Abstract
Background:
Direct acting antivirals (DAAs) have revolutionized management of hepatitis C virus (HCV), but treatment uptake remains low among persons who inject drugs (PWID). We report the continuum of care for HCV and describe predictors of treatment with DAAs among PWID in Seattle.
Methods:
We analyzed data from the 2018 Seattle area National HIV Behavioral Surveillance (NHBS) survey of PWID. Persons ≥18 years of age who injected drugs in the past year and completed the core NHBS survey, a local survey supplement, and rapid HCV antibody testing were included. Among those who screened HCV antibody positive, we calculated proportions and 95% confidence intervals for self-reported steps along the HCV care continuum. Multivariable logistic regression was used to calculate the adjusted odds (AOR) of having received DAA therapy.
Results:
The sample included 533 PWID, 376 (71%) of whom tested positive for antibodies to HCV. Among those who were HCV antibody positive, 94% reported any prior HCV test, 81% reported a prior confirmatory test, and 68% reported a prior HCV diagnosis. Of those diagnosed, 26% had undergone treatment and 18% had been cured. In a multivariate model, being one year older (AOR 1.05 per year, 1.01-1.08) was predictive of DAA treatment, while homelessness (AOR 0.39, 0.19-0.80) and female gender (AOR 0.36, 0.16-0.78) were associated with a lower odds of DAA therapy.
Conclusions:
Despite widespread HCV testing among PWID in Seattle, treatment uptake remains low in the DAA era. In particular, treatment of women, younger adults and persons living homeless is lagging behind.
Keywords: hepatitis c, people who inject drugs, continuum of care, DAA therapy
1. INTRODUCTION
Direct acting antivirals (DAAs) have revolutionized the landscape of hepatitis C virus (HCV) care, allowing for shorter, safer, and more effective treatment of chronic HCV infection. Regardless of genotype, cure rates for chronic HCV now exceed 95%, both for treatment naïve and treatment experienced patients in the clinical trial literature (Afdhal et al., 2014; Asselah et al., 2018; Bourlière et al., 2017; Corcorran and Scott, 2018; Feld et al., 2015; Forns et al., 2017; Foster et al., 2015; Jacobson et al., 2017; Zeuzem et al., 2018), with real world effectiveness trials showing sustained virologic response (SVR) rates of 87-90% among historically undertreated populations such as people who inject drugs (PWID) (Hajarizadeh et al., 2018). This success has led to a shift in the approach to treating HCV, with U.S. and international guidelines now recommending treatment of all adults with chronic HCV infection, including those with an active substance use disorder (WHO, 2018; AASLD, 2019). The advent of DAA therapy has similarly created an unprecedented opportunity to eliminate HCV, and in 2016, the World Health Organization (WHO) outlined strategic targets to help achieve global elimination of viral hepatitis by 2030 (WHO, 2016). However, despite these ambitious targets, the success of elimination efforts remains predicated on the ability to test and treat key populations at highest risk for contracting and transmitting HCV, a task that has historically been challenging.
In the United States, injection drug use remains the most common risk factor for ongoing HCV transmission, and from 2009 to 2017, rates of acute HCV increased rapidly among persons aged 20 – 29 years and 30 – 39 years, two age groups most affected by the ongoing opioid epidemic (CDC, 2019; Gomes et al., 2018; Zibbell et al., 2018). Despite the high burden of HCV among people who inject drugs, U.S. based data on the continuum of HCV care for PWID in the DAA era are limited, and what data do exist suggest HCV treatment uptake remains low among PWID, well below the 80% target set forth by WHO (Brown et al., 2017; Falade-Nwulia et al., 2019; Morris et al., 2019; Tsui et al., 2019).
Using data from the 2015 Seattle area National HIV Behavioral Surveillance (NHBS) survey of PWID, we previously published one of the few U.S based analyses of the hepatitis C continuum of care among PWID in the DAA era (interferon was last routinely used in Washington state in 2013) (Tsui et al., 2019). In that study we found a high prevalence of prior testing among HCV-positive PWID in Seattle and King County, but large gaps in the continuum of care for HCV treatment. Since data collection in 2015, there have been several changes to the landscape of HCV care, including the approval of pan-genotypic regimens (e.g. sofosbuvir-velpatasvir and glecaprevir-pibrentasvir) and the introduction of simplified treatment guidelines (AASLD, 2019). Similarly, there have been several advances in the public health response to HCV at the local, national and international level, most notably the creation of WHO’s global viral hepatitis elimination targets (WHO, 2016), the development of a national strategy for the elimination of hepatitis B and C (National Academies of Sciences, 2016), elimination of sobriety and advanced fibrosis requirements for Washington state Medicaid patients (AASLD, 2019; Silverman, 2016), and the establishment of a statewide plan for HCV elimination in Washington state, which has streamlined and reduced DAA prior authorization requirements for Washington Medicaid patients (WSDOH, 2019). These changes, paired with broader experience treating PWID with DAAs over the past several years (Bouscaillou et al., 2018; Dore et al., 2016; Grebely et al., 2018; Hajarizadeh et al., 2018; Morris et al., 2017), make updated care continuum data of particular interest.
In this study we used data from the 2018 Seattle area NHBS survey of PWID to describe the updated HCV continuum of care among PWID in the Seattle metropolitan area. As part of this analysis we compared steps along the continuum of care between 2015 and 2018, and we evaluated differences in the care continuum by age and gender. Additionally, we evaluated predictors of prior receipt of DAA therapy, versus no treatment or failed interferon-based therapy, in an effort to better understand persistent gaps in care delivery 5 years into the DAA era.
2. METHODS
2.1. Study Sample
This study analyzed data from the 2018 Seattle area NHBS survey of PWID. NHBS is a national survey, administered by the Centers for Disease Control and Prevention (CDC), that conducts behavioral surveillance among persons at high risk for HIV infection. The NHBS survey is currently administered in 22 metropolitan areas and focuses on three distinct risk groups for HIV infection: men who have sex with men (MSM), PWID, and persons at increased risk for heterosexually acquired HIV. Additional details on NHBS survey methodology have been previously published (Lansky et al., 2007).
Study participants were recruited via respondent driven sampling (RDS), a form of snowball sampling where participants are paid a small sum of money to recruit their peers. Persons were eligible to complete the NHBS survey if they were ≥18 years of age, injected drugs in the past year, lived in King or Snohomish County, and were able to complete the survey in English or Spanish. In addition to completing the core NHBS survey, participants were asked to complete a local survey supplement focusing on hepatitis C and rapid HCV and HIV antibody tests (OraSure Technologies). For the purposes of this study, the analytic sample was restricted to those persons who had completed the core NHBS survey, the local survey supplement, and a rapid HCV antibody test.
2.2. Data Collection
All surveys were administered by trained interviewers at community sites in King County, Washington from June to November 2018. Details of the local study procedures have been previously published (Burt et al., 2017; Tsui et al., 2019). To confirm history of injection drug use, participants were screened for physical evidence of recent drug use and asked detailed questions about drug preparation and injection practices. After providing informed consent, participants were given an interviewer-administered survey, which included information on medical history, sociodemographic characteristics, and sexual and drug-use practices. Questions on prior HCV testing, diagnosis and treatment were split between the core NHBS survey and the local survey supplement. Following survey completion, all participants were offered rapid HCV and HIV antibody testing (OraSure Technologies), for which a separate consent was provided. All participants received $25 for completing the survey, $25 for completing HIV testing, and $20 for each enrollment referral (up to 5).
No personal identifiers were collected as part of the NHBS and local surveys. This analysis was conducted for the purposes of public health surveillance by the HIV/STD Program at Public Health – Seattle & King County and did not require approval by an institutional review board.
2.3. Measures
The primary outcomes of interest were self-reported steps along the HCV care continuum and predictors of DAA therapy. Steps along the HCV care continuum included prior receipt of:
Any HCV test (“Have you ever been tested for hepatitis C infection?”);
A confirmatory HCV test (“Have you ever had a blood draw for hepatitis C? This is also known as a confirmatory test, RNA or viral load test.”);
A diagnosis of HCV (“Has a doctor, nurse, or other health care provider ever told you that you had hepatitis C?”);
HCV treatment (“Have you ever taken medicine to treat your hepatitis C infection?”);
HCV cure (“Were you told you were cured of hepatitis C?”).
Receipt of any HCV test, a confirmatory HCV test, and a diagnosis of HCV were assessed among all participants who screened HCV antibody positive. HCV treatment and cure were assessed only among those who endorsed a prior diagnosis of HCV. Results were subsequently stratified by age and gender. Additionally, care continuum data from the current 2018 NHBS PWID survey were compared to unadjusted estimates from the prior Seattle area NHBS PWID survey cycle performed in 2015 (NHBS-IDU4), the results of which have previously been published (Tsui et al., 2019). Due to questions on confirmatory HCV testing differing significantly between the 2015 and 2018 survey, comparisons were not possible for this step of the care cascade.
Self-reported DAA therapy was measured using a question asked to participants who reported any history of HCV treatment (“The most recent time you were treated, did you receive the new treatment that consists of taking pills for a few months and no interferon shots?”). Participants who answered “no” to this question were assumed to have received interferon-containing regimens, and participants who reported no history of HCV treatment were presumed to not have taken DAAs. Predictors of DAA therapy were assessed among participants who reported a prior diagnosis of HCV. Participants who had not received DAAs but reported being cured by interferon-based therapy were excluded from the comparator arm. Predictors included: age, gender, race and ethnicity, homelessness, health insurance status, visit with a healthcare provider in the past year, place of usual healthcare, drug of choice, injection frequency, HIV status, receipt of buprenorphine in the past year, and receipt of methadone in the past year.
2.4. Statistical Analysis
We calculated proportions and 95% confidence intervals for steps along the HCV care continuum, including for care continuum data stratified by age (< 40 years of age vs. ≥ 40 years of age) and gender (male vs. female). An age cutoff of less than 40 years of age and greater than or equal to 40 years of age was selected based on 2017 CDC data showing a higher incidence of acute HCV infections among PWID ages 20-29 and 30-39 in comparison to older age cohorts (CDC, 2019). We similarly performed a sensitivity analysis using an age cutoff of less than 30 years of age and greater than or equal to 30 years of age. Chi-squared tests were used to compare care continuum proportions by gender and age, as well as between data collected in 2015 and the current 2018 NHBS PWID cycle.
We used multivariable logistic regression to calculate the crude and adjusted odds ratio (AOR) of having received DAA therapy among those with a prior diagnosis of HCV. This multivariable model included variables that were statistically significant in bivariate analyses based on a p-value of <0.05. Exact logistic regression was used for bivariate analyses where cell sizes were less than 5. For this analysis, participants who reported prior treatment with DAAs were coded as “exposed,” and participants who reported a diagnosed of HCV but had not received DAAs and had not been cured by non-DAA regimens (e.g. interferon-based therapy) were coded as “unexposed.”
To investigate assumptions about RDS sampling, we used diagnostic tools in RDS Analyst including estimating homophily for major variables, bottleneck plots, and convergence plots (Gile et.al., 2015; Handcock et.al., 2014). We determined that the characteristics of some RDS chains resulted in unstable adjusted estimates. Data were therefore analyzed as a convenience sample without RDS adjustment. All survey questions required a response, which minimized missing data. Although participants could refuse to answer any individual question, most variables had no missing data, and none had ≥5% missing data. As such, no adjustments for missing data were performed. All data were analyzed using STATA SE 16 (Stata Corp, College Station TX).
3. RESULTS
3.1. Sample and Characteristics
A total of 555 PWID completed the core NHBS survey, 12 of whom were RDS seeds. Of these 555 study participants, 9 were excluded from our analytic sample because they did not complete the local survey supplement, 4 because they did not undergo rapid HCV antibody testing, and 9 because their HCV antibody status was incorrectly coded by the interviewer. This yielded an analytic sample of 533 PWID, of whom 376 (71%) screened antibody positive for HCV.
The demographic characteristics of study participants are presented in Table 1, stratified by HCV antibody status. Among HCV antibody positive individuals, 60.6% were male, 58.5% were 40 years of age or older, 72.6% were white, and 58.5% were currently homeless. Nearly 71% of HCV antibody positive participants reported heroin as their primary drug of choice, with over half (58.5%) reporting use of medications for opioid use disorder (e.g. methadone or buprenorphine) in the past 12 months. Among the analytic sample, the prevalence of HCV antibody increased with age, with 60% of those ages 18 – 29 years, 63% of those ages 30 – 39 years, 74% of those ages 40 – 49 years, and 82% of those 50 years and older screening positive for antibodies to HCV.
TABLE 1.
Characteristics of PWID by HCV Antibody Status, 2018 Seattle area NHBS (n=533)
| HCV+ (n=376) | HCV− (n=157) | |||
|---|---|---|---|---|
| N | Column % | N | Column % | |
| Age | ||||
| 18-29 | 58 | 15.4 | 39 | 24.8 |
| 30-39 | 98 | 26.1 | 58 | 36.9 |
| 40-49 | 90 | 23.9 | 32 | 20.4 |
| 50+ | 130 | 34.6 | 28 | 17.8 |
| Gender | ||||
| Male | 228 | 60.6 | 92 | 58.6 |
| Female | 145 | 38.6 | 61 | 38.9 |
| Transgender | 3 | 0.8 | 4 | 2.6 |
| Race / Ethnicity* | ||||
| Am. Indian / Native Am. | 96 | 25.5 | 23 | 14.7 |
| Asian / Pacific Islander | 23 | 6.1 | 11 | 7.0 |
| Black / African Am. | 66 | 17.6 | 39 | 24.8 |
| Hispanic / Latinx | 46 | 12.2 | 20 | 12.7 |
| White | 273 | 72.6 | 107 | 68.2 |
| Homeless, current | 220 | 58.5 | 103 | 65.6 |
| Primary Drug | ||||
| Heroin | 266 | 70.7 | 101 | 64.3 |
| Methamphetamine | 39 | 10.4 | 38 | 24.2 |
| Heroin + Meth | 45 | 12.0 | 9 | 5.7 |
| Other | 26 | 6.9 | 9 | 5.7 |
| Medication for Opioid Use Disorder, past 12 months | 217 | 58.5 | 73 | 51.8 |
Select all, can sum to >100%
3.2. HCV Continuum of Care Data
2018 Continuum of Care
Among the 376 persons who screened antibody positive for HCV, 94% reported being previously tested for HCV, 81% reported a prior confirmatory test for HCV, and 68% (n = 254) reported that a doctor, nurse or other healthcare professional had previously told them they had HCV. An additional 7% reported being told they had cleared HCV. Among the 254 people who had received a formal diagnosis of HCV, 26% had been treated for HCV, and 18% had been cured.
Comparison of 2018 and 2015 HCV Continuum of Care Data
When comparing the unadjusted 2018 and 2015 HCV care cascade data, similar proportions of PWID reported a prior test for HCV (94% vs. 91%, p=0.16) and a prior diagnosis of HCV (68% vs. 73%, p=0.11). However, comparing 2018 to 2015, a significantly higher percentage of those with a confirmed HCV diagnosis reported prior treatment (26% vs. 16%; p=0.01). Similarly, a higher proportion of PWID with a prior diagnosis of HCV reported being cured in 2018 when compared to the proportion who reported completing HCV treatment (used as a surrogate for cure) in 2015 (18% vs. 6%; p<0.001).
2018 Continuum of Care Stratified by Age
There were 220 PWID ≥40 years of age and 156 PWID <40 years of age who screened positive for antibodies against HCV. In comparison to PWID less than 40 years of age, PWID 40 years of age and older were more likely to report prior testing for HCV (96% vs. 91%, p=0.05), including prior confirmatory testing (85% vs. 75%, p=0.02), and were more likely to report a prior diagnosis of HCV (72% vs. 61%, p=0.02). Among the 159 PWID ≥40 years of age and the 95 PWID <40 years of age with a prior diagnosis of HCV, older PWID were more likely to report prior treatment for HCV (33% vs. 13%, p<0.001) and report achieving a sustained virologic response or cure (25% vs. 6%, p<0.001), when compared to their younger counterparts.
There were only 58 PWID under the age of 30 years who screened positive for antibodies to HCV; 51 (88%) reported a prior test for HCV, 41 (71%) reported a prior confirmatory test for HCV, and 32 (55%) reported a prior diagnosis of HCV. Among the 32 PWID less than 30 years of age who had previously been diagnosed with HCV, only 2 (6%) had been treated for HCV, and none had been cured.
2018 Continuum of Care Stratified by Gender
In our analytic sample there were 228 men and 145 women who screened positive for antibodies against HCV. Similar proportions of men and women reported prior HCV screening (94% vs. 93%, p=0.64), prior confirmatory testing (81% vs. 80%, p=0.79), and a prior diagnosis of HCV (70% vs. 63%, p=0.18). Among the 160 men and 92 women who reported a diagnosis of HCV, men were more likely to report prior HCV treatment (31% vs. 16%, p=0.009) and cure (22% vs. 12%, p=0.05), when compared to women.
3.3. Predictors of DAA treatment
Among the 254 participants who reported a prior diagnosis of HCV, 48 self-reported prior treatment with DAAs, while 196 had neither received DAAs nor been cured by interferon-based therapy. In a multivariate model comparing these two groups, being one year older was associated with a 5% higher odds of having received treatment with DAAs (AOR 1.05 per year, 1.01-1.08), while homelessness (AOR 0.39, 0.19-0.80) and female gender (AOR 0.36, 0.16-0.78) were associated with a 61% and 64% lower odds of having received treatment with DAAs, respectively. When comparing those who had and had not been treated with DAAs, there was no statistically significant difference in race and ethnicity, health insurance status, having visited a healthcare provider in the past year, usual place of healthcare, drug of choice, injection frequency, HIV status, having received buprenorphine in the past year, or having received methadone in the past year.
4. DISCUSSION
This study found that despite high levels of HCV testing among Seattle area PWID and significant gains in HCV treatment between 2015 and 2018, treatment uptake remains well below targets set by Washington state and WHO elimination plans. In particular, treatment of women is significantly lagging behind that of men, with women having a 64% lower odds of prior receipt of DAA therapy. Treatment of younger adults and persons experiencing homelessness is similarly falling behind in this high-risk urban cohort. Our findings suggest that increased efforts are needed to link PWID into care, with targeted outreach efforts aimed at women, younger PWID and persons living homeless.
In our analysis, we observed a large gap in the HCV care continuum for treatment, a finding that is reflective of other recent HCV care continuum studies in North America. In a 2019 study of 124 HCV positive PWID from Baltimore, Falade-Nwulia et al. reported that only 20% of the study cohort had started or completed HCV treatment, despite 91% identifying HCV as a major health concern, and 89% reporting awareness of new treatments for HCV (Falade-Nwulia et al., 2019). Similarly, a study performed at five federally qualified health centers (FQHCs) in Philadelphia from October 2012 through June 2016 found high rates of linkage to care (82.7% referred to an HCV provider) among 885 patients with chronic HCV infection; however, only 15% of patients in this cohort initiated HCV treatment (Coyle et al., 2019). In addition, recent data from Canada similarly report low rates of HCV treatment uptake among PWID. A study by Socías et al., published in 2019, showed that among 915 HCV-positive PWID living in Vancouver, British Columbia, only 16% initiated DAA therapy between April 2015 and November 2017, despite DAAs being included in the government drug formulary (Socías et al., 2019).
To the best of our knowledge, this is the first study specific to PWID in the United States to show a gender-based disparity in receipt of DAA therapy for HCV-mono-infected persons. A recent study examining rates of HCV testing and treatment initiation at 57 federally qualified health centers found that women were less likely than men to be tested for HCV and to initiate HCV treatment (AOR 0.76); however, only 6% of study participants had a history of any drug use, with only 3% reporting a history of opioid use disorder (Assoumou et al., 2020). Similarly, a previous study by Kanwal et al. examined nationwide data on HCV treatment within the Veterans Administration (VA) system. In this retrospective cohort, the overall odds of receiving DAA therapy was similar for men and women; however, women younger than 50 years of age had a 29% lower odds of receiving DAA therapy in comparison to men less than 50 years of age (Kanwal et al., 2016). While the reasons behind this age and gender based disparity in receipt of DAA therapy were not known, authors speculated that young women may have higher competing priorities, such as child and elder care (Kanwal et al., 2016). A similar study by Rojas Rojas et al., based on data from the French national healthcare reimbursement database, showed that, in comparison to men, HCV-positive women receiving medications for opioid use disorder were 41% less likely to have received pegylated-interferon (peg-INF) and 28% less likely to have received treatment with DAAs (Rojas Rojas et al., 2019). Conversely, in a cohort of HIV and HCV coinfected patients from the Kaiser Permanente system in Northern California, Lam et al. found no significant difference in DAA initiation based on gender; although women comprised only 12.8% of the cohort (Lam et al., 2019).
Why women in our study, when compared to men, had a 64% lower odds of receiving treatment with DAAs remains unclear. Prior studies of PWID have suggested that, in comparison to men, women suffer from higher rates of depression, have higher rates of self-reported medical conditions, are less educated, and are more likely to have been the victim of physical or sexual abuse (United Nations Office on Drugs and Crime, 2004; Olszewski et al., 2009; Back et al., 2011; Rojas Rojas et al., 2019). These factors, paired with high rates of unintended pregnancy, transactional sex work, and unstable housing among Seattle area women who inject drugs (Stewart et al., 2020), may contribute to the local disparity in receipt of DAA therapy. Additional factors, including the stigma associated with injection drug use, which may be particularly strong for women who have historically comprised a minority of PWID, and implicit bias within the healthcare system may also be contributing (Bourgois et al., 2004; Hammarlund et al., 2018). Additional research is needed to better understand the multiple factors driving lower rates of DAA uptake among female PWID with chronic HCV infection.
Our finding that younger PWIDs were less likely than older PWIDs to have received HCV treatment, including treatment with DAAs, is concerning, especially given the rapidly rising incidence of acute HCV among PWID less than 40 years of age (CDC, 2019). While this finding may reflect a component of length time bias, with older individuals having had more time to access treatment, our results are consistent with findings from other published studies (Morris et al., 2019; Rojas Rojas et al., 2019), including a recent prospective study of young PWID (<30 years of age) in San Francisco, published by Morris et al. In this study, 60 young PWID with a new HCV infection were identified between February 2015 and January 2018. Of these 60 individuals, 49 (82%) remained viremic. Thirty individuals, or 61% of those who remained viremic, accepted a referral to HCV treatment, with only 5 PWID (10% of viremic individuals) initiating HCV treatment (Morris et al., 2019). These findings, paired with the results of our study and recent CDC data showing a rapid rise in the incidence of acute HCV among persons less than 40 years of age, indicate there is an unmet need for increased harm reduction service and HCV treatment among this demographic, and if we are unable to successfully treat younger PWID, a group on the forefront of HCV transmission, we will be unable to achieve HCV elimination goals.
In the past several years, Washington state has made strides to increase access to DAAs, both through the elimination of sobriety and advanced fibrosis requirements for HCV treatment (CHLPI, 2016; Mooney DeBoy, 2015; Silverman, 2016), and through the creation of a statewide elimination plan, including the development of a novel purchasing agreement with AbbVie Inc., aimed at lowering the cost of HCV treatment for Washington Medicaid patients (WSDOH, 2019). While the Washington state HCV elimination plan and novel DAA purchasing agreement were developed and implemented after data collection for this study occurred, advanced fibrosis requirements were eliminated in 2016, and policies requiring a period of sobriety prior to HCV treatment were similarly eliminated in 2015 - 2016 (CHLPI, 2016; AASLD, 2019; Mooney DeBoy, 2015). Given these policy changes, active injection drug use should, in theory, not be a barrier to treatment; however, the only modest gains made in HCV treatment of Seattle area PWID between 2015 and 2018 suggest that removing such criteria does little to increase access to HCV treatment for PWID when systems are not in place to provide treatment in community settings and at other clinical care access points, such as addiction treatment programs.
The success of community-based HCV treatment has been well documented in the global literature, with studies showing an increase in treatment uptake and similar, if not higher, sustained virologic response (SVR) rates when treatment is provided in community versus tertiary settings (Benitez et al., 2020; Wade et al., 2016; Eckhardt et al., 2018; Kattakuzhy et al., 2017; Morris et al., 2017; Wade et al., 2020). Indeed, recent reports from across the United States demonstrate modest uptake of DAAs and high rates of cure when HCV treatment is provided in community-based and non-traditional sites such as syringe service programs (Eckhardt et al., 2018; Winetsky et al., 2020), addiction treatment programs (Akiyama et al., 2019), and neighborhood clinics serving unhoused individuals (Benitez et al., 2020). Nevertheless, despite these successes, PWID continue to experience substantial structural and individual level barriers to HCV treatment including stigma within the healthcare system, difficulty in navigating the healthcare system, competing needs, and limited knowledge regarding the availability and tolerability of newer DAA regimens (Falade-Nwulia et al., 2020; Falade-Nwulia et al., 2019; Jones et al., 2014; Zeremski et al., 2013). Similarly, provider hesitancy to treat people with active substance use disorders, and concerns regarding the risk of HCV reinfection, remain barriers at the prescriber level (Zeremski et al., 2013). Nevertheless, one could argue that, in addition to strengthening harm reduction services, as called for by WHO (WHO, 2016), treating more people who actively inject drugs may in fact be the best strategy to prevent reinfection, through a treatment as prevention approach (Grebely et al., 2013; Martin et al., 2013; Zelenev et al., 2018).
There are several limitations to our study. Patients were categorized as having HCV based on HCV antibody results, and HCV RNA testing was not performed as part of this study. Similarly, information on completion of fibrosis staging was not obtained, and all information on prior testing, diagnosis, and treatment was based on self-report and not substantiated by review of medical records. The comparison between 2015 and 2018 care continuum data is further limited by the fact that there was a different order and wording for certain questions across the two study years. In 2015, “Did you get a confirmatory hepatitis C RNA test, also known as a viral load test?” was only asked of those participants who reported a doctor, nurse or other healthcare professional had told them they had hepatitis C. By contrast, in 2018, “Have you ever had a blood draw for hepatitis C? This is also known as a confirmatory test, RNA, or viral load test” was asked of all participants who screened HCV antibody positive. This discrepancy precluded us from comparing receipt of HCV RNA testing across years. Furthermore, in 2015 we did not ask participants directly about being cured of HCV, but rather about completing treatment for HCV. While cure rates among PWID who complete DAA therapy are excellent (Dore et al., 2016; Grebely et al., 2018; Norton et al., 2017), asking about treatment completion in 2015 may have misclassified some participants who completed interferon therapy but did not achieve a sustained virologic response as being cured. In the 2018 study, we asked participants directly about cure to better ascertain the proportion of PWID who achieved an SVR. If this misclassification related to self-reported HCV cure did occur, it should strengthen the conclusion that the proportion who achieved an HCV cure in 2018 is significantly higher when compared to the proportion achieving a cure in 2015. Finally, this study analyzes data from a single geographic region, and the findings may not be generalizable to other populations of PWID. Given that data were analyzed as a convenience sample and not RDS-adjusted, these estimates should not be interpreted as being entirely representative of all Seattle area PWID. Emblematic of this, the high proportion of buprenorphine and methadone use observed in our sample may suggests that study participants were more engaged in harm reduction services and treatment for opioid use disorder than the general population of Seattle area PWID.
5. CONCLUSIONS
Our study of Seattle area PWID highlights the inadequate scale-up of HCV treatment within this highly affected urban population. Although gains in HCV treatment were made between 2015 and 2018, current treatment delivery is far short of the 80% target for treatment uptake set forth by WHO (WHO, 2016; WHO, 2017). While falling drug costs and recent reductions in prior authorization requirements for Washington state Medicaid patients (WSDOH, 2019) may improve treatment uptake in the coming years, immediate efforts are needed to expand HCV treatment services for PWID if Seattle and King County are to reach elimination targets by 2030 (WHO, 2016; National Academies of Sciences, 2016; WSDOH, 2019). In particularly, there is a need to expand treatment programs aimed at women, younger adults, and persons living homeless, three groups who are falling behind in the DAA era.
Figure 1. Hepatitis C Continuum of Care Among Seattle Area PWID, 2018 vs. 2015 NHBS Data.
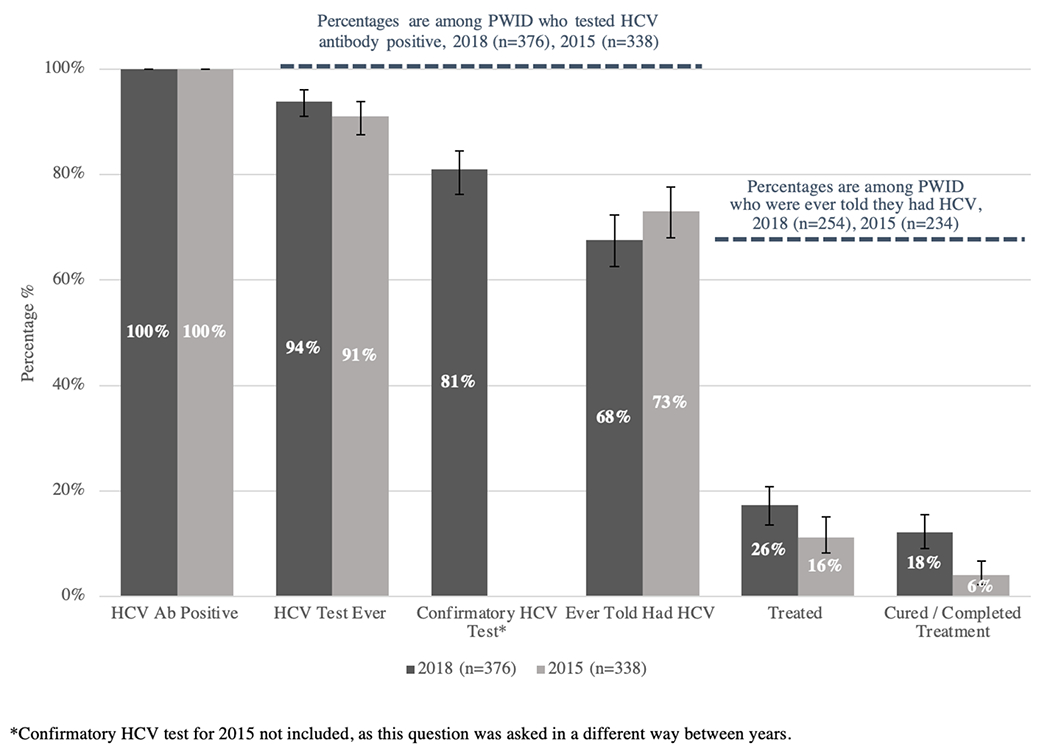
Data from the 2018 NHBS survey are depicted in the dark gray columns. Data from the 2015 NHBS survey are depicted in the light gray columns. Column 1: Total number of HCV antibody positive PWID (2018 n=376, 100%; 2015 n=338, 100%). Column 2: Proportion of HCV antibody positive PWID who report any prior test for HCV (2018 n=353, 94%; 2015 n=308, 91%). Column 3: Proportion of HCV antibody positive PWID who report a prior confirmatory test for HCV (2018 n=303, 81%; 2015 n/a). Column 4: Proportion of HCV antibody positive PWID who report a prior diagnosis of HCV (2018 n=254, 68%; 2015 n=247, 73%). Column 5: Proportion of HCV antibody positive PWID with a prior diagnosis of HCV who report treatment for HCV (2018 n=65, 26%; 2015 n=38, 16%). Column 6: Proportion of HCV antibody positive PWID with a prior diagnosis of HCV who report being cured of HCV (2018 n=46, 18%, 2015 n=14, 6%). Black error bars represent 95% confidence intervals for column percentages.
Figure 2. Hepatitis C Continuum of Care Among Seattle Area PWID by Age, 2018 NHBS.
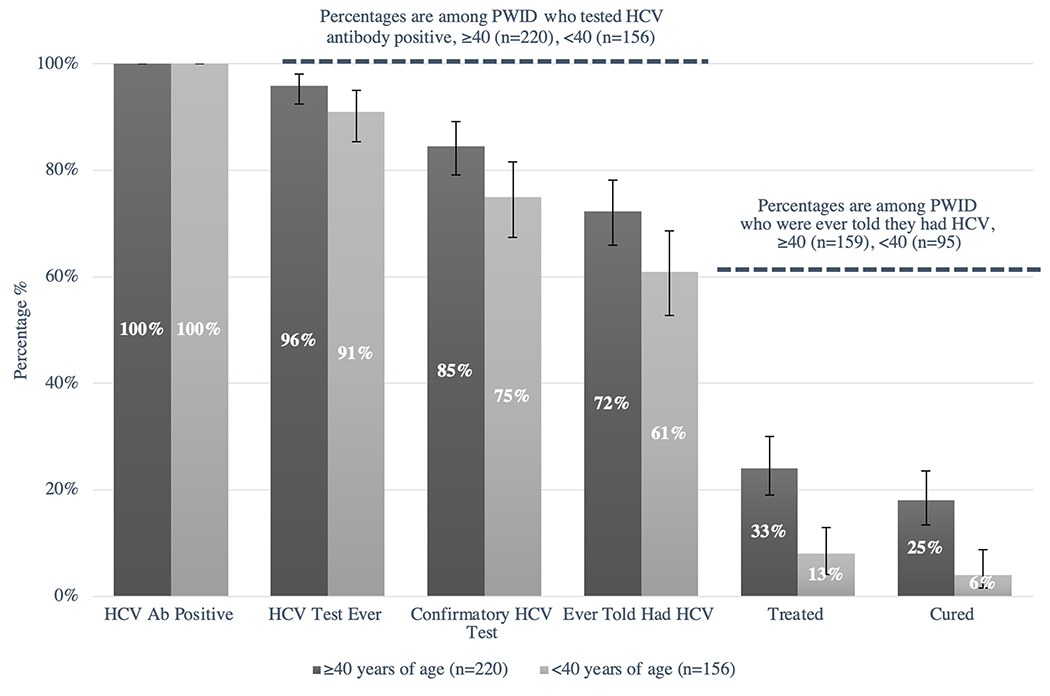
PWID 40 years of age and older are depicted in the dark gray columns. PWID less than 40 years of age are depicted in the light gray columns. Column 1: Total number of HCV antibody positive PWID ≥40 years of age (n= 220, 100%) and <40 years of age (n=156, 100%). Column 2: Proportion of HCV antibody positive PWID who report any prior test for HCV (≥40 years of age n=211, 96%; <40 years of age n=142, 91%). Column 3: Proportion of HCV antibody positive PWID who report a prior confirmatory test for HCV (≥40 years of age n=186, 85%; <40 years of age n=117, 75%). Column 4: Proportion of HCV antibody positive PWID who report a prior diagnosis of HCV (≥40 years of age n=159, 72%; ,40 years of age n=95, 61%). Column 5: Proportion of HCV antibody positive PWID with a prior diagnosis of HCV who report treatment for HCV (≥40 years of age n= 53, 33%; <40 years of age n=12, 13%). Column 6: Proportion of HCV antibody positive PWID with a prior diagnosis of HCV who report being cured of HCV (≥40 years of age n=40, 25%; <40 years of age n=6, 6%). Black error bars represent 95% confidence intervals for column percentages.
Figure 3. Hepatitis C Continuum of Care Among Seattle Area PWID by Gender, 2018 NHBS.
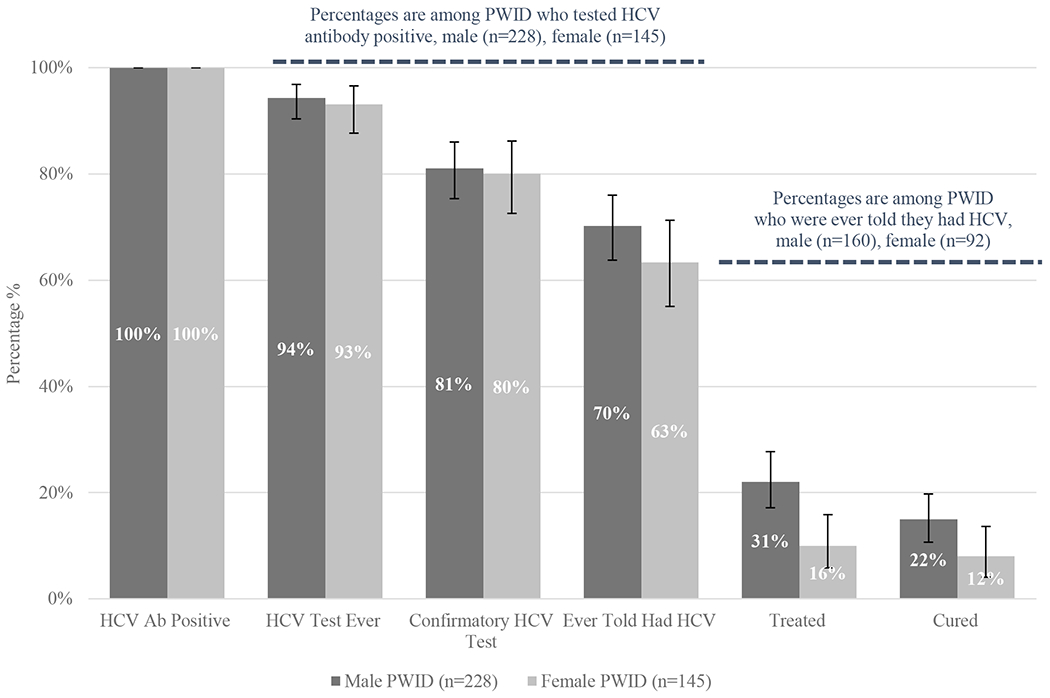
Male PWID are depicted in the dark gray columns. Female PWID are depicted in the light gray columns. Column 1: Total number of HCV antibody positive male PWID (n= 228, 100%) and female PWID (n=145, 100%). Column 2: Proportion of HCV antibody positive PWID who report any prior test for HCV (males n=215, 94%; females n=135, 93%). Column 3: Proportion of HCV antibody positive PWID who report a prior confirmatory test for HCV (males n=185, 81%; females n=116, 80%). Column 4: Proportion of HCV antibody positive PWID who report a prior diagnosis of HCV (males n=160, 70%; females n=92, 63%). Column 5: Proportion of HCV antibody positive PWID with a prior diagnosis of HCV who report treatment for HCV (males n=50, 31%; females n=15, 16%). Column 6: Proportion of HCV antibody positive PWID with a prior diagnosis of HCV who report being cured of HCV (males n=35, 22%, females n=11, 12%). Black error bars represent 95% confidence intervals for column percentages.
TABLE 2.
Predictors of Treatment with Direct Acting Antiviral Therapy Among PWID Who Reported a Prior Diagnosis of HCV, 2018 Seattle area NHBS
| Characteristic | DAA Treatment n=48 #(%) | No DAA Treatment n=196 #(%) | OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|---|---|
| Age, mean (SD) | 50.1 (10.8) | 42.4 (11.4) | 1.06 (1.03-1.10) | 1.05 (1.01-1.08) |
| Gender | ||||
| Men | 38 (79.2) | 116 (59.2) | ref | ref |
| Women | 10 (20.8) | 78 (39.8) | 0.39 (0.16-0.86) | 0.36 (0.16-0.78) |
| Trans | 0 (0.0) | 2 (1.0) | -- | -- |
| Race/Ethnicity (can select ≥1)* | ||||
| Am. Indian / Native Am. | 7 (14.6) | 52 (26.5) | 0.47 (0.20-1.12) | |
| Asian | 1 (2.1) | 5 (2.6) | 0.81 (0.17-7.52) | |
| Black / African Am. | 10 (20.8) | 36 (18.4) | 1.17 (0.53-2.56) | |
| Hispanic / Latinx | 4 (8.3) | 28 (14.3) | 0.55 (0.18-1.64) | |
| Nat. Hawaiian / Pacific Is. | 3 (6.3) | 9 (4.6) | 1.38 (0.23-5.84) | |
| White | 34 (70.8) | 144 (73.5) | 0.88 (0.42-1.92) | |
| Housing status | ||||
| Currently homeless | 17 (35.4) | 125 (63.8) | 0.31 (0.16-0.60) | 0.39 (0.19-0.80) |
| Not currently homeless | 31 (65.6) | 71 (36.2) | ref | ref |
| Health insurance status | ||||
| Insured | 46 (95.8) | 187 (95.4) | 1.11 (0.23-5.30) | |
| Not insured | 2 (4.2) | 9 (4.6) | ref | |
| Visited HCP in last year | ||||
| Yes | 47 (97.9) | 175 (89.3) | 5.84 (0.74-43.02) | |
| No | 1 (2.1) | 21 (10.7) | ref | |
| Primary drug | ||||
| Heroin | 36 (75.0) | 135 (68.9) | ref | |
| Methamphetamine | 3 (6.3) | 21 (10.7) | 0.54 (0.10-1.95) | |
| Other | 9 (18.8) | 40 (20.4) | 0.84 (0.33-1.98) | |
| Injection frequency | ||||
| Injects daily | 42 (87.5) | 178 (91.3) | 0.67 (0.25-1.80) | |
| Injects less than daily | 6 (12.5) | 17 (8.7) | ref | |
| HIV status | ||||
| Negative | 41 (85.4) | 156 (79.6) | ref | |
| Positive | 2 (4.2) | 6 (3.1) | 1.27 (0.12-7.42) | |
| Unknown | 5 (10.4) | 34 (17.4) | 0.56 (0.16-1.57) | |
| Buprenorphine use^ | ||||
| Used in the past year | 7 (15.2) | 43 (23.2) | 0.59 (0.25-1.42) | |
| Did not use in past year | 39 (84.8) | 142 (76.8) | ref | |
| Methadone use^ | ||||
| Used in past year | 22 (47.8) | 81 (43.8) | 1.18 (0.62-2.25) | |
| Did not use in past year | 24 (52.2) | 104 (56.2) | ref |
Each race/ethnicity variable compares people who selected that race/ethnicity to people who did not.
Among those who reported any opioid use.
HIGHLIGHTS.
We describe the HCV care continuum and predictors of DAA therapy among PWID in Seattle.
71% of PWID tested HCV Ab+, 68% of whom had previously been diagnosed with HCV.
Of those diagnosed, 26% had undergone treatment for HCV and 18% had been cured.
Compared to 2015, a higher proportion of PWID in 2018 reported HCV treatment and cure.
Female gender, younger age and homelessness were associated with a lower odds of DAA therapy.
ACKNOWLEDGEMENTS
Authors would like to acknowledge the participants and staff who responded to and conducted the 2018 National HIV Behavioral Surveillance survey.
ROLE OF FUNDING SOURCE: This work was supported by the National HIV Behavioral Surveillance with funding from cooperative agreements with the Centers for Disease Control and Prevention (5U1BPS003250, 6NU62PS005094). The Centers for Disease Control and Prevention had no involvement in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. Additionally, Dr. Maria Corcorran was supported in this work by a training grant from the National Institute of Diabetes and Digestive and Kidney Diseases [5T32DK007742-22].
CONFLICT OF INTEREST
Dr. John Scott has served on an advisory board for Gilead Sciences. Dr. Julia Dombrowski conducted research supported by grants to the University of Washington from Hologic, Inc. All other authors have nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P, Foster GR, Bräu N, Buti M, Jacobson IM, Subramanian GM, Ding X, Mo H, Yang JC, Pang PS, Symonds WT, McHutchison JG, Muir AJ, Mangia A, Marcellin P, Investigators, ION-I Investigators, 2014. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 370, 1889–1898. [DOI] [PubMed] [Google Scholar]
- Akiyama MJ, Norton BL, Arnsten JH, Agyemang L, Heo M, Litwin AH, 2019. Intensive Models of Hepatitis C Care for People Who Inject Drugs Receiving Opioid Agonist Therapy: A Randomized Controlled Trial. Ann Intern Med 170, 594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Association for the Study of Liver Disease (AASLD), 2019. Infectious Diseases Society of America (IDSA). HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. https://www.hcvguidelines.org/. (Accessed July 3 2020).
- Asselah T, Kowdley KV, Zadeikis N, Wang S, Hassanein T, Horsmans Y, Colombo M, Calinas F, Aguilar H, de Ledinghen V, Mantry PS, Hezode C, Marinho RT, Agarwal K, Nevens F, Elkhashab M, Kort J, Liu R, Ng TI, Krishnan P, Lin CW, Mensa FJ, 2018. Efficacy of Glecaprevir/Pibrentasvir for 8 or 12 Weeks in Patients With Hepatitis C Virus Genotype 2, 4, 5, or 6 Infection Without Cirrhosis. Clin Gastroenterol Hepatol 16, 417–426. [DOI] [PubMed] [Google Scholar]
- Assoumou SA, Wang J, Nolen S, Eftekhari Yazdi G, Mayer KH, Puro J, Salomon JA, Linas BP, 2020. HCV Testing and Treatment in a National Sample of US Federally Qualified Health Centers during the Opioid Epidemic. J Gen Intern Med 35, 1477–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Payne RL, Wahlquist AH, Carter RE, Stroud Z, Haynes L, Hillhouse M, Brady KT, Ling W, 2011. Comparative profiles of men and women with opioid dependence: results from a national multisite effectiveness trial. Am J Drug Alcohol Abuse 37, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez TM, Fernando SM, Amini C, Saab S, 2020. Geographically Focused Collocated Hepatitis C Screening and Treatment in Los Angeles’s Skid Row. Dig Dis Sci 65, 3023–3031. [DOI] [PubMed] [Google Scholar]
- Bourgois P, Prince B, Moss A, 2004. The Everyday Violence of Hepatitis C Among Young Women Who Inject Drugs in San Francisco. Hum Organ 63, 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourlière M, Gordon SC, Flamm SL, Cooper CL, Ramji A, Tong M, Ravendhran N, Vierling JM, Tran TT, Pianko S, Bansal MB, de Lédinghen V, Hyland RH, Stamm LM, Dvory-Sobol H, Svarovskaia E, Zhang J, Huang KC, Subramanian GM, Brainard DM, McHutchison JG, Verna EC, Buggisch P, Landis CS, Younes ZH, Curry MP, Strasser SI, Schiff ER, Reddy KR, Manns MP, Kowdley KV, Zeuzem S, Investigators, POLARIS-1 and POLARIS-4 Investigators., 2017. Sofosbuvir, Velpatasvir, and Voxilaprevir for Previously Treated HCV Infection. N Engl J Med 376, 2134–2146. [DOI] [PubMed] [Google Scholar]
- Bouscaillou J, Kikvidze T, Butsashvili M, Labartkava K, Inaridze I, Etienne A, Le Pluart D, Kamkamidze G, Gamezardashvili A, Kharshiladze D, Avril E, Luhmann N, 2018. Direct acting antiviral-based treatment of hepatitis C virus infection among people who inject drugs in Georgia: A prospective cohort study. Int J Drug Policy 62, 104–111. [DOI] [PubMed] [Google Scholar]
- Brown JL, Gause NK, Lewis D, Winhusen T, 2017. Examination of the Hepatitis C Virus care continuum among individuals with an opioid use disorder in substance use treatment. J Subst Abuse Treat 76, 77–80. [DOI] [PubMed] [Google Scholar]
- Burt RD, Tinsley J, Glick SN, 2017. A Decline in HIV Testing Among Persons Who Inject Drugs in the Seattle Area, 2004-2015. J Acquir Immune Defic Syndr 75 Suppl 3, S346–S351. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC), 2019. Viral Hepatitis Surveillance - United States, 2017. Atlanta, Georgia. https://www.cdc.gov/hepatitis/statistics/2017surveillance/pdfs/2017HepSurveillanceRpt.pdf [Google Scholar]
- Center for Health Law and Policy Innovation (CHLPI): Harvard Law School, 2016. Federal Court Requires WA Medicaid to Provide HCV Cure to Patients. https://www.chlpi.org/federal-court-requires-wamedicaid-to-provide-hcv-cure-to-patients/. (Accessed June 23 2020).
- Corcorran M, Scott J, 2018. Treatment of DAA-Experienced Patients with Chronic Hepatitis C. Current Hepatology Reports 17, 121–129. [Google Scholar]
- Coyle C, Moorman AC, Bartholomew T, Klein G, Kwakwa H, Mehta SH, Holtzman D, 2019. The Hepatitis C Virus Care Continuum: Linkage to Hepatitis C Virus Care and Treatment Among Patients at an Urban Health Network, Philadelphia, PA. Hepatology 70, 476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore GJ, Altice F, Litwin AH, Dalgard O, Gane EJ, Shibolet O, Luetkemeyer A, Nahass R, Peng CY, Conway B, Grebely J, Howe AY, Gendrano IN, Chen E, Huang HC, Dutko FJ, Nickle DC, Nguyen BY, Wahl J, Barr E, Robertson MN, Platt HL, C-EDGE CO-STAR Study Group, 2016. Elbasvir-Grazoprevir to Treat Hepatitis C Virus Infection in Persons Receiving Opioid Agonist Therapy: A Randomized Trial. Ann Intern Med 165, 625–634. [DOI] [PubMed] [Google Scholar]
- Eckhardt BJ, Scherer M, Winkelstein E, Marks K, Edlin BR, 2018. Hepatitis C Treatment Outcomes for People Who Inject Drugs Treated in an Accessible Care Program Located at a Syringe Service Program. Open Forum Infect Dis 5, ofy048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falade-Nwulia O, Gicquelais RE, Astemborski J, McCormick SD, Kirk G, Sulkowski M, Thomas DL, Mehta SH, 2020. Hepatitis C Treatment Uptake among People who Inject Drugs in the Oral Direct-Acting Antiviral Era. Liver Int. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falade-Nwulia O, Irvin R, Merkow A, Sulkowski M, Niculescu A, Olsen Y, Stoller K, Thomas DL, Latkin C, Mehta SH, 2019. Barriers and facilitators of hepatitis C treatment uptake among people who inject drugs enrolled in opioid treatment programs in Baltimore. J Subst Abuse Treat 100, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feld JJ, Jacobson IM, Hézode C, Asselah T, Ruane PJ, Gruener N, Abergel A, Mangia A, Lai CL, Chan HL, Mazzotta F, Moreno C, Yoshida E, Shafran SD, Towner WJ, Tran TT, McNally J, Osinusi A, Svarovskaia E, Zhu Y, Brainard DM, McHutchison JG, Agarwal K, Zeuzem S, ASTRAL-1 Investigators, 2015. Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. N Engl J Med 373, 2599–2607. [DOI] [PubMed] [Google Scholar]
- Forns X, Lee SS, Valdes J, Lens S, Ghalib R, Aguilar H, Felizarta F, Hassanein T, Hinrichsen H, Rincon D, Morillas R, Zeuzem S, Horsmans Y, Nelson DR, Yu Y, Krishnan P, Lin CW, Kort JJ, Mensa FJ, 2017. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial. Lancet Infect Dis 17, 1062–1068. [DOI] [PubMed] [Google Scholar]
- Foster GR, Afdhal N, Roberts SK, Bräu N, Gane EJ, Pianko S, Lawitz E, Thompson A, Shiffman ML, Cooper C, Towner WJ, Conway B, Ruane P, Bourlière M, Asselah T, Berg T, Zeuzem S, Rosenberg W, Agarwal K, Stedman CA, Mo H, Dvory-Sobol H, Han L, Wang J, McNally J, Osinusi A, Brainard DM, McHutchison JG, Mazzotta F, Tran TT, Gordon SC, Patel K, Reau N, Mangia A, Sulkowski M, ASTRAL-2 Investigators, ASTRAL-3 Investigators, 2015. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N Engl J Med 373, 2608–2617. [DOI] [PubMed] [Google Scholar]
- Gile KJ, Johnston LG, Salganik MJ, 2015. Diagnostics for Respondent-driven Sampling. J R Stat Soc Ser A Stat Soc 178, 241–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes T, Tadrous M, Mamdani MM, Paterson JM, Juurlink DN, 2018. The Burden of Opioid-Related Mortality in the United States. JAMA Netw Open 1, e180217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebely J, Dalgard O, Conway B, Cunningham EB, Bruggmann P, Hajarizadeh B, Amin J, Bruneau J, Hellard M, Litwin AH, Marks P, Quiene S, Siriragavan S, Applegate TL, Swan T, Byrne J, Lacalamita M, Dunlop A, Matthews GV, Powis J, Shaw D, Thurnheer MC, Weltman M, Kronborg I, Cooper C, Feld JJ, Fraser C, Dillon JF, Read P, Gane E, Dore GJ, Group SS, 2018. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol 3, 153–161. [DOI] [PubMed] [Google Scholar]
- Grebely J, Matthews GV, Lloyd AR, Dore GJ, 2013. Elimination of hepatitis C virus infection among people who inject drugs through treatment as prevention: feasibility and future requirements. Clin Infect Dis 57, 1014–1020. [DOI] [PubMed] [Google Scholar]
- Hajarizadeh B, Cunningham EB, Reid H, Law M, Dore GJ, Grebely J, 2018. Direct-acting antiviral treatment for hepatitis C among people who use or inject drugs: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 3, 754–767. [DOI] [PubMed] [Google Scholar]
- Hammarlund R, Crapanzano KA, Luce L, Mulligan L, Ward KM, 2018. Review of the effects of self-stigma and perceived social stigma on the treatment-seeking decisions of individuals with drug- and alcohol-use disorders. Subst Abuse Rehabil 9, 115–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handcock MS, Fellows IE, Gile KJ, 2014. RDS Analyst: Software for the Analysis of Respondent-Driven Sampling Data, Version 0.42, URL http://hpmrg.org.
- Jacobson IM, Lawitz E, Gane EJ, Willems BE, Ruane PJ, Nahass RG, Borgia SM, Shafran SD, Workowski KA, Pearlman B, Hyland RH, Stamm LM, Svarovskaia E, Dvory-Sobol H, Zhu Y, Subramanian GM, Brainard DM, McHutchison JG, Bräu N, Berg T, Agarwal K, Bhandari BR, Davis M, Feld JJ, Dore GJ, Stedman CAM, Thompson AJ, Asselah T, Roberts SK, Foster GR, 2017. Efficacy of 8 Weeks of Sofosbuvir, Velpatasvir, and Voxilaprevir in Patients With Chronic HCV Infection: 2 Phase 3 Randomized Trials. Gastroenterology 153, 113–122. [DOI] [PubMed] [Google Scholar]
- Jones L, Atkinson A, Bates G, McCoy E, Porcellato L, Beynon C, McVeigh J, Bellis MA, 2014. Views and experiences of hepatitis C testing and diagnosis among people who inject drugs: systematic review of qualitative research. Int J Drug Policy 25, 204–211. [DOI] [PubMed] [Google Scholar]
- Kanwal F, Kramer JR, El-Serag HB, Frayne S, Clark J, Cao Y, Taylor T, Smith D, White D, Asch SM, 2016. Race and Gender Differences in the Use of Direct Acting Antiviral Agents for Hepatitis C Virus. Clin Infect Dis 63, 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattakuzhy S, Gross C, Emmanuel B, Teferi G, Jenkins V, Silk R, Akoth E, Thomas A, Ahmed C, Espinosa M, Price A, Rosenthal E, Tang L, Wilson E, Bentzen S, Masur H, Kottilil S, Providers, a.t.A., 2017. Expansion of Treatment for Hepatitis C Virus Infection by Task Shifting to Community-Based Nonspecialist Providers: A Nonrandomized Clinical Trial. Ann Intern Med 167, 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam JO, Hurley LB, Chamberland S, Champsi JH, Gittleman LC, Korn DG, Lai JB, Quesenberry CP, Ready J, Saxena V, Seo SI, Witt DJ, Silverberg MJ, Marcus JL, 2019. Hepatitis C treatment uptake and response among human immunodeficiency virus/hepatitis C virus-coinfected patients in a large integrated healthcare system. Int J STD AIDS 30, 689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansky A, Abdul-Quader AS, Cribbin M, Hall T, Finlayson TJ, Garfein RS, Lin LS, Sullivan PS, 2007. Developing an HIV behavioral surveillance system for injecting drug users: the National HIV Behavioral Surveillance System. Public Health Rep 122 Suppl 1, 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin NK, Vickerman P, Grebely J, Hellard M, Hutchinson SJ, Lima VD, Foster GR, Dillon JF, Goldberg DJ, Dore GJ, Hickman M, 2013. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology 58, 1598–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney DeBoy A, 2015. Assuring Medicaid Beneficiaries Access to Hepatitis C (HCV) Drugs. Centers for Medicare and Medicaid Services, pp. 1–4. [Google Scholar]
- Morris L, Smirnov A, Kvassay A, Leslie E, Kavanagh R, Alexander N, Davey G, Williams O, Gilks C, Najman J, 2017. Initial outcomes of integrated community-based hepatitis C treatment for people who inject drugs: Findings from the Queensland Injectors’ Health Network. Int J Drug Policy 47, 216–220. [DOI] [PubMed] [Google Scholar]
- Morris MD, Mirzazadeh A, Evans JL, Briceno A, Coffin P, Hahn JA, Page KA, 2019. Treatment cascade for hepatitis C virus in young adult people who inject drugs in San Francisco: Low number treated. Drug Alcohol Depend 198, 133–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine, 2016. Eliminating the Public Health Problem of Hepatitis B and C in the United States: Phase One Report. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Norton BL, Fleming J, Bachhuber MA, Steinman M, DeLuca J, Cunningham CO, Johnson N, Laraque F, Litwin AH, 2017. High HCV cure rates for people who use drugs treated with direct acting antiviral therapy at an urban primary care clinic. Int J Drug Policy 47, 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski D, Giraudon I, Hedrich D, Montanari L, 2009. Women’s Voices: Experiences and perceptions of women who face drug-related problems in Europe. European Monitoring Centre for Drugs and Drug Addiction. https://www.emcdda.europa.eu/system/files/publications/549/EMCDDATP_women%27s_voices_133363.pdf [Google Scholar]
- Rojas Rojas T, Di Beo V, Delorme J, Barre T, Mathurin P, Protopopescu C, Bailly F, Coste M, Authier N, Carrieri MP, Rolland B, Marcellin F, 2019. Lower HCV treatment uptake in women who have received opioid agonist therapy before and during the DAA era: The ANRS FANTASIO project. Int J Drug Policy 72, 61–68. [DOI] [PubMed] [Google Scholar]
- Silverman E, 2016. Washington state told to lift restrictions on hepatitis C medicines. https://www.statnews.com/pharmalot/2016/05/27/washington-state-hepatitis-drug-prices/. (Accessed July 3 2020).
- Socías ME, Ti L, Wood E, Nosova E, Hull M, Hayashi K, Debeck K, Milloy MJ, 2019. Disparities in uptake of direct-acting antiviral therapy for hepatitis C among people who inject drugs in a Canadian setting. Liver Int 39, 1400–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J, Stadeli KM, Green ML, Etter-Carlson L, Dahl E, Davidson GH, Golden M, Dhanireddy S, 2020. A Co-Located Continuity Clinic Model to Address Healthcare Needs of Women Living Unhoused With Opioid Use Disorder, Who Engage in Transactional Sex in North Seattle. Sex Transm Dis 47, e5–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui JI, Miller CM, Scott JD, Corcorran MA, Dombrowski JC, Glick SN, 2019. Hepatitis C continuum of care and utilization of healthcare and harm reduction services among persons who inject drugs in Seattle. Drug Alcohol Depend 195, 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime, 2004. Substance abuse treatment and care for women: Case studies and lessons learned. Vienna, Austria. https://www.unodc.org/pdf/report_2004-08-30_1.pdf [Google Scholar]
- Wade AJ, Doyle JS, Gane E, Stedman C, Draper B, Iser D, Roberts SK, Kemp W, Petrie D, Scott N, Higgs P, Agius PA, Roney J, Stothers L, Thompson AJ, Hellard ME, 2020. Outcomes of Treatment for Hepatitis C in Primary Care, Compared to Hospital-based Care: A Randomized, Controlled Trial in People Who Inject Drugs. Clin Infect Dis 70, 1900–1906. [DOI] [PubMed] [Google Scholar]
- Wade AJ, Veronese V, Hellard ME, Doyle JS, 2016. A systematic review of community based hepatitis C treatment. BMC Infect Dis 16, 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington State Department of Health (WSDOH), 2019. Hep C Free Washington: Plan to Eliminate Hepatitis C in Washington State by 2030. https://www.doh.wa.gov/Portals/1/Documents/Pubs/150nonDOH-HepCFreeWAPlanJuly2019.pdf
- Winetsky D, Burack D, Antoniou P, Garcia B, Gordon P, Scherer M, 2020. Psychosocial Factors and the Care Cascade for Hepatitis C Treatment Colocated at a Syringe Service Program. J Infect Dis 222, S392–S400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO), 2016. Global Health Sector Strategy on Viral Hepatitis 2016-2021: Towards Ending Viral Hepatitis. Geneva, Switzerland. https://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HFV-2016.06-eng.pdf;jsessionid=D7A1400CD873080DAF06F57D03424D3C?sequence=1 [Google Scholar]
- World Health Organization (WHO), 2017. Global Hepatitis Report. Geneva, Switzerland, pp. 7–17. https://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455-eng.pdf?sequence=1 [Google Scholar]
- World Health Organization (WHO), 2018. Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection. Geneva, Switzerland. https://apps.who.int/iris/bitstream/handle/10665/273174/9789241550345-eng.pdf?ua=1 [PubMed] [Google Scholar]
- Zelenev A, Li J, Mazhnaya A, Basu S, Altice FL, 2018. Hepatitis C virus treatment as prevention in an extended network of people who inject drugs in the USA: a modelling study. Lancet Infect Dis 18, 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeremski M, Zibbell JE, Martinez AD, Kritz S, Smith BD, Talal AH, 2013. Hepatitis C virus control among persons who inject drugs requires overcoming barriers to care. World J Gastroenterol 19, 7846–7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuzem S, Foster GR, Wang S, Asatryan A, Gane E, Feld JJ, Asselah T, Bourlière M, Ruane PJ, Wedemeyer H, Pol S, Flisiak R, Poordad F, Chuang WL, Stedman CA, Flamm S, Kwo P, Dore GJ, Sepulveda-Arzola G, Roberts SK, Soto-Malave R, Kaita K, Puoti M, Vierling J, Tam E, Vargas HE, Bruck R, Fuster F, Paik SW, Felizarta F, Kort J, Fu B, Liu R, Ng TI, Pilot-Matias T, Lin CW, Trinh R, Mensa FJ, 2018. Glecaprevir-Pibrentasvir for 8 or 12 Weeks in HCV Genotype 1 or 3 Infection. N Engl J Med 378, 354–369. [DOI] [PubMed] [Google Scholar]
- Zibbell JE, Asher AK, Patel RC, Kupronis B, Iqbal K, Ward JW, Holtzman D, 2018. Increases in Acute Hepatitis C Virus Infection Related to a Growing Opioid Epidemic and Associated Injection Drug Use, United States, 2004 to 2014. Am J Public Health 108, 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]


