
Fig. 1.
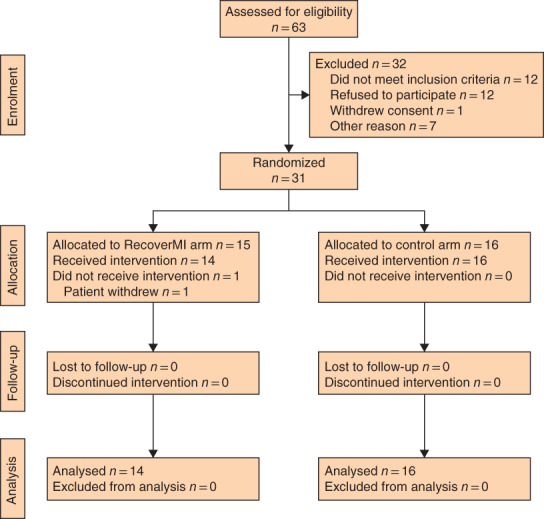
CONSORT diagram for the trial
Patients' demographic, co-morbidity, surgical details and AJCC cancer stage are shown in Table 1; there were no differences between the groups. Throughout the study, there was high compliance with the ERAS protocol, with 15 of 16 patients in the control arm and all 14 patients in the RecoverMI arm receiving preoperative analgesic medication according the MDACC ERAS programme (Table S1, supporting information). In addition, there was no significant difference in goal-directed intraoperative fluid administration between participants in the two study arms.
Table 1.
Demographic and surgical details of patients
| RecoverMI arm (n = 14) | Control arm (n = 16) | |
|---|---|---|
| Age (years) * | 58·7(12·6) | 59·3(10·2) |
| Sex ratio (F : M) | 8 : 6 | 6 : 10 |
| White Caucasian ethnicity | 10 | 10 |
| ASA fitness grade | ||
| II | 0 | 2 |
| III | 14 | 14 |
| Charlson/Deyo co-morbidity score * | 4·6(1·7) | 5·0(1·9) |
| Type of surgery | ||
| Laparoscopic | 4 | 5 |
| Robotic | 10 | 11 |
| Surgical procedure | ||
| Right colectomy | 8 | 8 |
| Left colectomy | 3 | 1 |
| Low anterior resection | 3 | 7 |
| AJCC stage | ||
| I | 6 | 5 |
| II | 4 | 6 |
| III | 2 | 4 |
| IV | 1 | |
| Unresectable polyp | 1 | 1 |
Values are mean(s.d.).
Primary outcome
Median total 30-day LOS was 28·3 (i.q.r. 23·7–43·6) h in the RecoverMI arm compared with 51·5 (43·8–67·0) h in the control arm (P = 0·041). Similarly, median initial LOS was also shorter in the RecoverMI arm: 27·1 (23·0–29·1) h versus 51·5 (43·0–67·0) h in the control arm (P = 0·002) (Table 2).
Table 2.
Primary outcomes
| RecoverMI arm (n = 14) | Control arm (n = 16) | P | |
|---|---|---|---|
| Initial LOS (h) | 0·002† | ||
| Mean(s.d.) (95 per cent c.i.) | 28·2(10·7) (22·6, 33·8) | 53·8(17·8) (45·1, 62·5) | |
| Median (i.q.r.) | 27·1 (23·0–29·1) | 51·5 (43·8–67·0) | |
| Total LOS (h) | 0·041† | ||
| Mean(s.d.) (95 per cent c.i.) | 80·4(142·6) (5·7, 155·1) | 53·8(17·8) (45·1, 62·5) | |
| Median (i.q.r.) | 28·3 (23·7–43·6) | 51·5 (43·8–67·0) | |
| Clavien–Dindo grade* | 0·209‡ | ||
| ≥ III | 2 | 0 | |
| I–II | 3 | 0 |
The highest grade of complication is reported; both patients with grade III complications requiring reoperation also had grade II complications (total of 3 patients with adverse events). LOS, length of hospital stay.
Wilcoxon rank sum test;
Fisher's exact test.
Secondary outcomes
Four patients in the RecoverMI arm returned to the hospital for an ED visit (2 patients) or readmission (2). In the control arm, one patient returned to the ED for evaluation of leg swelling, but was discharged after excluding deep vein thrombosis. In the RecoverMI arm, one patient attended the ED with increased abdominal pain more than 1 week after discharge, but was subsequently discharged home. Another patient developed Clostridium difficile colitis and required observation. One patient developed an anastomotic leak after total mesorectal excision without diversion, and required readmission and reoperation. Finally, one further patient developed postoperative bowel obstruction owing to a port-site hernia, and required readmission and reoperation. None of these complications resulting in an ED visit or readmission was deemed to be related to accelerated discharge. Throughout the study period, no patient required outpatient parenteral fluid therapy.
Telemedicine outcomes
The scheduled TeleRecovery assessment in the RecoverMI arm was completed successfully for 13 of the 14 patients (93 per cent). One patient was discharged without the appropriate device and was unable to complete the telemedicine encounter. Throughout the trial, contact with providers was available to patients in the RecoverMI arm on demand, via text messaging using the iPad or through televideoconferencing (Table 3).
Table 3.
Telemedicine contact rate (available to RecoverMI arm only)
| No. of patients (n = 14) | |
|---|---|
| Successful completion of scheduled televideoconference | 13 |
| Use of unscheduled telemedicine contact | 10 |
| Patient-initiated message | |
| Clinical question | 6 |
| Non-clinical question | 2 |
| Patient-initiated televideoconference | |
| Clinical question | 2 |
| Non-clinical question | 1 |
Quality-of-life and pain score outcomes
Neither the EQ-5D-5L™ index score nor the score on the visual analogue scale (VAS) part of the instrument was significantly different between patients in the RecoverMI arm and those in the control arm (Fig. 2). The BPI score was similar at baseline, initial postoperative clinic visits and 30 days after surgery, but was significantly higher in the RecoverMI arm compared with the control arm at discharge: mean(s.d.) score 3·23(1·86) versus 1·80(1·63) respectively (P = 0·052) (Fig. 3).
Fig. 2.
EQ-5D-5L™ results for RecoverMI and control arms of the trial
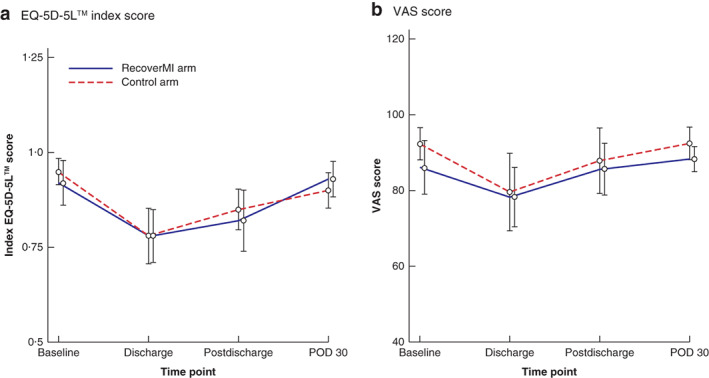
Mean trend plots for a EQ-5D-5L™ index and b visual analogue scale (VAS) summary scores in questionnaire EQ-5D-5L™. Error bars denote 95 per cent confidence intervals.
Fig. 3.
Brief Pain Inventory results for RecoverMI and control arms of the trial

Mean trend plots for a severity and b interference summary scores on the Brief Pain Inventory (BPI). Error bars denote 95 per cent confidence intervals. *P = 0·052 (Wilcoxon rank sum test).
Patient satisfaction
Patient satisfaction was assessed at POD 30 using a 20-question custom-designed survey addressing multiple aspects of the perioperative care pathway (Table S2, supporting information). The response rate was 93 per cent (13 of 14) and 88 per cent (14 of 16) in the RecoverMI and control arms respectively. There were no significant differences between the treatment arms for any of the questionnaire items. Nearly all respondents in both arms did not feel they needed to be kept in the hospital for a longer period of time to recover from surgery (P = 0·462).
Discussion
The RecoverMI trimodal strategy of MIS, an ERAS protocol and TeleRecovery reduced both 30-day total and index LOS after colorectal cancer resection. This was achieved without compromising patient satisfaction or QoL, and with no increase in severe complications. This confirms the feasibility and potential efficacy of short-stay colorectal cancer resection.
The 30-day LOS for the control arm of just over 2 days was shorter than expected based on historical data at study design, but reflects the combination of MIS and implementation of a structured ERAS programme for colorectal surgery in the unit before study activation. Although it could be argued that the 2-day postoperative stay in the control arm is already short, the further reduction in LOS observed in the RecoverMI arm could potentially open pathways to ambulatory short-stay surgery after MIS for colorectal cancer. There are many potential advantages to ambulatory short-stay colorectal surgery, including a more rapid return to normalcy, reduced resource utilization, potential for lower risk of exposure to hospital pathogens, and the ability to stay with family locally or at home while recovering. Although the discharge pain scores were higher in the RecoverMI than in the control arm, overall the scores were low. In addition, the difference is probably because discharge, and hence the measurement of the pain score at discharge, occurred 1 day earlier in the RecoverMI arm.
The potential for selected patients to be discharged on the first day after colorectal surgery has been reported in retrospective studies7–8,19. However, prospective randomized trial data are important to eliminate the potential bias of retrospective studies. Based on a clinically meaningful 50 per cent reduction in overall LOS, this phase II study required enrolment of just over 30 patients to demonstrate that short-stay colectomy could be accomplished in a selected population with controllable morbidity.
Most previous studies of the use of telemedicine in the postoperative setting have focused on replacing or supplementing clinic visits11,20. The goal of telemedicine use in the present study was to evaluate telemedicine as a mechanism for supporting patients during their early postoperative recovery, and serving as an adjunct to an early discharge. Although no therapy was offered, TeleRecovery enhanced communication between the patient and surgical team. This scheduled communication served as a mechanism for psychological support to patients participating in an accelerated discharge programme. The ability to provide connectivity through televideoconferencing and text messaging between the patient and surgical team provided a unique mechanism for verbal and visual interaction with the patient after discharge that probably facilitated patient acceptance. As the technology continues to expand, the ability to incorporate remote monitoring, such as fitness trackers, remote vital-sign monitoring and wound monitoring, may further increase the value of telemedicine in postsurgical care.
This study is not without limitations. Although the inclusion criteria were designed to be broad and patients were randomized, the operating surgeon ultimately determined whether the patient was eligible for randomization. However, this determination was made before surgery, and thus before randomization. Additionally, unconscious bias to later discharge in the control arm could have been possible, although patients in both study arms were assessed at least twice daily by a surgeon, and during additional visits throughout the day by a mid-level provider whose primary responsibility was inpatient care. However, the number of visits a patient was required to have was not specified, as the protocol was designed to minimize disruption to the routine workflow. Patients were enrolled only if they had surgery from Monday to Wednesday, owing to the need for the subsequent TeleRecovery visit during the working week. However, if telemedicine allows accelerated discharge, the cost savings could be used to support TeleRecovery consultation outside standard working hours. In this study, patients in the RecoverMI arm used their iPad® to ensure secure Health Insurance Portability and Accountability Act (HIPAA)-compliant connectivity. In the future, given the ubiquity of personal smartphones, such an approach may be unnecessary and personal devices may be used. Although there were more postoperative readmissions, ED visits and adverse events in the RecoverMI arm, the difference was not statistically significant. It must, however, be noted that the study was not powered to detect any difference in this outcome, and that no adverse events or readmissions were believed to be due to use of the accelerated recovery protocol.
The results of this prospective RCT demonstrate the feasibility of short-stay approaches with next-day discharge after colorectal resection. Future studies in a multi-institutional setting are needed to confirm the application and cost-effectiveness of this trimodal approach to accelerated recovery in a variety of hospital and practice settings.
Supplementary Material
Appendix S1. Supporting Information.
Acknowledgements
The RecoverMI trial was supported by an American Society of Colon and Rectal Surgeons Research Foundation Limited Project Grant (to G.J.C.) and in part by the National Cancer Institute of the National Institutes of Health under award number CA016672 (The University of Texas MD Anderson Cancer Center Support Grant), the Andrews Family Fund for Colorectal Cancer Research (to G.J.C.), and the Aman Trust for Colorectal Cancer Research and Education (to G.J.C.).
Disclosure: The authors declare no conflict of interest.
References
- 1. Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi Aet al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017; 67: 177–193. [DOI] [PubMed] [Google Scholar]
- 2. Chang GJ, Kaiser AM, Mills S, Rafferty JF, Buie WD; Standards Practice Task Force of the American Society of Colon and Rectal Surgeons . Practice parameters for the management of colon cancer. Dis Colon Rectum 2012; 55: 831–843. [DOI] [PubMed] [Google Scholar]
- 3. Monson JR, Weiser MR, Buie WD, Chang GJ, Rafferty JF, Buie WDet al. ; Standards Practice Task Force of the American Society of Colon and Rectal Surgeons . Practice parameters for the management of rectal cancer (revised). Dis Colon Rectum 2013; 56: 535–550. [DOI] [PubMed] [Google Scholar]
- 4. Gash KJ, Greenslade GL, Dixon AR. Enhanced recovery after laparoscopic colorectal resection with primary anastomosis: accelerated discharge is safe and does not give rise to increased readmission rates. Colorectal Dis 2012; 14: 1287–1290. [DOI] [PubMed] [Google Scholar]
- 5. Kennedy RH, Francis EA, Wharton R, Blazeby JM, Quirke P, West NPet al. Multicenter randomized controlled trial of conventional versus laparoscopic surgery for colorectal cancer within an enhanced recovery programme: EnROL. J Clin Oncol 2014; 32: 1804–1811. [DOI] [PubMed] [Google Scholar]
- 6. Lovely JK, Maxson PM, Jacob AK, Cima RR, Horlocker TT, Hebl JRet al. Case-matched series of enhanced versus standard recovery pathway in minimally invasive colorectal surgery. Br J Surg 2012; 99: 120–126. [DOI] [PubMed] [Google Scholar]
- 7. Delaney CP. Outcome of discharge within 24 to 72 hours after laparoscopic colorectal surgery. Dis Colon Rectum 2008; 51: 181–185. [DOI] [PubMed] [Google Scholar]
- 8. Levy BF, Scott MJ, Fawcett WJ, Rockall TA. 23-hour-stay laparoscopic colectomy. Dis Colon Rectum 2009; 52: 1239–1243. [DOI] [PubMed] [Google Scholar]
- 9. Gignoux B, Pasquer A, Vulliez A, Lanz T. Outpatient colectomy within an enhanced recovery program. J Visc Surg 2015; 152: 11–15. [DOI] [PubMed] [Google Scholar]
- 10. Beaver K, Campbell M, Williamson S, Procter D, Sheridan J, Heath Jet al. An exploratory randomized controlled trial comparing telephone and hospital follow-up after treatment for colorectal cancer. Colorectal Dis 2012; 14: 1201–1209. [DOI] [PubMed] [Google Scholar]
- 11. Hwa K, Wren SM. Telehealth follow-up in lieu of postoperative clinic visit for ambulatory surgery: results of a pilot program. JAMA Surg 2013; 148: 823–827. [DOI] [PubMed] [Google Scholar]
- 12. Viers BR, Lightner DJ, Rivera ME, Tollefson MK, Boorjian SA, Karnes RJet al. Efficiency, satisfaction, and costs for remote video visits following radical prostatectomy: a randomized controlled trial. Eur Urol 2015; 68: 729–735. [DOI] [PubMed] [Google Scholar]
- 13. Katz MH, Slack R, Bruno M, McMillan J, Fleming JB, Lee JEet al. Outpatient virtual clinical encounters after complex surgery for cancer: a prospective pilot study of ‘TeleDischarge’. J Surg Res 2016; 202: 196–203. [DOI] [PubMed] [Google Scholar]
- 14. Price BA, Bednarski BK, You YN, Manandhar M, Dean EM, Alawadi ZMet al. Accelerated enhanced Recovery following Minimally Invasive colorectal cancer surgery (RecoverMI): a study protocol for a novel randomised controlled trial. BMJ Open 2017; 7: e015960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg 2017; 152: 292–298. [DOI] [PubMed] [Google Scholar]
- 16. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RDet al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg 2009; 250: 187–196. [DOI] [PubMed] [Google Scholar]
- 17. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994; 23: 129–138. [PubMed] [Google Scholar]
- 18. Pickard AS, De Leon MC, Kohlmann T, Cella D, Rosenbloom S. Psychometric comparison of the standard EQ-5D to a 5 level version in cancer patients. Med Care 2007; 45: 259–263. [DOI] [PubMed] [Google Scholar]
- 19. Brandt E, Poulsen M, Lykke J, Jess P, Ovesen H. A minority of patients discharged within 24 hours after laparoscopic colon resection. Dan Med J 2013; 60: A4658. [PubMed] [Google Scholar]
- 20. Keeping-Burke L, Purden M, Frasure-Smith N, Cossette S, McCarthy F, Amsel R. Bridging the transition from hospital to home: effects of the VITAL telehealth program on recovery for CABG surgery patients and their caregivers. Res Nurs Health 2013; 36: 540–553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.


