Abstract
The SARS-CoV-2 infection, which causes the coronavirus disease (COVID-19), has affected lives, with very adverse outcomes in specific populations in the United States of America (USA), a high-income country, and two middle-income countries, Brazil and South Africa. This paper aims to discuss the relationship of race/ethnicity with COVID-19-associated factors in the three countries. The information is based on data collected from infectious disease/epidemiological centers in the USA, Brazil, and South Africa. Adverse COVID-19 outcomes have been associated with the burden of exposure and disease, linked to socioeconomic determinants, among specific ethnicities in all three countries. The prevalence of comorbidities before and the likelihood of work-related exposure in the context of COVID-19 infection puts ethnic minorities in the USA and some ethnic majorities and minorities in Brazil and South Africa at greater risk. We envisage that this work will contribute to ongoing discussions related to addressing socioeconomic determinants of health, and the need for stakeholders in various sectors to work on addressing observed health disparities for overall improvement in health and healthcare given the current pandemic.
Keywords: COVID-19, Comorbidities, Racial/ethnic disparity, South Africa, Brazil, United States of America
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, which causes the coronavirus disease (COVID-19), is a threat to global health. In this extended commentary, racial disparities in health and COVID-19 outcomes are compared across three countries: South Africa, Brazil, and the United States of America (USA). At the time of writing (September 2020), the USA, a high-income country, and two middle-income countries, Brazil and South Africa, were among the top ten countries with the highest number of cumulative COVID-19 confirmed cases (6,662,003, 4,495,183, and 659,656, respectively) and deaths (197,442, 135,793, and 15,940, respectively) in their corresponding continents [1]. In earlier studies, these countries have been used to present cases of racial stratifications [2–6]. For this extended commentary, we selected these countries, as examples where inequalities exist along ethnic lines in their respective continents, to explore disparities in COVID-19 outcomes using available data. These characteristics and experiences of the coronavirus pandemic provide a platform on which comparisons can be made. Such comparison does not negate the fact that differences exist in history and context across the three countries and such differences may have roles in the respective COVID-19-related outcomes.
Factors related to demographics and comorbid conditions are linked to the vulnerability of specific ethnicities to COVID-19 complications [7–11]. In these populations, the burden of other diseases has been attributed to socioeconomic determinants of health [12–14], which are thought to have some bearing on adverse COVID-19 outcomes (10). Comorbidities such as diabetes mellitus, hypertension, obesity, the human immunodeficiency virus (HIV), and tuberculosis (TB) infections have been shown to increase the risk of severe COVID-19 disease and mortality in the three countries [7–9, 15]. These comorbidities, which predispose to COVID-19 complications, had earlier been noted in individuals of African descent and some minority groups in the USA, Brazil, and South Africa [16–18], who also have poorer health outcomes, higher overall mortality, and lower life expectancy compared to Caucasians/Whites [13, 16–19]. These preexisting health disparities, in addition to exposure at home or at work, are believed to contribute to their disproportionately higher COVID-19-related deaths in the three countries.
Prepandemic Health Disparities
The relatively higher burden of chronic diseases, among specific ethnicities in the three countries, is believed to reflect lower access to healthcare, primary care providers, or disease prevention strategies [12, 13, 18, 20]. In the USA, the 2018 data depicting uninsured rates among non-elderly individuals (up to 64 years old) were higher for minority populations such as American Indians and Alaska Natives (AIAN) at 21.8%, Hispanics (19%), and Blacks (11.5%) compared with Whites (7.5%) [21]. In the 2018 General Household Survey in South Africa, 9.9% of Black South Africans were noted to have medical insurance cover compared to 17.1% of Coloreds, 52% of Indians/Asians, and 72.9% of Whites [22]. In Brazil, analysis of the 2010 data on elderly access to health services in São Paulo showed that populations classified as Black (74.3%) or Brown (69.8%) were more likely to be uninsured compared to Whites (46.9%) [2]. The COVID-19 virus outbreak is the most recent in a list of health issues that disproportionately affect these specific vulnerable populations in the USA, Brazil, and South Africa. The disparities previously existing in these countries have the potential to worsen during the coronavirus pandemic. Against this background, we reflect on the possible relationships between ethnicity and COVID-19 outcomes, and factors influencing these outcomes such as exposure and disease-predisposing comorbidities across the three countries.
In Table 1, the top ten leading causes of death in 2019 are presented for the three countries as reported by the Institute for Health Metrics and Evaluation [23]. Worth noting is that some of the causes of death which predate the pandemic also feature as comorbidities of the COVID-19 virus infection. Across the three countries, populations of specific ethnicities are prone to the burden of communicable and noncommunicable diseases [16–18, 24–26]. On the infectious disease front, HIV and TB constitute a considerable fraction especially in South Africa. South Africa had an estimated 7.7 million people living with HIV in 2019 [27]. In Brazil and the USA, about 1.1 million and 920,000 people, respectively, live with HIV [28, 29]. South Africa also features among the four countries that contribute to more than 40% of the global TB burden [30], with an estimated 2017 TB incidence and mortality of 567 and 39 per 100,000, respectively [31]. Brazil’s cases were estimated to be 44 and 2.4 per 100 000 for incidence and mortality, respectively [32] while the incidence rate in the USA for the same year was 2.8 per 100,000 [33].
Table 1.
Leading causes of death (2019) across South Africa, Brazil, and the USA [23]
| Leading causes of death (year: 2019) | |||
|---|---|---|---|
| South Africa | Brazil | USA | |
| 1 | HIV/AIDS | Ischemic heart disease | Ischemic heart disease |
| 2 | Ischemic heart disease | Stroke | Lung cancer |
| 3 | Stroke | Lower respiratory infections | COPD |
| 4 | Lower respiratory infections | COPD | Stroke |
| 5 | Diabetes | Interpersonal violence | Alzheimer’s disease |
| 6 | Tuberculosis | Diabetes | Chronic kidney disease |
| 7 | Road injuries | Alzheimer’s disease | Colorectal cancer |
| 8 | Interpersonal violence | Road injuries | Lower respiratory infection |
| 9 | Neonatal disorders | Chronic kidney disease | Diabetes |
| 10 | Diarrheal diseases | Cirrhosis | Cirrhosis |
The COVID-19 Experience
Across the three countries, there were similarities and differences in the populations confirmed to be infected with the coronavirus, as noted in hospitalization data. More females were infected in the USA (51.7%) and South Africa (58.3%) while in Brazil, more males were infected [7, 8, 11]. In South Africa, the higher number of female cases may be due to exposure in roles related to healthcare and teaching, along with their health-seeking behaviors [34]. In the USA and Brazil, confirmed infections were highest in the age groups 18–29 years (21.8%) and 60–69 years (20.5%), respectively [8, 11]. In South Africa, the age groups 30–34 and 35–39 years accounted for 12.5 and 12.8% of cases, respectively, which collectively comprised 25.3% of cases [34].
While more cases of confirmed infection were recorded among females in South Africa and the USA, there were more deaths among males. Across all three countries, those who were more likely to develop severe disease were older individuals, males, and those with comorbidities [7–9]. Hypertension, diabetes mellitus, cardiovascular disease, and obesity were some of the comorbidities reported with COVID-19 hospitalization and death [7–9, 11]. In South Africa, hypertension (29.1%) and diabetes (22.5%) were the most common conditions in COVID-9 admissions. They were also the most often reported comorbidities among individuals who died aged 40 years and older while HIV was the most common comorbidity among those between 20 and 39 years old at the time of death. In individuals between 20 and 60 years old at the time of death, HIV, TB, and obesity were commonly reported [7]. In Brazil, more than half (53.2%) of those hospitalized for severe acute respiratory syndrome (SARS) were positive for the COVID-19 virus. A minimum of one comorbidity was reported by almost two thirds (63.9%) of those who died over a period of 30 weeks [8]. Heart disease (45.4% and 9.2% in individuals above and below 60 years, respectively) and diabetes mellitus (33.6% and 9.0% in individuals above and below 60 years, respectively) were the most frequent comorbidities among individuals who died [8]. In the USA, hypertension (59.3%), obesity (47.0%), metabolic disease (42.9%), and cardiovascular disease (34.0%) were the most frequently reported comorbidities among hospitalized adult patients over a 3-month period [9]. The prevalence of some infectious diseases such as HIV or TB, and their effect on the pandemic has been noted. An increased prevalence of underlying health conditions has been noted to contribute to higher COVID-19 mortality in people living with HIV in the USA [35]. In South Africa, research also noted HIV as a risk factor for COVID-19-related complication and mortality [36].
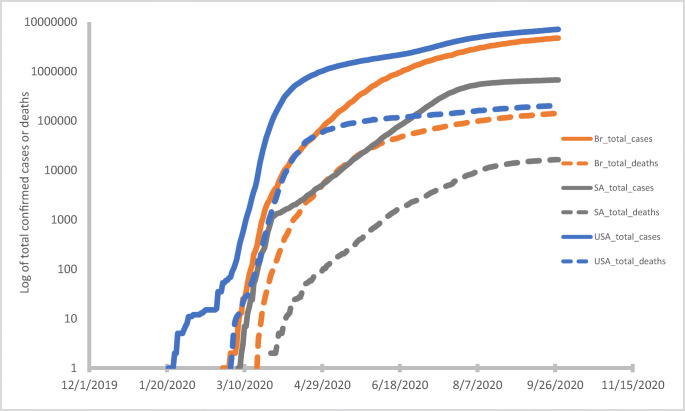
Figure 1 shows the cumulative number of confirmed infection cases (solid lines) and deaths (broken lines), since the start of the pandemic till September 2020, across the three countries. Data for Fig. 1 were generated from the coronavirus pandemic (COVID-19) data repository of Our World in Data, a collaborative effort of different people and organizations [37]. As shown in the figure, the USA had the highest record of cases and deaths and South Africa the least. This was noted in absolute as well as proportional values (for instance, cases per million population).
Fig. 1.
Confirmed COVID-19 virus infection cases (solid lines) and deaths (broken lines) up till 28 September 2020 in South Africa, Brazil, and the USA
African countries, including South Africa, had earlier been noted as outliers as it related to the pandemic course—case fatalities did not earlier match expectations, especially considering the continent’s generally fragile healthcare system [38, 39]. Among the possible reasons for this are testing capacity which likely lags infections, and the non-presentation (for testing) of some infected/exposed individuals who may not have access to healthcare or the wherewithal to isolate/quarantine should such be indicated. More recent reports have noted a seroprevalence of about 40% in specific populations in South Africa [40]; further research will be helpful in providing more insight on COVID-19 virus prevalence in the general population. Reports of excess deaths across the three countries further supports the notion that confirmed infections are a fraction of the actual pandemic scale [41–46].
The data on COVID-19 hospitalization and fatalities noted some similarities across the three countries. In South Africa, factors associated with mortality reflected age, gender, health sector where admitted, comorbidity, and ethnic classifications. Within the ethnic factors, being of Black African, Colored, or Indian ethnicity was associated with in-hospital mortality [7]. In Brazil, an ethnicity effect was noted, where individuals of Brown and Black ethnicity had increased risk of mortality [8, 10]. In the USA, rates for hospitalization (which can be taken as indication of severe disease) were considerably higher for minority populations: the prevalence ratio1 was higher for those classified as American Indians/Alaskan Natives (1.9), African Americans (1.8), and Hispanics/Latinos (1.6) compared to Whites (0.5) [9].
The workplace has been noted to pose considerable risk to African Americans and other minority populations in the USA, where exposure has been considerable among low-wage earners who are involved in essential work and services in the current pandemic [47]. Many structural and social factors expose these workers to the vulnerability of the coronavirus infection which is compounded by their preexisting underlying medical conditions: for instance, the need to work multiple jobs at different facilities with high exposure potential for low wages contributes to increased exposure, illustrating the contribution of social insecurity to coronavirus transmission [47]. In Brazil, Black and Brown Brazilians have been noted to comprise a considerable portion of essential workers who are occupationally more exposed to infection [10]. South Africa’s healthcare workers, like other health care workers globally, have been noted to be among those occupationally more exposed to the viral infection [48]. A considerable proportion of workers also transitioned to work-from-home settings in South Africa, further highlighting the importance of the workplace as a risk for transmission [49]. The workplace therefore needs to be evaluated as a potential key transmission venue, with interventions in place to minimize transmission especially in common work areas.
The spread of the viral infection across the three countries share some similarities. Index cases were generally from residents who had travelled to a country with confirmed infections. Over time, cases spread from more affluent areas to higher concentration among the socioeconomically vulnerable who live in places and conditions where distancing, as advocated for public health promotion and pandemic management, may pose challenges. A consideration of such contextualities will improve the application of public health models for infection mitigation.
The Way Forward
Further studies on overall case fatality among different ethnic populations in all three countries can contribute to research-informed findings. Worth noting in these considerations is the age group with the highest prevalence of infections in Brazil (20.5% in the 60 to 69 years group), along with other possible associations [8]. It is very likely that across all three countries, not all infected individuals presented to a healthcare facility and this may limit the generalizability of study findings, both within and outside the three countries. Data is also not available on the health seeking behavior, incidence of medical pluralism, and type of supplementary treatment and remedies, used by the infected or exposed individuals across the different population and ethnic groups, perhaps even prior to hospitalization, and how these may have influenced outcomes, if at all. More research on these is required to inform mitigation efforts, in this and similar future pandemics.
The COVID-19 mortality among people of African descent and some other minorities in the USA, Brazil and South Africa may reflect their increased level of exposure to the virus, burden of comorbidities, or challenges with access to healthcare resources. These, in addition to other risk factors that may have influenced pandemic outcomes, also require further investigation. The pandemic seems to pose added pressure on people of lower socioeconomic status (largely classified along ethnic lines) and those who were already battling with socioeconomic pressures and chronic diseases in the time preceding the pandemic. Public health campaigns during and in the wake of the pandemic need to engage the population, and effectively target those at increased risk due to age, co-morbidities, ethnicity, socioeconomic status, and other pertinent factors, for sustainable and publicly owned infection prevention and health promotion behaviors.
While work-related exposure, in addition to preexisting comorbidities, likely increases the risk of COVID-19 infection and mortality among vulnerable populations, many of those affected work in informal jobs where social and health security may not be available. Their remuneration also does not afford them the luxury to attend to pressing health matters, as they may only be paid for worked hours. As such, their presentation for healthcare attention, even in the context of COVID-19 disease, could be relatively late, which again delays diagnosis and appropriate management, increasing infection in vulnerable communities, along with morbidity and mortality.
Through public health messages, the cost-effectiveness of interventions such as physical distancing and sanitation in flattening the curve of infection transmission to reduce adverse outcomes in all settings have been highlighted. However, these measures have been noted as privileges for households who live in small spaces [50], and where access to clean water supply, soap, and other sanitary measures may be limited. Among these populations, the need to work for sustenance may also override the need for compliance with physical distancing, especially for many informal workers who head food-insecure households. Food and other related insecurities in these families were a reality that predated the current pandemic and has only been magnified by the current pandemic circumstances [51]. Social and economic inequity may also result in multigenerational use of small living environments, further exposing some in the higher-risk group (the elderly) to the risk of infection. This risk may be obtained in low-income areas (mostly classified along ethnic lines) across all three countries.
Findings from studies in the USA indicate that underlying health conditions alone may not be solely responsible for the disparity between racial/ethnic groups in infection rate and mortality related to COVID-19. These and some other reports from South Africa and Brazil have suggested differences in exposure, age-specific incidence rates, and structural health determinants associated with specific ethnicities, as significant factors driving incidence and mortality [50, 52–56]. The larger proportion of positive cases among African-descending individuals and some minority populations in the three countries may be due to pervasive social inequalities that increase exposure. These include but are not limited to challenges with implementing social distancing in lower socioeconomic status communities [50, 52–56].
While infections may not yet have been fully contained, data exist for preliminary analyses of infection, morbidity, and mortality according to context. As the countries with relatively high numbers of confirmed infections and mortality across their respective continents, and with socioeconomic differences along ethnic lines, there may be room for findings that can inform pandemic control measures in other similar settings. Recommendations by the Centers for Disease Control and Prevention (CDC) on health equity considerations [57] can serve as a guide for governments to apply towards improving the life of vulnerable people in times of crisis. Tracking of COVID-19 case and mortality data for disparities in socioeconomic, gender, ethnic, geographic, and other characteristics will help to improve the management of patient groups as well as in resource allocation and design of targeted public health information and interventions. In multiracial societies where inequities exist along ethnic lines, data disaggregation by ethnicity and other pertinent characteristics can be used to promote health equity.
An opportunity now exists to prepare for the next epidemic, utilizing current experiences for health and disaster management, with due consideration of disparities and how these can be addressed. The ongoing pandemic has highlighted the importance of addressing social health determinants, the importance of basic functional amenities in different sectors, and the need for collective global effort in infection control. Furthermore, it highlights the need for all economies to address attainment of the Sustainable Development Goals (SDGs) in member states. Accomplishing these would help to narrow disparities in various sectors of the economy and the population. The current pandemic has also emphasized the benefits of multidisciplinary and multisectoral coordination for pandemic containment and mitigation. Essentially, all stakeholders, including the public, should be made aware of the probability that the COVID-19 virus infection may not be a once-off occurrence but may reoccur over seasons. As such, long-term sustainable actions are required for future containment and mitigation.
Limitations of the Study
This extended commentary presents data on COVID-19-related hospitalization and mortality among different ethnicities across three countries in high- and middle-income settings. Its findings are subject to at least four limitations which must be considered in the presented inferences. Firstly, the information presented on hospitalization and mortality (“The COVID-19 Experience” section) is based on data from hospitalization cases, with data on ethnicity not necessarily available for all hospitalized patients. Secondly, because the findings on confirmed cases and mortality are informed by hospitalization-level data, such findings may not necessarily account for data on infected individuals attended to in other healthcare facilities (non-hospital settings) or those who may have had COVID-19-related complications outside of hospital settings, for instance, in their homes. Thirdly, this manuscript excludes seroprevalence data across the three countries. While the records show a relatively lower number of confirmed coronavirus infections in South Africa, compared to Brazil and the USA, it is worth noting that the outbreak in South Africa commenced later. The differences in pandemic commencement dates may have influenced pandemic mitigation and hence epidemiological profiles. A fourth limitation stems from the presentation of information on the first wave of infections in each country. Subsequent waves have arisen, as in many other parts of the world, with outcomes which have not been accounted for in this commentary. In addition, while healthcare accessibility and COVID-19 virus exposure may differ among populations, contextual details could have further influenced COVID-19 outcomes in the three countries. However, in this study, we did not explore these aspects which poses further limitation.
Nevertheless, this manuscript presents some preliminary data which can be used to initiate discussions related to the influence of social health determinants on COVID-19 and other health outcomes. During and at the end of the pandemic, it will be necessary to evaluate how exposure, preexisting social factors, and interventions put in place, affected the epidemiological course, generally and among specific populations. The need also exists to explore continued health disparities which existed before, and which can be enhanced by pandemics such as the current coronavirus, and account for the effect of these to allow for contextual comparisons of infectious disease data where needed.
Conclusion/Recommendations
Individuals of African descent and some minority populations have been noted to be more vulnerable to adverse COVID-19 outcomes in multiracial societies. This is thought to reflect a combination of disparities in exposure, and health and health care access, as a result of their occupational and socioeconomic backgrounds. Social determinants of health need to be addressed to reduce health and related inequities, with the expansion of the public healthcare system for more focus on chronic disease prevention and control, especially in vulnerable populations. On the heels of public health and other measures for containment and mitigation of the COVID-19 virus pandemic, sustained discussions on social, political, economic, and ethnic health determinants among all stakeholders is necessary for overall improvement in health and healthcare. Adherence to the World Health Organization’s recommendation for social justice, as prerequisite for health [58], is crucial to reduce health disparities by addressing social inequality to transform health inequities. Failure to address these issues will leave current and new poor people and communities more vulnerable to public health crises in future pandemics.
Acknowledgements
The authors would like to acknowledge Jean Fourie for her editorial contributions.
Author Contribution
GDH came up with the idea and conceptualization of the study. All other authors contributed towards further conceptualization and study design. GDH, ONM, COO and TRP were responsible for drafts of the manuscript. All authors read and approved the final manuscript.
Declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Footnotes
“Prevalence ratio is calculated as the ratio of the proportion of COVID-NET hospitalizations over the proportion of population in the COVID-NET catchment area” (reference number 9)
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization (WHO). Coronavirus Disease (COVID-19). Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200921-weekly-epi-update-6.pdf. Accessed 25 Sept 2020.
- 2.Da Silva A, Rosa TE d C, Batista LE, Kalckmann S, Louvison MCP, Teixeira DS d C, et al. Racial inequities and aging: Analysis of the 2010 cohort of the health, welfare and aging study (SABE) Rev Bras Epidemiol. 2018;21(Suppl 2):1–14. doi: 10.1590/1980-549720180004.supl.2. [DOI] [PubMed] [Google Scholar]
- 3.Bucciferro RJ. Running in Place: Black-White Inequality in the United States and Brazil. 2017 (November):1–37. Available from: https://cbe.wwu.edu/files/Economics/Black White Ineq US Brazil-Bucciferro.pdf. Accessed 21 May 2020.
- 4.Assouad L, Chancel L, Morgan M. Extreme Inequality: Evidence from Brazil, India, the Middle East and South Africa. Working Paper N° 2018 (September). Available from: https://wid.world/document/extreme-inequality-evidence-brazil-india-middle-east-south-africa-wid-world-working-paper-2018-4/. Accessed 31 May 2020.
- 5.Hamilton C, Huntley L, Alexander N, Guimarães ASA, James W. Beyond racism: race and inequality in Brazil, South Africa, and the United States. Contemp Sociol. 2004;33(2):162–163. doi: 10.1177/009430610403300209. [DOI] [Google Scholar]
- 6.Gradín C. Race and income distribution: evidence from the USA, Brazil and South Africa. Rev Dev Econ. 2014;18(1):73–92. doi: 10.1111/rode.12070. [DOI] [Google Scholar]
- 7.National Institute for Communicabel Diseases (NICD). COVID-19 Sentinel Hospital Surveillance Update. 2020. Available from: https://www.nicd.ac.za/wp-content/uploads/2020/09/NICD-COVID-19-Weekly-Sentinel-Hospital-Surveillnace-update-Week-38-2020-updated.pdf. Accessed 4 Oct 2020.
- 8.Brasil, Ministério da Saúde S de V em S. Boletim Epidemiológico Especial: Semana Epidemiológica 38 (13 a 19/09/2020). 2020. Available from: http://portalarquivos2.saude.gov.br/images/pdf/2020/September/23/Boletim-epidemiologico-COVID-32-final-23.09_18h30.pdf. Accessed 4 Oct 2020.
- 9.Centers for Disease Control and Prevention (CDC). COVIDView Summary ending on September 19, 2020. CDC. 2021;1–13. Available from: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/past-reports/09252020.html. Accessed 19 Sept 2020.
- 10.Baqui P, Bica I, Marra V, Ercole A, van der Schaar M. Ethnic and regional variations in hospital mortality from COVID-19 in Brazil: a cross-sectional observational study. Lancet Glob Health. 2020;8(8):e1018–e1026. doi: 10.1016/S2214-109X(20)30285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Demographic trends of COVID-19 cases and deaths in the US reported to CDC. Centers Dis Control Prev. 2020;5–7. Available from: covid.cdc.gov/covid-data-tracker. Accessed 4 Oct 2020.
- 12.Harris B, Goudge J, Ataguba JE, McIntyre D, Nxumalo N, Jikwana S, Chersich M. Inequities in access to health care in South Africa. Vol. 32. J Public Health Policy. 2011;32(Suppl 1):S102–S123. doi: 10.1057/jphp.2011.35. [DOI] [PubMed] [Google Scholar]
- 13.De Oliveira BLCA, Luiz RR. Mortality by skin color/race, urbanicity, and metropolitan region in Brazil. J Public Health (Berl.). 2019;27:309–320.
- 14.Forrester S, Jacobs D, Zmora R, Schreiner P, Roger V, Kiefe CI. Racial differences in weathering and its associations with psychosocial stress: the CARDIA study. Vol. 7, SSM - Popul Health. 2019. p. 7. Available from https://www.sciencedirect.com/science/article/pii/S2352827318302246. Accessed 22 Jan 2021. [DOI] [PMC free article] [PubMed]
- 15.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, and the Northwell COVID-19 Research Consortium. Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. J Am Med Assoc. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pillay-van Wyk V, Msemburi W, Laubscher R, Dorrington RE, Groenewald P, Glass T, Nojilana B, Joubert JD, Matzopoulos R, Prinsloo M, Nannan N, Gwebushe N, Vos T, Somdyala N, Sithole N, Neethling I, Nicol E, Rossouw A, Bradshaw D. Mortality trends and differentials in South Africa from 1997 to 2012: second National Burden of Disease Study. Lancet Glob Health. 2016;4(9):e642–e653. doi: 10.1016/S2214-109X(16)30113-9. [DOI] [PubMed] [Google Scholar]
- 17.Malta DC, Bernal RTI, De Souza MDFM, Szwarcwald CL, Lima MG, Barros MBDA. Social inequalities in the prevalence of self-reported chronic non-communicable diseases in Brazil: national health survey 2013. Int J Equity Health. 2016;15(1):1–11. doi: 10.1186/s12939-016-0427-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunningham TJ, Croft JB, Liu Y, Lu H, Eke PI, Giles WH. Vital signs: racial disparities in age-specific mortality among Blacks or African Americans — United States, 1999–2015. Morb Mortal Wkly Rep. 2017;66:444–456. doi: 10.15585/mmwr.mm6617e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olshansky SJ, Antonucci T, Berkman L, Binstock RH, Boersch-Supan A, Cacioppo JT, Carnes BA, Carstensen LL, Fried LP, Goldman DP, Jackson J, Kohli M, Rother J, Zheng Y, Rowe J. Differences in life expectancy due to race and educational differences are widening, and many may not catch up. Health Aff (Millwood) 2012;31(8):1803–1813. doi: 10.1377/hlthaff.2011.0746. [DOI] [PubMed] [Google Scholar]
- 20.Kon ZR, Lackan N. Ethnic disparities in access to care in post-apartheid South Africa. Am J Public Health. 2008;98(12):2272–2277. doi: 10.2105/AJPH.2007.127829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Artiga S, Orgera K, Damico A. Changes in health coverage by race and ethnicity since the ACA, 2010-2018. KFF. 2020;(March). Available from: https://www.kff.org/racial-equity-and-health-policy/issue-brief/changes-in-health-coverage-by-race-and-ethnicity-since-the-aca-2010-2018/. Accessed 25 Sept 2020.
- 22.Statistics South Africa. General Household Survey 2018. Available from: http://www.statssa.gov.za/?page_id=1854&PPN=P0318&SCH=7652. Accessed 25 Sept 2020.
- 23.Institute for Health Metrics and Evaluation (IHME). Available from: http://www.healthdata.org/. Accessed October 23, 2020.
- 24.Cohen RV, Drager LF, Petry TBZ, Santos RD. Metabolic health in Brazil: trends and challenges. Lancet Diabetes Endocrinol. 2020;8(12):937–938. doi: 10.1016/S2213-8587(20)30370-3. [DOI] [PubMed] [Google Scholar]
- 25.Barber S. Death by racism. Lancet Infect Dis. 2020;20(8):903. doi: 10.1016/S1473-3099(20)30567-3. [DOI] [PubMed] [Google Scholar]
- 26.David TN. Left Behind. Black America: A Neglected Priority in the Global AIDS Epidemic. Los Angeles: Black AIDS Institute; 2008. [Google Scholar]
- 27.Parker A, Koegelenberg CFN, Moolla MS, Louw EH, Mowlana A, Nortjé A, Ahmed R, Brittain N, Lalla U, Allwood BW, Prozesky H, Schrueder N, Taljaard JJ. High HIV prevalence in an early cohort of hospital admissions with COVID-19 in Cape Town, South Africa. S Afr Med J. 2020;110(10):982–987. doi: 10.7196/SAMJ.2020.v110i10.15067. [DOI] [PubMed] [Google Scholar]
- 28.Daniels JP. COVID-19 threatens HIV care continuity in Brazil. Lancet HIV. 2020;7(12):e804–e805. doi: 10.1016/S2352-3018(20)30312-X. [DOI] [PubMed] [Google Scholar]
- 29.Kerani RP, Satcher Johnson A, Buskin SE, Rao D, Golden MR, Hu X, Hall HI. The Epidemiology of HIV Among People Born Outside the United States, 2010-2017. Public Health Rep. 2020;135(5):611–620. doi: 10.1177/0033354920942623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization (WHO). Global Tuberculosis Report 2020. Geneva: World Health Organization; 2020. Available from: https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf. Accessed January 23, 2021.
- 31.Centers for Disease Control and Prevention (CDC). South Africa Country Profile. Available from: https://www.cdc.gov/globalhivtb/where-we-work/southafrica/southafrica.html. Accessed 23 Jan 2021.
- 32.Centers for Disease Control and Prevention (CDC). Brazil Country Profile. Available from: https://www.cdc.gov/globalhivtb/where-we-work/zimbabwe/zimbabwe.html. Accessed January 23, 2021.
- 33.Stewart RJ, Tsang CA, Pratt RH, Price SF, Langer AJ. Tuberculosis — United States, 2017. Morb Mortal Wkly Rep. 2018;67(11):317–323. doi: 10.15585/mmwr.mm6711a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Institute for Communicable Diseases (NICD). COVID-19 Weekly Epidemiology Brief. 2020. Available from: https://www.nicd.ac.za/wp-content/uploads/2020/09/COVID-19-Weekly-Epidemiology-Brief-week-38.pdf. Accessed October 4, 2020.
- 35.Hadi YB, Naqvi SFZ, Kupec JT, Sarwari AR. Characteristics and outcomes of COVID-19 in patients with HIV: a multicentre research network study. AIDS. 2020;34(13):F3–F8. Available from 10.1097/QAD.0000000000002666. [DOI] [PMC free article] [PubMed]
- 36.Boulle AA, Davies M, Hussey H, Morden E, Vundle Z, Zweigenthal V, et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. 2020. Available from 10.1093/cid/ciaa1198.
- 37.Roser M, Ritchie H, Ortiz-Ospina E, Hasell J. Coronavirus Pandemic (COVID-19). Published Online at OurWorldInData.org. Available from: https://ourworldindata.org/coronavirus. Accessed 20 Sept 2020.
- 38.Binagwaho A, Frisch MF, Ntawukuriryayo JT, Hirschhorn LR. Changing the covid-19 narrative in Africa: using an implementation research lens to understand successes and plan for challenges ahead. Ann Glob Heal. 2020;86(1):1–5. [DOI] [PMC free article] [PubMed]
- 39.Harding A. Coronavirus in South Africa: scientists explore surprise theory for low death rate. BBC News (Online). 2020 (September 2). Available from: https://www.bbc.com/news/world-africa-53998374. Accessed 27 Sept 2020.
- 40.Baleta A. Covid-19: High Prevalence found in Cape Town Antibody Study. Daily Maverick. 2020 (September 4). Available from: https://www.dailymaverick.co.za/article/2020-09-04-covid-19-high-prevalence-found-in-cape-town-antibody-study/?tl_inbound=1&tl_groups%5B0%5D=80895&tl_period_type=3&utm_medium=email&utm_campaign=. Accessed 23 Jan 2021.
- 41.South African Medical Research Council. Report on Weekly Deaths in South Africa. 2020. Available from: https://www.samrc.ac.za/reports/report-weekly-deaths-south-africa. Accessed 23 Jan 2021.
- 42.Dyer O. Covid-19: Excess deaths point to hidden toll in South Africa as cases surge. BMJ. 2020;370(July):26886846. doi: 10.1136/bmj.m3038. [DOI] [PubMed] [Google Scholar]
- 43.Carvalho TA, Boschiero MN, Marson FAL. COVID-19 in Brazil: 150,000 deaths and the Brazilian underreporting. Diagn Microbiol Infect Dis. 2021;99(3):115258. doi: 10.1016/j.diagmicrobio.2020.115258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freitas ARR, de Medeiros NM, Frutuoso LCV, Beckedorff OA, de Martin LMA, de Coelho MMM, et al. Tracking excess deaths associated with the COVID-19 epidemic as an epidemiological surveillance strategy-preliminary results of the evaluation of six Brazilian capitals. Rev Soc Bras Med Trop. 2020;53:e20200558. [DOI] [PMC free article] [PubMed]
- 45.Rossen LM, Branum AM, Ahmad FB, Sutton P, Anderson RN. Excess Deaths Associated with COVID-19, by Age and Race and Ethnicity — United States, January 26–October 3, 2020. Morb Mortal Wkly Rep. 2020;69(42):1522–1527. doi: 10.15585/mmwr.mm6942e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woolf SH, Chapman DA, Sabo RT, Weinberger DM, Hill L. Excess deaths from COVID-19 and other causes, March-April 2020. JAMA. 2020;324:510–513. doi: 10.1001/jama.2020.11787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McClure ES, Vasudevan P, Bailey Z, Patel S, Robinson WR. Racial capitalism within public health-how occupational settings drive covid-19 disparities. Am J Epidemiol. 2020;189(11):1244–1253. doi: 10.1093/aje/kwaa126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dramowski A, Zunza M, Dube K, Parker M, Slogrove A. South African healthcare workers and COVID-19: a shared responsibility to protect a precious and limited resource. S Afr Med J. 2020;110(7):567–56849. doi: 10.7196/SAMJ.2020.v110i7.14903. [DOI] [PubMed] [Google Scholar]
- 49.International Labour Organization (ILO). The impact of the COVID-19 pandemic on jobs and incomes in G20 economies. Presentation of the ILO-OECD Covid-19 report, presented at the 3rd Employment Working Group (EWG) meeting under the Saudi G20 Presidency (virtual meeting, 19-20 August 2020). Available from: https://www.ilo.org/global/docs/WCMS_753607/lang%2D%2Den/index.htm. Accessed 25 Jan 2021.
- 50.Yancy CW. COVID-19 and African Americans. JAMA. 2020;323(19):1891–1892. doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- 51.High Level Panel of Experts (HLPE). Impacts of COVID-19 on food security and nutrition: developing effective policy responses to address the hunger and malnutrition pandemic. HLPE Issues Paper. 2020 (September). Available from: 10.4060/cb1000en. www.fao.org/cfs/cfs-hlpe. Accessed 24 Jan 2021.
- 52.Zelner J, Trangucci R, Naraharisetti R, Cao A, Malosh R, Broen K, et al. Racial disparities in coronavirus disease 2019 (COVID-19) mortality are driven by unequal infection risks. Clin Infect Dis. 2019;2020:1–8. doi: 10.1093/cid/ciaa1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rentsch CT, Kidwai-Khan F, Tate JP, Park LS, King JT, Skanderson M, et al. Patterns of COVID-19 testing and mortality by race and ethnicity among United States veterans: a nationwide cohort study. PLoS Med. 2020;17(9):1–17. doi: 10.1371/journal.pmed.1003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ogedegbe G, Ravenell J, Adhikari S, Butler M, Cook T, Francois F, Iturrate E, Jean-Louis G, Jones SA, Onakomaiya D, Petrilli CM, Pulgarin C, Regan S, Reynolds H, Seixas A, Volpicelli FM, Horwitz LI. Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. JAMA Netw Open. 2020;3(12):e2026881. doi: 10.1001/jamanetworkopen.2020.26881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shaw JA, Meiring M, Cummins T, Chegou NN, Claassen C, du Plessis N, et al. Higher SARS-CoV-2 Seroprevalence in Workers with Lower Socioeconomic Status in Cape Town, South Africa. PLoS One. 2020;16(2):e0247852. 10.1371/journal.pone.0247852. [DOI] [PMC free article] [PubMed]
- 56.Li SL, Pereira RHM, Prete Jr CA, Zarebski AE, Emanuel L, Alves PJH, et al. Social and racial inequalities in COVID-19 risk of hospitalisation and death across São Paulo state, Brazil. 2020 [Preprint]. Available from: https://www.medrxiv.org/content/10.1101/2020.12.09.20246207v2. Accessed 24 Jan 2021.
- 57.Centers for Disease Control and Prevention (CDC). COVID-19 in Racial and Ethnic Minority Groups. Vol. 2019, CDC - Coronavirus Disease 2019 (COVID-19). 2020. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/racial-ethnic-minorities.html. Accessed 22 Sept 2020.
- 58.World Health Organization (WHO). Closing the Gap In A Generation: Health Equity through Action on the Social Determinants of Health – Final Report of the Commission on Social Determinants of Health 2008 (August 27). Available from: https://www.who.int/publications/i/item/WHO-IER-CSDH-08.1. Accessed 24 Jan 2021.