Abstract
Background
Although perihepatic lymph node metastases (PLNMs) are known to be a poor prognosticator for patients with colorectal liver metastases (CRLMs), optimal management remains unclear. This study aimed to determine the risk factors for PLNMs, and the survival impact of their number and location in patients with resectable CRLMs.
Methods
Data on patients with CRLM who underwent hepatectomy during 2003–2014 were analysed retrospectively. Recurrence-free (RFS) and overall (OS) survival were calculated according to presence, number and location of PLNMs. Risk factors for PLNM were evaluated by logistic regression analysis.
Results
Of 1485 patients, 174 underwent lymphadenectomy, and 54 (31·0 per cent) had PLNM. Ten patients (5·7 per cent) who had lymphadenectomy and 176 (13·4 per cent) who did not underwent repeat hepatectomy. Survival of patients with PLNM was significantly poorer than that of patients without (RFS: 5·3 versus 13·8 months, P < 0·001; OS: 20·5 versus 71·3 months; P < 0·001). Median OS was significantly better in patients with para-aortic versus hepatoduodenal ligament PLNMs (58·2 versus 15·5 months; P = 0·011). Patients with three or more PLNMs had significantly worse median OS than those with one or two (16·3 versus 25·4 months; P = 0·039). The presence of primary tumour lymph node metastases (odds ratio 2·35; P = 0·037) and intrahepatic recurrence requiring repeat hepatectomy (odds ratio 5·61; P = 0·012) were significant risk factors for PLNM on multivariable analysis.
Conclusion
Patients undergoing repeat hepatectomy and those with primary tumour lymph node metastases are at significant risk of PLNM. Although PLNM is a poor prognostic factor independent of perihepatic lymph node station, patients with one or two PLNMs have a more favourable outcome than those with more PLNMs.
Bad sign of advanced disease
Introduction
For patients with unresectable metastatic colorectal cancer, recent progress in chemotherapy and molecular targeted agents has improved survival, with 5-year overall survival (OS) rates as high as 25 per cent1–3. Despite progress in systemic therapies, hepatic resection remains the only curative option for patients with colorectal liver metastases (CRLMs). The 5-year OS rate after resection of CRLMs ranges between 33 and 55 per cent, depending on clinicopathological risk factors4–6. Coexistence of extrahepatic metastases is a poor prognostic factor in patients with CRLM, and had been considered a contraindication to resection. However, concomitant resection of CRLMs and extrahepatic metastases, including lung and peritoneal metastases, can be associated with a 5-year OS rate of 19–36 per cent in well selected patients if all metastatic sites can be resected7,8.
Perihepatic lymph node metastases (PLNMs), defined as metastases located along the hepatoduodenal ligament, common hepatic artery, coeliac trunk and in the aortocaval space, are considered to result from metastatic spread of the CRLMs themselves (rather than the colorectal primary) through perihepatic lymphatic channels9. Several previous studies10–12 have reported the incidence of PLNM in patients treated with hepatectomy for CRLM to range from 6 to 28 per cent. Studies in the 1990s13–15 (before the introduction of modern systemic therapy) found that metastasis in perihepatic lymph node (LN) stations was one of the worst prognostic factors among patients with extrahepatic metastases; no more than 10 per cent of patients were alive 5 years after resection of CRLM. Therefore, it had been proposed that the coexistence of PLNM with CRLM represents a contraindication to resection10–12,16. Recent studies17–20 incorporating modern chemotherapy and molecular targeted agents have reported encouraging outcomes after hepatectomy with perihepatic lymphadenectomy for PLNM, achieving 5-year OS rates of 13–21 per cent.
One study21 reported no influence of the location of PLNM on survival between metastases on the portal vein versus hepatic artery side. Although several researchers22,23 found that the number of metastatic LNs has an impact on survival after resection of other hepatobiliary cancers (such as intrahepatic cholangiocarcinoma, ICC), there are no reports on the association between number of PLNMs in patients with resectable CRLM and survival. The aim of this study was to determine the optimal treatment strategy for PLNM, risk factors for PLNM, and the survival impact of the number and location of PLNMs in patients with resectable CRLM in the era of modern chemotherapy.
Methods
This study was approved by the Institutional Review Board of the University of Texas MD Anderson Cancer Center. Patients with CRLM who underwent hepatectomy at this centre between January 2003 and December 2014 were identified from a prospectively maintained institutional database. Patients who had non-curative resection were excluded. Perihepatic LNs were classified into three groups: nodes located along the hepatoduodenal ligament (along the proper hepatic artery, portal vein, and bile duct and retropancreatic head); nodes located along the common hepatic artery and coeliac artery (along the common hepatic, coeliac, left gastric and superior mesenteric arteries); and para-aortic LNs.
Treatment
Hepatectomy was undertaken when it was predicted to result in an adequate standardized future liver remnant, to spare two continuous hepatic segments, and to maintain vascular inflow/outflow and biliary drainage. In patients with an anticipated insufficient standardized future liver remnant, two-stage hepatectomy and/or preoperative portal vein embolization (PVE) was performed following multidisciplinary tumour board discussion. Postoperative chemotherapy was administered routinely to complete a total of 12 cycles, including cycles administered before operation.
Perihepatic lymph node assessment
All patients routinely underwent preoperative CT of the chest and abdomen. MRI and PET–CT were performed if CRLMs were undetectable on CT and/or extrahepatic metastases were suspected but not obvious on CT. PLNM positivity on preoperative imaging was determined from the radiological report by experienced radiologists specializing in abdominal imaging for this study. If any preoperative imaging study indicated PLNM, the patient was considered to have PLNM; all such patients underwent hepatectomy with perihepatic lymphadenectomy or LN biopsy even if other imaging studies did not support this interpretation. Patients with extensive PLNM on preoperative imaging (multiple bulky LNs with extension beyond the lymph node on preoperative CT, MRI or PET–CT) were not considered for curative resection. If a prominent or firm perihepatic LN was detected during surgery in a patient with no evidence of PLNM before surgery, perihepatic lymphadenectomy or LN biopsy was carried out. When PLNMs were identified on intraoperative frozen-section analysis of a perihepatic LN biopsy, perihepatic lymphadenectomy was performed. Patients who underwent perihepatic lymphadenectomy or LN biopsy for staging purposes at the discretion of the surgeon (no evidence of PLNM on preoperative imaging or during intraoperative assessment) were excluded from this analysis to minimize selection bias.
The diagnostic performance of preoperative imaging in the detection of PLNM was determined using pathological diagnosis as the reference. Similarly, in patients without evidence of PLNM on preoperative imaging, the diagnostic performance of intraoperative assessment was determined using pathological diagnosis as the reference.
Data collection
The following data were collected from patients' medical records: age, sex, primary tumour location, presence or absence of primary LN metastases, CRLM characteristics (number of tumours, largest tumour diameter and timing of detection of metastases), RAS mutation status, serum carcinoembryonic antigen (CEA) level before hepatectomy, presence or absence of extrahepatic metastases other than PLNMs, prehepatectomy chemotherapy, perioperative data (type of hepatectomy, resection margin, duration of operation, intraoperative blood loss, blood transfusion, postoperative morbidity, duration of hospital stay after hepatic resection, and unplanned readmission within 45 days after discharge24), PLNM characteristics (location and number), and whether perihepatic lymphadenectomy was performed. Major resection was defined as resection of three or more liver segments. A positive surgical margin was defined as a tumour-free margin less than 1 mm25. Major complication was defined according to the Dindo–Clavien classification (above grade III)26.
Statistical analysis
Continuous variables are presented as median (range). Data were analysed using Wilcoxon signed-rank test, χ2 test or Fisher's exact test, as appropriate. Recurrence-free survival (RFS) and OS were calculated from the date of hepatic resection and estimated using the Kaplan–Meier method. The log rank test was used to compare survival curves. A multivariable analysis based on the logistic regression model was used to identify risk factors for PLNM. All variables with P < 0·100 in univariable analysis were included in the logistic regression model for multivariable analysis. All tests were two-tailed, and P < 0·050 was considered statistically significant. Statistical computations were performed using JMP® pro 12.1 (SAS Institute, Cary, North Carolina, USA).
Results
A total of 1485 patients were included in the study, of whom 174 (11·7 per cent) underwent perihepatic lymphadenectomy and 1311 (88·3 per cent) did not. The patients who had perihepatic lymphadenectomy had significantly more liver metastases, larger hepatic tumour diameter, a higher serum CEA level, and a greater likelihood of preoperative chemotherapy administration and primary tumour located in the colon (not rectum). RAS mutation status was similar in the two groups (Table 1).
Table 1.
Preoperative characteristics of patients who did or did not undergo perihepatic lymphadenectomy
| No perihepatic lymphadenectomy | Perihepatic lymphadenectomy | ||
|---|---|---|---|
| (n = 1311) | (n = 174) | P * | |
| Age (years) | 0·231 | ||
| < 75 | 1228 (93·7) | 167 (96·0) | |
| ≥ 75 | 83 (6·3) | 7 (4·0) | |
| Sex ratio (M : F) | 794 : 517 | 91 : 83 | 0·037 |
| Primary tumour location | 0·030 | ||
| Colon | 1004 (76·6) | 146 (83·9) | |
| Rectum | 307 (23·4) | 28 (16·1) | |
| Primary tumour lymph node metastasis | 0·927 | ||
| Negative | 414 (33·6) | 53 (34·0) | |
| Positive | 818 (66·4) | 103 (66·0) | |
| Unknown | 79 | 18 | |
| No. of hepatic metastases | < 0·001 | ||
| Solitary | 620 (47·3) | 47 (27·0) | |
| Multiple | 691 (52·7) | 127 (73·0) | |
| Largest hepatic tumour diameter (mm) | < 0·001 | ||
| < 30 | 866 (66·1) | 82 (47·1) | |
| ≥ 30 | 445 (33·9) | 92 (52·9) | |
| Timing of hepatic metastases | 0·569 | ||
| Metachronous | 366 (27·9) | 45 (25·9) | |
| Synchronous | 945 (72·1) | 129 (74·1) | |
| RAS mutation status | 1·000 | ||
| Wild type | 400 (58·7) | 64 (58·7) | |
| Mutant | 281 (41·3) | 45 (41·3) | |
| Unknown | 630 | 65 | |
| CEA level (ng/ml) | 0·166 | ||
| < 10 | 976 (74·4) | 121 (69·5) | |
| ≥ 10 | 335 (25·6) | 53 (30·5) | |
| Extrahepatic metastases other than perihepatic lymph node | 0·079 | ||
| No | 1211 (92·4) | 154 (88·5) | |
| Yes | 100 (7·6) | 20 (11·5) | |
| Preoperative chemotherapy | 0·028 | ||
| No | 239 (18·2) | 20 (11·5) | |
| Yes | 1072 (81·8) | 154 (88·5) | |
| Intrahepatic recurrence requiring repeat hepatectomy | 0·004 | ||
| No | 1135 (86·6) | 164 (94·3) | |
| Yes | 176 (13·4) | 10 (5·7) |
Values in parentheses are percentages. CEA, carcinoembryonic antigen.
χ2 test.
Reflecting their higher liver tumour burden, more patients with perihepatic lymphadenectomy were treated with aggressive hepatectomy (two-stage hepatectomy, preoperative PVE and major hepatectomy). Consistent with the higher incidence of aggressive procedures, duration of operation was longer among patients with perihepatic lymphadenectomy and they had a longer hospital stay (Table 2).
Table 2.
Perioperative characteristics of patients who did or did not undergo perihepatic lymphadenectomy
| No perihepatic lymphadenectomy | Perihepatic lymphadenectomy | ||
|---|---|---|---|
| (n = 1311) | (n = 174) | P * | |
| Two-stage hepatectomy | < 0·001 | ||
| No | 1256 (95·8) | 149 (85·6) | |
| Yes | 55 (4·2) | 25 (14·4) | |
| Preoperative PVE | < 0·001 | ||
| No | 1213 (92·5) | 122 (70·1) | |
| Yes | 98 (7·5) | 52 (29·9) | |
| Resection with RFA | < 0·001 | ||
| No | 1116 (85·1) | 163 (93·7) | |
| Yes | 195 (14·9) | 11 (6·3) | |
| Major hepatectomy (≥ 3 segments) | < 0·001 | ||
| No | 642 (49·0) | 48 (27·6) | |
| Yes | 669 (51·0) | 126 (72·4) | |
| Surgical margin | 0·088 | ||
| Negative | 1174 (89·8) | 147 (85·5) | |
| Positive | 134 (10·2) | 25 (14·5) | |
| Unknown | 3 | 2 | |
| Duration of operation (min) | < 0·001 | ||
| < 180 | 701 (53·5) | 31 (17·8) | |
| ≥ 180 | 610 (46·5) | 143 (82·2) | |
| Intraoperative blood loss (ml) | 0·797 | ||
| < 1000 | 1249 (95·3) | 165 (94·8) | |
| ≥ 1000 | 62 (4·7) | 9 (5·2) | |
| Blood transfusion | 0·356 | ||
| No | 1210 (92·3) | 164 (94·3) | |
| Yes | 101 (7·7) | 10 (5·7) | |
| Major postoperative complication | 0·109 | ||
| No | 1136 (86·7) | 143 (82·2) | |
| Yes | 175 (13·3) | 31 (17·8) | |
| Major liver-related complication | 0·280 | ||
| No | 1220 (93·1) | 158 (90·8) | |
| Yes | 91 (6·9) | 16 (9·2) | |
| Duration of hospital stay (days) | 0·002 | ||
| ≤ 6 | 755 (57·6) | 79 (45·4) | |
| > 6 | 556 (42·4) | 95 (54·6) | |
| Unplanned readmission within 45 days | 0·071 | ||
| No | 1206 (92·0) | 153 (87·9) | |
| Yes | 105 (8·0) | 21 (12·1) |
Values in parentheses are percentages. PVE, portal vein embolization; RFA, radiofrequency ablation.
χ2 test.
Of the 174 patients who underwent perihepatic lymphadenectomy, 54 (31·0 per cent) had pathologically confirmed PLNM and 120 (69·0 per cent) did not. Details of neoadjuvant and adjuvant chemotherapy are shown in Table 3. Forty-eight patients (89 per cent) with PLNM and 114 (95·0 per cent) patients without received neoadjuvant and/or adjuvant chemotherapy with a modern chemotherapy regimen (oxaliplatin or irinotecan with/without molecular target agent).
Table 3.
Details of perioperative chemotherapy in patients who underwent perihepatic lymphadenectomy
| Without perihepatic lymph node metastases | With perihepatic lymph node metastases | |
|---|---|---|
| (n = 120) | (n = 54) | |
| Neoadjuvant chemotherapy | ||
| No | 12 (10·0) | 8 (15) |
| Yes | 108 (90·0) | 46 (85) |
| No. of cycles of chemotherapy* | 6 (3–30) | 6 (2–14) |
| Oxaliplatin-based | 90 of 108 (83·3) | 35 of 46 (76) |
| Irinotecan-based | 26 of 108 (24·1) | 15 of 46 (33) |
| Use of bevacizumab | 78 of 108 (72·2) | 32 of 46 (70) |
| Use of cetuximab/panitumumab | 12 of 108 (11·1) | 6 of 46 (13) |
| Adjuvant chemotherapy | ||
| Unknown | 5 | 4 |
| No | 33 of 115 (28·7) | 15 of 50 (30) |
| Yes | 82 of 115 (71·3) | 35 of 50 (70) |
| No. of cycles of chemotherapy* | 6 (1–12) | 6 (1–12) |
| Oxaliplatin-based | 57 of 78 (73) | 16 of 34 (47) |
| Irinotecan-based | 14 of 78 (18) | 11 of 34 (32) |
| Use of bevacizumab | 29 of 77 (38) | 13 of 34 (38) |
| Use of cetuximab/panitumumab | 9 of 77 (12) | 6 of 34 (18) |
Values in parentheses are percentages unless indicated otherwise;
values are median (range).
Of 35 patients who underwent perihepatic lymphadenectomy or LN biopsy for staging purposes (no evidence of PLNM on preoperative imaging or during intraoperative assessment), two had pathologically confirmed PLNM.
Incidence of PLNM, and performance of preoperative imaging and intraoperative assessment in detection of PLNM
A flow diagram summarizing the detection of PLNM during the treatment course is shown in Fig. 1. Thirty-nine (40 per cent) of 98 patients who had suspected PLNM on preoperative imaging had this confirmed by pathological examination. A further 76 patients who did not have suspected PLNM on preoperative imaging had suspicion of PLNM during intraoperative assessment. Of these, 15 (20 per cent) actually had PLNM confirmed by pathological examination.
Fig. 1.
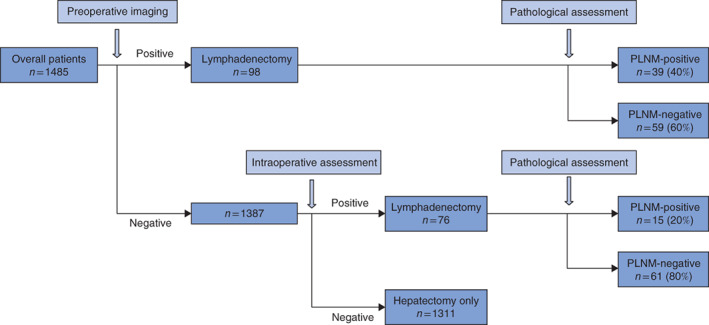
Flow diagram showing detection of perihepatic lymph node metastases during the course of treatment
Risk factors for PLNM
Results of logistic regression analyses to identify risk factors for PLNM are shown in Table 4. On univariable analysis, presence of primary tumour LN metastases (odds ratio (OR) 2·63, 95 per cent c.i. 1·15 to 5·99; P = 0·016) and intrahepatic recurrence requiring repeat hepatectomy (OR 5·80, 1·44 to 23·42; P = 0·009) were identified as significant risk factors for PLNM. Multivariable analysis indicated that presence of primary tumour LN metastases (OR 2·35, 1·04 to 5·60; P = 0·037) and intrahepatic recurrence requiring repeat hepatectomy (OR 5·61, 1·45 to 27·41; P = 0·012) were significant independent risk factors for PLNM.
Table 4.
Results of univariable and multivariable regression analyses to identify factors related to perihepatic lymph node metastases
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Odds ratio | P | Odds ratio | P | |
| Age (years) | ||||
| < 75 | 1·00 (reference) | |||
| ≥ 75 | 3·12 (0·67, 16·32) | 0·145 | ||
| Sex | ||||
| M | 1·00 (reference) | |||
| F | 1·42 (0·75, 2·71) | 0·288 | ||
| Primary tumour location | ||||
| Colon | 1·00 (reference) | |||
| Rectum | 0·87 (0·36, 2·12) | 0·757 | ||
| Primary tumour lymph node metastasis | ||||
| Negative | 1·00 (reference) | 1·00 (reference) | ||
| Positive | 2·63 (1·15, 5·99) | 0·016 | 2·35 (1·04, 5·60) | 0·037 |
| No. of hepatic metastases | ||||
| Solitary | 1·00 (reference) | |||
| Multiple | 1·25 (0·59, 2·61) | 0·556 | ||
| Largest hepatic tumour diameter (mm) | ||||
| < 30 | 1·00 (reference) | |||
| ≥ 30 | 1·05 (0·55, 2·00) | 0·883 | ||
| Timing of hepatic metastases | ||||
| Metachronous | 1·00 (reference) | |||
| Synchronous | 0·99 (0·48, 2·07) | 0·990 | ||
| RAS status | ||||
| Wild type | 1·00 (reference) | |||
| Mutant | 0·81 (0·36, 1·81) | 0·600 | ||
| CEA level (ng/ml) | ||||
| < 10 | 1·00 (reference) | |||
| ≥ 10 | 0·73 (0·35, 1·49) | 0·379 | ||
| Extrahepatic metastases other than perihepatic lymph node | ||||
| No | 1·00 (reference) | |||
| Yes | 1·98 (0·77, 5·11) | 0·163 | ||
| Preoperative chemotherapy | ||||
| No | 1·00 (reference) | |||
| Yes | 0·81 (0·31, 2·18) | 0·687 | ||
| Two-stage hepatectomy | ||||
| No | 1·00 (reference) | |||
| Yes | 1·05 (0·42, 2·62) | 0·910 | ||
| Intrahepatic recurrence requiring repeat hepatectomy | ||||
| No | 1·00 (reference) | 5·61 (1·45, 27·41) | ||
| Yes | 5·80 (1·44, 23·42) | 0·009 | 0·012 | |
| Preoperative PVE | ||||
| No | 1·00 (reference) | |||
| Yes | 0·65 (0·32, 1·37) | 0·256 | ||
Values in parentheses are 95 per cent confidence intervals. CEA, carcinoembryonic antigen; PVE, portal vein embolization.
Survival according to perihepatic lymphadenectomy and presence or absence of PLNM
Median follow-up was 42·2 months for patients without perihepatic lymphadenectomy, 43·0 months for those who had lymphadenectomy without identification of PLNM, and 37·0 months for patients who had lymphadenectomy with PLNM. In an analysis of survival that included only patients who underwent perihepatic lymphadenectomy, median RFS and OS were significantly worse in patients with PLNM than in those without (RFS: 5·3 versus 13·8 months, P < 0·001; OS: 20·5 versus 71·3 months, P < 0·001) (Fig. 2a,b).
Fig. 2.
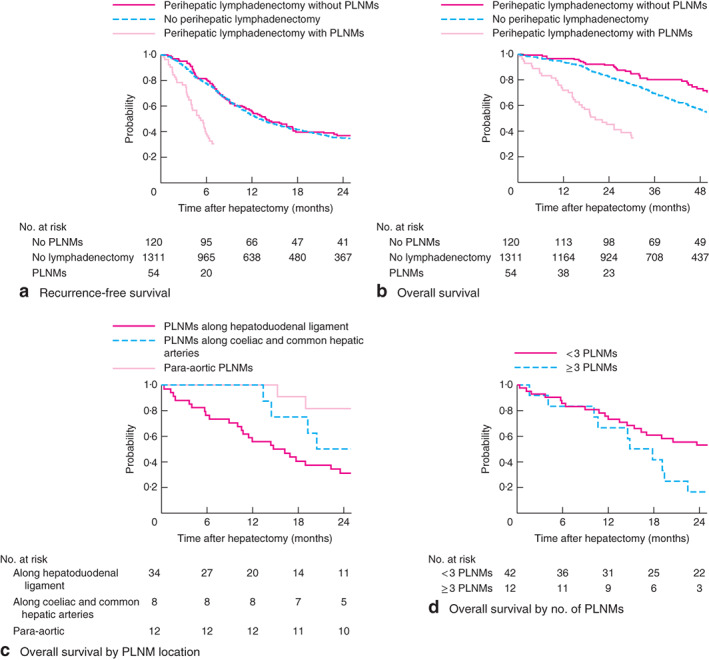
a Recurrence-free survival and b overall survival in patients who did not undergo perihepatic lymphadenectomy and in patients who had lymphadenectomy with or without identification of perihepatic lymph node metastases (PLNMs). c Overall survival according to location of PLNMs. d Overall survival in patients with PLNMs according to number of metastases. aP = 0·542 (no lymphadenectomy versus lymphadenectomy without PLNMs), P < 0·001 (no lymphadenectomy versus lymphadenectomy with PLNMs), P < 0·001 (lymphadenectomy with versus without PLNMs); bP = 0·030 (no lymphadenectomy versus lymphadenectomy without PLNMs), P < 0·001 (no lymphadenectomy versus lymphadenectomy with PLNMs), P < 0·001 (lymphadenectomy with versus without PLNMs); cP = 0·012 (PLNMs along hepatoduodenal ligament versus para-aortic PLNMs), dP = 0·039 (log rank test)
Comparison of survival between patients who did not have perihepatic lymphadenectomy (no evidence of PLNM on preoperative imaging and intraoperative assessment) and patients who underwent lymphadenectomy without PLNM (suspicion of PLNM on preoperative imaging or intraoperative assessment, but no evidence on pathological analysis) showed that RFS was similar in both groups (median RFS 13·0 versus 13·8 months respectively; P = 0·542) (Fig. 2a). However, OS in patients without PLNM on lymphadenectomy was significantly better than that among patients who did not undergo lymphadenectomy (median 71·3 versus 56·0 months; P = 0·030) (Fig. 2b).
Survival according to location of PLNMs
Of the 54 patients with PLNM, the metastases were located along the hepatoduodenal ligament in 34 patients, coeliac and common hepatic arteries in eight, and 12 patients had para-aortic PLNMs. Median RFS and OS were 4·5 and 15·5 months respectively in patients with perihepatoduodenal ligament metastases, 6·1 and 23·0 months in those with pericoeliac and common hepatic artery metastases, and 5·1 and 58·2 months in patients with para-aortic metastases (Fig. 2c). There was no significant difference in RFS among the three groups (P = 0·233). OS of patients with para-aortic LN metastases was significantly better than that in patients with perihepatoduodenal ligament metastases (P = 0·012).
Survival according to number of PLNMs
Of the 54 patients with PLNM, 42 had one or two PLMNs and 12 had three or more. The patients were dichotomized (fewer than 3 versus 3 or more PLNMs) on the basis of a previous report on ICC22. Median RFS was similar in the two groups (5·1 versus 5·8 months respectively; P = 0·911). However, patients with one or two PLNMs had significantly better median OS than patients with three or more (25·4 versus 16·3 months; P = 0·039) (Fig. 2d).
Discussion
In patients with resectable CRLM, PLNMs have been considered a poor prognostic factor, and any therapeutic benefit of perihepatic lymphadenectomy remains controversial11,13. However, starting in the 2000s, studies7,12,19–21 showed acceptable survival outcomes after the combination of hepatic resection for CRLM and lymphadenectomy for PLNM. Although randomized trials comparing hepatectomy and perihepatic lymphadenectomy with chemotherapy alone are not available, these results suggested a therapeutic benefit of perihepatic lymphadenectomy in selected patients. The present results support a benefit of lymphadenectomy for well selected patients with PLNM.
At present, there is no consensus regarding the optimal treatment strategy for patients with CRLM who are suspected of having PLNM on preoperative imaging. Previous studies27,28 reported the poor diagnostic accuracy of preoperative CT, with a low positive predictive value (30–56 per cent) and sensitivity (33–40 per cent). The positive predictive value in the present study (40 per cent) confirms that preoperative CT can lead to overdiagnosis of PLNM. A positive predictive value for PET of 100 per cent has been reported27, but the number of patients with increased fluorodeoxyglucose uptake was small. The diagnostic accuracy of PET–CT was not assessed in the present study because not all patients underwent such imaging, an institutional decision following the PETCAM (Impact of Positron Emission Tomography Imaging Prior to Liver Resection for Colorectal Adenocarcinoma Metastases) trial29.
Previously reported risk factors for PLNM include number and location of CRLMs, CRLMs present at initial diagnosis (synchronous metastases), CEA level and peritoneal metastases10,30. Data from the present analysis show that primary tumour LN metastases and intrahepatic recurrence requiring repeat hepatectomy are significant risk factors for PLNM. Although RAS mutation is an established poor prognostic factor for patients with colorectal cancer31,32, it was not associated with PLNM here.
Several previous studies10,12,17 showed a significant survival impact of PLNM location (coeliac trunk or aortocaval). In an analysis of patients with PLNM in the context of modern chemotherapy, survival did not differ by PLNM location (periportal versus coeliac trunk LNs)21, consistent with the present findings. Here, OS was significantly better in patients with para-aortic LN metastases compared with patients with PLNM along the hepatoduodenal ligament. This might be because para-aortic metastases may develop from the primary tumour rather than CRLMs. In this context, resection of para-aortic LN metastases with the primary tumour may improve survival33.
Although it was reported previously that the ratio of metastatic to total number of resected perihepatic LNs is an independent predictor of poor RFS and OS after hepatectomy with lymphadenectomy for CRLM21, no significant differences in RFS or OS were observed in the present cohort when the patients were dichotomized according to LN ratio as in the previous report (data not shown). A previous study22 of patients with ICC indicated that the number of PLNMs (less than 3 versus 3 or more) was a significant prognostic factor for OS. Therefore, this grouping was used in the present analysis, which showed that OS was significantly better in patients with one or two PLNMs. Consistent with data from other hepatobiliary cancers (such as perihilar cholangiocarcinoma and ICC)22,34–35, the number of PLNMs but not the location was found to be prognostic.
Limitations of this study include the retrospective nature of the analysis and that the cohort was treated in a single institution. However, the study reports on one of the largest cohorts of patients with PLNM who underwent perihepatic lymphadenectomy. Patients who had extensive PLNM on preoperative imaging, and who did not undergo hepatectomy as a result of this finding, were not included in the analysis. This might have reduced the positive predictive value of preoperative modalities. Multiple testing for survival in patients with PLNMs (number and location of metastases) is a potential issue, and multivariable analysis for survival among patients with PLNM was not performed because of the small sample size.
Acknowledgements
The authors thank S. Deming for language editing support. The University of Texas MD Anderson Cancer Center is supported in part by the National Institutes of Health/National Cancer Institute under award number P30CA016672.
Disclosure: The authors declare no conflict of interest.
Snapshot quiz 18/10

References
- 1. Stintzing S, Modest DP, Rossius L, Lerch MM, von Weikersthal LF, Decker Tet al. . FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol 2016; 17: 1426–1434. [DOI] [PubMed] [Google Scholar]
- 2. Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel Met al. . Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010; 28: 4697–4705. [DOI] [PubMed] [Google Scholar]
- 3. Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi Set al. . FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol 2015; 16: 1306–1315. [DOI] [PubMed] [Google Scholar]
- 4. Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RDet al. . Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 2002; 235: 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andreou A, Aloia TA, Brouquet A, Dickson PV, Zimmitti G, Maru DMet al. . Margin status remains an important determinant of survival after surgical resection of colorectal liver metastases in the era of modern chemotherapy. Ann Surg 2013; 257: 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cummings LC, Payes JD, Cooper GS. Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer 2007; 109: 718–726. [DOI] [PubMed] [Google Scholar]
- 7. Leung U, Gonen M, Allen PJ, Kingham TP, DeMatteo RP, Jarnagin WRet al. . Colorectal cancer liver metastases and concurrent extrahepatic disease treated with resection. Ann Surg 2017; 265: 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chua TC, Saxena A, Liauw W, Chu F, Morris DL. Hepatectomy and resection of concomitant extrahepatic disease for colorectal liver metastases – a systematic review. Eur J Cancer 2012; 48: 1757–1765. [DOI] [PubMed] [Google Scholar]
- 9. Christophi C, Nguyen L, Muralidharan V, Nikfarjam M, Banting J. Lymphatics and colorectal liver metastases: the case for sentinel node mapping. HPB 2014; 16: 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jaeck D, Nakano H, Bachellier P, Inoue K, Weber JC, Oussoultzoglou Eet al. . Significance of hepatic pedicle lymph node involvement in patients with colorectal liver metastases: a prospective study. Ann Surg Oncol 2002; 9: 430–438. [DOI] [PubMed] [Google Scholar]
- 11. Beckurts KT, Holscher AH, Thorban S, Bollschweiler E, Siewert JR. Significance of lymph node involvement at the hepatic hilum in the resection of colorectal liver metastases. Br J Surg 1997; 84: 1081–1084. [PubMed] [Google Scholar]
- 12. Adam R, de Haas RJ, Wicherts DA, Aloia TA, Delvart V, Azoulay Det al. . Is hepatic resection justified after chemotherapy in patients with colorectal liver metastases and lymph node involvement? J Clin Oncol 2008; 26: 3672–3680. [DOI] [PubMed] [Google Scholar]
- 13. Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJet al. . Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 2000; 343: 905–914. [DOI] [PubMed] [Google Scholar]
- 14. Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek Pet al. . Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 2000; 355: 1041–1047. [DOI] [PubMed] [Google Scholar]
- 15. Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SKet al. . A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004; 22: 23–30. [DOI] [PubMed] [Google Scholar]
- 16. Kokudo N, Sato T, Seki M, Ohta H, Azekura K, Ueno Met al. . Hepatic lymph node involvement in resected cases of liver metastases from colorectal cancer. Dis Colon Rectum 1999; 42: 1285–1290. [DOI] [PubMed] [Google Scholar]
- 17. Pulitano C, Bodingbauer M, Aldrighetti L, Choti MA, Castillo F, Schulick RDet al. . Colorectal liver metastasis in the setting of lymph node metastasis: defining the benefit of surgical resection. Ann Surg Oncol 2012; 19: 435–442. [DOI] [PubMed] [Google Scholar]
- 18. Adam R, de Haas RJ, Wicherts DA, Vibert E, Salloum C, Azoulay Det al. . Concomitant extrahepatic disease in patients with colorectal liver metastases: when is there a place for surgery? Ann Surg 2011; 253: 349–359. [DOI] [PubMed] [Google Scholar]
- 19. Nanji S, Tsang ME, Wei X, Booth CM. Regional lymph node involvement in patients undergoing liver resection for colorectal cancer metastases. Eur J Surg Oncol 2017; 43: 322–329. [DOI] [PubMed] [Google Scholar]
- 20. Hodgson R, Sethi H, Ling AH, Lodge P. Combined hepatectomy and hepatic pedicle lymphadenectomy in colorectal liver metastases is justified. HPB (Oxford) 2017; 19: 525–529. [DOI] [PubMed] [Google Scholar]
- 21. Oussoultzoglou E, Romain B, Panaro F, Rosso E, Pessaux P, Bachellier Pet al. . Long-term survival after liver resection for colorectal liver metastases in patients with hepatic pedicle lymph nodes involvement in the era of new chemotherapy regimens. Ann Surg 2009; 249: 879–886. [DOI] [PubMed] [Google Scholar]
- 22. Nakagawa T, Kamiyama T, Kurauchi N, Matsushita M, Nakanishi K, Kamachi Het al. . Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma. World J Surg 2005; 29: 728–733. [DOI] [PubMed] [Google Scholar]
- 23. Tamandl D, Kaczirek K, Gruenberger B, Koelblinger C, Maresch J, Jakesz Ret al. . Lymph node ratio after curative surgery for intrahepatic cholangiocarcinoma. Br J Surg 2009; 96: 919–925. [DOI] [PubMed] [Google Scholar]
- 24. Brudvik KW, Mise Y, Conrad C, Zimmitti G, Aloia TA, Vauthey JN. Definition of readmission in 3041 patients undergoing hepatectomy. J Am Coll Surg 2015; 221: 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng Cet al. . Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg 2005; 241: 715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grobmyer SR, Wang L, Gonen M, Fong Y, Klimstra D, D'Angelica Met al. . Perihepatic lymph node assessment in patients undergoing partial hepatectomy for malignancy. Ann Surg 2006; 244: 260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rau C, Blanc B, Ronot M, Dokmak S, Aussilhou B, Faivre Set al. . Neither preoperative computed tomography nor intra-operative examination can predict metastatic lymph node in the hepatic pedicle in patients with colorectal liver metastasis. Ann Surg Oncol 2012; 19: 163–168. [DOI] [PubMed] [Google Scholar]
- 29. Moulton CA, Gu CS, Law CH, Tandan VR, Hart R, Quan Det al. . Effect of PET before liver resection on surgical management for colorectal adenocarcinoma metastases: a randomized clinical trial. JAMA 2014; 311: 1863–1869. [DOI] [PubMed] [Google Scholar]
- 30. Elias D, Saric J, Jaeck D, Arnaud JP, Gayet B, Rivoire Met al. . Prospective study of microscopic lymph node involvement of the hepatic pedicle during curative hepatectomy for colorectal metastases. Br J Surg 1996; 83: 942–945. [DOI] [PubMed] [Google Scholar]
- 31. Vauthey JN, Zimmitti G, Kopetz SE, Shindoh J, Chen SS, Andreou Aet al. . RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg 2013; 258: 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Denbo JW, Yamashita S, Passot G, Egger M, Chun YS, Kopetz SEet al. . RAS mutation is associated with decreased survival in patients undergoing repeat hepatectomy for colorectal liver metastases. J Gastrointest Surg 2017; 21: 68–77. [DOI] [PubMed] [Google Scholar]
- 33. Wong JS, Tan GH, Teo MC. Management of para-aortic lymph node metastasis in colorectal patients: a systemic review. Surg Oncol 2016; 25: 411–418. [DOI] [PubMed] [Google Scholar]
- 34. Aoba T, Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Yet al. . Assessment of nodal status for perihilar cholangiocarcinoma: location, number, or ratio of involved nodes. Ann Surg 2013; 257: 718–725. [DOI] [PubMed] [Google Scholar]
- 35. Guglielmi A, Ruzzenente A, Campagnaro T, Valdegamberi A, Bagante F, Bertuzzo Fet al. . Patterns and prognostic significance of lymph node dissection for surgical treatment of perihilar and intrahepatic cholangiocarcinoma. J Gastrointest Surg 2013; 17: 1917–1928. [DOI] [PubMed] [Google Scholar]


