Abstract
Purpose:
To study the possible changes in Scheimpflug corneal densitometry 6 months after mitomycin C–augmented trabeculectomy and to compare these measurements with healthy controls.
Methods:
Corneal densitometry was monitored with the Pentacam HR3 before and 6 months after first-time uncomplicated mitomycin C–augmented trabeculectomy in 42 eyes of 42 white patients with open-angle glaucoma and in 22 healthy age-matched controls. Preoperative intraocular pressure (IOP), central corneal thickness, known duration of the disease, gender, the type and number of substances, applications and amount of benzalkonium chloride per day, and postoperative topical cortisone use were tested for possible correlations in the trabeculectomy group.
Results:
There was a statistically significant reduction of mean diurnal IOP from 19.0 ± 7.7 to 11.1 ± 7.7 mm Hg (P = 0.003) and the amount of pressure-lowering substances from 3.7 ± 1.0 to 0.1 ± 0.5 (P < 0.001). Densitometry measurements decreased in the entire cornea from 25.5 ± 5.7 to 23.1 ± 5.8 grayscale units (P = 0.001) with emphasis in the anterior layer. They returned close to normal 6 months after trabeculectomy and were not statistically significantly different compared with a healthy control group (22.8 ± 3.4 grayscale unit; P = 0.824). No correlations could be found with these observations and possible causing factors studied.
Conclusions:
Corneal densitometry, an objective and sensitive measure of corneal transparency, returned close to normal 6 months after trabeculectomy. Although the observations cannot be associated with any causing factor in this study, the significant IOP reduction and the nearly complete cessation of topical antiglaucomatous substances including benzalkonium chloride seem to be the most plausible reasons for this finding.
Key Words: open-angle glaucoma, trabeculectomy, IOP reduction, corneal densitometry, antiglaucomatous therapy, benzalkonium chloride
Clarity or transparency is one of the most important signs of a healthy cornea. Usually, transparency is clinically assessed by standard slit-lamp examination, which, however, represents a rather subjective evaluation. Scheimpflug imaging and analysis of the anterior segment with the Pentacam HR3 is one of the methods to simultaneously measure the transmission of light through the cornea and the amount of backscattered light. This allows a more reliable, reproducible, and objective evaluation of corneal transparency. Even clinically clear corneas have been identified with higher levels of corneal backscatter,1–3 which might be an indicator for subclinical structural changes caused by pathological insults.
It is a well-accepted standard that glaucoma patients are initially treated with topical intraocular pressure (IOP)–lowering substances. Moreover, there is quite a huge amount of literature confirming the benefits of IOP lowering to stop or slow down progression of the disease.4–7 Although IOP reduction is highest with initial filtration surgery,8 possible complications associated with glaucoma surgery prevented the breakthrough of this strategy.
Many patients, however, do not tolerate topical medication well and develop severe cardiopulmonary side effects9 or ocular surface disease with tear film instability, conjunctival and corneal damage, and progressive ocular discomfort.10 Compliance issues11–13 represent another problem with topical IOP-lowering medications. Whenever medical or laser treatment seems unlikely to stop progression, or side effects are severe and compliance is reduced, incisional glaucoma surgery is recommended.
Reduction in corneal clarity is a sensitive response to a wide range of corneal insults. Because IOP-lowering substances and their preservatives are known to irritate the ocular surface,10 corneal densitometry is a possibility of assessing and monitoring the cornea's condition and structural integrity. Successful trabeculectomy usually allows a nearly complete reduction of IOP-lowering substances and their preservatives.
The objective of the present study was, therefore, to investigate changes of corneal transparency and backscattered light measured with a rotating Scheimpflug imaging system 6 months after uncomplicated trabeculectomy with mitomycin C (MMC) and to compare the results with healthy age-matched controls. Furthermore, to examine clinical and demographic factors that might be associated with possible changes.
MATERIALS AND METHODS
In this prospective clinical study, 42 eyes (17 right and 25 left) of 42 white patients with primary open-angle glaucoma undergoing first time MMC-augmented trabeculectomy were included for analysis as well as 22 healthy controls matched for age and central corneal thickness (CCT) were included. Inclusion criteria in the glaucoma group were patients older than 18 years with clinically normal corneas showing glaucoma progression after maximal effective medical treatment had failed to control IOP. Exclusion criteria were patients using contact lenses, suffering from diabetes mellitus, being treated with corticosteroids, and intraocular laser therapy or ocular surgery within 6 months before trabeculectomy. The necessity of more than 1 bleb needling, more than 1 injection of viscoelastic (Healon GV, AMO Germany GmbH) to stabilize the anterior chamber in the early postoperative period, phacoemulsification during the 6-month follow-up, or insufficient quality of densitometry measurements also led to exclusion.
Healthy controls were recruited from patients scheduled for phacoemulsification or retinal surgery because of a macular hole or macular pucker.
All trabeculectomies were performed by either of 2 surgeons (K.R.P. and L.E.P.) using the same standardized technique, which has been described previously.14,15 After the preparation of a fornix-based conjunctival flap, two 9 × 4 mm Merocel sponges soaked with 0.2 mg/mL of MMC 0.02% were applied below the conjunctiva for 3 minutes. The area exposed to MMC was irrigated with 10 mL of balanced salt solution.
Postoperative treatment was also standardized. Patients received preservative-free topical steroids (dexamethasone; Dexa EDO; Dr. Mann Pharma GmbH, Germany) 5 times a day for 4 weeks, with gradual tapering thereafter. Furthermore, preservative-free topical antibiotics (ofloxacin; Floxal EDO, Dr. Mann Pharma GmbH) were given 3 times a day for a week and a preservative-free mydriatic (cyclopentolat; Zyklolat EDO, Dr. Mann Pharma GmbH) twice a day for a week.
Baseline recordings included age, sex, known duration of the disease, number of IOP-lowering substances, applications and the amount of benzalkonium chloride (BAC) per day, and previous surgeries. A comprehensive ophthalmic examination included refraction, best spectacle corrected visual acuity (VA) with Snellen high-contrast VA, slit-lamp biomicroscopy of the anterior segment, Goldmann applanation tonometry (average of 6 measurements at 1 ,4 , 7 ,10 pm, at midnight in a supine position, and at 7 am; Haag-Streit, Koeniz, Switzerland), gonioscopy, and fundus examination with a 90-diopter lens. A complete glaucoma workup included automated perimetry (Swedish interactive threshold algorithm standard 30-2 program; Carl Zeiss Meditec, Dublin, CA), confocal scanning laser ophthalmoscopy (HRT II, Heidelberg Engineering Inc, Heidelberg, Germany), and scanning laser polarimetry (Nerve Fibre Analyzer GDxPRO, Carl Zeiss Meditec). Lens thickness (Echograph B-scan-Cinescan S; Quantel Medical, Clermont-Ferraud, France), if applicable, was measured with ultrasound and axial length with optical biometry (IOL-Master; Carl Zeiss Meditec AG, Jena, Germany). The Pentacam HR 3 (Oculus, Wetzlar, Germany), a noncontact 3D Scheimpflug imaging system, was used for objective analysis of the anterior segment and to evaluate corneal densitometry with the densitometry analysis software (version 1.21r25) before and 6 months after uncomplicated trabeculectomy. This software creates a map of the amount of backscattered light in different regions and depths of the cornea and allows a full-thickness evaluation of corneal clarity without any extent of the duration of the anterior segment examination of 2 seconds. Four annular zones are thereby analyzed. The central annular zone reaches from 0 to 2 mm, followed by concentric zones from 2 to 6 mm, 6 to 10 mm, and 10 to 12 mm from the apex to the periphery of the cornea. Four corneal layers are analyzed as well, including an anterior layer (AL; anterior 120 μm), a posterior layer (PL; posterior 60 μm), a central layer (CL; between AL and PL; 120 μm posterior of the epithelium and 60 μm anterior of the endothelium), and the total layer (TL; between epithelium and endothelium). Entire cornea represents all annular zones and layers. The most peripheral annular zone (10–12 mm) was not included in the analysis because its reproducibility has been shown to be weak.16 Moreover, benign corneal limbal degenerations, which might alter corneal transparency, are quite common in the age group of the study cohort. Pentacam grayscale units (GSUs) range from 0, representing maximum corneal transparency, to 100, representing complete opacity. The built-in Pentacam Nucleus Staging (PNS) software was used as an objective measure of nuclear cataracts. The PNS cataract grading score ranges from 0 to 5, with 5 indicating most advanced nuclear cataract. An experienced operator performed Pentacam measurements with uniform dark ambient light conditions at the same time of day between 9 am and 12 pm with patients and controls in a nonmydriatic state.17
Postoperative examinations at the 6-month follow-up included refraction, best spectacle corrected VA with Snellen high-contrast VA, slit-lamp examination of the anterior and posterior segment, Goldmann applanation tonometry (average of 6 measurements at 1, 4, 7, 10 pm, at midnight in a supine position, and at 7 am), and a full glaucoma workup. Any substances necessary at 6 months postoperatively, as well as any interventions necessary to keep the filtering bleb functioning, were recorded.
The study was approved by the ethics committee of the Medical Faculty Carl Gustav Carus of the Technische Universität Dresden, Germany, and followed the tenets of the Declaration of Helsinki. All participants signed a written informed consent form.
Based on means and SD of the total layer in a preliminary investigation, a sample size of at least 38 patients (alpha = 0.05; power = 0.80) was required (G Power 3.1.9.2. sample size software; University of Duesseldorf, Germany).
For data analysis, the SPSS software (version 25; IBM Statistics, New York, NY) was used. Normal distribution was tested with the Shapiro–Wilk test and Q-Q plots. Normally distributed data were expressed as means ± SD. Linear mixed models were applied to compare preoperative and postoperative differences of IOP, number of medications, VA, and densitometry. Comparison of preoperative and postoperative densitometry values with healthy controls were analyzed using the independent t test. The Pearson's correlation coefficient was used to identify clinical and demographic factors associated with possible changes in corneal backscatter. Because of multiple testings, the P value was adjusted by Bonferroni correction. Therefore, the level of significance was set to P < 0.004.
RESULTS
Demographics of patients are shown in Table 1. Mean age of the controls was 66 ± 8 years (P = 0.384), and mean CCT was 531.2 ± 13.1 μm (P = 0.36).
TABLE 1.
Demographics of Patients
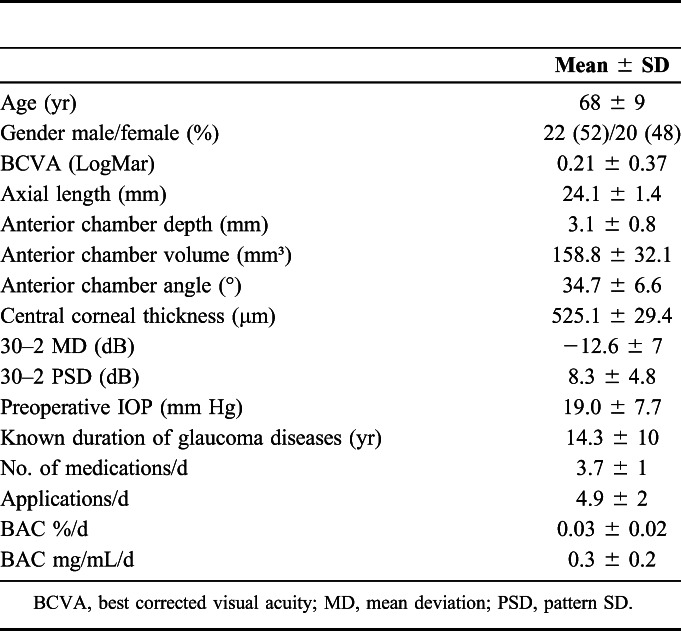
| Mean ± SD | |
| Age (yr) | 68 ± 9 |
| Gender male/female (%) | 22 (52)/20 (48) |
| BCVA (LogMar) | 0.21 ± 0.37 |
| Axial length (mm) | 24.1 ± 1.4 |
| Anterior chamber depth (mm) | 3.1 ± 0.8 |
| Anterior chamber volume (mm³) | 158.8 ± 32.1 |
| Anterior chamber angle (°) | 34.7 ± 6.6 |
| Central corneal thickness (μm) | 525.1 ± 29.4 |
| 30–2 MD (dB) | −12.6 ± 7 |
| 30–2 PSD (dB) | 8.3 ± 4.8 |
| Preoperative IOP (mm Hg) | 19.0 ± 7.7 |
| Known duration of glaucoma diseases (yr) | 14.3 ± 10 |
| No. of medications/d | 3.7 ± 1 |
| Applications/d | 4.9 ± 2 |
| BAC %/d | 0.03 ± 0.02 |
| BAC mg/mL/d | 0.3 ± 0.2 |
BCVA, best corrected visual acuity; MD, mean deviation; PSD, pattern SD.
Thirty-three patients (78.6%) had a diagnosis of high pressure and 9 (21.4%) of normal pressure open-angle glaucoma and had been treated for the disease with topical IOP-lowering medications for a mean of 14.3 ± 10 years before surgery. They used a mean of 3.7 ± 1 substances in different combinations and application frequencies. Prostaglandins were used in 41 (98%), ß-blockers in 33 (79%), α-agonists in 37 (88%), carboanhydrase inhibitors in 38 (90%), and pilocarpine in 6 (14%) of the cases. Thirty-six patients (85.7%) received IOP-lowering medications preserved with BAC, and only 6 (14.3%) were treated completely preservative free. IOP was statistically significantly reduced from 19.0 ± 7.7 to 11.1 ± 7.7 mm Hg (P = 0.003) postoperatively (Fig. 1), as well as the need for IOP-lowering substances from 3.7 ± 1.0 to 0.1 ± 0.5 (Fig. 2, P < 0.001).
FIGURE 1.

Box plot of mean diurnal IOP, mean (X) ± SD, and median (quartile 25%, quartile 75%) before and 6 months after trabeculectomy. *Marks significance with P < 0.004.
FIGURE 2.

Bar graph of mean number of IOP-lowering substances before and 6 months after trabeculectomy. Significance with P < 0.004.
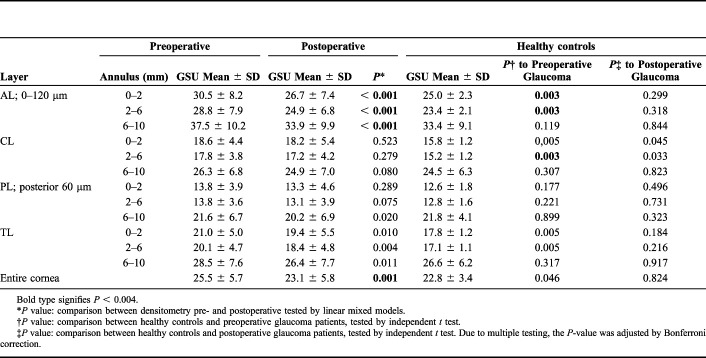
Corneal densitometry measurements before and after trabeculectomy and in age-matched healthy controls are shown in Table 2. Corneal backscatter was reduced in the entire cornea from 25.5 ± 5.7 to 23.1 ± 5.8 GSUs (P = 0.001) 6 months after trabeculectomy with emphasis in the AL (Fig. 3 and Table 2). Densitometry values of the AL and the entire cornea returned close to normal 6 months postoperatively and were not statistically significantly different compared with an age-matched healthy control group (23.1 ± 5.8 vs. 22.8 ± 3.4 GSU; P = 0.824) (Fig. 4). Furthermore, patients with glaucoma showed preoperatively significantly higher corneal backscatter in the optically significant 0 to 2 and 2 to 6 mm annular zone of the AL compared with healthy controls (P = 0.003, Table 2). This difference was not statistically significant after trabeculectomy anymore (P > 0.004, Table 2). In the entire cornea, which means all layers and annular zones, preoperative densitometry was higher compared with the healthy control group (P = 0.046), which was not statistically significant after Bonferroni correction anymore.
TABLE 2.
Scheimpflug Densitometry Measuring Corneal Backscatter Before and After Trabeculectomy and in Healthy Age-Matched Controls
| Layer | Preoperative | Postoperative | Healthy controls | ||||
| Annulus (mm) | GSU Mean ± SD | GSU Mean ± SD | P* | GSU Mean ± SD | P† to Preoperative Glaucoma | P‡ to Postoperative Glaucoma | |
| AL; 0–120 μm | 0–2 | 30.5 ± 8.2 | 26.7 ± 7.4 | < 0.001 | 25.0 ± 2.3 | 0.003 | 0.299 |
| 2–6 | 28.8 ± 7.9 | 24.9 ± 6.8 | < 0.001 | 23.4 ± 2.1 | 0.003 | 0.318 | |
| 6–10 | 37.5 ± 10.2 | 33.9 ± 9.9 | < 0.001 | 33.4 ± 9.1 | 0.119 | 0.844 | |
| CL | 0–2 | 18.6 ± 4.4 | 18.2 ± 5.4 | 0.523 | 15.8 ± 1.2 | 0,005 | 0.045 |
| 2–6 | 17.8 ± 3.8 | 17.2 ± 4.2 | 0.279 | 15.2 ± 1.2 | 0.003 | 0.033 | |
| 6–10 | 26.3 ± 6.8 | 24.9 ± 7.0 | 0.080 | 24.5 ± 6.3 | 0.307 | 0.823 | |
| PL; posterior 60 μm | 0–2 | 13.8 ± 3.9 | 13.3 ± 4.6 | 0.289 | 12.6 ± 1.8 | 0.177 | 0.496 |
| 2–6 | 13.8 ± 3.6 | 13.1 ± 3.9 | 0.075 | 12.8 ± 1.6 | 0.221 | 0.731 | |
| 6–10 | 21.6 ± 6.7 | 20.2 ± 6.9 | 0.020 | 21.8 ± 4.1 | 0.899 | 0.323 | |
| TL | 0–2 | 21.0 ± 5.0 | 19.4 ± 5.5 | 0.010 | 17.8 ± 1.2 | 0.005 | 0.184 |
| 2–6 | 20.1 ± 4.7 | 18.4 ± 4.8 | 0.004 | 17.1 ± 1.1 | 0.005 | 0.216 | |
| 6–10 | 28.5 ± 7.6 | 26.4 ± 7.7 | 0.011 | 26.6 ± 6.2 | 0.317 | 0.917 | |
| Entire cornea | 25.5 ± 5.7 | 23.1 ± 5.8 | 0.001 | 22.8 ± 3.4 | 0.046 | 0.824 | |
Bold type signifies P < 0.004.
P value: comparison between densitometry pre- and postoperative tested by linear mixed models.
P value: comparison between healthy controls and preoperative glaucoma patients, tested by independent t test.
P value: comparison between healthy controls and postoperative glaucoma patients, tested by independent t test. Due to multiple testing, the P-value was adjusted by Bonferroni correction.
FIGURE 3.
Areas of the densitometry map (gray color), where GSU (grayscale units) changed significantly.
FIGURE 4.
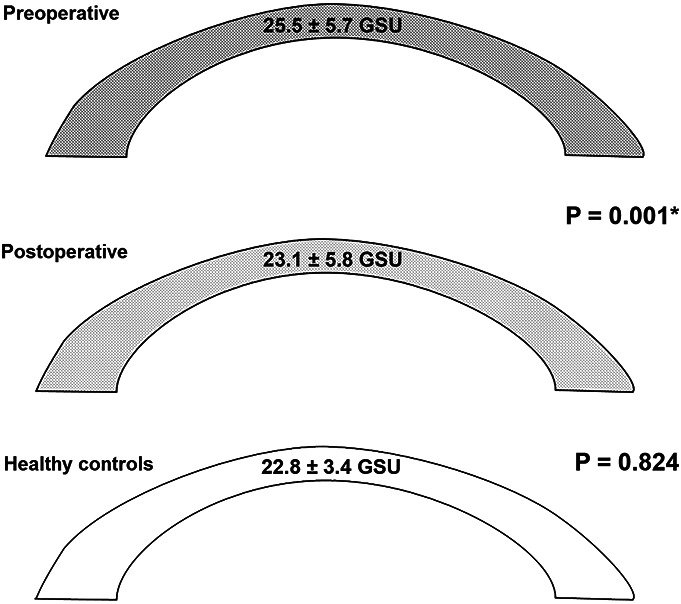
Densitometry (GSU; grayscale units) in the entire cornea before and after trabeculectomy and in healthy controls. *Marks significance with P < 0.004.
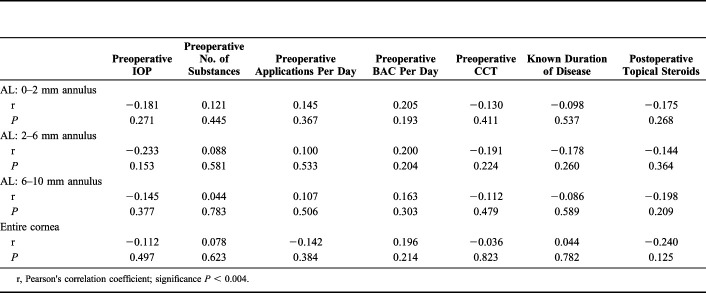
To find possible correlations, preoperative IOP, CCT, known duration of the disease, sex, the number of substances, applications and amount of BAC per day, and postoperative topical steroid use were tested in the 3 radial zones of the AL because the greatest differences were seen in the AL (Table 2 and Fig. 3). No significant associations were found for the parameters investigated (Table 3).
TABLE 3.
Correlation Between Preoperative and Postoperative Changes of Corneal Densitometry Values and Different Baseline Factors in the Anterior Layer and the Entire Cornea
| Preoperative IOP | Preoperative No. of Substances | Preoperative Applications Per Day | Preoperative BAC Per Day | Preoperative CCT | Known Duration of Disease | Postoperative Topical Steroids | |
| AL: 0–2 mm annulus | |||||||
| r | −0.181 | 0.121 | 0.145 | 0.205 | −0.130 | −0.098 | −0.175 |
| P | 0.271 | 0.445 | 0.367 | 0.193 | 0.411 | 0.537 | 0.268 |
| AL: 2–6 mm annulus | |||||||
| r | −0.233 | 0.088 | 0.100 | 0.200 | −0.191 | −0.178 | −0.144 |
| P | 0.153 | 0.581 | 0.533 | 0.204 | 0.224 | 0.260 | 0.364 |
| AL: 6–10 mm annulus | |||||||
| r | −0.145 | 0.044 | 0.107 | 0.163 | −0.112 | −0.086 | −0.198 |
| P | 0.377 | 0.783 | 0.506 | 0.303 | 0.479 | 0.589 | 0.209 |
| Entire cornea | |||||||
| r | −0.112 | 0.078 | −0.142 | 0.196 | −0.036 | 0.044 | −0.240 |
| P | 0.497 | 0.623 | 0.384 | 0.214 | 0.823 | 0.782 | 0.125 |
r, Pearson's correlation coefficient; significance P < 0.004.
Best spectacle corrected VA with Snellen high-contrast VA remained stable. It changed insignificantly from 0.21 ± 0.37 to 0.23 ± 0.33 LogMAR 6 months after trabeculectomy (P = 0.474).
Thirty-one (74%) patients were phakic and 11 (26%) pseudophakic. Lens status did not change during follow-up. PNS remained stable in 27 patients (88%). PNS increased by 2 units in one patient and by one unit in 3 patients. Mean change of PNS was 0.9 ± 0.7 to 0.9 ± 0.7 (P = 0.818).
DISCUSSION
In this study, Scheimpflug corneal densitometry monitored with the Pentacam HR3 densitometry module was used to examine the amount of corneal backscattered light, before and 6 months after uncomplicated MMC-augmented trabeculectomy and compared with an age-matched healthy control group. A statistically significant reduction of densitometry measurements in the entire cornea with emphasis in the AL, which turned close to normal after surgery, was seen.
The normal cornea scatters light mainly in the AL (air/tear film/cornea interface), followed by the CL and PL.16,18 An explanation for this finding might be that deeper layers of corneal lamellae are more strictly organized than more superficial layers.19 Light scattering increases if the space between collagen fibrils becomes less regular. Furthermore, keratocytes, which have the highest density in the anterior stroma,20 are an important source of light scatter.21 Disruption of the uniform spacing between collagen lamellae or a change of keratocyte shape under stress induce an increase in corneal backscatter. Keratocytes in a wounded area for example, as well as increased space between the collagen lamellae in corneal edema, increase backscatter and corneal opacification because of changes in the refractive index.22 Pentacam densitometry has been used to objectively monitor the progress or response to therapy of corneal infections.23 Furthermore, Koc et al2 presented evidence that increasing corneal backscatter may be a sensitive method to identify subclinical keratoconus, even earlier than topographic, topometric, and tomographic analyses. This might allow the hypothesis that corneal densitometry is a very sensitive indicator of corneal structural integrity, of corneal health or disease.3,24 One explanation of the current findings might be a better endothelial cell and metabolic pump function at lower IOP levels, thereby normalizing corneal hydration, keratocyte activity, and corneal transparency. A study investigating the effect of topical prostaglandins on corneal clarity found statistically significantly reduced corneal densitometry measurements starting at 3 months after initiating IOP-lowering treatment.25 Another possibility might be that chronic and long-term topical IOP-lowering medication stimulates keratocyte proliferation and activity, which leads to a decreased cellular transparency21 and an increased stromal reflectivity especially in the anterior stromal layer, which was shown by in vivo confocal micoscopy.26 Keratocytes are the major cell type of the stroma and responsible for providing the extracellular matrix and maintaining stromal homeostasis. Most of these corneal keratocytes are located in the anterior stroma.20 Under normal conditions, keratocytes are transparent except for the nuclei,21 when studied with in vivo confocal microscopy.27 Although we did not find any correlations with possible causing factors, it seems plausible that after nearly complete cessation of chronic topical therapy with IOP-lowering substances and the complete cessation of exposure to preservatives, the cornea recovers.
The reduction of backscatter of light and improvement of transparency mainly in the anterior section of the cornea might be an indirect proof for a “healthier” cornea after IOP-lowering surgery. At lower IOP levels, the pump function of the corneal endothelium improves. Moreover, topical IOP lowering is rendered unnecessary and eliminates the cumulative toxic effect of the active compounds and BAC.28 It is likely that the refractive index changes in a “healing” cornea.
VA remained stable 6 months after uncomplicated trabeculectomy. However, routine VA examinations with Snellen high-contrast VA may not reveal a better visual quality caused by decreased light backscattering. Although high-contrast VA remained stable, low-contrast VA might have shown a positive impact on VA.29 There is significant literature on VA loss after trabeculectomy, often implicating cataract formation,30,31 which was not seen in the current study. One reason might be that cataracts were mild before surgery and another that the follow-up of 6 months might be too short for cataracts to progress.
It is one of the limitations of the study that low-contrast VA testing, which might have picked up small changes in VA, was not performed. Furthermore, we did not ask for or assess the quality of vision such as blurring, contrast sensitivity, or glare. Another limitation is that endothelial cell counts were not assessed in all patients. In the 11 cases with preoperative and postoperative endothelial cell counts (CEM-530 Specular Microscope; Nidek, Japan), they changed insignificantly from 2091 ± 344 to 2056 ± 340 cells/mm2 (P = 0.64). In addition, the time course of change in Pentacam corneal densitometry cannot be addressed because we only examined corneal densitometry before and 6 months after trabeculectomy. In vivo confocal microscopy would be a good method to detect changes in number of keratocytes located in the anterior stroma. This should be addressed in a future study. Finally, the cohort might have not been large enough to find correlations with possible causing factors.
Assessing Scheimpflug corneal densitometry or transparency and its changes might be a sensitive and objective method to evaluate the overall “health” or structural integrity of the cornea. Corneal densitometry in the AL returned close to normal 6 months after trabeculectomy. Although the observations could not be associated with any causing factor in this study, the significant drop in IOP and the complete cessation of topical antiglaucomatous medications including BAC seem to be the most plausible reasons for this finding. This indicates, on one hand, that corneal changes caused by higher IOP, and glaucoma medication and its preservatives are probably reversible. On the other hand, the results give evidence of the benefit of earlier glaucoma surgery in the course of the disease.
Footnotes
The authors have no funding or conflicts of interest to disclose.
Clinical trial registration: NCT02959242.
REFERENCES
- 1.Patel SV, McLaren JW, Hodge DO, et al. The effect of corneal light scatter on vision after penetrating keratoplasty. Am J Ophthalmol. 2008;146:913–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koc M, Tekin K, Tekin MI, et al. An early finding of keratoconus: increase in corneal densitometry. Cornea. 2018;37:580–586. [DOI] [PubMed] [Google Scholar]
- 3.Chan TCY, Wong ES, Chan JCK, et al. Corneal backward scattering and higher-order aberrations in children with vernal keratoconjunctivitis and normal topography. Acta Ophthalmol. 2018;96:e327–e333. [DOI] [PubMed] [Google Scholar]
- 4.Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998;126:487–497. [DOI] [PubMed] [Google Scholar]
- 5.The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998;126:498–505. [DOI] [PubMed] [Google Scholar]
- 6.Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. [DOI] [PubMed] [Google Scholar]
- 7.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. [DOI] [PubMed] [Google Scholar]
- 8.Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–1953. [DOI] [PubMed] [Google Scholar]
- 9.Korte JM, Kaila T, Saari KM. Systemic bioavailability and cardiopulmonary effects of 0.5% timolol eyedrops. Graefes Arch Clin Exp Ophthalmol. 2002;240:430–435. [DOI] [PubMed] [Google Scholar]
- 10.Mathews PM, Ramulu PY, Friedman DS, et al. Evaluation of ocular surface disease in patients with glaucoma. Ophthalmology. 2013;120:2241–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quigley HA, Friedman DS, Hahn SR. Evaluation of practice patterns for the care of open-angle glaucoma compared with claims data: the Glaucoma Adherence and Persistency Study. Ophthalmology. 2007;114:1599–1606. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv Ophthalmol. 2008;53 (suppl 1):S57–S68. [DOI] [PubMed] [Google Scholar]
- 13.Tsai JC, McClure CA, Ramos SE, et al. Compliance barriers in glaucoma: a systematic classification. J Glaucoma. 2003;12:393–398. [DOI] [PubMed] [Google Scholar]
- 14.Pillunat KR, Spoerl E, Terai N, et al. Corneal biomechanical changes after trabeculectomy and the impact on intraocular pressure measurement. J Glaucoma. 2017;26:278–282. [DOI] [PubMed] [Google Scholar]
- 15.Waibel S, Spoerl E, Furashova O, et al. Bleb morphology after mitomycin-C augmented trabeculectomy: comparison between clinical evaluation and anterior segment optical coherence tomography. J Glaucoma. 2019;28:447–451. [DOI] [PubMed] [Google Scholar]
- 16.Ni Dhubhghaill S, Rozema JJ, Jongenelen S, et al. Normative values for corneal densitometry analysis by Scheimpflug optical assessment. Invest Ophthalmol Vis Sci. 2014;55:162–168. [DOI] [PubMed] [Google Scholar]
- 17.Bahar A, Pekel G. How does light intensity of the recording room affect the evaluation of lens and corneal clarity by Scheimpflug tomography? Cornea. 2020;39:137–139. [DOI] [PubMed] [Google Scholar]
- 18.Cankaya AB, Tekin K, Kiziltoprak H, et al. Assessment of corneal backward light scattering in the healthy cornea and factors affecting corneal transparency. Jpn J Ophthalmol. 2018;62:335–341. [DOI] [PubMed] [Google Scholar]
- 19.Hart WM. Adler's Physiology of the Eye. St. Louis, MO: Missouri Mosby-Year-Book, Inc.; 1992. [Google Scholar]
- 20.Patel S, McLaren J, Hodge D, et al. Normal human keratocyte density and corneal thickness measurement by using confocal microscopy in vivo. Invest Ophthalmol Vis Sci. 2001;42:333–339. [PubMed] [Google Scholar]
- 21.Karring H, Thogersen IB, Klintworth GK, et al. Proteomic analysis of the soluble fraction from human corneal fibroblasts with reference to ocular transparency. Mol Cell Proteomics. 2004;3:660–674. [DOI] [PubMed] [Google Scholar]
- 22.Jester JV, Moller-Pedersen T, Huang J, et al. The cellular basis of corneal transparency: evidence for “corneal crystallins”. J Cell Sci. 1999;112(pt 5):613–622. [DOI] [PubMed] [Google Scholar]
- 23.Otri AM, Fares U, Al-Aqaba MA, et al. Corneal densitometry as an indicator of corneal health. Ophthalmology. 2012;119:501–508. [DOI] [PubMed] [Google Scholar]
- 24.Tekin K, Sekeroglu MA, Kiziltoprak H, et al. Corneal densitometry in healthy corneas and its correlation with endothelial morphometry. Cornea. 2017;36:1336–1342. [DOI] [PubMed] [Google Scholar]
- 25.Sen E, Inanc M, Elgin U. The effect of topical latanoprost on corneal clarity; 1-year prospective study (dagger). Cutan Ocul Toxicol. 2019;38:253–257. [DOI] [PubMed] [Google Scholar]
- 26.Martone G, Frezzotti P, Tosi GM, et al. An in vivo confocal microscopy analysis of effects of topical antiglaucoma therapy with preservative on corneal innervation and morphology. Am J Ophthalmol. 2009;147:725–735.e1. [DOI] [PubMed] [Google Scholar]
- 27.Moller-Pedersen T. Keratocyte reflectivity and corneal haze. Exp Eye Res. 2004;78:553–560. [DOI] [PubMed] [Google Scholar]
- 28.Baudouin C, Pisella PJ, Fillacier K, et al. Ocular surface inflammatory changes induced by topical antiglaucoma drugs: human and animal studies. Ophthalmology. 1999;106:556–563. [DOI] [PubMed] [Google Scholar]
- 29.Akkaya Turhan S, Dizdar Yigit D, Toker E. Impact of changes in the optical density of postlens fluid on the clinical performance of miniscleral lenses. Eye Contact Lens 2019. [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 30.AGIS (Advanced Glaucoma Intervention Study) Investigators. The advanced glaucoma intervention study: 8. risk of cataract formation after trabeculectomy. Arch Ophthalmol. 2001;119:1771–1779. [DOI] [PubMed] [Google Scholar]
- 31.Mathew RG, Murdoch IE. The silent enemy: a review of cataract in relation to glaucoma and trabeculectomy surgery. Br J Ophthalmol. 2011;95:1350–1354. [DOI] [PubMed] [Google Scholar]