Abstract
Purpose:
To report the outcome of unilateral small incision lenticule extraction (SMILE) in a patient with granular corneal dystrophy type 2 (GCD2).
Methods:
Slit-lamp photography and Fourier domain optical coherence tomography were used to document the clinical course and appearance of the corneas in a patient with genetically determined GCD2 who underwent unilateral SMILE in the right eye.
Results:
Slit-lamp examination of a 23-year-old woman revealed 2 faint opacities at the surgical interface approximately 2 months after the SMILE procedure had been performed on her right eye. Nine and 3 typical GCD2 deposits located immediately beneath the Bowman layer were observed in the right and left corneas, respectively. Over time, the deposits at the interface increased in size, density, and number in the right eye. Fourier domain optical coherence tomography performed 33 months after the SMILE procedure revealed deposits at the SMILE interface that were distinct from those located immediately beneath the Bowman layer. The severity of disease exacerbation was less in this patient than what is typically observed in others who have undergone laser-assisted in situ keratomileusis or photorefractive keratectomy.
Conclusions:
SMILE is contraindicated in patients with GCD2, as are other corneal refractive surgical procedures. This case highlights the importance of genetic testing before the performance of refractive corneal procedures—especially for patients with corneal opacities on preoperative slit-lamp examination or a family history of corneal disease compatible with that of a corneal dystrophy.
Key Words: granular corneal dystrophy type 2, small incision lenticule extraction, Fourier domain optical coherence tomography, transforming growth factor-β
Granular corneal dystrophy type 2 (GCD2) is an autosomal-dominant corneal stromal dystrophy caused by a missense mutation in the transforming growth factor-β–induced gene (TGFBI), which results in an Arg124His mutation in the encoded protein.1 Trauma to the central cornea is known to induce the transforming growth factor-β (TGF-β),2 which induces the TGFBI gene and the production of TGF-β–induced protein (TGFBIp).3,4 Iatrogenic damage to the central cornea caused by laser-assisted in situ keratomileusis (LASIK), photorefractive keratectomy (PRK), and laser-assisted subepithelial keratectomy has been reported to exacerbate corneal deposits in patients with GCD2.5–9 Radial keratotomy has been reported to exacerbate granular corneal dystrophy type 1 in 1 patient.10
Small incision lenticule extraction (SMILE) is a relatively new refractive surgical procedure that uses a femtosecond laser to create a stromal lenticule of the desired shape within the corneal stroma. The lenticule is extracted through a small (2.0–3.0 mm) incision, which causes less damage to the corneal epithelium than LASIK, PRK, or laser-assisted subepithelial keratectomy. We report the exacerbation of GCD2 after SMILE for the first time, discuss the mechanisms by which this might occur, and describe possible strategies for its management.
CASE REPORT
A 23-year-old Korean woman was referred to Severance Eye Hospital at Yonsei University for evaluation and management of corneal opacities after unilateral SMILE, OD, which had been performed elsewhere 2 months previously. She had no relevant medical history. There was also no history of trauma or surgery, except for the SMILE procedure in her right eye. There was a vague family history of corneal disorders, but no definite diagnosis of GCD in any family members.
Review of medical records obtained from the SMILE surgeon revealed that her cornea was described as “within normal limits” at the preoperative visit. Her manifest refraction before SMILE was −3.00 −0.25 × 80 OD and −2.75 −0.25 × 180 OS, yielding 20/20 corrected distance vision (CDVA) in both eyes. No genetic testing was performed preoperatively.
On examination at our institution, uncorrected vision was 20/20 OD and 20/70 OS. Manifest refraction was +0.50 0.00 ×180 OD and −2.25 −0.50 × 180 OS. CDVA was 20/20 OD and 20/20 OS. CDVA and manifest refraction for both eyes were unchanged at all subsequent visits.
Slit-lamp examination revealed 9 small, faint deposits in the anterior corneal stroma of the right eye immediately beneath the Bowman layer (Fig. 1A). Two very faint deposits were also observed at the SMILE interface by slit-lamp microscopy, but these could not be documented photographically or with Fourier domain optical coherence tomography (FD-OCT; RTvue-100, Optovue Inc, Fremont, CA). The appearance of these deposits suggested early exacerbation of GCD2. In the left cornea, 3 granular deposits were observed in the anterior stroma immediately beneath the Bowman layer (Fig. 1B). The ocular examination was otherwise unremarkable. Genetic testing confirmed heterozygosity for GCD2.
FIGURE 1.
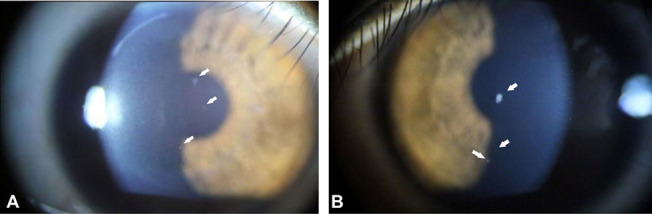
Corneal deposits seen at the initial visit, 2 months after SMILE, OD. A, Slit-lamp photograph showing granular deposits in the anterior stroma immediately beneath Bowman's layer in the right eye (arrows). Very small interface deposits were seen by slit-lamp examination, but these could not be documented photographically. B, Slit-lamp photograph showing granular deposits in the anterior stroma beneath Bowman's layer in the left eye (arrows).
Slit-lamp examination and FD-OCT performed 5 months after SMILE revealed more prominent deposits at the SMILE interface in the right eye and deposits beneath the Bowman layer in both eyes similar to those commonly observed in GCD2 corneas that have not undergone refractive surgery (Fig. 2).11 Interface deposits could not be documented by FD-OCT.
FIGURE 2.
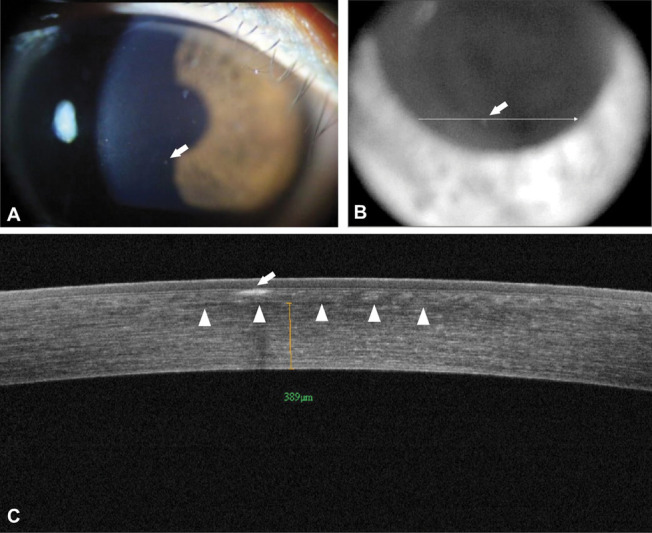
Corneal deposits 5 months after SMILE, OD. A, Slit-lamp photograph showing granular deposits in the right eye. B, FD-OCT video image of the same eye. The arrow points to the same deposit shown in panels A and B. C, FD-OCT image showing the anterior sub-epithelial location of the deposit (arrow), which is clearly separate from the SMILE interface (arrowheads). The distance measuring tool in panel C indicates that the interface is 389 μm from the corneal endothelium.
From 8 to 15 months after SMILE, the size and number of corneal deposits beneath the Bowman layer increased gradually in the right eye, whereas only the size of the deposits increased in the left eye (Fig. 3). At 15 months after SMILE, 1 deposit located at the SMILE interface could be captured by photography, but not by FD-OCT imaging (Fig. 3E, arrowhead).
FIGURE 3.
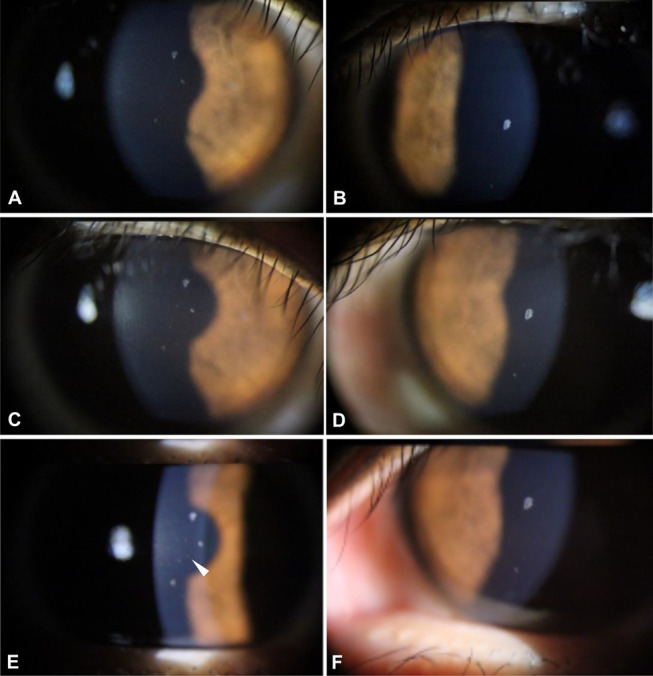
Corneal deposits 8, 12, and 15 months after SMILE, OD. Panels A and B show deposits in the right and left eyes, respectively, at 8 months. Panels C and D show deposits in the right and left eyes, respectively, at 12 months. Panels E and F show deposits in the right and left eyes, respectively, at 15 months. One faint deposit in panel E (arrowhead) is located at the interface, but it could not be documented by FD-OCT. All other deposits were located in the anterior stroma immediately beneath Bowman's layer.
Thirty-three months after SMILE, 3 deposits were observed along the SMILE interface in the right cornea by slit-lamp examination (Fig. 4A). The size and number of both interface and subepithelial deposits had increased noticeably since the initial visit. The left cornea, which served as an internal unoperated control, showed 3 granular deposits that had increased slightly in size, but not in number, since the initial visit. Subepithelial and interface deposits were imaged simultaneously on 1 FD-OCT sagittal section (Figs. 4B, C). One deposit can be seen in the anterior stroma immediately beneath the Bowman layer, and the other can be seen at the SMILE interface (Fig. 4C).
FIGURE 4.
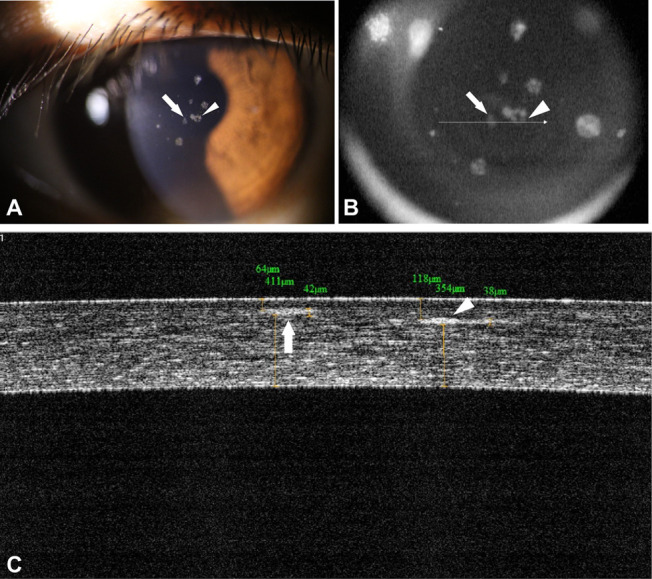
Corneal deposits 33 months after SMILE, OD. A, Slit-lamp photograph showing granular deposits in the right eye in the anterior stroma (arrow) and at the SMILE interface (arrowhead). B, FD-OCT video image of the same eye. The arrow and arrowhead point to deposits that are similarly marked in panel A. C, FD-OCT image showing that one of these deposits is located in the anterior sub-epithelial stroma (arrow), and the other is located at the SMILE interface (arrowhead). The distance measuring tool in C indicates that there is 354 μm between the SMILE interface, where the deposit is located, and the corneal endothelium (this newly formed and more centrally located interface deposit is different from the deposits seen in Fig. 2).
To compare the location of deposits in this case with those appearing after LASIK in patients with GCD2, we obtained the amputated flap from a 40-year-old woman with GCD2 who had undergone LASIK 20 years previously and stained the specimen with Masson's trichrome stain. As can be seen, deposits are located beneath the Bowman layer and adjacent to the flap internal surface of the amputated flap (Fig. 5), similar to what we observed after SMILE (Fig. 4C).
FIGURE 5.
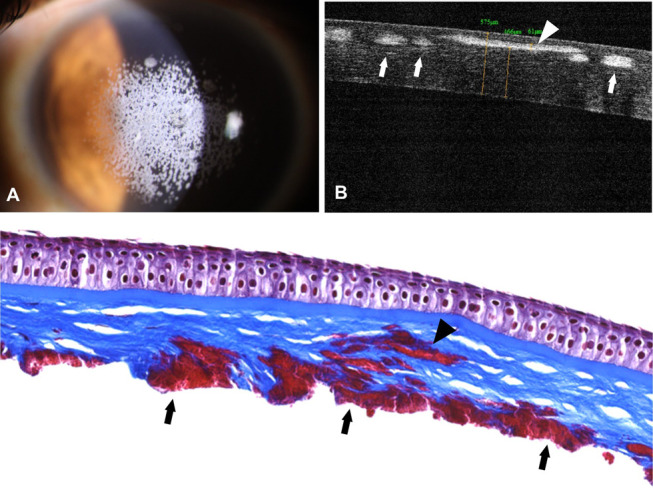
Corneal deposits after LASIK in a patient with GCD2. A, Slit-lamp photograph showing numerous granular deposits in the anterior stroma. B, FD-OCT image showing a deposit in the anterior stroma (arrowhead) and deposits at the LASIK flap interface (arrows). C, Histological section of the amputated LASIK flap showing a deposit at the anterior stroma (arrowhead) and deposits at the LASIK flap interface (arrows; Masson's trichrome stain; original magnification ×200).
This study followed the tenets of the Declaration of Helsinki and was approved by the Severance Hospital Institutional Review Board, Seoul, South Korea (4-2020-0600).
DISCUSSION
We report herein a patient with GCD2 in whom corneal deposits appeared at the surgical interface 2 months after SMILE. These deposits increased in size, density, and number through 33 months postoperatively. Both the operative and unoperated eyes displayed subepithelial granular deposits, typical of those seen in the corneas of patients with GCD2 who have not undergone corneal surgery.11 The number and size of these subepithelial deposits increased more in the eye that had undergone SMILE than in the opposite, unoperated eye.
Although increasing in severity, the interface deposits were few in number and small in size, even 33 months postoperatively, suggesting that exacerbation of GCD2 after SMILE is not as severe as it is after other corneal refractive surgical procedures.5,9,12 This conclusion may be premature, however, because this is the first reported case of the results of SMILE performed on a patient with GCD2, and other factors might influence the phenotypic expression of the GCD2 mutation, as discussed below.
Previous studies of eyes in which there was an exacerbation of GCD2 after LASIK revealed that the corneal deposits appear at the flap interface, as seen in Figure 5, and increase in severity over time.8,13,14 We previously demonstrated by immunohistochemistry that the interface deposits seen after LASIK contain TGFBIp.14 Using scanning electron microscopy, we found that these deposits adhere to exposed, cut edges of collagen fibers, which are produced by LASIK,13 and we hypothesize that GCD2 deposits at the SMILE interface will be similarly localized.
Han et al15 reported a broad variation in the severity of phenotypic expression of corneal deposits among patients with heterozygous GCD2. Choi et al16 reported twofold differences in the expression of 555 genes between primary cultured wild-type and homozygous GCD2 corneal fibroblasts. They demonstrated that primary cultured fibroblasts from individuals who were heterozygous or homozygous for GCD2 were more strongly adherent to collagen-I, collagen-IV, fibronectin, and laminin in comparison to wild-type cells.16
Recent molecular studies showed that the features and function of the mitochondria, as well as cellular oxidative stress, were altered in cultured corneal fibroblasts expressing mutant TGFBIp.17,18 Choi et al19,20 demonstrated that TGFBIp causes a delay in autophagic clearance because of impaired lysosomal function in cultured GCD2 corneal fibroblasts. Based on these studies, we hypothesize that the phenotypic expression of the GCD2 Arg124His mutation might be modified by other influential genes. Future studies will be necessary to determine whether the relatively mild exacerbation of disease in our case might be related to the characteristics of genes other than TGFBI or to the fact that SMILE is inherently less likely to exacerbate GCD2 than LASIK.15,21
TGF-β, which plays an essential role in wound healing via its pleotropic effects on cell proliferation, cell differentiation, extracellular matrix production, and immune modulation,22,23 activates TGFBI, leading to the production of TGFBIp.24 Exacerbation of corneal deposits in patients with GCD2 is undoubtedly related to the role that TGF-β plays in wound healing.3 The corneal epithelium produces more TGF-β than stromal keratocytes.25–27 The SMILE procedure causes less epithelial and subepithelial trauma than LASIK. This may help to explain why exacerbation of CGD2 might be less severe after SMILE than it is after LASIK.
Currently, there are no universally effective long-term strategies to manage visually significant exacerbations of GCD2 after SMILE. Phototherapeutic keratectomy (PTK) can postpone keratoplasty in patients with GCD2 whose corneal deposits are exacerbated after LASIK, although the deposits eventually recur. PTK performed on the surface of the remaining posterior stroma after amputation of the flap was reported to be superior to PTK performed with flap retention for visual acuity, recurrence, and complications.28 PTK performed with flap retention involves excimer ablation on both the posterior surface of the flap and the surface of the posterior remaining stroma. Amputation of the cornea anterior to the SMILE interface followed by PTK on the surface of the remaining stroma may be a reasonable treatment strategy if deposits become visually significant. A 40 to 60 μm PTK might be sufficient to remove most visually significant deposits from the remaining corneal stroma.28
Another strategy might be to perform a second SMILE to remove the additional stromal tissue containing opaque deposits from both sides of the interface. This strategy is theoretically feasible, given that conventional SMILE has high accuracy and predictability in creating incisions at the intended depth, with a minimum lenticule thickness at its edge of 15 μm (range 10–30 μm) in a clear cornea.29,30 Additional accumulation of deposits beneath the Bowman layer and near the interface may, however, impair transmission of laser energy, resulting in inaccurate cuts if SMILE is repeated. This and other procedures that involve removal of the opacities by excimer laser ablation will undoubtedly be temporary because we anticipate further accumulation of deposits postoperatively. Further studies are needed to determine the safety and efficacy of these potential treatments for managing visually significant exacerbations of GCD2 after SMILE.
In summary, we report the appearance of corneal deposits at the interface after SMILE in a patient with GCD2. The severity of these deposits was less than those typically seen after LASIK. Further studies will be required to determine whether this difference in severity is due to reduced epithelial trauma from SMILE compared with that of LASIK, the effect of modulating genes, or other factors. This case highlights the importance of genetic testing before the performance of refractive corneal procedures—especially for patients with corneal opacities on preoperative slit-lamp examination or a family history of corneal disease compatible with that of a corneal dystrophy. We conclude that SMILE is contraindicated in patients with GCD2, just like LASIK and PRK are contraindicated in these patients.
Footnotes
E. K. Kim is a member of the medical advisory board of Avellino Lab USA. The remaining authors have no funding or conflicts of interest to disclose.
Contributor Information
Jay Jiyong Kwak, Email: jkwak@yuhs.ac.
Sook Hyun Yoon, Email: sookhyuny@yuhs.ac.
Kyoung Yul Seo, Email: seoky@yuhs.ac.
Tae-im Kim, Email: tikim@yuhs.ac.
Hyung Keun Lee, Email: shadik@yuhs.ac.
R. Doyle Stulting, Email: dstulting@woolfsoneye.com.
REFERENCES
- 1.Holland EJ, Daya SM, Stone EM, et al. Avellino corneal dystrophy. Clinical manifestations and natural history. Ophthalmology. 1992;99:1564–1568. [DOI] [PubMed] [Google Scholar]
- 2.Tandon A, Tovey JC, Sharma A, et al. Role of transforming growth factor beta in corneal function, biology and pathology. Curr Mol Med. 2010;10:565–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maeng YS, Lee GH, Choi SI, et al. Histone methylation levels correlate with TGFBIp and extracellular matrix gene expression in normal and granular corneal dystrophy type 2 corneal fibroblasts. BMC Med Genomics. 2015;8:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi SI, Kim BY, Dadakhujaev S, et al. Inhibition of TGFBIp expression by lithium: implications for TGFBI-linked corneal dystrophy therapy. Invest Ophthalmol Vis Sci. 2011;52:3293–3300. [DOI] [PubMed] [Google Scholar]
- 5.Wan XH, Lee HC, Stulting RD, et al. Exacerbation of Avellino corneal dystrophy after laser in situ keratomileusis. Cornea. 2002;21:223–226. [DOI] [PubMed] [Google Scholar]
- 6.Jun RM, Tchah H, Kim TI, et al. Avellino corneal dystrophy after LASIK. Ophthalmology. 2004;111:463–468. [DOI] [PubMed] [Google Scholar]
- 7.Banning CS, Kim WC, Randleman JB, et al. Exacerbation of Avellino corneal dystrophy after LASIK in North America. Cornea. 2006;25:482–484. [DOI] [PubMed] [Google Scholar]
- 8.Chao-Shern C, Me R, DeDionisio LA, et al. Post-LASIK exacerbation of granular corneal dystrophy type 2 in members of a Chinese family. Eye (Lond). 2018;32:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantelli F, Lambiase A, Di Zazzo A, et al. Sands of Sahara after LASIK in Avellino corneal dystrophy. Case Rep Ophthalmol Med. 2012;2012:413010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feizi S, Pakravan M, Baradaran-Rafiee AR, et al. Granular corneal dystrophy manifesting after radial keratotomy. Cornea. 2007;26:1267–1269. [DOI] [PubMed] [Google Scholar]
- 11.Hong JP, Kim TI, Chung JL, et al. Analysis of deposit depth and morphology in granular corneal dystrophy type 2 using Fourier domain optical coherence tomography. Cornea. 2011;30:729–738. [DOI] [PubMed] [Google Scholar]
- 12.Kim TI, Kim T, Kim SW, et al. Comparison of corneal deposits after LASIK and PRK in eyes with granular corneal dystrophy type II. J Refract Surg. 2008;24:392–395. [DOI] [PubMed] [Google Scholar]
- 13.Roh MI, Grossniklaus HE, Chung SH, et al. Avellino corneal dystrophy exacerbated after LASIK: scanning electron microscopic findings. Cornea. 2006;25:306–311. [DOI] [PubMed] [Google Scholar]
- 14.Poulsen ET, Nielsen NS, Jensen MM, et al. LASIK surgery of granular corneal dystrophy type 2 patients leads to accumulation and differential proteolytic processing of transforming growth factor beta-induced protein (TGFBIp). Proteomics. 2016;16:539–543. [DOI] [PubMed] [Google Scholar]
- 15.Han KE, Choi SI, Chung WS, et al. Extremely varied phenotypes in granular corneal dystrophy type 2 heterozygotes. Mol Vis. 2012;18:1755–1762. [PMC free article] [PubMed] [Google Scholar]
- 16.Choi SI, Yoo YM, Kim BY, et al. Involvement of TGF-{beta} receptor- and integrin-mediated signaling pathways in the pathogenesis of granular corneal dystrophy II. Invest Ophthalmol Vis Sci. 2010;51:1832–1847. [DOI] [PubMed] [Google Scholar]
- 17.Kim TI, Kim H, Lee DJ, et al. Altered mitochondrial function in type 2 granular corneal dystrophy. Am J Pathol. 2011;179:684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi SI, Kim TI, Kim KS, et al. Decreased catalase expression and increased susceptibility to oxidative stress in primary cultured corneal fibroblasts from patients with granular corneal dystrophy type II. Am J Pathol. 2009;175:248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi SI, Kim BY, Dadakhujaev S, et al. Impaired autophagy and delayed autophagic clearance of transforming growth factor β-induced protein (TGFBI) in granular corneal dystrophy type 2. Autophagy. 2012;8:1782–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi SI, Woo JH, Kim EK. Lysosomal dysfunction of corneal fibroblasts underlies the pathogenesis of granular corneal dystrophy type 2 and can be rescued by TFEB. J Cell Mol Med. 2020;24:10343–10355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Na KS, Kim MS. An unusual form of Avellino dystrophy after laser in situ keratomileusis: a late onset or recurrence? J Clin Exp Ophthalmol. 2011;2:1–3. [Google Scholar]
- 22.Finnson KW, McLean S, Di Guglielmo GM, et al. Dynamics of transforming growth factor beta signaling in wound healing and scarring. Adv Wound Care (New Rochelle). 2013;2:195–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF-beta family in wound healing, burns and scarring: a review. Int J Burns Trauma. 2012;2:18–28. [PMC free article] [PubMed] [Google Scholar]
- 24.Han KE, Choi SI, Kim TI, et al. Pathogenesis and treatments of TGFBI corneal dystrophies. Prog Retin Eye Res. 2016;50:67–88. [DOI] [PubMed] [Google Scholar]
- 25.Torricelli AA, Santhanam A, Wu J, et al. The corneal fibrosis response to epithelial-stromal injury. Exp Eye Res. 2016;142:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson SE, Schultz GS, Chegini N, et al. Epidermal growth factor, transforming growth factor alpha, transforming growth factor beta, acidic fibroblast growth factor, basic fibroblast growth factor, and interleukin-1 proteins in the cornea. Exp Eye Res. 1994;59:63–71. [DOI] [PubMed] [Google Scholar]
- 27.Poulsen ET, Runager K, Nielsen NS, et al. Proteomic profiling of TGFBI-null mouse corneas reveals only minor changes in matrix composition supportive of TGFBI knockdown as therapy against TGFBI-linked corneal dystrophies. FEBS J. 2018;285:101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jun I, Jung JW, Choi YJ, et al. Long-term clinical outcomes of phototherapeutic keratectomy in corneas with granular corneal dystrophy type 2 exacerbated after LASIK. J Refract Surg. 2018;34:132–139. [DOI] [PubMed] [Google Scholar]
- 29.Titiyal JS, Kaur M, Shaikh F, et al. Small incision lenticule extraction (SMILE) techniques: patient selection and perspectives. Clin Ophthalmol. 2018;12:1685–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozgurhan EB, Agca A, Bozkurt E, et al. Accuracy and precision of cap thickness in small incision lenticule extraction. Clin Ophthalmol. 2013;7:923–926. [DOI] [PMC free article] [PubMed] [Google Scholar]


