Abstract
Zoledronic acid (ZA) is an effective agent in osteoporosis and malignancy-related bone disease but may be associated with increased risk of atrial fibrillation (AF), although current studies disagree on this risk. To examine the risk of incident AF among patients receiving ZA compared with denosumab in the first year of treatment, we performed a new-user, active comparator cohort study including privately insured Americans between January 1, 2010, and June 30, 2019. Individuals aged ≥50 years without known arrhythmia or advanced kidney disease who initiated ZA were 1:1 propensity score (PS)-matched to individuals initiating denosumab in separate osteoporosis and malignancy cohorts. The primary outcome was incident diagnosis of AF (≥1 inpatient or ≥2 outpatient diagnostic codes) over 1 year. Secondary outcomes included stroke/transient ischemic attack (TIA) and nonvertebral fracture. In the osteoporosis cohort (n = 16,235 pairs), mean age was 71 years, and 93% were female. There was higher risk of AF with ZA compared with denosumab over 1 year (incidence rate [IR] = 18.6 versus 14.9 per 1000 person-years; hazard ratio [HR] = 1.25; 95% confidence interval [CI] 1.04 to 1.50). In the malignancy cohort (n = 7732 pairs), mean age was 70 years, and 66% were female. There was a numerically higher, albeit not statistically significant, risk of AF with ZA compared with denosumab over 1 year (IR = 46.9 versus 39.0 per 1000 person-years; HR = 1.19; 95% CI 1.00 to 1.43; p = 0.06). No difference in stroke/TIA rates occurred. In the malignancy cohort, ZA was less effective than denosumab at preventing nonvertebral fractures (HR = 1.32; 95% CI 1.01 to 1.74). Compared with denosumab, ZA treatment for osteoporosis and possibly for malignancy-related bone disease is associated with modestly increased risk of incident AF in the first year of treatment.
Keywords: ZOLEDRONIC ACID, DENOSUMAB, ATRIAL FIBRILLATION, OSTEOPOROSIS
Introduction
Bisphosphonates, such as intravenous zoledronic acid (ZA) or oral alendronate, are commonly used as initial therapy for osteoporosis and malignancy-related bone disease.(1) Although bisphosphonates are highly efficacious and generally safe, there remain specific safety concerns, including atypical femoral fractures, osteonecrosis of the jaw, and increased atrial fibrillation (AF).(2–4) In the HORIZON Pivotal Fracture Trial (PFT), a randomized controlled trial (RCT) of ZA versus placebo in postmenopausal women with osteoporosis, an unexpected and significant increase in arrhythmias driven by rates of serious AF (deemed life-threatening or resulting in hospitalization or disability) was found in those treated with ZA annually for 3 years.(2) Overall rates of AF were similar in the two groups, but serious AF events were documented more commonly in the ZA group (1.3% ZA versus 0.5% placebo; p < 0.001), most often occurring at least 30 days after the infusion.(2)
By contrast, neither the 3-year extension of the HORIZON-PFT nor the HORIZON Recurrent Fracture Trial (RFT) showed a significant increase in AF or arrhythmias with ZA.(5,6) Additionally, a meta-analysis of seven observational studies showed an overall pooled odds ratio of 1.04 (95% confidence interval [CI] 0.92 to 1.16) for atrial fibrillation with bisphosphonate use.(7)
Given conflicting results from prior placebo-controlled RCTs and observational studies, we examined the risk of incident AF in patients initiating ZA compared with denosumab, a monoclonal antibody to receptor activator of NF-κB ligand (RANKL), which is not known to have an association with AF, in osteoporosis and malignancy cohorts using a large private insurance claims database. We compared new users of ZA to new users of an active comparator (ie, denosumab), a pharmacoepidemiologic study design that reduces the risks of confounding and selection bias.(8,9)
Materials and Methods
Data source
We conducted a cohort study using claims data from the Clinformatics Data Mart (Optum, Inc., Eden Prairie, MN, USA) from January 1, 2010, to June 30, 2019. This database contains longitudinal claims from UnitedHealth Group and Medicare Advantage members, including diagnosis codes, procedure codes, pharmacy dispensing, and outpatient and inpatient claims. Approximately 75.5 million fully insured (both medical and pharmacy coverage) individuals are included, with representation from across the United States (US). The data were deidentified before analysis, and informed consent was therefore not required. The study protocol was approved by the Institutional Review Board of Brigham and Women’s Hospital (2011P002580–158).
Study cohort
We included patients aged ≥50 years who newly initiated ZA or denosumab. The cohort entry date was the first date of ZA or denosumab administration. Because ZA is given approximately every 365 days and denosumab is given every 180 days, subjects were required to have a minimum of 455 days of continuous insurance eligibility without record of ZA or denosumab administration before the index date.(10) For the osteoporosis cohort (Fig. 1), patients were required to have ≥1 International Classification of Diseases 9th or 10th revision (ICD-9 or ICD-10) code for osteoporosis (ICD-9: 733.0; ICD-10: M80, M81) but no active malignancy (defined as a malignancy diagnosis code associated with oncology provider claim), bone marrow transplant, or radiation therapy during the covariate assessment period. For the malignancy cohort (Fig. 2), patients were required to have ≥1 diagnosis code for multiple myeloma or solid organ malignancies commonly associated with bone metastases (breast, prostate, thyroid, lung, bladder, renal, and pancreatic cancers or melanoma) from oncology provider claims, bone marrow transplant, or radiation therapy (diagnosis and procedure codes in Supplemental Table S1). Patients in the active malignancy cohort were permitted to also have a diagnosis of osteoporosis. For both cohorts, exclusion criteria included exposure to both ZA and denosumab at the same time, prior exposure to ZA or denosumab, ineligibility to receive ZA (chronic kidney disease [CKD] stage 4 or 5, end-stage renal disease, dialysis) within the 455-day covariate assessment period, or prior AF or suspicion for prior AF (based on diagnosis, procedure, or medication dispensing codes suggestive of underlying arrhythmia) in all available data before the cohort entry date.
Fig 1.
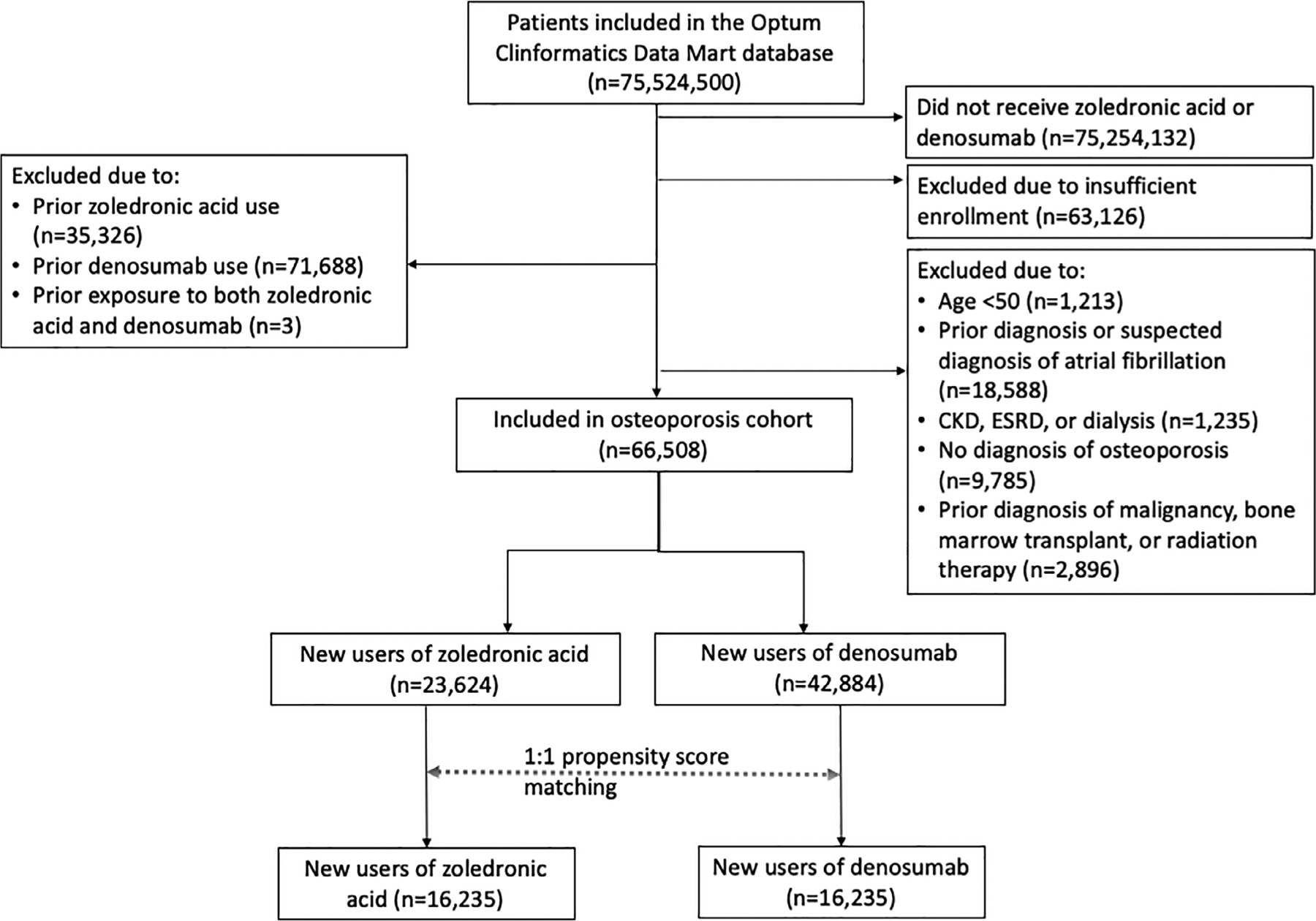
Osteoporosis cohort selection. The final osteoporosis study cohort included a total of 16,235 propensity score–matched pairs of denosumab and zoledronic acid initiators. CKD = chronic kidney disease; ESRD = end-stage renal disease.
Fig 2.
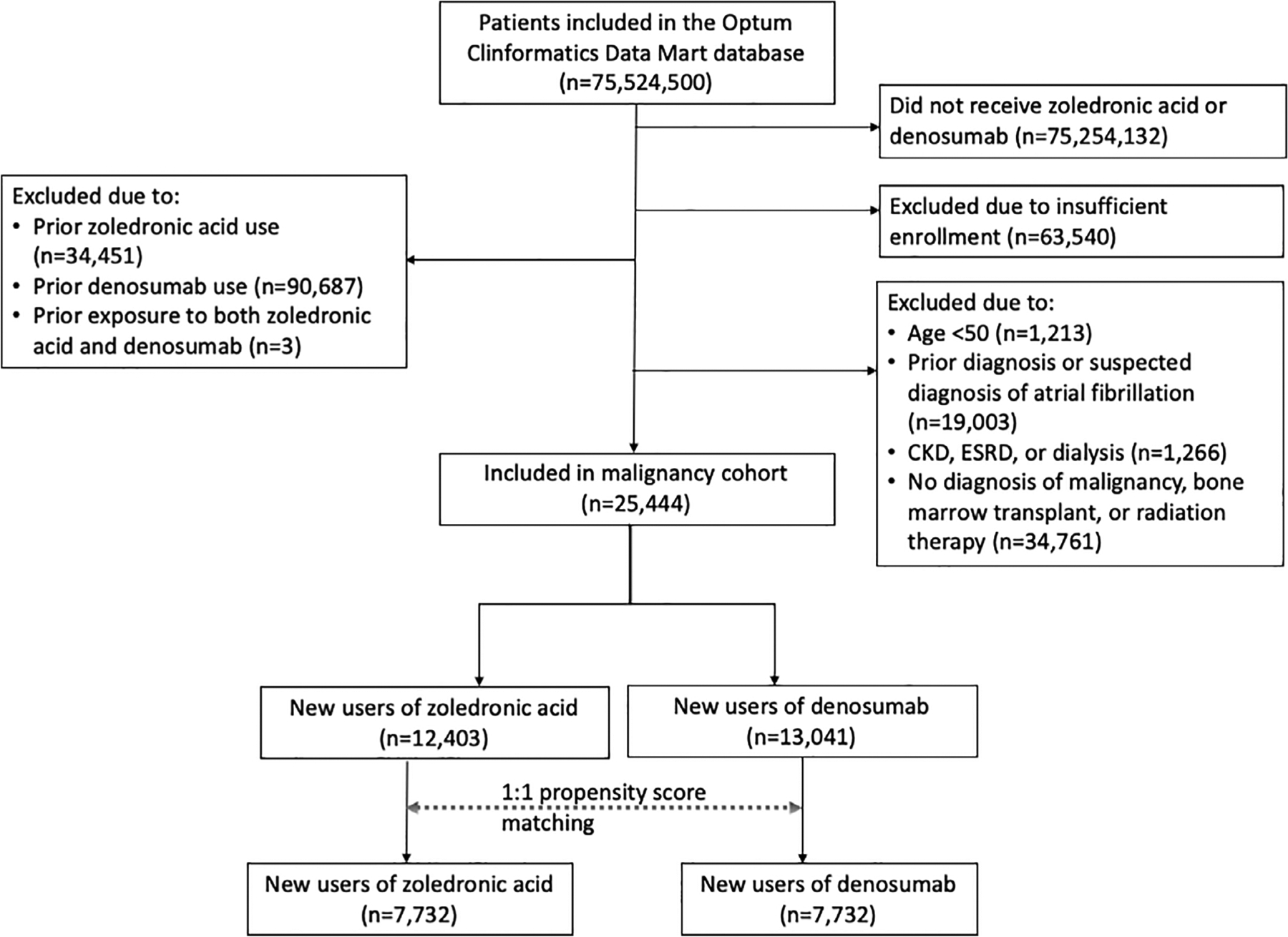
Malignancy cohort selection. The final malignancy study cohort included a total of 7732 propensity score–matched pairs of denosumab and zoledronic acid initiators. CKD = chronic kidney disease; ESRD = end-stage renal disease.
Outcome definition
The primary outcome was incident AF based on a diagnosis code for AF (ICD-9: 427.3; ICD-10: I48.0, I48.1, I48.2, I48.3, I48.4, or I48.9) from either ≥1 inpatient visit or ≥2 outpatient visits (henceforth called “AF diagnosis;” Supplemental Table S1), a previously defined measure to assess incident AF in claims databases using at least 1 inpatient and 2 outpatient claims to improve the specificity of AF classification.(11,12) Secondary outcomes included an inpatient diagnosis code for AF (henceforth called “AF hospitalization”), a previously validated metric for identifying incident AF hospitalization in claims databases with sensitivity 95% and specificity 99%,(13) and any AF diagnosis code combined with dispensing of AF medications (henceforth “AF + medication dispensing”).(14,15) Non-cardiac secondary outcomes included a safety outcome of occurrence of stroke or transient ischemic attack (stroke/TIA), considered a possible downstream consequence of AF, and an effectiveness outcome of occurrence of nonvertebral osteoporotic and pathologic fracture of the humerus, wrist, hip, or pelvis (henceforth “nonvertebral fractures”) by diagnosis codes and/or procedure codes, a previously validated measure with a positive predictive value more than 93%.(16–18)
Follow-up started on the day immediately after the cohort entry date and continued through the earliest of the following: occurrence of the study outcome, switch to the alternate medication, 365th day of follow-up, insurance disenrollment, death, or end of study period.
Covariates
Baseline variables potentially related to initiation of denosumab or ZA and/or development of AF were examined during the 455-day covariate assessment period before the cohort entry date. These variables included demographic factors (age, sex, region, year of cohort entry), markers of health care utilization, comorbidities, and medications (Table 1). Smoking and tobacco use were measured as previously described.(19) To further quantify the burden of comorbidities, we calculated a combined comorbidity score and claims-based frailty index using diagnostic codes for the 365-day period before the cohort entry date.(20,21)
Table 1.
Baseline Characteristics of Study Patients in the 455 Days Before Receipt of Zoledronic Acid or Denosumab Over 455-Day Covariate Assessment Period: 1:1 Propensity Score Matched
| Osteoporosis cohort | Malignancy cohort | |||||
|---|---|---|---|---|---|---|
| Characteristic | Zoledronic acid (n = 16,235) | Denosumab (n = 16,235) | Standardized difference | Zoledronic acid (n = 7732) | Denosumab (n = 7732) | Standardized difference |
| Demographic | ||||||
| Age (years) | 71.7 ± 8.6 | 71.6 ± 9.0 | −0.01 | 70.1 ± 8.7 | 70.1 ± 8.9 | <0.001 |
| Female sex | 15,147 (93.3) | 15,112 (93.1) | −0.01 | 5131 (66.4) | 5171 (66.9) | 0.01 |
| United Health | 2821 (17) | 3706 (23) | 0.15 | 1998 (26) | 2170 (28) | 0.05 |
| Medicare Advantage | 13,414 (83) | 12,529 (77) | −0.15 | 5734 (74) | 5562 (72) | −0.05 |
| Health care utilization | ||||||
| Primary care office visits | 6.7 ± 6.8 | 6.7 ± 6.7 | <0.001 | 6.5 ± 7.1 | 6.6 ± 7.4 | 0.01 |
| Cardiology office visits | 0.7 ± 1.7 | 0.7 ± 1.7 | <0.001 | 0.8 ± 1.8 | 0.8 ± 1.8 | <0.001 |
| Endocrine office visits | 0.3 ± 1.1 | 0.4 ± 1.2 | 0.09 | 0.2 ± 0.9 | 0.2 ± 1.1 | <0.001 |
| Emergency room visits | 0.5 ± 1.0 | 0.5 ± 1.0 | <0.001 | 0.5 ± 0.9 | 0.5 ± 1.0 | <0.001 |
| Hospitalizations | 0.2 ± 0.6 | 0.2 ± 0.6 | <0.001 | 0.3 ± 0.6 | 0.3 ± 0.6 | <0.001 |
| Prescription claims | 22.8 ± 20.5 | 22.7 ± 20.6 | <0.001 | 20.9 ± 18.4 | 20.8 ± 18.3 | −0.01 |
| Electrocardiogram performed | 7115 (43.8) | 7026 (43.3) | −0.01 | 4456 (57.6) | 4433 (57.3) | −0.01 |
| Echocardiogram performed | 2390 (14.7) | 2417 (14.9) | 0.01 | 1785 (23.1) | 1793 (23.2) | <0.001 |
| Bone density scan performed | 12,519 (77.1) | 12,479 (76.9) | <0.001 | 2960 (38.3) | 2978 (38.5) | <0.001 |
| History of comorbidities | ||||||
| Combined comorbidity scorea | 0.9 ± 1.5 | 0.9 ± 1.6 | <0.001 | 2.7 ± 2.2 | 2.7 ± 2.1 | <0.001 |
| Frailty scoreb | 0.2 ± 0.1 | 0.2 ± 0.1 | <0.001 | 0.1 ± 0.1 | 0.1 ± 0.1 | <0.001 |
| Hypertension | 9569 (58.9) | 9535 (58.7) | <0.001 | 5021 (64.9) | 5016 (64.9) | <0.001 |
| Hyperlipidemia | 10,050 (61.9) | 10,061 (62.0) | <0.001 | 4592 (59.4) | 4572 (59.1) | −0.01 |
| Obesity | 970 (6.0) | 969 (6.0) | <0.001 | 917 (11.9) | 942 (12.2) | 0.01 |
| Diabetes mellitus | 2586 (15.9) | 2573 (15.8) | <0.001 | 1904 (24.6) | 1909 (24.7) | <0.001 |
| Coronary artery disease | 1893 (11.7) | 1898 (11.7) | <0.001 | 1255 (16.2) | 1245 (16.1) | <0.001 |
| Coronary artery bypass graft | 154 (0.9) | 159 (1.0) | 0.01 | 159 (2.1) | 147 (1.9) | −0.01 |
| Cerebrovascular disease | 1454 (9.0) | 1433 (8.8) | −0.01 | 800 (10.3) | 798 (10.3) | <0.001 |
| Peripheral vascular disease | 1897 (11.7) | 1896 (11.7) | <0.001 | 1020 (13.2) | 1032 (13.3) | <0.001 |
| Congestive heart failure | 583 (3.6) | 582 (3.6) | <0.001 | 405 (5.2) | 415 (5.4) | 0.01 |
| Mitral or aortic valve disease | 1003 (6.2) | 1001 (6.2) | <0.001 | 550 (7.1) | 543 (7.0) | <0.001 |
| Congenital heart disease | 95 (0.6) | 103 (0.6) | <0.001 | 42 (0.5) | 42 (0.5) | <0.001 |
| Obstructive sleep apnea | 1095 (6.7) | 1067 (6.6) | <0.001 | 606 (7.8) | 600 (7.8) | <0.001 |
| COPD | 1847 (11.4) | 1730 (10.7) | −0.02 | 1264 (16.3) | 1257 (16.3) | <0.001 |
| Hyperthyroidism | 326 (2.0) | 316 (1.9) | −0.01 | 106 (1.4) | 108 (1.4) | <0.001 |
| Liver disease | 635 (3.9) | 642 (4.0) | 0.01 | 809 (10.5) | 798 (10.3) | −0.01 |
| Stage 2 CKD | 307 (1.9) | 310 (1.9) | <0.001 | 178 (2.3) | 171 (2.2) | −0.01 |
| Stage 3 CKD | 828 (5.1) | 850 (5.2) | <0.001 | 529 (6.8) | 541 (7.0) | 0.01 |
| Tobacco use | 1582 (9.7) | 1561 (9.6) | <0.001 | 1406 (18.2) | 1436 (18.6) | 0.01 |
| Alcohol use | 198 (1.2) | 199 (1.2) | <0.001 | 122 (1.6) | 125 (1.6) | <0.001 |
| Medication use | ||||||
| Amphetamines | 189 (1.2) | 176 (1.1) | −0.01 | 60 (0.8) | 68 (0.9) | 0.01 |
| Beta blockers | 2808 (17.3) | 2746 (16.9) | −0.01 | 1550 (20.0) | 1542 (19.9) | <0.001 |
| Calcium channel blocker | 392 (2.4) | 379 (2.3) | −0.01 | 154 (2.0) | 152 (2.0) | <0.001 |
| ACE inhibitor or ARB | 5040 (31.0) | 5039 (31.0) | <0.001 | 2816 (36.4) | 2828 (36.6) | <0.001 |
| Anticoagulants | 370 (2.3) | 374 (2.3) | <0.001 | 388 (5.0) | 388 (5.0) | <0.001 |
| ≥2 NSAID prescriptions | 2831 (17.4) | 2819 (17.4) | <0.001 | 1242 (16.1) | 1248 (16.1) | <0.001 |
| ≥2 glucocorticoid prescriptions | 4611 (28.4) | 4556 (28.1) | −0.01 | 3721 (48.1) | 3760 (48.6) | 0.01 |
| ≥2 opioid prescriptions | 4115 (25.3) | 4006 (24.7) | −0.01 | 3431 (44.4) | 3418 (44.2) | <0.001 |
| Prior bisphosphonate usec | 3643 (22.4) | 3631 (22.4) | <0.001 | 750 (9.7%) | 782 (10.1) | 0.01 |
COPD = chronic obstructive pulmonary disease; CKD = chronic kidney disease; ACE inhibitor = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; NSAID = nonsteroidal anti-inflammatory drug.
Values are number (percentage) for categorical or mean ± SD for continuous variables. In addition to variables listed here, region and year of drug administration were also included in propensity score matching.
Assessed over 365-day covariate assessment period.(20)
Assessed over 365-day covariate assessment period.(21)
Prior bisphosphonate use, not including zoledronate.
Statistical analyses
To control for potential confounding, we performed a propensity score (PS) analysis in each cohort using a multivariable logistic regression model with the treatment (ZA versus denosumab) as the dependent variable and the aforementioned covariates from Table 1 as independent variables (osteoporosis cohort matched on 43 variables, malignancy cohort matched on 44 including the presence of osteoporosis). We used a PS analysis with 1:1 matching without replacement and nearest neighbor matching with a caliper of 0.02 on the PS scale. We assessed covariate balance between the PS-matched cohorts using standardized differences, with a value below 0.1 indicating negligible differences between treatment groups.(22)
After PS matching, we estimated the incidence rate (IR) with 95% CIs for ZA and denosumab users for each outcome. Cox proportional hazards models were used to estimate the hazard ratio (HR) and 95% CIs of the outcomes within 365 days after the initiation of ZA versus denosumab. The primary analysis was an as-treated design, which allowed the ability to control for variable lengths of follow-up, up to 1 year. A Kaplan–Meier plot was used to visualize the time to outcome separately by group.
A prespecified subgroup analysis was performed for patients in each cohort who had cardiovascular disease (defined as diagnostic codes for coronary artery disease, heart failure, peripheral vascular disease, or cerebrovascular disease). In addition, we conducted several sensitivity analyses to assess the robustness of our findings. For osteoporosis, the recommended frequency of administration of ZA is annually and denosumab twice annually, whereas for malignancy, ZA and denosumab can be administered up to weekly. Given the potential cumulative dose-related differences between the osteoporosis and malignancy cohorts, we applied as-treated analyses with variable follow-up times of 30, 60, 90, and 180 days. Second, as patients with multiple myeloma disproportionately received ZA over denosumab during the study period, we evaluated the findings in the subgroup of patients with active malignancies not including multiple myeloma.
All analyses were conducted using the validated Aetion Platform V3.12 (Aetion, New York, NY, USA) and R version 3.1.2 (The R Foundation, Vienna, Austria).(23) The p values were two-sided, and a significance level was set at 0.05.
Results
Osteoporosis cohort
There were 21,332 ZA and 38,124 denosumab new initiators with osteoporosis and without active malignancy in the study database (Fig. 1; Supplemental Table S2). After 1:1 PS matching, 16,235 pairs of ZA and denosumab initiators were identified, with well-balanced baseline characteristics (Table 1). Both groups had a mean age of 71 years and were 93% female.
The incidence rate of AF was 18.60 and 14.91 per 1000 person-years in the ZA and denosumab users, respectively (Table 2). When compared with PS-matched denosumab initiators, ZA initiators had higher risk of incident AF diagnosis (HR = 1.25; 95% CI 1.04 to 1.50). Risk of AF hospitalization (HR = 1.38; 95% CI 1.03 to 1.85) and AF + medication dispensing (HR = 1.30; 95% CI 1.02 to 1.67) were also higher with ZA compared with denosumab (Table 2; Fig. 3A). There was no difference in risk of strokes/TIA or nonvertebral fractures between groups. In the cardiovascular disease subgroup (n = 4318 PS-matched pairs), there was a trend toward increased AF diagnosis in the ZA group (HR = 1.22; 95% CI 0.93 to 1.61) (Supplemental Table S3).
Table 2.
Incidence Rates of Primary (AF Diagnosis) and Secondary Outcomes Within 365 Days After Initiation of Zoledronic Acid or Denosumab in the Propensity Score–Matched Study Patients
| Osteoporosis cohort | |||||||
|---|---|---|---|---|---|---|---|
| Zoledronic acid (n = 16,235) | Denosumab (n = 16,235) | ||||||
| n | PY | IR/1000 PY (95% CI) | n | PY | IR/1000 PY (95% CI) | HR (95% CI)a | |
| AF diagnosisb | 251 | 13,496 | 18.60 (16.37, 21.05) | 201 | 13,483 | 14.91 (12.92, 17.12) | 1.25 (1.04, 1.50) |
| AF hospitalization | 105 | 13,552 | 7.75 (6.34, 9.38) | 76 | 13,528 | 5.62 (4.43, 7.03) | 1.38 (1.03, 1.85) |
| AF + medication dispensingc | 146 | 13,534 | 10.79 (9.11, 12.69) | 112 | 13,516 | 8.29 (6.82, 9.97) | 1.30 (1.02, 1.67) |
| Stroke/transient ischemic attack | 69 | 13,568 | 5.09 (3.96, 6.44) | 60 | 13,538 | 4.43 (3.38, 5.70) | 1.15 (0.81, 1.62) |
| Nonvertebral osteoporotic fracture | 233 | 13,480 | 17.28 (15.14, 19.65) | 252 | 13,428 | 18.77 (16.52, 21.23) | 0.92 (0.77, 1.10) |
| Malignancy cohort | |||||||
| Zoledronic acid (n = 7732) | Denosumab (n = 7732) | ||||||
| n | PY | IR/1000 PY (95% CI) | n | PY | IR/1000 PY (95% CI) | HR (95% CI)a | |
| AF diagnosisb | 254 | 5419 | 46.87 (41.28, 53.01) | 222 | 5688 | 39.03 (34.06, 44.52) | 1.19 (1.00, 1.43) |
| AF hospitalization | 84 | 5468 | 15.36 (12.25, 19.02) | 81 | 5724 | 14.15 (11.24, 17.59) | 1.08 (0.79, 1.46) |
| AF + medication dispensingc | 120 | 5457 | 21.99 (18.23, 26.29) | 110 | 5717 | 19.24 (15.81, 23.19) | 1.14 (0.88, 1.47) |
| Stroke/transient ischemic attack | 41 | 5488 | 7.47 (5.36, 10.14) | 53 | 5737 | 9.24 (6.92, 12.08) | 0.80 (0.53, 1.21) |
| Nonvertebral osteoporotic fracture | 118 | 5444 | 21.68 (17.94, 25.96) | 92 | 5703 | 16.13 (13.00, 19.78) | 1.32 (1.01, 1.74) |
PY = person-years; IR = incidence rate; IRR = incidence rate ratio; CI = confidence interval; HR = hazard ratio; AF = atrial fibrillation.
Denosumab users were the reference group.
AF diagnosis = 1 inpatient or 2 outpatient diagnosis codes for AF.
Medication dispensing = prescription claim for beta blocker, non-dihydropyridine calcium channel blocker, or anticoagulant within 30 days of inpatient or outpatient AF diagnostic code.
Fig 3.
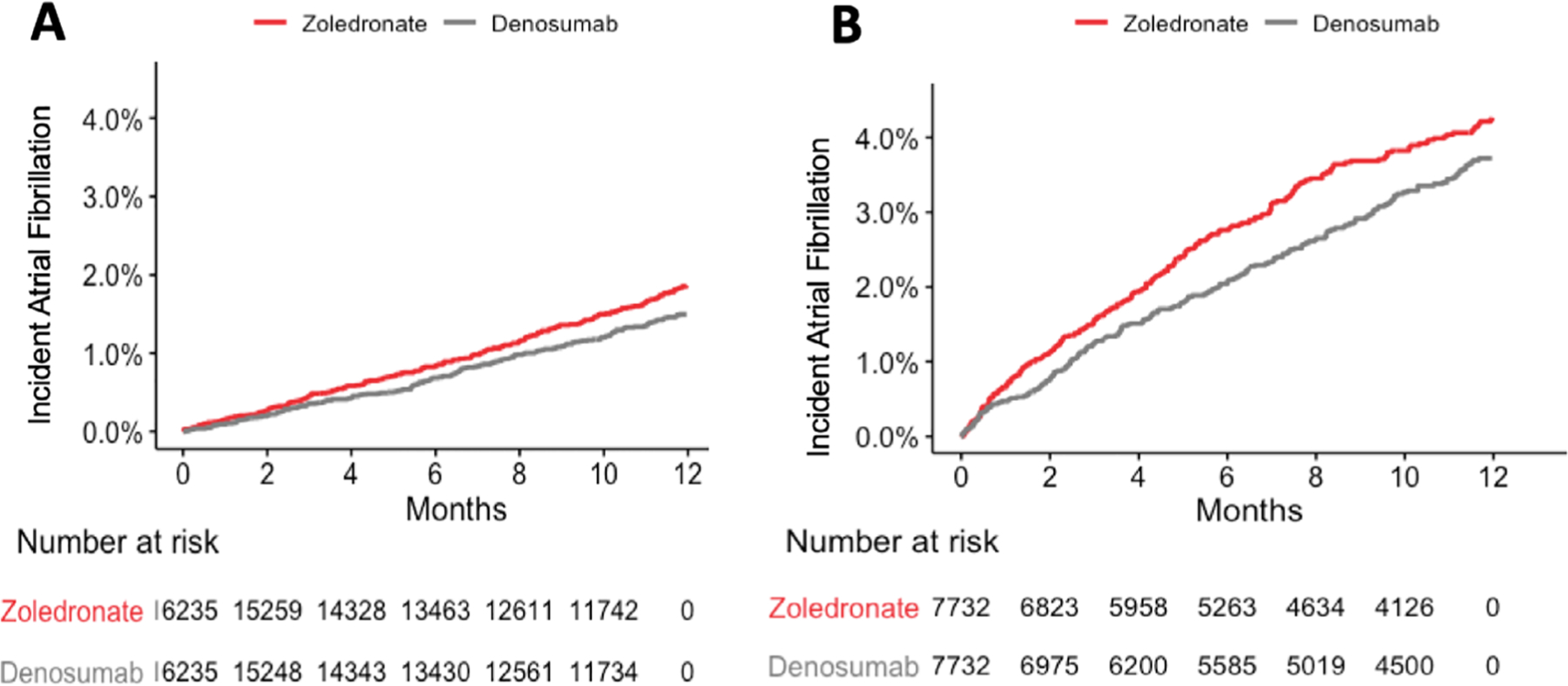
Kaplan–Meier curves for primary outcome (one inpatient or two outpatient codes for atrial fibrillation) in osteoporosis cohort (A) and malignancy cohort (B) with zoledronic acid versus denosumab. AF = atrial fibrillation.
Malignancy cohort
There were 11,195 ZA and 11,761 denosumab new initiators with active malignancy in the study database (Fig. 2; Supplemental Table S2). After 1:1 PS matching, 7732 pairs of ZA and denosumab initiators were identified, with well-balanced baseline characteristics (Table 1). Both groups had a mean age of 70 years and were 66% female. In general, malignancies were distributed roughly evenly between the ZA and denosumab groups (within ±10%), except for multiple myeloma (19.2% in the ZA group versus 3.2% in the denosumab group; Supplemental Table S4). Compared with the osteoporosis cohort, the malignancy cohort had higher Charlson comorbidity indices (2.7 versus 0.9), diabetes (25% versus 16%), coronary artery disease (16% versus 12%), chronic obstructive pulmonary disease (16% versus 11%), and evaluation of cardiac status based on electrocardiograms (57% versus 43%) and echocardiograms (23% versus 15%).
Incident AF rates in the malignancy cohort were higher than in the osteoporosis cohort, with IRs of 46.87 and 39.03 per 1000 person-years in the ZA and denosumab users, respectively. At 365 days, there was a trend toward higher risk of incident AF diagnosis with ZA compared with denosumab (HR = 1.19; 95% CI 1.00 to 1.43; p = 0.06) (Table 2; Fig. 3B). In contrast to the osteoporosis cohort, there was no significantly higher risk of AF hospitalization or AF + medication dispensing in the malignancy cohort. Similar to the osteoporosis cohort, there was no increased risk of stroke/TIA. Importantly, in contrast to the osteoporosis cohort, ZA was less effective at preventing nonvertebral fractures than denosumab (HR = 1.32 for fracture; 95% CI 1.01 to 1.74). In the cardiovascular disease subgroup of the malignancy cohort (n = 2468 PS-matched pairs), there was a significantly higher risk of AF diagnosis (HR = 1.36; 95% CI 1.05 to 1.76) and AF hospitalization (HR = 1.60; 95% CI 1.05 to 2.46) (Supplemental Table S3). In the sensitivity analysis of the malignancy cohort excluding multiple myeloma patients, we noted similar findings compared with the primary analysis findings; specifically, there was a trend toward increased incident AF in ZA users (HR = 1.20; 95% CI 0.98 to 1.46) (Supplemental Tables S5 and S6; Supplemental Figs. S1 and S2).
The median number of doses of ZA received in the initial 180 days of follow-up was higher in the malignancy cohort (median 2.0; IQR 1.0 to 5.0) compared with the osteoporosis cohort (median 1.0; IQR 1.0 to 1.0) (Supplemental Table S7). Given suspicion for a dose-related effect at early time points related to more frequent dosing after the initial diagnosis of malignancy-related bone complications, we analyzed the risk of AF at multiple time points including 30, 60, 90, 180, and 365 days from the cohort entry date. In both the osteoporosis and malignancy cohorts, the estimated magnitude of the increased AF risk with ZA was numerically highest at 30 to 60 days and decreased gradually over time (Supplemental Table S8); however, wide confidence intervals limit the interpret-ability of this finding.
Discussion
In this large cohort representative of the privately insured population in the US, we found that ZA was associated with a 25% higher relative risk (absolute risk difference 3.69 events per 1000 person-years) of incident AF in patients treated for osteoporosis compared with denosumab at 1 year. A similar trend existed in patients treated for malignancy-related bone complications, despite limited precision and power given the smaller size of the malignancy cohort. We also noted an increased risk of AF in the malignancy cohort within the cardiovascular subgroup and at shorter follow-up times. This could reflect the higher total dose and dosing frequency of ZA and denosumab at early time points for malignancy-related bone complications compared with osteoporosis. Compared with the osteoporosis cohort, the malignancy cohort had higher rates of observed AF, which may be due to the higher rates of comorbidities and cardiac monitoring in the malignancy cohort.
This study informs and extends from prior literature. The HORIZON-PFT trial of ZA versus placebo showed an increased rate of arrhythmias including serious AF in patients treated with ZA.(2) However, increased AF risk was not consistently observed in other RCTs and post-marketing observational studies.(5–7,24–28) Further, neither randomized trials nor observational studies have examined the risk of AF in ZA users relative to an active comparator, decreasing generalizability from randomized trials and raising the possibility of confounding by indication in observational studies. Similar to HORIZON-PFT, our study using real-world data and an active comparator shows a modestly higher risk of AF and AF hospitalization in patients who receive ZA for osteoporosis.(2) The Food and Drug Administration’s (FDA) post-marketing surveillance report in 2008 showed no significant increase in AF among 19,687 bisphosphonate-treated and 18,358 placebo-treated patients from prior clinical trials, followed for up to 3 years.(29) However, our study follows a larger number of patients in a real-world rather than a post-clinical trial setting, and we examined zoledronic acid alone rather than a combination of oral and intravenous bisphosphonates, which may account for the observed differences from the FDA findings.
Prior authors have postulated several mechanisms for the increased risk of AF with ZA, including calcium shifts after the infusion, cytokine release, and deposition of bisphosphonates in myocardial tissue.(30–33) Our finding of an increased risk of AF with ZA relative to denosumab, an active comparator that also affects calcium levels, suggests that transient calcium shifts are unlikely to be the primary mechanism behind increased AF risk with ZA. Additionally, the distribution of AF events throughout the year after initiation in our study seems inconsistent with an acute cytokine-mediated effect. Further studies are needed to better understand the mechanism underlying AF risk with ZA.
Importantly, we observed no increased risk of strokes or TIA. Consistent with these findings, a comparative safety study of ZA versus denosumab previously showed no significant difference in stroke, myocardial infarction, or heart failure, although this study did not assess for incident arrhythmias and AF.(10) Additionally, two meta-analyses showed no increase in cardiovascular ischemic events with bisphosphonate use,(34,35) and a RCT of ZA versus placebo in women with osteopenic bone mineral density at the hip showed a trend toward decreased myocardial infarctions and all-cause mortality over 6 years of follow-up.(36) Growing evidence suggests that bisphosphonate use may be associated with lower all-cause mortality in eligible patients treated for osteoporosis.(6,35,37–39) Furthermore, ZA may be associated with higher disease-free survival in breast cancer.(40–44) Given this evidence and the substantial benefit in reducing skeletal-related events, we believe that the benefits of ZA use continue to outweigh the modest increased risk of incident AF for most patients. However, clinicians should be aware of the modestly increased risk of AF with ZA versus denosumab within the first year of treatment.
Of note, we found that rates of nonvertebral fractures were lower with denosumab than ZA in the malignancy cohort, but not the osteoporosis cohort, which is consistent with prior studies.(10,45–47) A prior cohort study, which also used the Clinformatics Data Mart, showed no difference in risk of nonvertebral osteoporotic fractures in patients with osteoporosis treated with ZA versus denosumab, although patients treated for malignancy-related bone complications were not assessed.(10) Since then, several studies have indicated that denosumab may be modestly more potent than ZA in reducing bone-related events in patients being treated for malignancy. An RCT of ZA versus denosumab in men with bone metastases from castration-resistant prostate cancer showed that denosumab was superior in delaying the onset of new pathologic fractures.(45) Two meta-analyses of RCTs comparing ZA to denosumab in malignancy also indicated that denosumab was superior to ZA in delaying time to first skeletal-related event by 17%.(46,47) This was consistent with our finding of greater malignancy-related fracture prevention efficacy with denosumab than with ZA.
Our results must be interpreted in the context of the study design. Because this study relies on observational data, our results cannot prove a causal relationship between ZA use and atrial fibrillation. Although we restricted our study to patients eligible for both treatment strategies and controlled for many potential confounders using PS matching, it is possible that unmeasured confounding still exists. For example, denosumab may be preferentially used in patients with mild CKD; because mild CKD may not be coded by physicians, this covariate may represent a source of residual confounding. Additionally, secular trends over the 8-year study period may have influenced both prescribing and risk of outcomes; however, we attempt to address this by matching on year of first use of ZA or denosumab in the propensity score. Further, this study was not designed to examine other potential safety events such as atypical femoral fractures, osteonecrosis of the jaw, or all-cause mortality. Within the malignancy group, we did not have sufficiently large sample sizes to evaluate individual malignancies, and further studies using larger sample sizes are needed to examine whether individual malignancies are associated with AF in patients treated with ZA versus denosumab. Finally, although there is no known association between denosumab and risk of AF, the possibility that denosumab is protective against AF cannot be entirely excluded based on the current study. Despite the limitations, our study has several strengths, including large cohort sizes for both osteoporosis and malignancy. Unlike prior observational studies of ZA and AF risk, we utilized an active comparator arm and restricted the study population to new users, which reduces the risk of confounding in pharmacoepidemiologic studies.(8,9) Finally, our study assessed differences in AF-related and skeletal safety outcomes between the two treatment strategies.
In conclusion, our study found that ZA was associated with a 25% higher relative risk, or an absolute risk difference of 3.69 events per 1000 person-years, of incident AF within the first year of treatment in patients treated for osteoporosis compared with denosumab in a large PS-matched cohort study in the US. A similar trend was also noted with ZA in patients with malignancy, although further studies with larger sample sizes are needed in this population. This study validates an early finding from the HORIZON-PFT trial and clarifies the equivocal data from prior observational studies by using a large sample size and an active comparator. Given the modestly increased risk of AF with ZA versus denosumab in real-world data, clinicians may consider known AF risk factors, such as cardiovascular comorbidities, when choosing whether to initiate ZA or denosumab in high-risk patients.
Supplementary Material
Acknowledgments
KMD and SJC are supported by National Institutes of Health Ruth L. Kirschstein Institutional National Research Service Awards (grant numbers T32AR007258 and T32DK007028, respectively).
Disclosures
SCK has received research grants to the Brigham and Women’s Hospital from Pfizer, AbbVie, Roche, and Bristol-Myers Squibb for unrelated studies. A close family member of SJC is employed by a Johnson & Johnson company. EWY has received a research grant to the Massachusetts General Hospital from Amgen for unrelated studies. KMD and MF state that they have no conflicts of interest.
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- 1.Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2019;104(5):1595–622. [DOI] [PubMed] [Google Scholar]
- 2.Black DM, Delmas P, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356 (18):1809–22. [DOI] [PubMed] [Google Scholar]
- 3.Kohno N, Aogi K, Minami H, et al. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2005;23(15):3314–21. [DOI] [PubMed] [Google Scholar]
- 4.Smith MR, Halabi S, Ryan CJ, et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (Alliance). J Clin Oncol. 2014;32(11):1143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black DM, Reid IR, Boonen S, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-pivotal fracture trial (PFT). J Bone Miner Res. 2012; 27(2):243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyles KW, Colón-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SC, Kim MJ, Cadarette SM, Solomon DH. Bisphosphonates and risk of atrial fibrillation: a meta-analysis. Arthritis Res Ther. 2010; 12:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2(4):221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshida K, Solomon DH, Kim SC. Active-comparator design and new-user design in observational studies. Nat Rev Rheumatol. 2015;11(7): 437–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi NK, Solomon DH, Tsacogianis TN, Landon JE, Song HJ, Kim SC. Comparative safety and effectiveness of denosumab versus zoledronic acid in patients with osteoporosis: a cohort study. J Bone Miner Res. 2017;32(3):611–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee MP, Desai RJ, Jin Y, Brill G, Ogdie A, Kim SC. Association of usteki-numab vs TNF inhibitor therapy with risk of atrial fibrillation and cardiovascular events in patients with psoriasis or psoriatic arthritis. JAMA Dermatol. 2019;155(6):700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walkey AJ, Greiner MA, Heckbert SR, et al. Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am Heart J. 2013;165(6):949–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glazer NL. Newly detected atrial fibrillation and compliance with antithrombotic guidelines. Arch Intern Med. 2007;167(3):246–52. [DOI] [PubMed] [Google Scholar]
- 14.Kim SC, Liu J, Solomon DH. Risk of incident atrial fibrillation in gout: a cohort study. Ann Rheum Dis. 2016;75(8):1473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SC, Liu J, Solomon DH. The risk of atrial fibrillation in patients with rheumatoid arthritis. Ann Rheum Dis. 2014;73(6):1091–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desai RJ, Mahesri M, Abdia Y, et al. Association of osteoporosis medication use after hip fracture with prevention of subsequent nonvertebral fractures. JAMA Netw Open. 2018;1(3):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudson M, Avina-Zubieta A, Lacaille D, Bernatsky S, Lix L, Jean S. The validity of administrative data to identify hip fractures is high—a systematic review. J Clin Epidemiol. 2013;66(3):278–85. [DOI] [PubMed] [Google Scholar]
- 18.Ray WA, Griffin MR, Fought RL, Adams ML. Identification of fractures from computerized medicare files. J Clin Epidemiol. 1992;45(7): 703–14. [DOI] [PubMed] [Google Scholar]
- 19.Kim SC, Choudhry N, Franklin JM, et al. Patterns and predictors of persistent opioid use following hip or knee arthroplasty. Osteoarthritis Cartilage. 2017;25(9):1399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring frailty in Medicare data: development and validation of a claims-based frailty index. J Gerontol A Biol Sci Med Sci. 2018;73(7):980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S, Verpillat P, Rassen J, Patrick A, Garry E, Bartels D. Transparency and reproducibility of observational cohort studies using large healthcare databases. Clin Pharmacol Ther. 2016;99(3):325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heckbert SR, Li G, Cummings SR, Smith NL, Psaty BM. Use of alendronate and risk of incident atrial fibrillation in women. Arch Intern Med. 2008;168(8):826–31. [DOI] [PubMed] [Google Scholar]
- 25.Loke YK, Jeevanantham V, Singh S. Bisphosphonates and atrial fibrillation: systematic review and meta-analysis. Drug Saf. 2009;32: 219–28. [DOI] [PubMed] [Google Scholar]
- 26.Abrahamsen B, Eiken P, Brixen K. Atrial fibrillation in fracture patients treated with oral bisphosphonates. J Intern Med. 2009;265(5):581–92. [DOI] [PubMed] [Google Scholar]
- 27.Bunch TJ, Anderson JL, May HT, et al. Relation of bisphosphonate therapies and risk of developing atrial fibrillation. Am J Cardiol. 2009;103(6):824–8. [DOI] [PubMed] [Google Scholar]
- 28.Reid IR, Horne AM, Mihov B, et al. Fracture prevention with zoledronate in older women with osteopenia. N Engl J Med. 2018;379(25): 2407–16. [DOI] [PubMed] [Google Scholar]
- 29.Food and Drug Administration Update of safety review follow-up to the October 1, 2007 early communication about the ongoing safety review of bisphosphonates [Internet]. Updated Nov 12, 2008. Available at: https://wayback.archive-it.org/7993/20170405195601/https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm136201.htm.
- 30.Cummings S, Schwartz AV, Black DM. Alendronate and atrial fibrillation. N Engl J Med. 2007;356(18):1895–6. [DOI] [PubMed] [Google Scholar]
- 31.Pazianas M, Compston J, Huang CL. Atrial fibrillation and bisphosphonate therapy. J Bone Miner Res. 2010;25(1):2–10. [DOI] [PubMed] [Google Scholar]
- 32.Cipriani C, Castro C, Curione M, et al. Acute effect of zoledronic acid on the risk of cardiac dysrhythmias. Intern Emerg Med. 2015;10(2):151–6. [DOI] [PubMed] [Google Scholar]
- 33.Hewitt RE, Lissina A, Green AE, Slay ES, Price DA, Sewell AK. The bisphosphonate acute phase response: rapid and copious production of proinflammatory cytokines by peripheral blood gd T cells in response to aminobisphosphonates is inhibited by statins. Clin Exp Immunol. 2005;139(1):101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim DH, Rogers JR, Fulchino LA, Kim CA, Solomon DH, Kim SC. Bisphosphonates and risk of cardiovascular events: a meta-analysis. PLoS One. 2015;10(4):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kranenburg G, Bartstra JW, Weijmans M, et al. Bisphosphonates for cardiovascular risk reduction: a systematic review and meta-analysis. Atherosclerosis. 2016;252:106–15. [DOI] [PubMed] [Google Scholar]
- 36.Reid IR, Horne AM, Mihov B, et al. Effects of zoledronate on cancer, cardiac events, and mortality in osteopenic older women. J Bone Miner Res. 2020;35(1):20–7. [DOI] [PubMed] [Google Scholar]
- 37.Colón-Emeric CS, Mesenbrink P, Lyles KW, et al. Potential mediators of the mortality reduction with zoledronic acid after hip fracture. J Bone Miner Res. 2010;25(1):91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook PN, Cameron ID, Chen JS, et al. Oral bisphosphonates are associated with reduced mortality in frail older people: a prospective five-year study. Osteoporos Int. 2011;22(9):2551–6. [DOI] [PubMed] [Google Scholar]
- 39.Bolland MJ, Grey AB, Gamble GD, Reid IR. Effect of osteoporosis treatment on mortality: a meta-analysis. J Clin Endocrinol Metab. 2010;95 (3):1174–81. [DOI] [PubMed] [Google Scholar]
- 40.Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic zcid in premenopausal breast cancer. N Engl J Med. 2009; 360(7):679–91. [DOI] [PubMed] [Google Scholar]
- 41.Gnant M, Mlineritsch B, Stoeger H, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011;12(7):631–41. [DOI] [PubMed] [Google Scholar]
- 42.Perrone F, De Laurentiis M, De Placido S, et al. Adjuvant zoledronic acid and letrozole plus ovarian function suppression in premenopausal breast cancer: HOBOE phase 3 randomised trial. Eur J Cancer. 2019;118:178–86. [DOI] [PubMed] [Google Scholar]
- 43.Coleman R, Cameron D, Dodwell D, et al. Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet Oncol. 2014; 15(9):997–1006. [DOI] [PubMed] [Google Scholar]
- 44.Eidtmann H, De Boer R, Bundred N, et al. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST study. Ann Oncol. 2010;21 (11):2188–94. [DOI] [PubMed] [Google Scholar]
- 45.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lipton A, Fizazi K, Stopeck AT, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer. 2012; 48(16):3082–92. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Z, Pu F, Shao Z. The skeletal-related events of denosumab versus zoledronic acid in patients with bone metastases: a meta-analysis of randomized controlled trials. J Bone Oncol. 2017;9:21–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


