Abstract
Impaired fracture healing in patients with obesity-associated type 2 diabetes (T2D) is a significant unmet clinical problem that affects millions of people worldwide. However, the underlying causes are poorly understood. Additionally, limited clinical information is available on how pre-diabetic hyperglycemia in obese individuals impacts bone healing. Here, we use the diet-induced obesity (DIO) mouse (C57BL/6J) model to study the impact of obesity-associated pre-diabetic hyperglycemia on bone healing and fibrillar collagen organization as healing proceeds from one phase to another. We show that DIO mice exhibit defective healing characterized by reduced bone mineral density, bone volume, and bone volume density. Differences in the healing pattern between lean and DIO mice occur early in the healing process as evidenced by faster resorption of the fibrocartilaginous callus in DIO mice. However, the major differences between lean and DIO mice occur during the later phases of endochondral ossification and bone remodelling. Comprehensive analyses of fibrillar collagen microstructure and expression pattern during these phases, using a set of complementary techniques that include histomorphometry, immunofluorescence staining, and second harmonic generation microscopy, demonstrate significant defects in DIO mice. Defects include strikingly sparse and disorganized collagen fibers, as well as pathological accumulation of unfolded collagen triple helices. We also demonstrate that DIO-associated changes in fibrillar collagen structure are attributable, at least in part, to the accumulation of advanced glycation end products, which increase the collagen-fiber crosslink density. These major changes impair fibrillar collagens functions, culminating in defective callus mineralization, remodelling, and strength. Our data extend the understanding of mechanisms by which obesity and its associated hyperglycemia impairs fracture healing and underline defective fibrillar collagen microstructure as a novel and important contributor.
1. Introduction
Fractures are among the most frequent trauma injuries of the musculoskeletal system [1]. In spite of the significant improvement achieved over the past decades in fracture treatment, impaired or delayed fracture healing remains a significant unmet clinical problem that affects millions of people worldwide [2]. Impaired/delayed fracture healing can result from several factors including comorbid diseases, among which T2D that associates obesity is a significant risk factor [3,4]. Obesity and its associated complications is currently a worldwide health concern that afflicts more than one-third of adults in the US, and this number is projected to climb significantly in the coming decades [5].
Under normal conditions, bone healing proceeds through a sequence of consecutive, yet overlapping, processes that can be divided into three phases: inflammation, repair, and remodeling [6,7]. The inflammatory phase is initiated as a result of blood vessel rupture at the fracture area and develops into a fracture hematoma rich in inflammatory cells, inflammatory cytokines, and angiogenic factors [3,7]. The inflammatory phase sets the stage for the initiation of the repair phase [7]. The nature of repair phase is dependent on the anatomical location as well as the mechanical stability of the fracture [7,8]. A cortical bone fracture with a low degree of stability (e.g. diaphyseal fractures stabilized by intramedullary nail) heals through indirect, secondary bone healing (endochondral ossification) and is typified by primary development of a fibrocartilaginous (soft) callus [9]. Chondrocytes within the soft callus initially proliferate and then undergo hypertrophy and mineralization, which harden the cartilaginous area that bridges the fracture gap to reduce interfragmentary movement, allowing new blood vessels to invade the calcified area [7,10,11]. This neovascularization starts a new round of recruitment of mesenchymal stem cells (MSCs) and monocytes, which differentiate into osteoblasts and osteoclasts, respectively. The coordinated processes of osteoclast-mediated resorption of calcified cartilage and osteoblasts-mediated generation of new bone result in bony bridging of the fracture gap and formation of a bony callus filled with woven bone [7,12]. This bony bridging is indicative of successful healing and allows for resumption of normal bone loading [9]. Once the callus is filled with woven bone, the remodeling phase ensues to re-establish the cortical bone structure [12].
Clinical studies revealed delayed fracture healing in obese individuals (BMI>30) and T2D patients [4]. However, stratifying nondiabetic obese individuals into normal and prediabetic groups is generally lacking in clinical studies. As a result, the impact of obesity-associated prediabetic hyperglycemia on fracture healing remains largely unknown in clinical settings. Animal studies bridge this gap in knowledge and indicate compromised fracture healing in the diet-induced obesity (DIO) animal model, which is a well-established murine model of obesity-associated pre-diabetic hyperglycemia and insulin insensitivity [13,14]. Impaired healing in the DIO model involves pathological adipogenesis of MSCs, which inhibits MSC osteogenic commitment and, thus, impedes fracture healing [13]. In spite of these reported defects, causes of impaired fracture healing in the DIO murine models remain poorly understood. Importantly, no studies thus far have thoroughly investigated the impact of the structure of fibrillar collagen on the healing process. Fibrillar collagen provide the template for mineral disposition in bone tissue and during fracture healing [15,16]. Therefore, the extent of tissue mineralization is highly dependent on the content, size and organization of collagen fibers [17]. Since adequate tissue mineralization is pivotal for successful fracture healing (see above), investigating the microstructure of fibrillar collagen during different phases of healing is essential for understanding the general process of healing and for gaining insights into disease-associated impaired healing. The significance of such studies is further highlighted in the context of obesity-associated hyperglycemia, given that chronic hyperglycemia results in accumulation of advanced glycation end products (AGEs), which alter the structure and reduce the elasticity of collagen fibers via formation of non-enzymatic crosslinks [18].
Second harmonic generation (SHG) microscopy has emerged as a powerful tool to quantitatively analyze the organization of fibrillar collagen [19,20]. SHG is a nonlinear optical process, in which two photons of the incident light combine to generate a single photon with double the energy (double the frequency) and half the wave length [20]. SHG occurs with a very limited number of highly ordered proteins that have no center of symmetry (harmonophores), among which fibrillar collagen is the most extensively studied [21–25]. The advantages of SHG include that it is a label-free technique and sensitive to changes that occur in fiber organization under different conditions such as disease states [20,21]. Importantly, SHG also provides structural information about collagen-fiber orientation (orientation index) [24,26] geometry (fiber diameter) [26], and crosslink density [27].
Here, we utilize SHG among other techniques to investigate the structure of fibrillar collagen throughout the duration of fracture healing in both lean and DIO mice.
2. Materials and Methods
2.1. Animals
All animal protocols were approved by the University Committee on Animal Resources (IACUC) at the University of Rochester Medical Center and the Pennsylvania State University College of Medicine. Male C57BL/6J mice at 3-week age were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and allowed to acclimate to the care facility for 2 weeks. Mice were group-housed in ventilated cages with bedding and provided with ad libitum access to pelleted feed and water. The mouse facility had a 12-hour light/dark cycle at 21.1°C to 22.8°C and 30% to 70% humidity. The investigators were not blinded during animal allocation.
2.2. Induction of obesity and hyperglycemia
To induce obesity, hyperglycemia, and glucose intolerance, the standard approach for diet-induced obesity was used [13]. Briefly, 6-week-old male C57BL/6J mice (12 mice/group) were fed high-fat diet (HFD) (60 kcal % fat; Open Source Diets, Research Diets Inc.) for 12 weeks. Lean, control mice received lean diet (10 kcal % fat; Open Source Diets, Research Diets Inc.). Each mouse was weighed preoperatively and at time of harvest. Glucose tolerance test was performed preoperatively as follows: each mouse was fasted for 6 h (daytime), anesthetized using 5% isoflurane, and blood was drawn from the tail vein to measure fasting glucose level using One Touch Ultra (Lifescan). Then, each mouse received an IP injection of 2g/kg glucose, and blood glucose levels were measured at 15, 30, 60, 90, and 120 min post-injection. Notably, only male mice were used in this study. Female C57BL/6 mice are protected against HFD-induced metabolic changes and, unlike male mice, do not develop glucose intolerance, hyperinsulinemia, or insulin resistance [28,29]. Further studies are required to investigate these differences between male and female DIO mice and how they affect fracture healing.
2.3. Mid-diaphysis tibial fracture surgery
Tibial fractures were induced in both lean and DIO mice in the right hindlimb according to the established protocol for the open tibial fracture osteotomy model [30]. X-ray images were collected post-operatively and at time of harvest using UltraFocus DXA system (Faxitron Bioptics). Based on previously published information on the time course of fracture healing in the DIO model [13], callus tissue from both lean and DIO mice were harvested 7, 10, 14, 21, and 35 days post-operatively (Supplementary Fig. S1). Animals were housed on a 12-h light/dark cycle.
2.4. Micro-Computed Tomography (μCT) analyses
Samples were scanned using a vivaCT 40 (Scanco) at 55 kVp, 145 mA, 300 ms integration time, and 10.5-micron isometric voxels. For each bone, a region spanning 2.1 mm proximal and distal to the center of the fracture was analyzed using the Scanco software. Nine axial image slices, spaced equidistantly (50 slices = 0.525 mm) from each other, were used for analysis. Manual contouring of the sampled axial slices was performed to carefully isolate the fracture callus from the remaining areas, i.e., cortex and intramedullary canal. Standard Gaussian filter was applied, and bone was segmented with a consistent threshold of 250. Scanco scripts were then applied to calculate the total area of the callus and the bone area within the callus for each slice. These areas were averaged across slices and multiplied by the slice spacing to estimate the total volume of the callus (TV) and the bone volume (BV), respectively. Callus BV/TV was then calculated. Following terminology used for trabecular bone analysis, callus bone mineral density (BMD) was calculated as the average density of all voxels within the contoured callus [31].
2.5. Biomechanical testing
Blinded biomechanical testing was performed on the healing tibia (i.e. of the right hindlimb) at day 35 post-fracture. The contralateral unbroken tibia (i.e. of the left hindlimb) was used for normalization. Testing was performed as previously described [32] using an EnduraTec TestBench TM System (200 Nmm torque cell; Bose Corporation). Torsion was applied at a rate of 1°/s until failure, and the maximum torque at the point of failure was recorded.
2.6. Safranin-O staining and histomorphometric analysis
Sagittal sections of 5 μm thickness were obtained from a 60-μm region spanning the centre of the fracture callus and stained with Hematoxylin/Safranin-O/Fast Green. Blinded histomorphometric analysis was performed using the OsteoMeasure system (OsteoMetrics Inc.), and relative Safranin-O (Saf-O)-stained area was calculated by normalizing Saf-O-stained area to the total callus area.
2.7. Multiphoton and SHG microscopy
The basic design of the multiphoton and SHG imaging system has been described previously [33]. The laser used for SHG was an InSight DS+ mode-locked single-box laser system with automated dispersion compensation, tunable between 680–1300 nm (Spectra-Physics, Mountain View). During imaging, the laser output was attenuated using acousto-optic tunable filters (AOTF) to avoid sample damage, and average power was consistently maintained throughout the imaging process. The attenuated laser was directed to a Nikon resonant scan head, coupled with the Nikon A1 MP+ Multi-Photon Upright Microscope system (Nikon Instruments), and imaging was performed using a Nikon Upright Microscope. The laser beam was focused onto the specimen through a water immersion objective lens (CFI75 Apo Water 25X/1.1 LWD 2.0-mm WD) for the collection of forward scattered SHG (FSHG) and backward scattered SHG (BSHG) signals, with 440 / 20 nm band pass filters. A 1.4 NA condenser was used to collect the FSHG signal.
For collagen crosslink density, to collect BSHG signal, an excitation wavelength of 950 nm and an emission range of 460–495 nm was used. Multi-photon excited fluorescence (MPEF) was simultaneously scanned up to 645 nm to define MPEF signal that overlaps with the corresponding BSHG signal. A 460 long-pass dichroic beam splitter (460 LPXR, Chroma Technology) was used to separate BSHG signal from MPEF signal. Reflected signals were collected under epi-illumination mode for both BSHG and MPEF imaging. All parameters (i.e. laser intensity, gain, voltage, dwell time, aspect ratio) were held constant across different samples.
2.7.1. SHG data acquisition and analysis
Nikon Confocal Software was used for acquisition of 3D images. Briefly, the multiphoton excitation beam was focused at the focal position of maximum signal intensity within each sample, and the beginning and end of the 3D stack were set based on signal level degradation. Line averaging and gain level were applied to minimize noise and avoid signal saturation, respectively. For visualization and quantitative metrics of 3D images, a series of 2D images were generated on Nikon software for each 3D stack and transferred to Volocity software (Version 6.3.1; Quorum Technologies Inc.) for 3D image restoration.
To analyze collagen volume fraction, SHG signal intensity, and crosslink density, SHG images were acquired using the settings of 344 × 344-μm field, 0.67 × 0.67 × 1-μm (x, y, z) resolution. To analyze fibrillar collagen diameter and orientation index, the settings used were 65 × 65-μm field, 0.126 × 0.126 × 1-μm (x, y, z) resolution. In all cases, a slow scan speed of 10 s per 1024 X 1024 pixels was applied.
2.7.2. Calculating SHG voxel count and voxel intensity
Volocity software was used to quantify voxel count and voxel intensity. A noise removal filter with a kernel size of 3 X 3 was applied to all 3D images, and the lower threshold was set to positive first standard deviation (+1σ) of the mean voxel intensity to exclude all possible background. Only voxels above this threshold were considered for analysis.
2.7.3. Analysis of collagen volume fraction
Collagen volume fraction was analyzed in 3D SHG data sets as previously described [34]. Briefly, the noise-removal filter (kernel size of 3X3) described above was applied, and collagen volume fraction within each region of interest (ROI) was determined by normalizing the sum of all voxels above the background threshold to the total ROI voxels using Volocity software. Collagen volume fraction was analyzed in callus tissues derived from 6 lean and 6 DIO mice. Three serial sections of each sample were analyzed, and enough ROIs were selected in each section to cover all the studied area (i.e. soft callus or woven bone).
2.7.4. Measurement of collagen-fiber diameter
Collagen fiber diameter was calculated from FSHG and BSHG images using Volocity and Origin 2018b Graphing and Analysis (OriginLab) software as described previously [26]. Briefly, SHG images were collected from 5 ROIs that sampled the studied area, and the Line Profile Tool in the XYZ Image View Mode of the Volocity program was used to draw two lines perpendicular to the direction of each collagen fiber. At the area of intersection between the line and fiber, pixels from each fiber were recorded and then plotted as number of pixels (x-axis) versus pixel intensity (y-axis) to produce a plot of intensity profile. Each peak on this plot represents one collagen fiber. The plot of intensity profile was then transferred to Origin software to calculate the full width half maximum (FWHM) of each fiber by fitting peaks to Gaussian distribution. Base-line correction was done using the peak analyzer feature in the Origin software. For each ROI, the average of FWHM values was calculated and fiber diameter in microns was obtained by multiplying FWHM by the pixel size. The analysis was performed on 6 lean and 6 DIO callus samples, with 3 serial sections from each sample analyzed. On average, five fibers were analyzed per each ROI.
2.7.5. Assessing collagen-fiber orientation index
Collagen-fiber orientation index was computed in 2D FSHG and BSHG images as detailed previously using MATLAB (The MathWorks) software [26]. Briefly, a custom-built MATLAB code was used to determine collagen-fiber orientation and anisotropy via Fast Fourier Transform (FFT) which converted the complex spatial patterns of collagen fibers within each image into directionally dependent frequency components [26]. The FT power spectrum was then plotted, and orientation index was computed as described [26]. Orientation index was analyzed in the same ROIs used to measure collagen-fiber diameter (see above).
2.7.6. Determining crosslink density
Using an excitation wavelength of 950 nm, BSHG signal and images were collected at half the excitation wavelength (475 nm), while MPEF images and emission spectra were acquired simultaneously. As expected, the SHG signal was dominant in the emission spectra. Collagen-positive voxels (i.e. SHG-positive voxels) were defined, and the total MPEF intensity within these voxels was computed for each ROI. The crosslink density (crosslink/collagen) was computed by normalizing the total MPEF intensity within each ROI to the corresponding total BSHG intensity as described previously [27]. Volocity was used to calculate total intensity. The analysis was performed on 6 lean and 6 DIO samples; 3 serial sections were analyzed per sample, and sufficient number of ROI within each section were selected to cover the entire studied area.
2.8. Immunofluorescent (IF) staining
Paraffin sections were deparaffinized in three changes of xylene (5 min each), rehydrated in ethanol (two changes of 100% ethanol for 5 min each, two changes of 95% ethanol for 5 min each, and finally one change of 70% ethanol for 2 min), and rinsed twice in deionized water (1 min each).
Antigen retrieval was performed according to the procedure optimized for each antigen (detailed in Supplementary Table 1) and was followed by permeabilization for 30 min at room temperature using 0.03% Triton in 1X TBS (TBST). Sections were then blocked for 2 h at room temperature in 10% normal goat or donkey serum (in 1X TBS) and incubated overnight at 4 °C with the primary antibody specific for each target protein (Supplementary Table 1).
For 2-step staining, three 5-min washes in 1X TBS were performed after incubation with the primary antibody, and slides were incubated for 1 h at room temperature with the secondary antibody (Supplementary Table 1). For 3-step staining, three 5-min washes in 1X TBS were performed, and slides were incubated with biotinylated goat anti-rabbit secondary antibody (Invitrogen), washed again with 3 changes of 1X TBS (5 min each), and incubated for 1 h at room temperature with Alexa Fluor 647-labelled Streptavidin (Invitrogen). For all staining experiments, slides incubated without primary antibody served as negative controls.
Finally, slides stained with either the 2-step or 3-step process were washed with three changes of 1X TBS (5 min each), followed by one wash in TBST (5 min), and a final 5-min wash in 1X TBS. Mounting and nuclear staining was performed using ProLongTM Gold antifade reagent with DAPI (Invitrogen). Imaging was performed using Zeiss Axio Observer 7 upright wide-field microscope (Carl Zeiss Microscopy GmbH). Image analysis for area and mean intensity was performed using Zen Blue advanced image analysis software.
2.9. Fluorescent staining with collagen hybridizing peptide
Staining was performed according to the manufacturer instructions and as described previously [18]. Briefly, the stock solution of the Collagen Hybridizing Peptide (CHP)-Cy3 conjugate (R-CHP; RED300, 3Helix Inc.) was diluted in 1X TBS just before use to the concentration of 5 μM, heated at 80 °C for 5 min to heat-dissociate self-assembled peptide monomers, and then quenched to room temperature by immersing in an ice-water bath for 30 s. Immediately, enough solution was added to cover each tissue sample. Tissues were incubated with the staining solution in a humidified chamber at 4 °C overnight. Finally, tissue slides were washed with three changes of 1X TBS (5 min each). Mounting, nuclear staining, imaging, and image analysis were performed as described under IF staining (see above).
2.10. RNA purification and quantitative reverse transcription polymerase chain reaction (RT-qPCR)
RNA was purified form callus tissues as previously described [35]. cDNAs were prepared using iScript cDNA Synthesis Kit (Bio-Rad), and then qPCR-amplified using TaqMan Fast Advanced Master Mix and TaqMan Gene-Expression Assays (ThermoFisher Scientific).
2.11. Statistical analysis
Unpaired student’s t test was used for statistical analysis (GraphPad PRISM 8 software). Statistical significance in figures is indicated using asterisks; (*) P < 0.05; (**) P < 0.01; P < 0.001(***); P < 0.0001(****).
3. Results
3.1. Mice with diet-induced obesity exhibit delayed bone healing
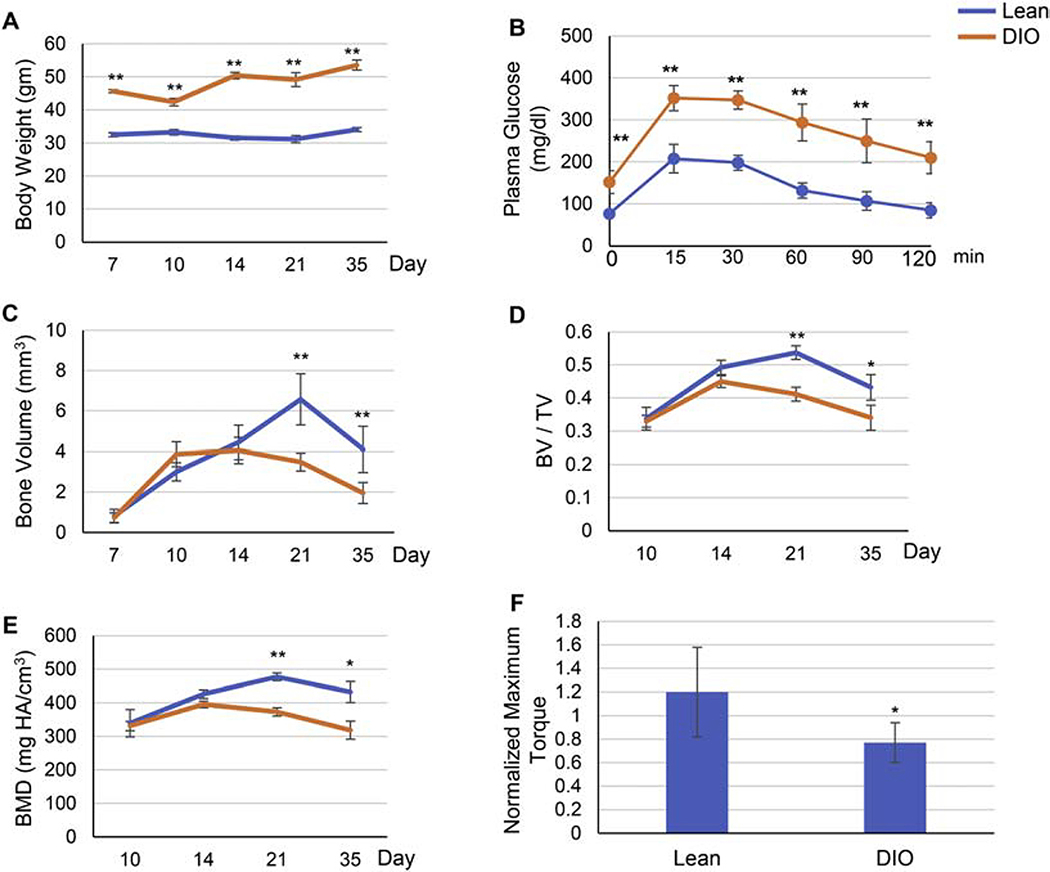
To induce obesity, we used the standard protocol for diet-induced obesity (DIO) mouse model [13]: we fed 5-week-old mice high-fat diet (60 kcal% fat) for 12 weeks, while control mice were fed lean diet (10% kcal% fat) over the same period. As expected, mice on high-fat diet (DIO mice) exhibited obesity (Fig. 1A), pre-diabetic hyperglycemia and glucose intolerance (Fig. 1B). We then administered tibial fractures to the right hind limb as previously described [30] (Supplementary Fig. S2A). Based on previously published data on the time course of fracture healing in the DIO mouse model [13], we harvested callus tissue at day 7, 10, 14, 21, and 35 post-fracture. Detailed Micro-Computed Tomography (μCT) analyses detected clear defective/delayed bone formation in DIO mice as evidenced by significantly lower bone volume (BV) (Fig. 1C), bone volume density (BV/TV) (Fig. 1D), and bone mineral density (BMD) (Fig. 1E) at day 21 (d21) and d35 post-fracture. Consistent with this, biomechanical torsional testing at d35 revealed significantly lower bone strength in DIO mice (Fig. 1F).
Fig. 1.
Fracture healing is delayed in DIO mice. (A) Body weight at time of harvest. Five-week-old mice were fed either lean or high-fat diet (denoted as Lean and DIO, respectively) for 12 weeks, subjected to mid-diaphysis tibial fracture, and harvested at the indicated post-fracture time points. (B) Pre-operative Glucose tolerance test (GTT). Mice were fasted for 6 h (daytime), received an IP injection of 2g/kg glucose, and blood glucose levels were measured at the specified post-injection time points. (C-E) Graphical presentation of μCT analysis of bone volume (C), bone volume density (BV/TV) (D), or bone mineral density (BMD) (E) performed at the specified post-fracture time points. (F) Graphical presentation of biomechanical torsional testing at day 35 post-fracture. Recorded maximum torque (N.mm) of the fractured right tibia was normalized to that of the contralateral unfractured left tibia to confirm that the observed decrease in biomechanical strength is specific to the healing tissue. For all analyses, n = 9. Data presented as average ± S.D. (*) P < 0.05; (**) P < 0.01 using student t-test and time-matched lean callus as a control.
3.2. The fibrocartilaginous callus undergoes accelerated resorption in DIO mice
The repair phase in diaphyseal fractures stabilized by intramedullary nail (Supplementary Fig. S2A) is initiated by the formation of a cartilaginous (soft) callus, which bridges the edges of the fractured bone [9]. To assess this early stage of healing, we used Safranin-O/Fast Green (Saf-O) staining to visualize the extracellular matrix (ECM) within the soft callus. Histomorphometric analysis indicated that both Saf-O-stained area and relative area (normalized to the total callus area) in lean mice plateaued between d7 and d14, and then rapidly resorbed to become hardly detectable by d21 (Supplementary Fig. S2B–D). In DIO mice, both Saf-O area and relative area were comparable to those of lean mice at d7, significantly larger at d10, and significantly smaller at d14 (Supplementary Fig. S2B–D). These results demonstrate that the soft callus attains a bigger size, but is resorbed earlier in DIO-mouse callus (DIO callus) as compared to lean callus.
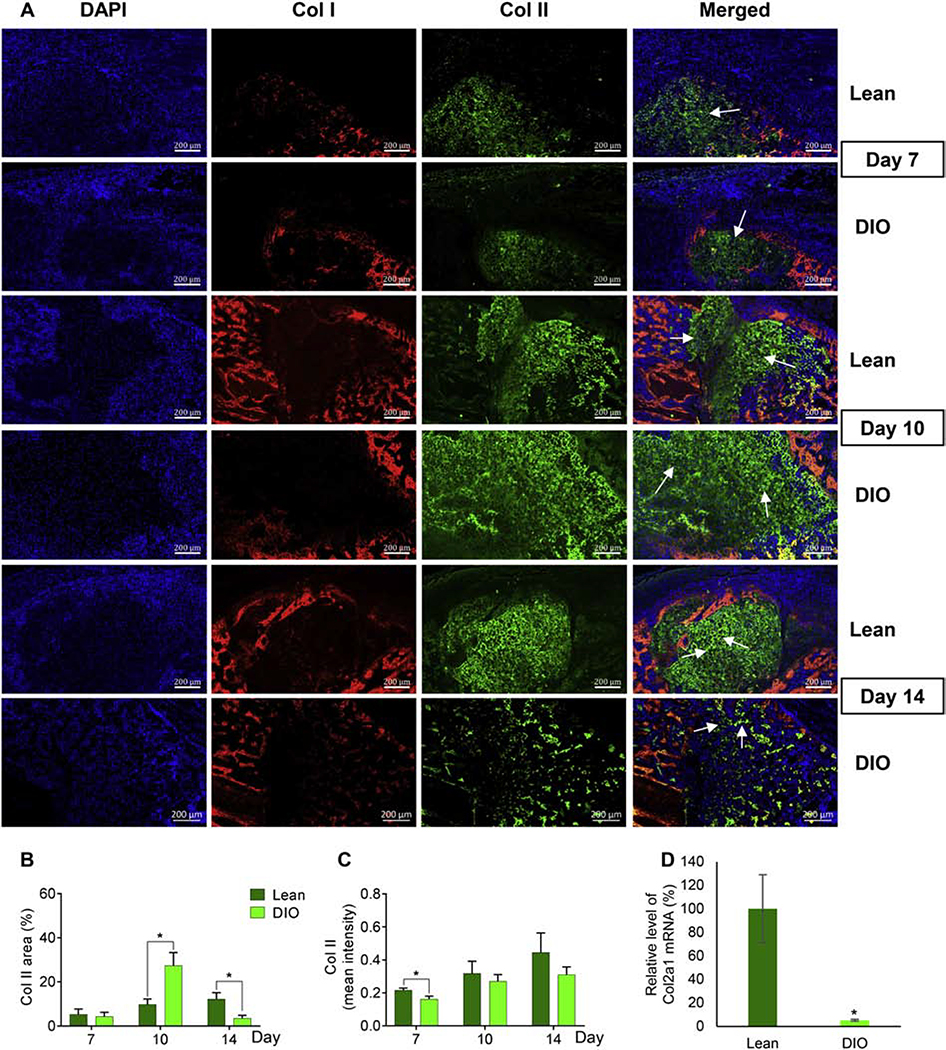
We then sought in-depth analysis of collagen (Col) II, the major form of collagen and the most abundant extracellular structural macromolecule within the cartilaginous soft callus [10]. To that end, we first performed IF staining of both Col I and Col II (Fig. 2A; co-staining of Col I and Col II; see below). As expected, we detected abundant expression of Col II at and around the fracture line (Fig. 2A; Supplementary Fig. S3A, B), while Col I was highly expressed in areas flanking the soft callus (Fig. 2A). For Col II, we analyzed the following two parameters to investigate expression and distribution within the callus: 1) Col II relative area, which is calculated by normalizing Col II-stained area to the total callus area, and 2) Col II mean intensity, which is calculated by normalizing total fluorescence intensity to the total number of fluorescent pixels. Compared to lean callus, Col II relative area in DIO callus was similar at d7, significantly larger at d10, and significantly smaller at d14 (Fig. 2B). On the other hand, Col II mean intensity was comparable in DIO and lean callus at d10 and d14 (Fig. 2C). Measuring the expression level of Col II transcript using RT-qPCR corroborated our IF data and confirmed significant and profound reduction in Col II expression in DIO callus at d14 (Fig. 2D). Notably, no difference was observed between lean and DIO callus with regards to chondrocytes morphology or arrangement (Fig. 2A).
Fig. 2.
The soft callus disappears earlier in DIO mice as compared to lean mice. (A) Representative images of IF staining of Col I (red) and Col II (green) in callus tissues isolated from either lean or DIO mice at the indicated post-fracture time points. DAPI stains nuclei (blue). Scale bar = 200μm. (B) Quantitation of Col II-stained area in IF images. Col II-stained area was normalized to the total-callus area and presented as area (%). (C) Quantitation of the mean intensity of Col II staining in IF images. Analysis was performed in 6 lean and 6 DIO callus tissues; 3 sections were analyzed in each. (D) RT-qPCR quantitation of Col2a1 mRNA (normalized to the level of β-actin mRNA). Quantitation was done on RNA purified from lean and DIO callus tissues at d14 post-fracture (n=3). Normalized level in lean callus is defined as 100.
All bar graphs represent average ± SEM. (*) P < 0.05 using student t-test and time-matched lean callus as a control.
3.3. Collagen fibers in DIO soft callus have normal orientation index but abnormally thin diameter
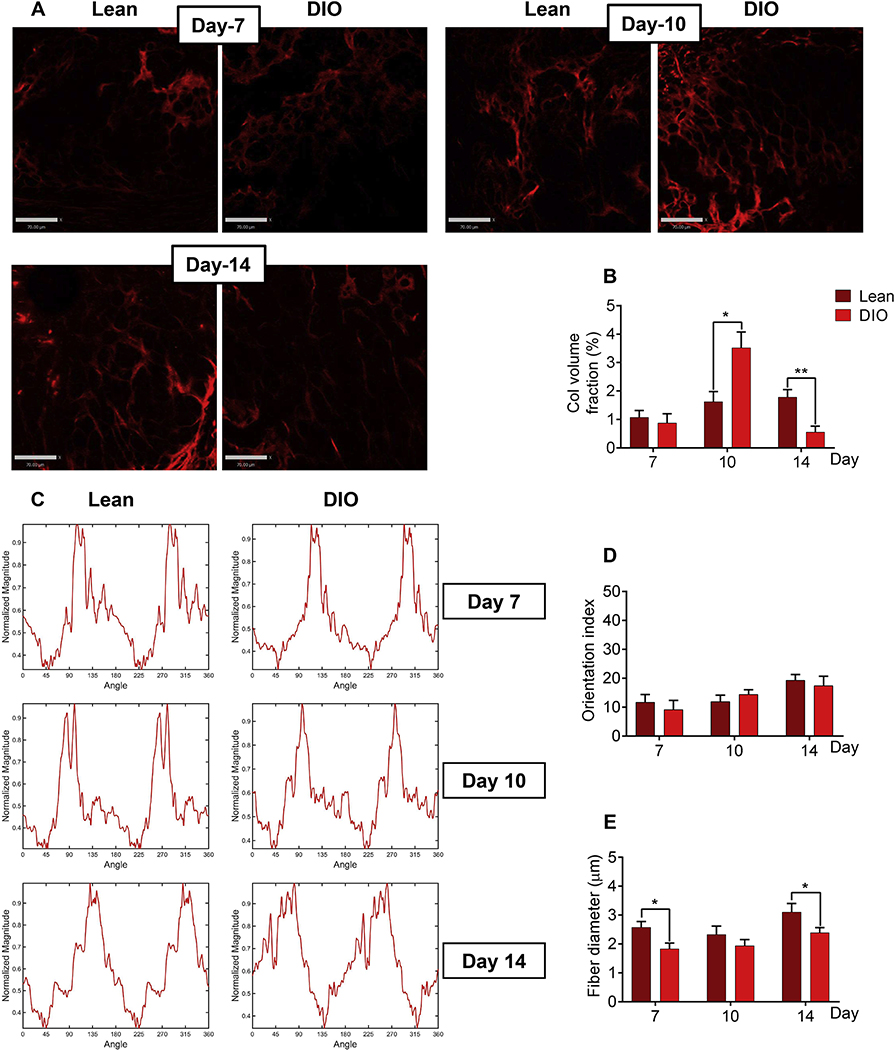
Col II is a protein of the fibrillar collagen family whose biological functions are dependent on not only its expression level, but also the structure of fibers it forms. Thus, we extended our studies and employed SHG microscopy to investigate the content and microstructural features of collagen fibers within the soft callus. As reported previously [36], and given that Col II is the major collagen type within the soft callus [10], we detected a strong SHG signal in the backward direction (BSHG) and a weaker signal in the forward direction (FSHG), which is attributed to the quasi-random organization of Col II fibrils at the μm scale [36]. Importantly, soft callus chondrocytes undergo hypertrophy and express Col I as healing proceeds [10]. To avoid interference from Col I fibers that also generate strong BSHG signal, we focused our analysis on soft-callus areas devoid of Col I expression, as determined by IF staining (Fig. 2A). Consistent with Col II IF data, analysis of fiber-generated BSHG demonstrated that the volume fraction of collagen fibers was significantly higher at d10 and significantly lower at d14 in DIO versus lean callus (Fig. 3A, B). These data support rapid disappearance of soft callus in DIO mice.
Fig. 3.
Collagen fibers in the soft callus of DIO mice have normal orientation index but aberrant fiber diameter. (A) Representative BSHG images of collagen fibers within the soft callus of either lean or DIO mice. Images were captured at the indicated post-fracture time points. Scale bar = 70μm. (B) Quantitation of collagen volume fraction in BSHG images. Collagen volume fraction within each region of interest (ROI) was determined by normalizing the sum of BSHG voxels to the total ROI voxels. Collagen fraction volume was determined in 6 lean and 6 DIO callus tissues; 3 sections were analyzed in each sample, and enough ROIs were selected within each section to cover the entire studied area. (C) Representative examples of Fourier Transform (FT) power spectra generated by analyzing BSHG images using MATLAB. (D) Quantitation of orientation index of the soft-callus fibrillar collagen computed from FT power spectra shown in (C). Orientation index was calculated in 6 lean and 6 DIO callus tissues; 3 sections were analyzed in each sample, and 5 ROIs were selected within each section. (E) Quantitation of collagen-fiber diameter in the same ROIs utilized to compute orientation index in (D).
For all analyses, IF images shown in Fig. 2A were utilized as a guide to select ROIs where Col II, but not Col I, was expressed (exemplified by white arrows in Fig. 2A). All bar graphs represent average ± SEM. (*) P < 0.05; (**) P < 0.01 using student t-test and time-matched lean callus as a control.
We then analyzed the SHG images to investigate the structure of soft-callus fibrillar collagen. We computed the fiber orientation index using the 2D Fourier transform [26], which quantifies the preferred orientation of collagen fibers (see Materials and Methods). The orientation index in lean callus was similar to that in DIO callus at all time points (Fig. 3C, D). On the other hand, measurement of the collagen-fiber diameter indicated that fibers were thinner in DIO callus at all time points, although the difference did not reach statistical significance at d10 (Fig. 3E).
3.4. Hypertrophic chondrocytes disappear in DIO callus earlier than lean callus
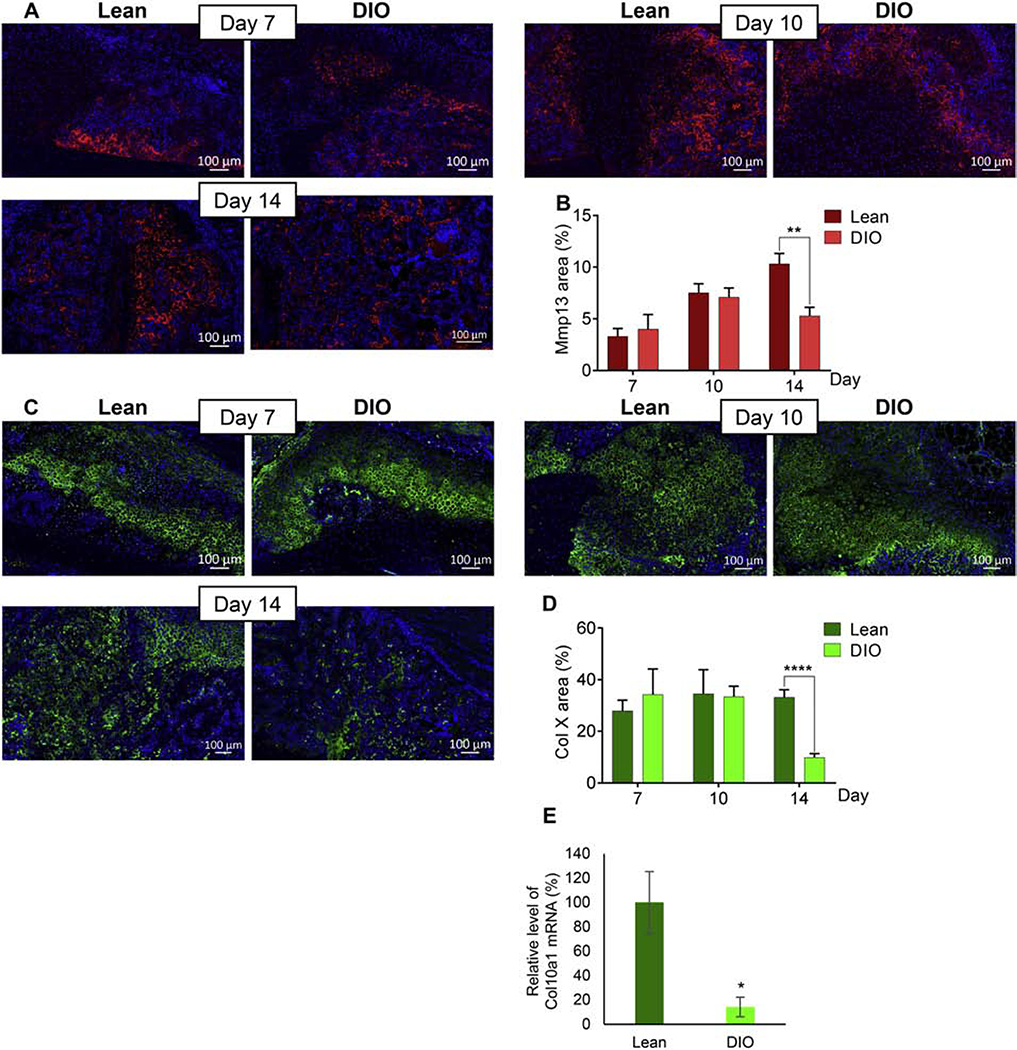
Within two weeks of the healing process, soft callus chondrocytes undergo hypertrophy and express matrix metalloproteinases (MMPs), an essential step that precedes endochondral ossification [6,10]. Consistent with this, we could easily detect hypertrophic chondrocytes as identified by their characteristic morphology in the soft callus of lean and DIO mice (Supplementary Fig. S4 A, B). To more accurately localize and quantify hypertrophic chondrocytes, we performed IF staining for the well-established chondrocyte-hypertrophy markers matrix metallopeptidase 13 (Mmp13) and Col X (Fig. 4A–D). Relative area of Mmp13 was comparable between lean and DIO callus at d7 and d10, but significantly lower in DIO callus at d14 (Fig. 4A, B). Same pattern was observed for Col X (Fig. 4C, D). RT-qPCR analysis confirmed reduced expression of Col10a1 mRNA in DIO callus at d14 (Fig. 4E). This earlier disappearance of hypertrophic chondrocytes provides further evidence for accelerated resorption of the soft callus in DIO mice.
Fig. 4.
Hypertrophic chondrocytes disappear rapidly in DIO callus
(A) Representative images of Mmp13 IF staining (red) in callus tissues isolated from either lean or DIO mice at the indicated post-fracture time points. DAPI stains nuclei (blue). Scale bar = 100μm. (B) Quantitation of Mmp13-stained area in IF images. Mmp13-stained area was normalized to the soft-callus area and presented as area (%). (C) As in (A) except Col X is stained (green). (D) As in (B) except Col X-stained area is quantitated. All IF analyses were performed in 6 lean and 6 DIO callus tissues; 3 sections were analyzed in each. (E) RT-qPCR quantitation of Col10a1 mRNA (normalized to the level of β-actin mRNA). Quantitation was done on RNA purified from lean and DIO callus tissues at d14 post-fracture (n=3). Normalized level in lean callus is defined as 100.All bar graphs represent average ± SEM. (*) P < 0.05; (**) P < 0.01; (****) P < 0.0001 using student t-test and time-matched lean callus as a control.
3.5. The woven-bone area in DIO callus has strikingly low content of Col I protein assembled into defective fibers
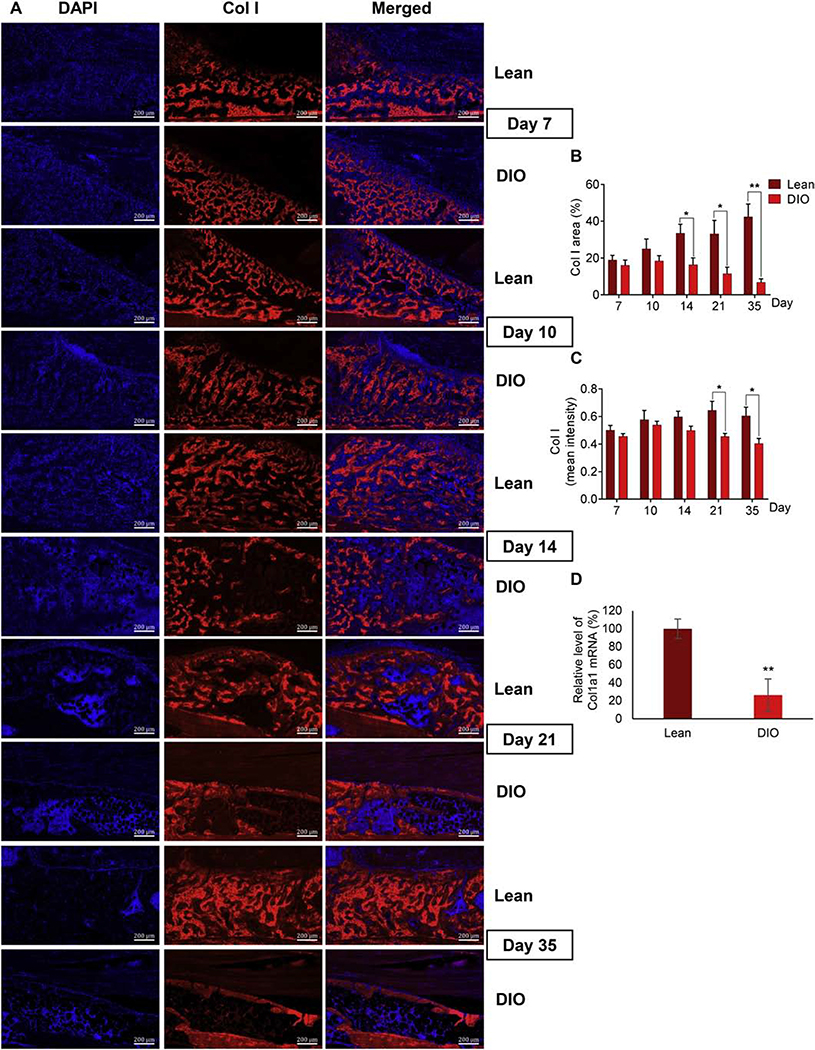
We used the same approaches described above to analyze the content of Col I protein (another fibrillar collagen) as well as the content and microstructure of collagen fibers in the woven-bone area. IF staining of Col I demonstrated the formation of woven bone as early as d7 in areas away from the fracture line in both lean and DIO calluses (Fig. 2A). Compared to lean callus, the relative area of Col I in DIO callus was significantly smaller at d14, d21, and d35, and Col I mean intensity was significantly lower at d21 and d35 (Fig. 5A–C). Consistent with this, Col I mRNA level was significantly reduced in DIO callus at d35 (Fig. 5D). Importantly, Col I in the lean callus appeared between d14 and d35 as a dense network, showing discernable areas of trabecular as well as lamellar-bone structure (Fig. 5A, Supplementary Fig. S5A). In a striking contrast, Col I expression in DIO callus was obviously scarce and disconnected, particularly at d21 and d35 (Fig. 5A; Supplementary Fig. S5A).
Fig. 5.
Col I content is lower in DIO callus than lean callus. (A) Representative images of IF staining of Col I (red) in callus tissues isolated from either lean or DIO mice at the indicated post-fracture time points. DAPI stains nuclei (blue). Scale bar = 200μm. (B) Quantitation of Col I-stained area in IF images. Col I-stained area was normalized to the total-callus area and presented as area (%). (C) Quantitation of the mean intensity of Col I staining in IF images. IF Analysis was performed in 6 lean and 6 DIO callus tissues; 3 sections were analyzed in each. (D) RT-qPCR quantitation of Col1a1 mRNA (normalized to the level of β-actin mRNA). Quantitation was done on RNA purified from lean and DIO callus tissues at d35 post-fracture (n=3). Normalized level in lean callus is defined as 100. All bar graphs represent average ± SEM. (*) P < 0.05; (**) P < 0.01 using student t-test and time-matched lean callus as a control.
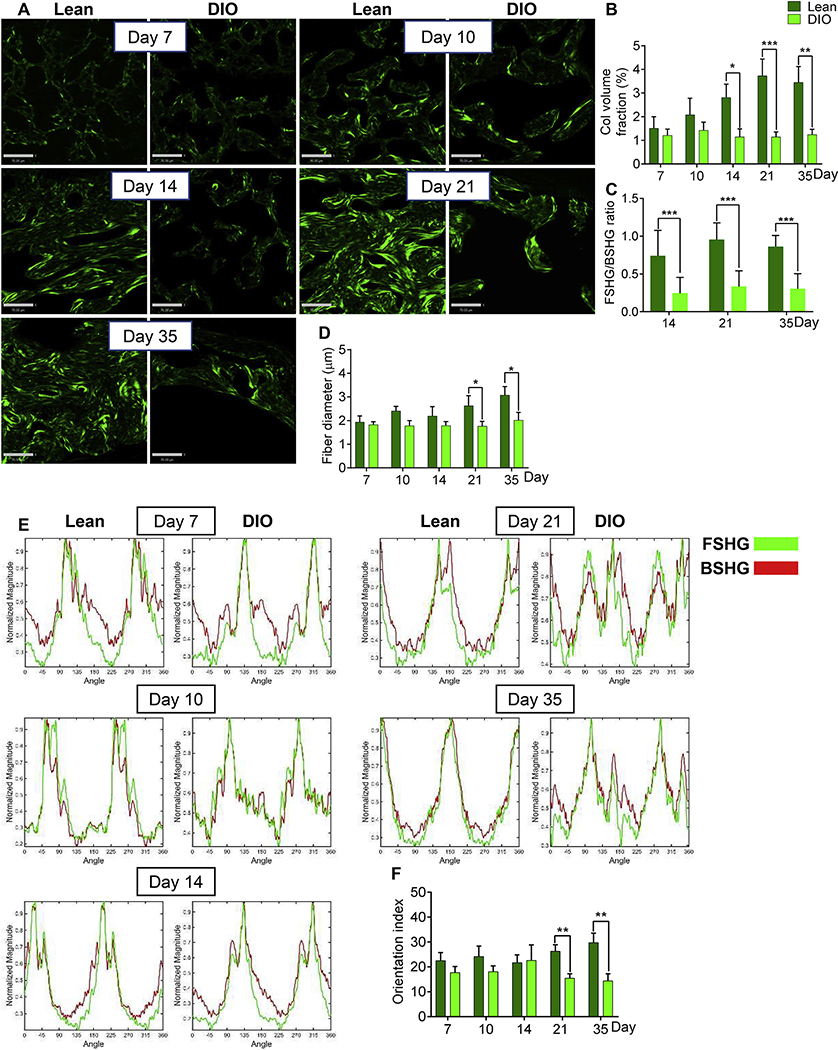
Mature Col I fibers, the main fibrillar collagen in the callus woven bone [37], generate a very strong FSHG signal [36]. Analysis of the woven-bone-derived FSHG signal indicated significantly lower collagen volume fraction in DIO callus as compared to lean callus at d14, d21, and d35 (Fig. 6A, B). Further, collagen fibers in the DIO-callus woven bone showed significantly lower FSHG/BSHG ratio at the same 3 time points (d14-d35) (Fig. 6C). Given that the FSHG/BSHG ratio increases as Col I fibers mature and become thicker [36], these data suggest faster assembly of mature Col I fibers in lean callus, or, alternatively, imparted assembly of Col I fibers in DIO callus. Consistent with this, analysis of the collagen-fiber diameter indicated smaller diameter in DIO callus, attaining statistical significance at d21 and d35 (Fig. 6D). Importantly, the orientation index of woven-bone collagen fibers in lean callus increased steadily from d14 to d35 (Fig. 6E, F), approaching the parallel, cable-like orientation of fibrillar collagen in the contralateral intact tibial diaphysis (Supplementary Fig. S5B). On the other hand, collagen fibers in DIO-callus woven bone remained disarrayed and showed no increase in parallelism between d14 and d35 (Fig. 6E, F), resulting in orientation indexes that were significantly lower than those of lean callus at d21 and d35 (Fig. 6E, F).
Fig. 6.
Collagen fibers in the woven-bone area of DIO callus have abnormally small diameter and low orientation index. (A) Representative FSHG images of fibrillar collagen in the woven-bone area of either lean or DIO callus. Images were captured at the indicated post-fracture time points. Scale bar = 70μm. (B) Quantitation of collagen volume fraction in FSHG images. Collagen volume fraction was determined as in Fig. 3B. (C) The FSHG/BSHG ratio was calculated at the specified post-fracture time points using the same ROIs utilized to generate data shown in (B). (D) Quantitation of collagen-fiber diameter in the woven-bone area. Fiber diameter was determined as in Fig. 3E. (E) Representative examples of FT power spectra generated as in Fig. 3C. (F) Quantitation of the orientation index of woven-bone fibrillar collagen. Orientation index was computed as in Fig. 3D.
For all analyses, IF images shown in Fig. 2A and Fig. 5A were used as a guide to choose ROIs. All bar graphs represent average ± SEM. (*) P < 0.05; (**) P < 0.01; (***) P < 0.001 using student t-test and time-matched lean callus as a control.
3.6. The level of unfolded collagen triple helices is pathologically elevated in DIO callus
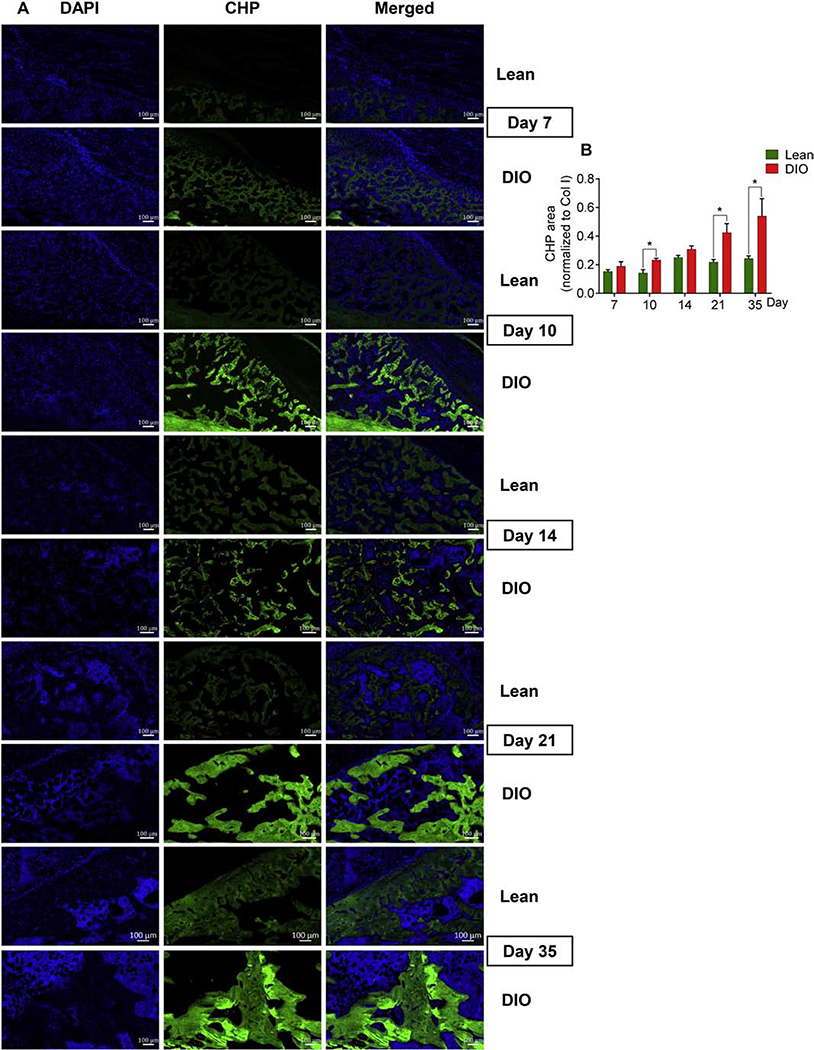
The triple helical structure is a hallmark of collagen proteins [38]. The triple helix becomes unfolded during tissue remodeling or as a pathological outcome of defective collagen fiber formation and/or degradation. To test whether the pronounced defects we observed in the DIO-callus woven bone might be attributed, at least in part, to denatured collagen structure, we visualized unfolded collagen helices using a synthetic collagen hybridizing probe (CHP) [39]. CHP shares the structural motif and the Gly-X-Y repeating sequence with natural collagen and, thus, hybridizes with unfolded collagen strands [39]. CHP is highly specific to unfolded collagen molecules with negligible binding to intact collagen triple helices and negligible non-specific binding to other proteins [39,40]. Our results confirmed CHP specificity to unfolded collagen strands by showing negligible hybridization with the intact contralateral tibial diaphysis (Supplementary Fig. S6). In the lean callus, areas that positively hybridized with CHP increased steadily as healing proceeded (Fig. 7A, B), consistent with active bone remodeling during later phases of healing (Fig. 7B). Interestingly, DIO callus showed a significantly higher content of damaged collagen throughout the healing process, which was most obvious and reached statistical significance at d10, d21, and d35 (Fig. 7A, B).
Fig. 7.
DIO callus has elevated levels of unfolded collagen helices. (A) Representative images of fluorescent staining (green) with collagen hybridizing peptide (CHP) that binds only to denatured, unfolded collagen strands. Staining was performed at the indicated post-fracture time points. Scale bar = 100μm. (B) Quantitation of CHP-stained area normalized to total Col I area.
Analysis was performed in 6 lean and 6 DIO callus tissues; 3 sections were analyzed in each. Bar graphs represent the average ± SEM. (*) P < 0.05 using student t-test and time-matched lean callus as a control.
3.7. DIO callus shows accumulation of AGEs within the woven-bone area and exhibits higher collagen crosslink density
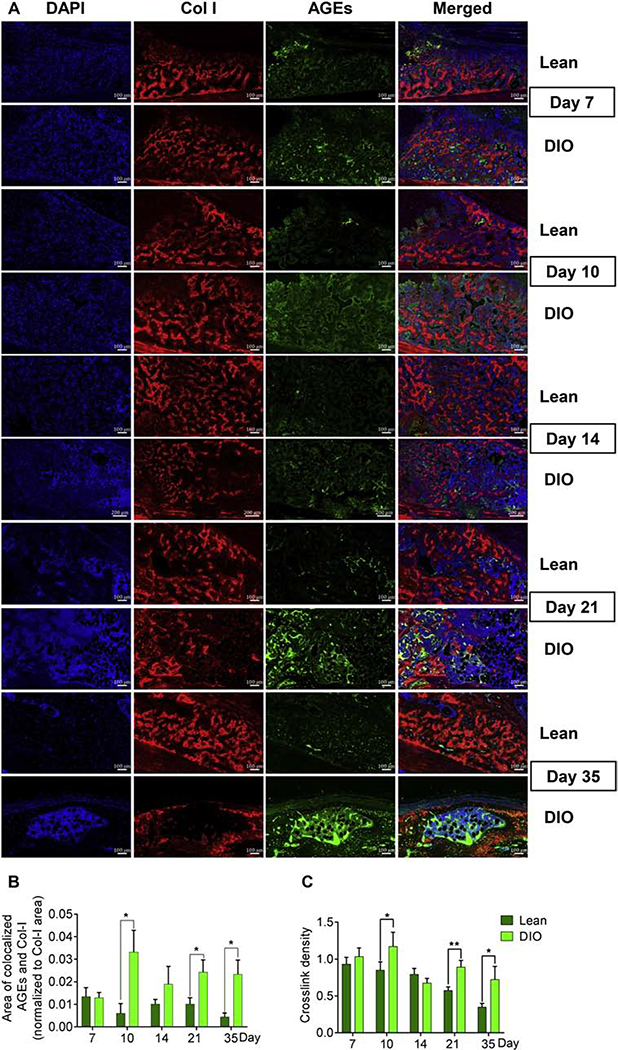
Accumulation of AGEs is a known factor of reduced bone quality in patients with diabetes/hyperglycemia [11,41] through formation of pathological crosslinks within collagen fibers [42]. AGEs-mediated, non-enzymatic crosslinks negatively impact the elasticity of collagen fibers as compared to the physiological, enzymatic, lysyl oxidase (Lox)-mediated crosslinks [42]. To detect AGEs accumulation in the fracture callus, we performed IF staining using a widely used polyclonal antibody that detects a wide range of AGEs [43–45] (further details are provided in Supplementary Table 1). The ability of this antibody to detect AGEs accumulation as a result of aging or disease conditions has been validated in different tissues, including musculoskeletal tissues [18,46–52]. Given that AGEs modify several proteins, and that our aim is to detect AGEs accumulation in Col I, we co-stained AGEs and Col I and limited our analysis to areas where they colocalize as detected by confocal microscopy (Supplementary Fig. S7). Compared to lean callus, the levels of AGEs at Col I-stained areas was significantly higher in DIO callus at d10, d21, and d35 (Fig. 8 A, B). On the other hand, analysis of Lox expression in the woven-bone area indicated no significant difference between lean and DIO calluses in either the relative area or mean intensity of Lox (Supplementary Fig. S8 A–C). To further investigate the possible impact of elevated AGEs levels in DIO callus, we measured the crosslink density of fibrillar collagen in the woven-bone area [27] (see Material and Methods). Consistent with AGEs IF data, the crosslink density was significantly higher in DIO callus as compared to lean callus at d10, d21, and d35 (Fig. 8C).
Fig. 8.
AGEs pathologically accumulate in DIO callus. (A) Representative images of IF co-staining of Col I (red) and AGEs (green) in callus tissues isolated from either lean or DIO mice at the indicated post-fracture time points. Scale bar = 70μm. (B) Quantitation of the area of Col I-colocalized AGEs normalized to the total area of Col I. Analysis was performed in 6 lean and 6 DIO callus tissues; 3 sections were analyzed in each. (C) Quantitation of collagen crosslink density by normalizing the intensity of MPEF signal to the intensity of the corresponding BSHG signal. Crosslink density was measured in 6 lean and 6 DIO callus tissues; 3 sections were analyzed in each, and enough ROIs were selected in each section to cover the entire studied area.
All bar graphs represent average ± SEM. (*) P < 0.05; (**) P < 0.01 using student t-test and time-matched lean callus as a control.
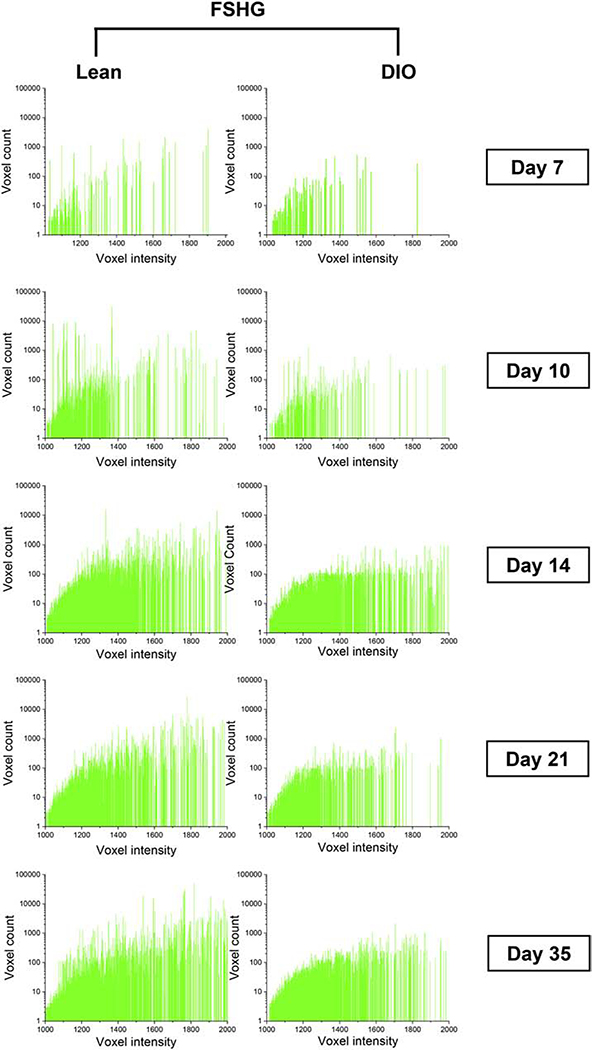
It has been previously reported that accumulation of AGEs reduces the intensity of fibrillar collagen SHG signal [18]. To investigate this, we performed a detailed analysis of the woven-bone-generated SHG signal by plotting SHG voxel intensity versus the voxel count. Results demonstrated a clear shift toward lower voxel intensity in DIO callus as compared to lean callus at d10, d21, and d35 (Fig. 9). Taken together, our data indicate pathological accumulation of AGEs in DIO callus, resulting in increased collagen-fiber crosslink density.
Fig. 9.
The SHG signal in DIO callus is weaker than lean callus. FSHG signal was analyzed in the woven-bone area of lean and DIO callus tissues at the indicated post-fracture time points. Plotting FSHG voxel intensity versus voxel count indicated a shift toward lower voxel intensity in DIO callus. Analysis of the corresponding BSHG signal generated comparable results (Supplementary Fig. S9).
4. Discussion
DIO mice exhibit obesity-associated pre-diabetic hyperglycemia and glucose intolerance (Fig. 1A, B). Given the limited clinical data on the impact of pre-diabetic hyperglycemia on fracture healing, studies in the DIO mouse model help bridge this knowledge gap [13]. The findings we are presenting here establish the healing phenotype of DIO mice and highlight similarities and differences between DIO and other murine models of obesity as well as models of diabetes mellitus (DM). Importantly, our results identify that defects in the content and microstructure of fibrillar collagen contribute to impaired fracture healing.
Fractures with low stability, like those stabilized with intramedullary nails (Supplementary Fig. S2A), heal through secondary bone healing [2]. Secondary healing occurs at and around the fracture line where blood vessels are damaged, generating ischemic and hypoxic conditions, which favor chondrogenic over osteogenic differentiation of MSCs [6,9]. Early in the healing process, chondrocytes proliferate to fill the fracture line and the surrounding area, generating a soft callus which then undergoes hypertrophy and terminal differentiation to mediate soft-callus mineralization, hardening of the fracture gap, and neovascularization [10]. These steps are temporally coordinated and precede endochondral ossification and bone remodeling [10]. The phenotype exhibited by the healing soft callus varies according to the DM model [53,54]. For example, in the spontaneously diabetic BB/OK rat, a model for T1D, the formation, ossification, and resorption of the soft callus proceed more slowly as compared to control rats [55]. In contrast, the streptozotocin-induced T1D mouse model shows accelerated loss of the soft callus [53,54], while the alloxan-induced T1D mouse model exhibits delayed chondrocyte maturation and hypertrophy, accompanied by reduced expression of vascular endothelial growth factor (VEGF) and delayed neovascularization [56]. Compared to T1D, less reports investigated fracture healing in models of obesity-associated prediabetic hyperglycemia and glucose intolerance, which includes a study that describes delayed soft callus formation and resorption in the genetically obese ob/ob mouse model [57]. Another study in the DIO mouse model utilizes hematoxylin/orange G staining to conclude normal size and morphology of soft callus at all time points [13]. Here, we present a comprehensive set of data that analyzes soft callus size and morphology (Fig. 2 and Supplementary Fig. S4), the microstructure of soft-callus fibrillar collagen (Fig. 3), as well as the expression of the well-established hypertrophy markers Mmp13 and Col X (Fig. 4). All our data support a model where DIO callus undergoes accelerated resorption, which is similar to the phenotype reported in streptozotocin-induced T1D mice [53,54]. However, the accelerated loss of soft callus in the streptozotocin-induced T1D mouse model is attributed to elevated levels of tumor necrosis factor (TNF)-α and increased apoptosis (as indicated by increased TUNEL staining) of hypertrophic chondrocytes [53,54]. We did not detect any significant difference between DIO and lean mice in either the expression level of TNF-α (Supplementary Fig. S10A) or the number of TUNEL-positive chondrocytes (Supplementary Fig. S10B), indicating different underlying molecular mechanisms of accelerated disappearance of soft callus. Interestingly, our SHG data show that DIO soft callus contains thinner-than-normal collagen fibers (Fig. 3E), which may contribute to their fast resorption; further studies are required to investigate this relationship.
Sites distal to the fracture gap (i.e., the far ends of the fracture callus) have intact blood vessels and normoxic conditions, which allow for direct osteogenic commitment of MSCs and generation of woven bone [9]. Woven bone undergoes mineralization early in the healing process before being fused with the soft callus [8]. Later in the healing process, woven bone matures and undergoes remodeling to generate lamellar bone [9]. Our IF and SHG data demonstrate woven bone formation at the two distal callus regions as early as d7 in both lean and DIO mice (Fig. 5A; 6A). At d7 and d10, Col I protein expression (Fig. 5A–C) and collagen fiber content (Fig. 6A, B) in the DIO-callus woven bone are normal and comparable to those in the lean. Callus mineralized volume, BV/TV, and volumetric bone mineral density are also comparable between lean and DIO mice at d10 (Fig. 1C–E). Beyond d10, the lean callus shows an obvious increase in the content of Col I and collagen fibers, and, during the late stages of healing, lamellar bone is easily detectable with its characteristic parallel-aligned collagen fibers (Fig. 5A, B; 6A, B). In contrast, the DIO-callus healing strikingly deteriorates beyond d10, as evidenced by a significant drop in the content of Col I (Fig. 5B) and collagen fibers (Fig. 6B) to ≤ 50% their levels in the lean callus. Abnormalities in the DIO callus also include clear defects in the microstructure of woven-bone fibrillar collagen, as evidenced by abnormally low FSHG/BSHG ratio, low orientation index, and small diameter (Fig. 6C–F). As a result, the bony callus of DIO mice consists of an immature and disconnected network of poorly oriented collagen fibers (Fig. 5A; 6A). These DIO-associated defects impede the normal functions of fibrillar collagen in bony tissue formation, organization, remodeling, and mechanics [17], culminating in reduced callus mineralization (Fig. 1E), bone volume (Fig. 1C), BV/TV (Fig. 1D), and bone strength (Fig. 1F).
Several studies on T1D, DIO, and ob/ob murine models report a reduction in bone content during the late stages of fracture healing [13,53,57,58]. The underlying causes of defective bone formation in these models are not well understood. In the DIO model, pathological adipogenic commitment of MSCs is suggested as a plausible contributor to impaired osteogenesis [13]. The data we present here identify defective microstructure of fibrillar collagen as another important contributor to impaired healing in DIO mice.
Fibrillar collagens include Col I, II, III, V, and XI, and the two recently characterized types Col XXIV and XXVII [59]. While Col I and II generate strong SHG signals, Col III and V generate very weak signals [60]. The expression levels of the remaining fibrillar collagen types (i.e. Col XI, XXIV, and XXVII) are very low in the callus compared to the levels of Col I and Col II (our unpublished data). Thus, the SHG signal we analyzed in the soft callus (away from hypertrophy zones) and woven bone is largely generated by Col II and Col I, respectively, with minimal contribution from other fibrillar collagens. Importantly, dysregulated expression of fibrillar (other than Col I and Col II) and non-fibrillar collagens in DIO callus might contribute to the detected defects in the microstructure of Col I and Col II fibers.
Our data also suggest that the abnormal microstructure of woven-bone fibrillar collagen is attributed, at least in part, to accumulation of AGEs (Fig. 8). It is noteworthy that AGEs accumulate in the callus at a higher rate than in the contralateral intact bone (Supplementary Fig. S11), probably due to the higher rate of deposition and resorption of new collagen fibers in the callus. AGEs modify many proteins in the callus in addition to Col I (Fig. 8A; Supplementary Fig. S7). Accumulation of AGEs in Col I fibers increases collagen MPEF signal and reduces the corresponding SHG signal [18] (Fig. 9), resulting in elevated collagen crosslink density (Fig. 8C). AGEs also inhibit the ECM remodeling via interfering with collagen degradation, resulting in pathological accumulation of old and partially degraded collagen fibers [61]. This is consistent with and explains, at least in part, our observation that DIO callus exhibits elevated levels of unfolded collagen triple helices (Fig. 7). Furthermore, AGEs attenuate MSCs differentiation and impair osteoblasts proliferation and function [62], which might be contributing to the reduced bone content in DIO callus that we and others observed [13,57,63] (Fig. 5A, B; 6A, B). Compounds that inhibit AGEs modification of fibrillar collagen are currently being researched [64]. Using such compounds in the future will address a limitation in this study by confirming a direct causal relationship between AGEs and changes in fibrillar collagens microstructures.
Taken together, the data we present here extend the understanding of delayed fracture healing that associates obesity and prediabetic hyperglycemia, and unravel defective fibrillar collagen microstructure as a novel and important contributor. Our findings are expected to help future studies aiming to address impaired healing in obese and pre-diabetic individuals.
Supplementary Material
HIGHLIGHTS.
Diet-induced obesity (DIO) mouse model, a well-established model of obesity and pre-diabetic hyperglycemia and glucose intolerance, exhibits delayed fracture healing.
The healing phenotype of DIO mice is characterized by accelerated soft callus resorption and defective bone formation.
Collagen I fibers in the DIO-mouse callus are strikingly sparse and have abnormal microstructure.
The DIO-mouse callus is typified by accumulation of advanced glycation end products and unfolded collagen helices.
DIO-associated collagen-fiber defects result in reduced callus mineralization and strength.
Acknowledgments
We thank Hwabok Wee for μCT scanning, Kaitlin Saloky for μCT analysis, Tzong Jen Sheu for help with fracture surgeries, Natalie Yoshioka for comments on the manuscript, and Irena Nowak for technical assistance. This work was supported by National Institutes of Health (NIH) R01 DK121327-01A1 to R.A.E and NIH S100D018124 to T.A.
Footnotes
Conflict of interests
The authors declare that they do not have any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Einhorn TA, Gerstenfeld LC, Fracture healing: Mechanisms and interventions, Nat. Rev. Rheumatol. 11 (2015) 45–54. 10.1038/nrrheum.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kostenuik P, Mirza FM, Fracture healing physiology and the quest for therapies for delayed healing and nonunion, J. Orthop. Res. 35 (2017) 213–223. 10.1002/jor.23460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Marin C, Luyten FP, Van der Schueren B, Kerckhofs G, Vandamme K, The impact of Type 2 diabetes on bone fracture healing, Front. Endocrinol. (Lausanne). 9 (2018) 1–15. 10.3389/fendo.2018.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hernandez RK, Do TP, Critchlow CW, Dent RE, Jick SS, Patient-related risk factors for fracture-healing complications in the United Kingdom General Practice Research Database, Acta Orthop. 83 (2012) 653–660. 10.3109/17453674.2012.747054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Flegal KM, Carroll MD, Ogden CL, Curtin LR, CLINICIAN ‘ S CORNER Among US Adults, 1999–2008, J. Am. Med. Assoc. 303 (2013) 235–241. 10.1001/jama.2009.2014. [DOI] [Google Scholar]
- [6].Bahney CS, Zondervan RL, Allison P, Theologis A, Ashley JW, Ahn J, Miclau T, Marcucio RS, Hankenson KD, Cellular biology of fracture healing, J. Orthop. Res. 37 (2019) 35–50. 10.1002/jor.24170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Claes L, Recknagel S, Ignatius A, Fracture healing under healthy and inflammatory conditions, Nat. Rev. Rheumatol. 8 (2012) 133–143. 10.1038/nrrheum.2012.1. [DOI] [PubMed] [Google Scholar]
- [8].Loi F, Córdova LA, Pajarinen J, hua Lin T, Yao Z, Goodman SB, Inflammation, fracture and bone repair, Bone. 86 (2016) 119–130. 10.1016/j.bone.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Marsell R, Einhorn TA, The biology of fracture healing, Injury. 42 (2011) 551–555. 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hu DP, Ferro F, Yang F, Taylor AJ, Chang W, Miclau T, Marcucio RS, Bahney CS, Cartilage to bone transformation during fracture healing is coordinated by the invading vasculature and induction of the core pluripotency genes, Dev. 144 (2017) 221–234. 10.1242/dev.130807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jiao H, Xiao E, Graves DT, Diabetes and Its Effect on Bone and Fracture Healing, Curr. Osteoporos. Rep. 13 (2015) 327–335. 10.1007/s11914-015-0286-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Williams JN, Kambrath AV, Patel RB, Kang KS, Mével E, Li Y, Cheng YH, Pucylowski AJ, Hassert MA, Voor MJ, Kacena MA, Thompson WR, Warden SJ, Burr DB, Allen MR, Robling AG, Sankar U, Inhibition of CaMKK2 Enhances Fracture Healing by Stimulating Indian Hedgehog Signaling and Accelerating Endochondral Ossification, J. Bone Miner. Res. 33 (2018) 930–944. 10.1002/jbmr.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Brown ML, Yukata K, Farnsworth CW, Chen DG, Awad H, Hilton MJ, O’Keefe RJ, Xing L, Mooney RA, Zuscik MJ, Delayed fracture healing and increased callus adiposity in a C57BL/6J Murine model of obesity-associated type 2 diabetes mellitus, PLoS One. 9 (2014). 10.1371/journal.pone.0099656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Williams LM, Campbell FM, Drew JE, Koch C, Hoggard N, Rees WD, Kamolrat T, Ngo HT, Steffensen IL, Gray SR, Tups A, The development of diet-induced obesity and glucose intolerance in C57Bl/6 mice on a high-fat diet consists of distinct phases, PLoS One. 9 (2014). 10.1371/journal.pone.0106159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ben Shoham A, Rot C, Stern T, Krief S, Akiva A, Dadosh T, Sabany H, Lu Y, Kadler KE, Zelzer E, Deposition of collagen type I onto skeletal endothelium reveals a new role for blood vessels in regulating bone morphology, Dev. 143 (2016) 3933–3943. 10.1242/dev.139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stock SR, The Mineral–Collagen Interface in Bone, Calcif. Tissue Int. 97 (2015) 262–280. 10.1007/s00223-015-9984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kim D, Lee B, Thomopoulos S, Jun YS, The role of confined collagen geometry in decreasing nucleation energy barriers to intrafibrillar mineralization, Nat. Commun. 9 (2018). 10.1038/s41467-018-03041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Krishnamoorthy D, Hoy RC, Natelson DM, Torre OM, Laudier DM, Iatridis JC, Illien-Jünger S, Dietary advanced glycation end-product consumption leads to mechanical stiffening of murine intervertebral discs, DMM Dis. Model. Mech. 11 (2018). 10.1242/dmm.036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Campagnola PJ, Loew LM, Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms, Nat. Biotechnol. 21 (2003) 1356–1360. 10.1038/nbt894. [DOI] [PubMed] [Google Scholar]
- [20].Zipfel WR, Williams RM, Christiet R, Nikitin AY, Hyman BT, Webb WW, Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation, Proc. Natl. Acad. Sci. U. S. A. 100 (2003) 7075–7080. 10.1073/pnas.0832308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mostaço-Guidolin L, Rosin NL, Hackett TL, Imaging collagen in scar tissue: Developments in second harmonic generation microscopy for biomedical applications, Int. J. Mol. Sci. 18 (2017). 10.3390/ijms18081772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Abraham T, Fong G, Scott A, Second harmonic generation analysis of early Achilles tendinosis in response to in vivo mechanical loading, BMC Musculoskelet. Disord. 12 (2011). 10.1186/1471-2474-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sharma A, Abraham T, Sampaio A, Cowan M, Underhill M, Scott A, Sodium cromolyn reduces expression of CTGF, ADAMTS1, and TIMP3 and modulates post-injury patellar tendon morphology, J. Orthop. Res. 29 (2011) 678–683. 10.1002/jor.21291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liu C, Cabahug-Zuckerman P, Stubbs C, Pendola M, Cai C, Mann KA, Castillo AB, Mechanical Loading Promotes the Expansion of Primitive Osteoprogenitors and Organizes Matrix and Vascular Morphology in Long Bone Defects, J. Bone Miner. Res. 34 (2019) 896–910. 10.1002/jbmr.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Scott A, Sampaio A, Abraham T, Duronio C, Underhill TM, Scleraxis expression is coordinately regulated in a murine model of patellar tendon injury, J. Orthop. Res. 29 (2011) 289–296. 10.1002/jor.21220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Abraham T, Kayra D, McManus B, Scott A, Quantitative assessment of forward and backward second harmonic three dimensional images of collagen Type I matrix remodeling in a stimulated cellular environment, J. Struct. Biol. 180 (2012) 17–25. 10.1016/j.jsb.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Marturano JE, Xylas JF, Sridharan GV, Georgakoudi I, Kuo CK, Lysyl oxidase-mediated collagen crosslinks may be assessed as markers of functional properties of tendon tissue formation, Acta Biomater. 10 (2014) 1370–1379. 10.1016/j.actbio.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pettersson US, Waldén TB, Carlsson PO, Jansson L, Phillipson M, Female Mice are Protected against High-Fat Diet Induced Metabolic Syndrome and Increase the Regulatory T Cell Population in Adipose Tissue, PLoS One. 7 (2012). 10.1371/journal.pone.0046057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hwang LL, Wang CH, Li TL, Chang SD, Lin LC, Chen CP, Chen CT, Liang KC, Ho IK, Yang WS, Chiou LC, Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice, Obesity. 18 (2010) 463–469. 10.1038/oby.2009.273. [DOI] [PubMed] [Google Scholar]
- [30].Kung MH, Yukata K, O’Keefe RJ, Zuscik MJ, Aryl hydrocarbon receptor-mediated impairment of chondrogenesis and fracture healing by cigarette smoke and benzo(α)pyrene, J. Cell. Physiol. 227 (2012) 1062–1070. 10.1002/jcp.22819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R, Guidelines for assessment of bone microstructure in rodents using micro-computed tomography, J. Bone Miner. Res. 25 (2010) 1468–1486. 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- [32].Reynolds DG, Hock C, Shaikh S, Jacobson J, Zhang X, Rubery PT, Beck CA, O’Keefe RJ, Lerner AL, Schwarz EM, Awad HA, Micro-computed tomography prediction of biomechanical strength in murine structural bone grafts, J. Biomech. 40 (2007) 3178–3186. 10.1016/j.jbiomech.2007.04.004. [DOI] [PubMed] [Google Scholar]
- [33].Abraham T, Clawson GA, Linton SS, McGovern CO, Tang X, Bio-distribution of near infrared imaging agent loaded targeted drug nanoparticle carriers in highly fibrotic pancreatic tumor determined using multiphoton and harmonic generation imaging, 1049729 (2018) 55. 10.1117/12.2300517. [DOI] [Google Scholar]
- [34].Abraham T, Hogg J, Extracellular matrix remodeling of lung alveolar walls in three dimensional space identified using second harmonic generation and multiphoton excitation fluorescence, J. Struct. Biol. 171 (2010) 189–196. 10.1016/j.jsb.2010.04.006. [DOI] [PubMed] [Google Scholar]
- [35].Le Bleu HK, Kamal FA, Kelly M, Ketz JP, Zuscik MJ, Elbarbary RA, Extraction of high-quality RNA from human articular cartilage, Anal. Biochem. 518 (2017) 134–138. 10.1016/j.ab.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Houle MA, Couture CA, Bancelin S, Van der Kolk J, Auger E, Brown C, Popov K, Ramunno L, Légaré F, Analysis of forward and backward Second Harmonic Generation images to probe the nanoscale structure of collagen within bone and cartilage, J. Biophotonics. 8 (2015) 993–1001. 10.1002/jbio.201500150. [DOI] [PubMed] [Google Scholar]
- [37].Miedel EL, Brisson BK, Hamilton T, Gleason H, Swain GP, Lopas L, Dopkin D, Perosky JE, Kozloff KM, Hankenson KD, Volk SW, Type III collagen modulates fracture callus bone formation and early remodeling, J. Orthop. Res. 33 (2015) 675–684. 10.1002/jor.22838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fidler AL, Boudko SP, Rokas A, Hudson BG, The triple helix of collagens - An ancient protein structure that enabled animal multicellularity and tissue evolution, J. Cell Sci. 131 (2018). 10.1242/jcs.203950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zitnay JL, Li Y, Qin Z, San BH, Depalle B, Reese SP, Buehler MJ, Yu SM, Weiss JA, Molecular level detection and localization of mechanical damage in collagen enabled by collagen hybridizing peptides, Nat. Commun. 8 (2017). 10.1038/ncomms14913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hwang J, San BH, Turner NJ, White LJ, Faulk DM, Badylak SF, Li Y, Yu SM, Molecular assessment of collagen denaturation in decellularized tissues using a collagen hybridizing peptide, Acta Biomater. 53 (2017) 268–278. 10.1016/j.actbio.2017.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yamamoto M, Sugimoto T, Advanced Glycation End Products, Diabetes, and Bone Strength, Curr. Osteoporos. Rep. 14 (2016) 320–326. 10.1007/s11914-016-0332-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Burr DB, Changes in bone matrix properties with aging, Bone. 120 (2019) 85–93. 10.1016/j.bone.2018.10.010. [DOI] [PubMed] [Google Scholar]
- [43].Horiuchi S, Araki N, Morino Y, Immunochemical approach to characterize advanced glycation end products of the Maillard reaction: Evidence for the presence of a common structure, J. Biol. Chem. 266 (1991) 7329–7332. [PubMed] [Google Scholar]
- [44].Reddy S, Bichler J, Wells-Knecht KJ, Thorpe SR, Baynes JW, Nε-(Carboxymethyl)lysine Is a Dominant Advanced Glycation End Product (AGE) Antigen in Tissue Proteins, Biochemistry. 34 (1995) 10872–10878. 10.1021/bi00034a021. [DOI] [PubMed] [Google Scholar]
- [45].Choi YG, Lim S, Characterization of anti-advanced glycation end product antibodies to nonenzymatically lysine-derived and arginine-derived glycated products, J. Immunoass. Immunochem. 30 (2009) 386–399. 10.1080/15321810903188136. [DOI] [PubMed] [Google Scholar]
- [46].Halushka MK, Selvin E, Lu J, Macgregor AM, Cornish TC, Use of human vascular tissue microarrays for measurement of advanced glycation endproducts, J. Histochem. Cytochem. 57 (2009) 559–566. 10.1369/jhc.2009.953273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Shcheglova T, Makker S, Tramontano A, Reactive immunization suppresses advanced glycation and mitigates diabetic nephropathy, J. Am. Soc. Nephrol. 20 (2009) 1012–1019. 10.1681/ASN.2008050555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Thomas SJ, Sarver JJ, Yannascoli SM, Tucker JJ, Kelly JD, Ahima RS, Barbe MF, Soslowsky LJ, Effect of isolated hyperglycemia on native mechanical and biologic shoulder joint properties in a rat model., J. Orthop. Res. 32 (2014) 1464–1470. 10.1002/jor.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rodriguez-Teja M, Gronau JH, Breit C, Zhang YZ, Minamidate A, Caley MP, McCarthy A, Cox TR, Erler JT, Gaughan L, Darby S, Robson C, Mauri F, Waxman J, Sturge J, AGE-modified basement membrane cooperates with Endo180 to promote epithelial cell invasiveness and decrease prostate cancer survival, J. Pathol. 235 (2015) 581–592. 10.1002/path.4485. [DOI] [PubMed] [Google Scholar]
- [50].Machahua C, Montes-Worboys A, Llatjos R, Escobar I, Dorca J, Molina-Molina M, Vicens-Zygmunt V, Increased AGE-RAGE ratio in idiopathic pulmonary fibrosis, Respir. Res. 17 (2016) 1–11. 10.1186/s12931-016-0460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hwang KR, Murrell GAC, Millar NL, Bonar F, Lam P, Walton JR, Advanced glycation end products in idiopathic frozen shoulders, J. Shoulder Elb. Surg. 25 (2016) 981–988. 10.1016/j.jse.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kanda A, Dong Y, Noda K, Saito W, Ishida S, Advanced glycation endproducts link inflammatory cues to upregulation of galectin-1 in diabetic retinopathy, Sci. Rep. 7 (2017) 1–15. 10.1038/s41598-017-16499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kayal RA, Siqueira M, Alblowi J, McLean J, Krothapalli N, Faibish D, Einhorn TA, Gerstenfeld LC, Graves DT, TNF-α mediates diabetes-enhanced chondrocyte apoptosis during fracture healing and stimulates chondrocyte apoptosis through FOXO1, J. Bone Miner. Res. 25 (2010) 1604–1615. 10.1002/jbmr.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Alharbi MA, Zhang C, Lu C, Milovanova TN, Yi L, Ryu JD, Jiao H, Dong G, Patrick O’Connor J, Graves DT, FOXO1 deletion reverses the effect of diabetic-induced impaired fracture healing, Diabetes. 67 (2018) 2682–2694. 10.2337/db18-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Follak N, Klöting I, Merk H, Influence of diabetic metabolic state on fracture healing in spontaneously diabetic rats, Diabetes. Metab. Res. Rev. 21 (2005) 288–296. 10.1002/dmrr.537. [DOI] [PubMed] [Google Scholar]
- [56].Wang DW, Du SL, Xu MT, Lu YT, Wang ZC, Wang LX, Effects of insulin therapy on fracture healing and expression of VEGF in diabetic rats, J. Appl. Biomed. 11 (2013) 33–40. 10.2478/v10136-012-0018-7. [DOI] [Google Scholar]
- [57].Gao F, Lv TR, Zhou JC, Qin XD, Effects of obesity on the healing of bone fracture in mice, J. Orthop. Surg. Res. 13 (2018) 1–8. 10.1186/s13018-018-0837-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ko KI, Coimbra LS, Tian C, Alblowi J, Kayal RA, Einhorn TA, Gerstenfeld LC, Pignolo RJ, Graves DT, Diabetes reduces mesenchymal stem cells in fracture healing through a TNFα-mediated mechanism, Diabetologia. 58 (2015) 633–642. 10.1007/s00125-014-3470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Exposito JY, Valcourt U, Cluzel C, Lethias C, The fibrillar collagen family, Int. J. Mol. Sci. 11 (2010) 407–426. 10.3390/ijms11020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ranjit S, Dvornikov A, Stakic M, Hong SH, Levi M, Evans RM, Gratton E, Imaging Fibrosis and Separating Collagens using Second Harmonic Generation and Phasor Approach to Fluorescence Lifetime Imaging, Sci. Rep. 5 (2015) 1–10. 10.1038/srep13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Asadipooya K, Uy EM, Advanced Glycation End Products (AGEs), Receptor for AGEs, Diabetes, and Bone: Review of the Literature, J. Endocr. Soc. 3 (2019) 1799–1818. 10.1210/js.2019-00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Meng HZ, Zhang WL, Liu F, Yang MW, Advanced glycation end products affect osteoblast proliferation and function by modulating autophagy via the receptor of advanced glycation end products/raf protein/mitogen-activated protein kinase/extracellular signalregulated kinase kinase/extracellular, J. Biol. Chem. 290 (2015) 28189–28199. 10.1074/jbc.M115.669499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, Woelk L, Fan H, Logan DW, Schürmann A, Saraiva LR, Schulz TJ, Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration, Cell Stem Cell. 20 (2017) 771–784.e6. 10.1016/j.stem.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Price DL, Rhett PM, Thorpe SR, Baynes JW, Chelating Activity of Advanced Glycation End-product Inhibitors, J. Biol. Chem. 276 (2001) 48967–48972. 10.1074/jbc.M108196200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.