Abstract
Epidemiological studies have been pivotal in advancing understanding of the etiology of food allergy and in guiding the development of evidence-based guidelines for food allergy prevention and clinical management. In recent years, as research into the population-level distribution and determinants of food allergy has accumulated, data indicate that substantial differences in food allergy outcomes and management exist across racial/ethnic and other socioeconomic strata. This clinical commentary aims to provide a review of existing epidemiological studies and shed valuable light on the disparate burden of food allergy. Emerging methods to quantify environmental exposure and food allergy outcomes are detailed, as are specific areas where future research is warranted. We also highlight the role that epidemiology plays in advancing health equity and provide a framework as to how it can effectively inform health policy at all phases of the policy cycle—from initial population health assessment to the evaluation and refinement of specific health policies (i.e. national guidelines to promote earlier introduction of peanut-containing foods for allergy prevention).
Keywords: food allergy, Epidemiology, population health, allergy prevention, health policy, ecological momentary assessment, health disparities, racial/ethnic differences, socioeconomic differences, food hypersensitivity, health equity, food allergy management
Epidemiology—The Cornerstone of Public Health
A core focus of epidemiology is the systematic characterization of disease distribution within specific populations. Epidemiology offers insights that inform disease management and prevention. For example, epidemiological data from the late 1980s and 1990s indicated that delayed introduction of allergens into the infant diet might increase the risk of food allergy. These findings led to a survey demonstrating a 10-fold greater prevalence of peanut allergy in children of Jewish ancestry in London compared to Israel, where peanut is consumed earlier in childhood and more frequently.(1) Subsequent interventional studies catalyzed a paradigm-shift in our scientific understanding of food allergy pathogenesis and prevention.(2)
As evidence has accumulated regarding the population health burden of food allergy, an emerging challenge is addressing the apparent disparities in its prevalence and outcomes. The US Department of Health and Human Services defines a health disparity as “a particular type of health difference that is closely linked with social, economic, and/or environmental disadvantage. Health disparities adversely affect groups of people who have systematically experienced greater obstacles to health based on characteristics historically linked to discrimination or exclusion” (e.g. race/ethnicity, socioeconomic status).(3, 4) In this review, we discuss social determinants of health contributing to observed disparities in food allergy outcomes. We consider how contemporary epidemiological methods can inform strategies to increase equity, expand access to healthcare, and improve food allergy management and outcomes.
Racial/Ethnic and Socioeconomic Disparities in Food Allergy Prevalence
Major US-based epidemiological studies from the past two decades reveal a consistent pattern: Black Americans experience a greater burden of food-allergic disease relative to White Americans. While these racial/ethnic differences are consistent with trends in other allergic diseases (e.g. asthma,(5) eczema(6, 7)), they run counter to public perception and media narratives that often frame food allergies as disproportionately impacting more affluent, White populations.
The first US-based epidemiological studies to estimate national prevalence of specific food allergies by race/ethnicity were conducted by Sicherer et al via random-digit telephone dialing(8),(9),(10). In their 2002 survey of ~15,000 Americans, dramatically higher rates of probable seafood allergy among Black respondents were observed relative to Whites (3.7% vs 1.9%).(8, 10) A subsequent 2008 survey estimated that tree nut and/or peanut allergy impact 1.9% of Black Americans compared to 1.4% of White Americans (a non-significant difference). Gupta et al recently replicated these findings in an even larger sample of US households observing significantly higher rates of seafood allergy among both Black children (2.5% vs. 1.2%) and Black adults (3.5% vs. 2.9%) as well as significantly higher rates of tree nut and/or peanut allergy among Black children (3.4% vs 2.3%) and Black adults (2.9% vs. 2.1%). (11) (12)
In 2011, a landmark parent-proxy survey of approximately 40,000 US children reported that the odds of any probable food allergy were nearly double among Black children relative to White children (OR=1.8), adjusting for household-income. At the same time, Black and low-income children that met strict symptom-report criteria for food allergy were significantly less likely to report a physician-diagnosis, potentially highlighting reduced access to specialty care.(13) Concomitantly published National Health Interview Survey data also reported racial/ethnic differences among food-allergic patients around access to specialists and follow-up healthcare, as well as food security (even after adjusting for income and parental education).(14)
A 2014 meta-regression(15) estimated that overall food allergy prevalence was increasing by 1.2 percentage points per decade among the general population—and by 2.1 percentage points per decade among Black Americans. The most recent national data indicate that the prevalence of food allergy amongst Black Americans now exceeds that for White Americans, both overall and for the most common allergens except wheat and soy (Figure 1).(11, 12) Of note, White Americans have a lower prevalence of food allergy than Hispanic- and Asian-Americans. While these data suggest that Black children are no longer less likely to receive a physician-diagnosis for a parent-reported “convincing” food allergy, a disparity remains among families earning <$50,000/year.
Figure 1.
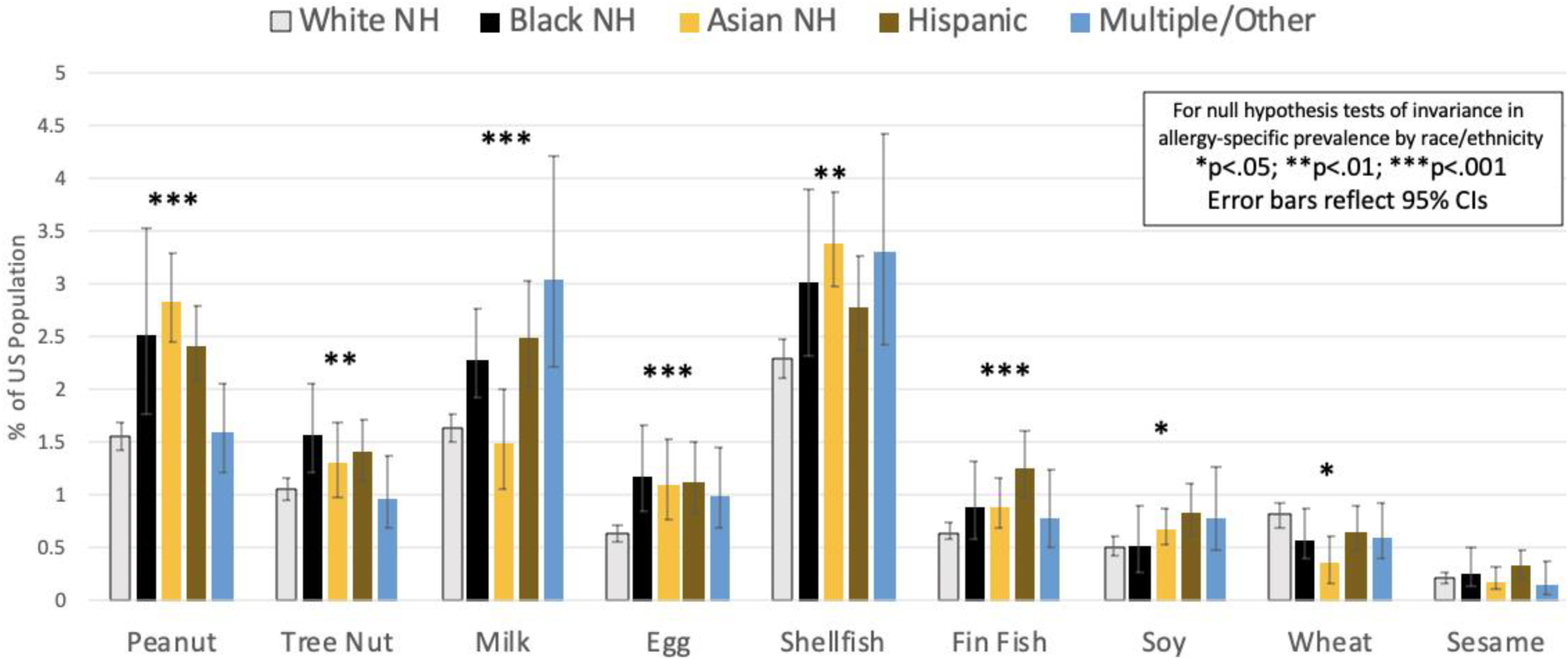
Estimated Prevalence of Current Food Allergy Among the US General Population by Race/Ethnicity reported by Gupta et al (2018, 2019)12,13
This pattern has also been observed elsewhere: a UK study (from the same authors who undertook the LEAP study) reported a significant increase in peanut-allergic children attending their allergy service amongst children from non-White backgrounds. This trend appeared to be specific for peanut and was not explained by improved awareness (and thus demand for referrals) among the non-White population.(16)
In the South African Food Allergy (SAFFA) study, there was a strikingly low rate of food allergy amongst Black 1–3 year-olds from rural communities, compared to their Black peers residing in the urban environment of Cape Town,(17) demonstrating the impact of urbanization on food allergy prevalence independent of race - a pattern that has also been reported in China.(18) This is consistent with 2011 US data identifying a dose-response relationship between residential population density and food allergy prevalence, which remained strong even after adjustment for respondent race/ethnicity and household income.(19) In the Australian HealthNuts study, food allergy was three times more common among infants of parents born in East Asia compared with those born to native Australians.(20) A related analysis of 60,000 Australian schoolchildren found that those born in Australia to Asian-born mothers were >2.5-fold more likely to have nut allergy by age five than non-Asian children. In contrast, children born in Asia who subsequently migrated to Australia had a dramatically reduced risk (aOR 0.1, 95% CI 0.03–0.31).(21) These data imply that gene-environment interactions can confound attempts to better understand the underlying causes of racial/ethnic differences in prevalence.
Defining Severity in Food Allergy
“Severe” outcomes (such as fatal and near-fatal anaphylaxis) are uncommon in food allergy.(22, 23) However, our inability to predict severity results in a risk-averse approach to management that can adversely impact food allergy-related quality of life (FAQL).(24) There is a lack of consensus, not only in what constitutes “severe” anaphylaxis, but also in the clinical criteria that define anaphylaxis itself.(23, 25) Further complicating this are the various perceptions of severity—food businesses and regulators will consider a severe reaction to be any food-related event which results in an unscheduled healthcare visit, although this might not equate to anaphylaxis and is affected by access to healthcare.(24)
An alternative approach defines severity in terms of the underlying purpose: is our aim to guide the treatment of acute reactions (in which case any definition must be straightforward and quick to assess)? To better monitor changes in the epidemiology of more severe reactions? Or to identify predictors of severity to help risk-stratify patients and improve their care? Unfortunately, attempts to resolve these issues have been hampered by overutilizing binary outcomes: severe/not severe, at-risk/not at-risk, whereas in reality, severity (and its impact on FAQL) is a continuum.(23) Perhaps it is time to ask our patients what they understand by “severity”, both in terms of the risk of severe reactions and how their perception of risk impacts on health-related quality-of-life (HRQL), the economic costs associated with food allergy, and how these might differ across socioeconomic strata.
Disparities in Food Allergy Outcomes associated with Race/Ethnicity
Unquestionably, the most severe food allergy outcome is fatal anaphylaxis. Data indicate that in the US, Black populations are at increased risk of fatal food-induced anaphylaxis relative to their White peers. An analysis of US mortality data from 1999–2010 found that annual rates of fatal food-induced anaphylaxis increased from 0.06 deaths per million (1999–2001) to 0.21 deaths per million (2008–2010) in Black males, but were stable among Whites, Hispanics, and Black females over the same period.(26) Whether these trends have continued in the last decade is unclear.
With respect to non-fatal anaphylaxis, a UK study reported a significantly higher incidence of anaphylaxis amongst British South Asians compared to Whites.(27) In the US, analysis of data from over 50,000 households found that Black food-allergic patients are 35% more likely to report a FA-related ED visit within the last 12 months, 14% more likely to report a “severe” food-allergic reaction history (indicated by multiple organ system involvement), and 7% more likely to report using an EAI to treat a food-allergic reaction, despite identical EAI prescription rates. After adjusting for household income and adult/caregiver educational attainment, Black patients remained 29%, 11%, and 7% more likely to report the aforementioned outcomes.(11, 12) Data from chart reviews of 817 food-allergic patients in Chicago and Cincinnati provide further evidence that allergic disease may disproportionately impact upon Black children (Figure 2).(28) Specifically, rates of food-induced anaphylaxis and FA-related ED visits were much higher among Black relative to White patients (33.9% and 39.7% vs 16.4% and 18.2%, respectively), even though these populations had similar age distributions. Moreover, White patients received follow-up allergy care for significantly longer (3.2 years vs. 4.3 years). Conversely, patients covered by Medicaid (a proxy for lower incomes) reported shorter duration of follow-up care, even after adjusting for race (3.2 years vs. 2.3 years). However, it is important to note that variable access to healthcare in the US makes emergency department utilization an imperfect proxy for food allergy severity.
Figure 2.
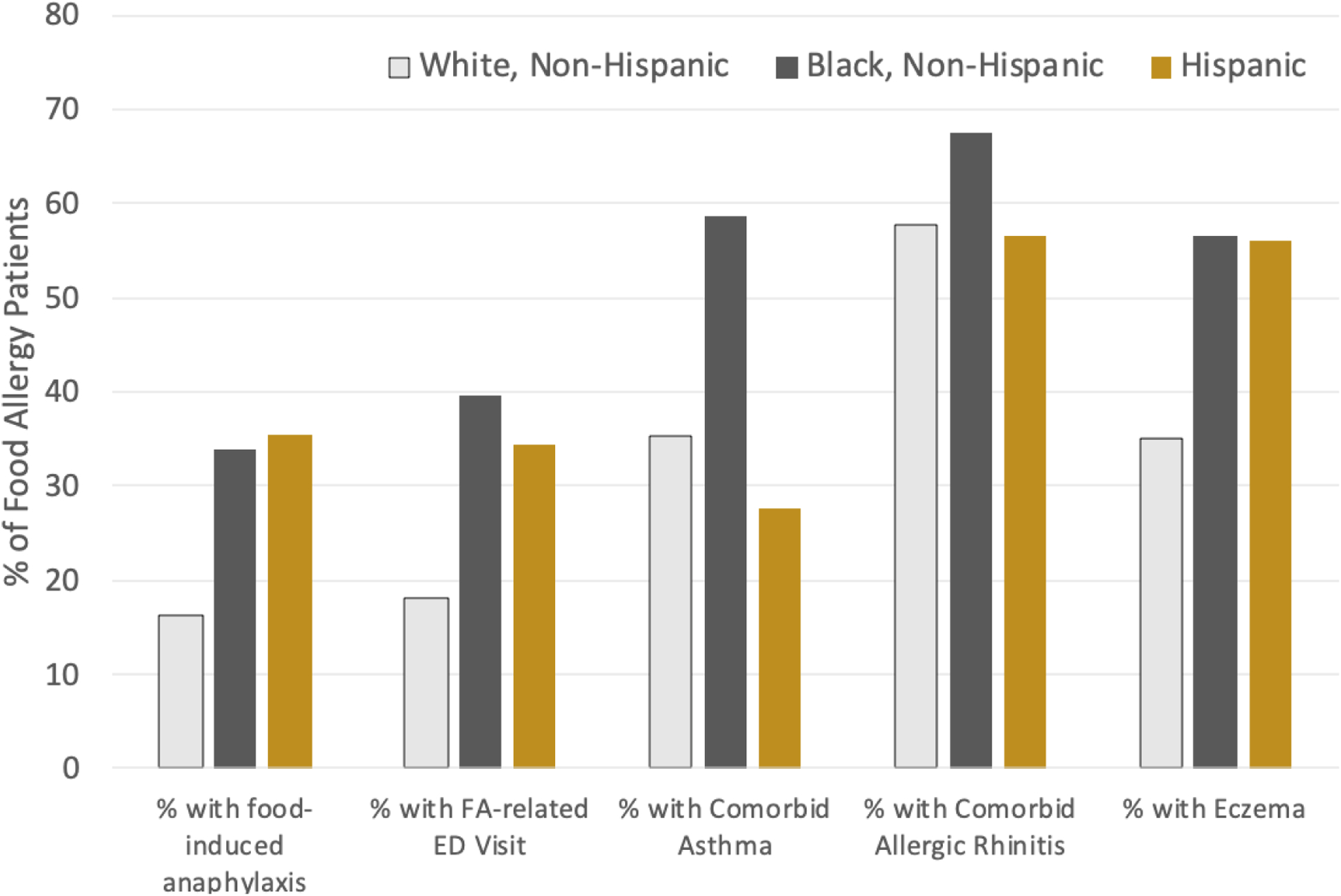
Estimated Racial/Ethnic Differences in Food Allergy Phenotypes and Health Care Utilization29
A different study examining the economic impact of pediatric food allergy found that families earning <$50,000 annually incurred 2.5 times the cost of FA-related emergency healthcare than higher-income families. These families also spent significantly less on specialist visits and out-of-pocket costs for preventive measures (e.g. epinephrine, allergen-free foods). These differences were robust to adjustment for numerous potential confounders, including reaction “severity” and race. Race was also an important determinant of out-of-pocket spending, which suggests that children in lower-income Black or Latinx families may be at greater risk for accidental ingestions and anaphylaxis by way of reduced access to specialty care, epinephrine, and allergen-free foods. A recent qualitative study of parents/caregivers of Medicaid-insured children with food allergy identified these same barriers to food allergy management. Parents/caregivers identified faulty perception of risk and insufficient education by food allergy care providers around the appropriate role of epinephrine in anaphylaxis management.(29)
On a related note, as evidence for the safety and efficacy of treatments and primary prevention strategies accumulates, it is important to ensure these are accessible to lower-income and racial/ethnic minority patients and avoid exacerbating the current disparities in population-level food allergy burden.(30, 31) Clinical trial participants must reflect the full diversity of the affected population, not only to ensure equity but also to provide sufficient data regarding the safety and efficacy of these emerging therapies within the populations that may benefit most. For example, in asthma, there is a clear difference in response to long-acting beta agonist (LABA) by race/ethnicity; LABA as a “step-up” strategy is less effective among Black patients than increasing their dose of inhaled corticosteroid.(32) Similar heterogeneity in treatment effects may exist for food allergy immunotherapies and must be considered during phase 3 studies and/or post-marketing surveillance.
The greatest change in food allergy management in the last 20 years is the acknowledgment that for many individuals, exposure - rather than avoidance - is likely the best strategy for food-allergic individuals who can tolerate the food when processed (such as for egg or dairy in baked foods) or as a primary prevention intervention for food allergy. In the End Allergies Together (EAT) Study, non-White ethnicity was associated with lower compliance with respect to the earlier introduction of food allergens, and delayed introduction of potential allergens in those participants randomized to standard introduction.(33) This variability might partially explain the observation that peanut allergy appears to be increasing in prevalence amongst non-White children.(16) Given the importance of earlier introduction as a primary prevention strategy, the impact of racial/ethnic disparities must be considered in guideline development.
Differences in Food Allergy-related Quality of Life and Psychosocial Burden
Even upon adjustment for household income and education, Black food-allergic patients are more likely to describe food insecurity and an inability to pay for medication. Both of which likely contribute to greater food allergy-related psychosocial burden.(14) Despite the importance of food allergy-related quality of life as a patient-reported outcome, there are no published epidemiological studies which have attempted to systematically compare food allergy-related psychosocial burden and/or quality of life across major racial/ethnic strata in the US. For example, the only large (N>1000) published study to date administering a validated HRQL instrument to a national sample of food allergy patients/caregivers did not assess participant race.(34) One study of 103 caregivers of food-allergic children recruited from an urban food allergy clinic observed remarkable racial/ethnic heterogeneity in food allergy perceptions and parent-proxy-reported psychosocial burden. African-American caregivers reported the lowest perceived risk of allergen exposure but the highest FA-related worry among all racial groups.(35) Further work is clearly needed to better characterize psychosocial burden within larger, more nationally-representative cohorts of racially diverse food allergy patients, which can inform future psychosocial interventions.
Learning from Ongoing NIH-supported Cohort Studies
There are two particularly notable NIH-supported cohort studies which are well-poised to help better understand racial and socioeconomic differences in food allergy phenotypes and possible endotypes by virtue of their targeted enrollment of large numbers of diverse Black and White families: the Detroit-based WHEALS Study and the multi-site FORWARD study.
The Wayne County Health, Environment, Allergy, and Asthma Longitudinal Study (WHEALS) established a birth cohort in 2003–2007 to identify environmental factors influencing the development of allergy and asthma in infancy and childhood. Interestingly, investigators did not observe an elevated risk of IgE-mediated allergy to milk, egg, or peanut among African-American children at age 3 (PBlack=7.4% vs Pnon-Black=7.2%; P=.98), although established differences in sensitization were observed (PBlack=43% vs. PWhite=32%; P=.01 to at least 1 of the three tested foods) and were robust to adjustment for household income, maternal education and Medicaid insurance.(36) These differences are consistent with previous analyses of NHANES data, which found substantial racial heterogeneity between food-allergic sensitization to peanut, egg, milk and shrimp.(37) Given that the most established racial differences in food allergy phenotypes between Black and White patients pertain to seafood allergy, it will be interesting to determine if these differences emerge within the WHEALS cohort. The investigators recently published data from follow-up at age 10 years.(38) While food allergy-specific outcomes were not reported, Black children were still more likely to have eczema, asthma, and allergic sensitization (including to numerous food allergens) compared to White children, even after adjusting for socioeconomic indicators (e.g. income, maternal education, difficulty making housing payments) and lifestyle variables (e.g. presence of pets, siblings, antibiotic use). The authors highlight the difficulties in teasing apart the independent effects of putative environmental determinants of childhood atopy (e.g. environmental tobacco smoke, diet, air pollution, dust mite and cockroach exposure) as they are highly correlated with race. Thus, it is critical to engaging larger, more socioeconomically, racially, and geographically diverse samples in future studies while leveraging emerging assessment modalities to better characterize relevant environmental exposures.
The Food Allergy Outcomes Related to White and African American Racial Differences (FORWARD) study is recruiting a sample of >950 Black, Latinx, and White families with food allergic-children from the metropolitan Chicago, Cincinnati, and Washington DC areas. Participants have physician-confirmed food allergy with EMR-linkage and are well-characterized with respect to sociodemographic, clinical, psychobehavioral, and community-level covariates, as well as repeated microbiome sampling of the gut and skin. As of July 2020, this cohort is still enrolling. Nonetheless, preliminary analyses on over 750 participants have already identified key racial differences in food allergy outcomes and comorbidities, as well as putative mechanisms—such as the recent finding of significant delays in the parent-reported timing of egg, peanut, and milk introduction among Black infants relative to their White peers—irrespective of household income strata(39)—a finding consistent with the EAT study.(33) Another recent manuscript (UNDER REVIEW) reported how Black FORWARD participants have higher rates of shellfish allergy than Whites, which was associated with an increased risk of asthma after adjustment for household income. These findings are consistent with a mechanism wherein allergic sensitization to tropomyosin—proteins which share similar structure within shellfish, house dust mites, and cockroaches—may increase risk of both shellfish allergy and asthma,(40) particularly among Black pediatric populations whose disproportionate exposure to these environmental allergens is well-established. (41, 42)
The Inter-related Social Determinants of Food Allergy Outcomes and Management
Table 1 summarizes key social determinants of health, which have previously been extensively detailed.(43–46) These social determinants are complex, inter-dependent, and often highly correlated with race/ethnicity —highlighting the need for sufficiently large and diverse samples in epidemiological studies that permit meaningful inference about the key determinants of food allergy outcomes. For example, a study based on Canadian census data identified low education and recent migration as being associated with reduced risk of self-reported food allergy.(47) It was unclear, however, whether this was a true lower prevalence (perhaps due to gene-environment interactions), a reflection of disparate healthcare access, or due to a lack of awareness/education that confounded self-report. Race and/or ethnicity can be important effect modifiers as well—as evidenced by the aforementioned meta-regression finding that food allergy prevalence rates have increased most rapidly among Black Americans in recent decades.(15)
Table 1.
Examples of how epidemiology can advance understanding and promote effective primary prevention of food allergy via a social determinants of health approach
| Social Determinant | Economic Stability | Neighborhood and Physical Environment | Education | Food | Community and Social Context | Health Care System |
|---|---|---|---|---|---|---|
| Specific domains |
|
|
|
|
|
|
| Epidemiological research questions |
Do access to high-quality preventive care and/or food allergy outcomes differ across employment, income, and/or wealth strata, independent of race/ethnicity? Do food allergy outcomes differ by insurance type or between insured vs. uninsured patients independent of race/ethnicity and SES? |
Does variability in neighborhood- and/or household-level environmental exposures associated with race/SES (e.g. dust mites, cockroach, environmental toxicants) influence the effectiveness of early introduction or otherwise influence atopy risk? Do patients have adequate transportation to access health care? Does variability in dust mite and cockroach exposure fully account for increased sensitization to shellfish, where targeted early introduction might be more critical? |
Do parents/caregivers with differing health literacy and/or educational attainment interpret the NIAID-sponsored peanut allergy prevention guidelines similarly? Does the timing/frequency/diversity of allergenic solids introduction differ across linguistic groups (e.g. English vs Spanish speakers), independent of race/ethnicity and SES? Do food allergy outcomes vary by patient/caregiver health literacy and/or educational attainment, independent of race/ethnicity and SES? Can patient education materials be modified (e.g. through inclusion of more visual/pictographic elements) to encourage understanding and guideline implementation among low literacy populations? |
Are diverse, allergenic solid foods accessible and affordable across racial/SES populations? Do different preparations of peanut (e.g. boiled vs. roasted peanuts) influence the feasibility and/or effectiveness of early introduction across racial/ethnic groups? What is the optimal dose, frequency and timing of peanut to introduce during infancy for maximal protection against allergy and does this differ by atopy status and//or across sociodemographic strata? Do baby formulas and foods provided by government-sponsored supplemental nutrition programs (e.g. WIC) support the implementation of food allergy prevention? |
Are parents/caregivers socially supported in their efforts to introduce allergenic solids per the PPA guidelines? How do differing community norms regarding infant feeding by race/SES impact adherence to PPA guidelines? Does stress stemming from community factors (e.g. exposure to community violence, structural racism) influence food allergy outcomes? How do racial/SES differences influence the home structure in ways that influence food allergy prevention (e.g. multi-generational, # of individuals in one home, breast-feeding practices, access to diverse foods, concepts and understanding of FA, cultural norms of feeding) |
Does pediatrician and allergist PPA guideline adherence vary depending on the sociodemographic characteristics of the patient populations they serve? Can high-risk infants with severe eczema/egg allergy access timely confirmatory testing to inform peanut introduction within the recommended window for “early” introduction? Is patient race/SES associated with:
Can the emergency department setting be better leveraged for epidemiological FA surveillance (via greater uniformity in coding practices) and/or promoting PPA guideline adherence (via improved patient education and linkage to follow-up care) Does access to specialty allergy care and preventive care differ regionally and/or between rural/urban areas? |
Improving Epidemiologic Assessment via Ecological Momentary Assessment and other emerging Mobile Data Collection Modalities
Well-conducted epidemiological studies can clarify these relationships and identify strategies to address not just underlying risk/protective factors but also health disparities. Given the high and increasing rates of smartphone ownership across all racial and income strata and their ubiquitous presence across our daily micro-environments,(48) these devices can improve epidemiological assessment of environmental exposures and food allergy outcomes in a way that is less burdensome and more ecologically valid than traditional approaches. Ecological momentary assessment (EMA) is utilized for the ambulatory assessment of a growing variety of health behaviors. Briefly, EMA involves using technology (e.g. mobile phone-based apps) to repeatedly sample participants’ current behavior and experiences as they take place. When well-implemented, EMA can reduce recall bias and improve external validity of collected data since target behaviors and psychological processes are assessed in near-real-time by “pinging” participants within the real-world microenvironments of interest.(49)
Event-contingent EMA, wherein brief mobile assessments are triggered by specific events, can help answer questions that are difficult to address using traditional epidemiological methods. For example, understanding previous allergic reaction symptomatology is crucial for clinicians and epidemiologists in their efforts to diagnose patients and assess disease severity. However, patient/caregiver-report of reaction symptoms is subject to bias, particularly when evaluated via survey-report long after the reaction. In contrast, event-contingent EMA approaches are designed to minimize recall bias by collecting relevant data immediately following the event. It is easy to imagine how such approaches could assist patients/caregivers, clinicians, and epidemiologists by triggering a reporting event on their phone once an allergic reaction resolves. Patients could document symptoms and other reaction information, including photos of suspected allergenic triggers and its packaging to ensure that detailed information is available for future clinical reference. Eventually, such events could potentially be triggered automatically by Bluetooth-enabled sensors integrated into EAIs.
While EMA has not yet been applied in the context of food allergy research, it is increasingly utilized for dietary assessment in the field of nutritional epidemiology. Dietary exposures are crucial determinants of food allergy outcomes(50),(51),(52),(53),(54),(55) and the timing and cumulative intake of specific dietary elements (e.g. allergenic foods, fiber) are important to assess. The inaccuracy and unreliability of traditional questionnaire-based dietary recall measures are well-documented within both self- and parent-proxy report paradigms.(56) A recent review summarizes how EMA is feasible and valid for dietary assessment in diverse settings(57)(58)(59)—including photography-based methods optimized for low literacy populations.(60),(61, 62) The evolution of these methods alongside advances in cloud-based image recognition promise to further reduce participant response burden—a crucial barrier to participation of under-represented communities and assessment of relevant exposures in epidemiological studies.(63)
Another advantage of EMA is its ability to effectively capture both within- and between-subject variability in patient-reported outcomes across space and time,(64) as well as associations with key covariates. Previous studies have described how stress and anxiety of food allergy management can adversely impact the psychosocial wellbeing of patients and their families.(65) However, these data are typically collected cross-sectionally and within clinical settings that can induce negative mood or anxiety in patients based on previous experiences (e.g. anaphylaxis, racism).(66, 67) Moreover, while we know that FA management and psychosocial burden are highly context-dependent, little is known about within-subject variability in FA-related psychosocial outcomes. Identifying psychosocial food allergy phenotypes (e.g. moderate stress/high variance, high stress/low variance) would provide useful epidemiological context regarding the population-level burden of food allergy and inform “just-in-time” interventions designed to deliver the appropriate supports via mobile devices when/where patients are most likely to benefit.(68) Importantly, in the context of improving the management of other chronic diseases (e.g. asthma), data indicate that such mobile technology interventions are feasible, effective, and enjoy high levels of user satisfaction among the urban racial/ethnic minority populations most affected.(69)(70)
Mobile technology-based approaches may also help support caregivers and clinicians in the timely introduction of allergenic solids—particularly to high-risk infants. Digital health efforts to promote early introduction, connect patients to local nutritionists, devise meal plans for weaning and food introduction, and track allergic reactions to introduced foods are currently available. However, little is currently known about their effectiveness in improving food allergy patient outcomes given the lack of systematic evaluation.
Summary: Epidemiology as a Tool to Drive and Monitor Change
The utility of epidemiology to address disparities in health extends much further than identifying the nature and extent of the disparities and the factors underlying them. Spasoff proposed five stages of policy development, all of which should be informed by epidemiology (Table 2).(71) Epidemiology thus represents an important tool to address disparities in health and healthcare. Figure 3 provides a conceptual framework, with reference to earlier introduction of food allergens as a prevention strategy. The effectiveness of early peanut introduction for the prevention of peanut allergy was clearly demonstrated by the LEAP study (where 75% of the cohort was White).(50) However, the extent to which the LEAP-informed NIAID-sponsored Guidelines for the Primary Prevention of Peanut Allergy (2) are being implemented by physicians and families across more socioeconomically diverse populations remains unclear, with one recent survey estimating that fewer than 1 in 3 pediatricians fully implement the guidelines in their clinical practice.(72) Furthermore, the extent to which these primary prevention strategies (i.e. modified feeding guidelines) are associated with population-level reductions in peanut allergy incidence is unknown.(73) Consequently, epidemiological surveillance characterizing the population-level burden of food allergy in the context of evolving guidelines and efforts to promote their widespread adoption - such as the ongoing NIH-supported “Intervention to Reduce Early (Peanut) Allergy in Children” study - remains critical. Moreover, by meeting patients where they are, including via emerging mobile assessment modalities, epidemiologists can potentially draw more valid and generalizable inferences about policy effectiveness and inform future efforts to address disparities in food allergy management and outcomes.
Table 2.
Five key steps of the policy cycle
|
Figure 3.
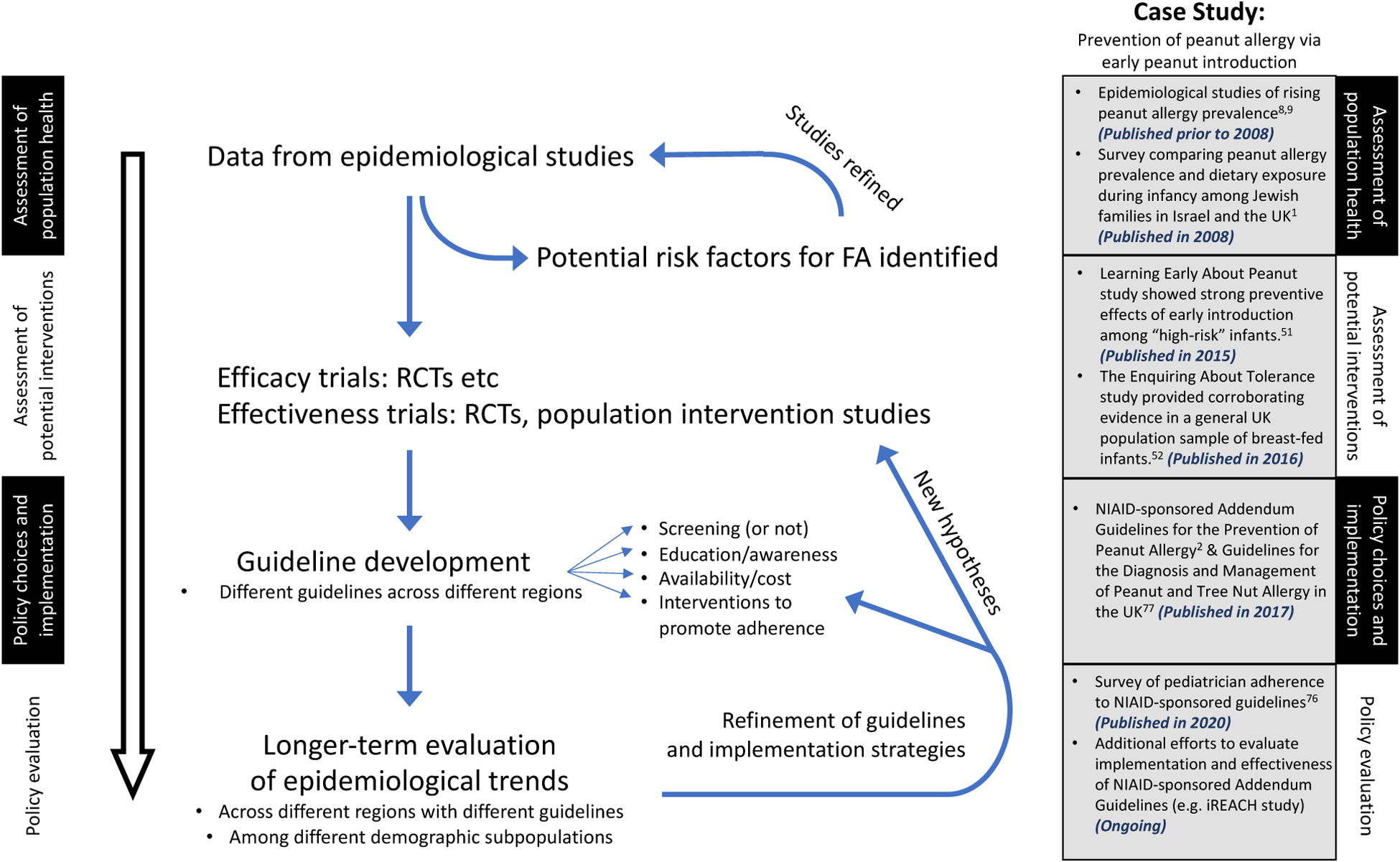
Role of epidemiology in informing policy related to earlier introduction of food allergens to prevent food allergy
Acknowledgements:
The authors would like acknowledge the contribution of Justin Zaslavsky BA for his assistance in revising the manuscript for publication.
PJT reports grants from the UK Medical Research Council, NIHR/Imperial Biomedical Research Centre, UK Food Standards Agency, Jon Moulton Charity Trust and End Allergies Together, outside the submitted work; personal fees from UK Food Standards Agency, DBV Technologies, Aimmune Therapeutics, Allergenis and ILSI Europe outside the submitted work.
RSC reports grants from NIAID, CoFAR, Aimmune, DBV Technologies, Astellas, Regeneron, and is an Advisory member for Alladapt, Genentech, Novartis, and receives personal fees from Before Brands, Nutricia outside the submitted work.
RSG reports grants from the National Institute of Health (NIH), the Stanford Sean N. Parker Center for Allergy Research, UnitedHealth Group, Thermo Fisher Scientific, Genentech, and the National Confectioners Association as well as personal fees from Before Brands, Kaléo Inc, Genentech, Institute for Clinical and Economic Review, Food Allergy Research & Education, Aimmune Therapeutics, and DBV Technologies outside the submitted work.
Footnotes
Conflict of Interest:
CMW has no conflicts to disclose
REFERENCES:
- 1.Du Toit G, Katz Y, Sasieni P, Mesher D, Maleki SJ, Fisher HR, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008;122(5):984–91. [DOI] [PubMed] [Google Scholar]
- 2.Togias A, Cooper SF, Acebal ML, Assa’ad A, Baker JR, Beck LA, et al. Addendum guidelines for the prevention of peanut allergy in the United States: Report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol. 2017;139(1):29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Secretary’s Advisory Committee on National Health Promotion and Disease Prevention Objectives for 2020. Phase I report: Recommendations for the framework and format of Healthy People 2020. Section IV: Advisory Committee findings and recommendations: U.S. Department of Health and Human Services.; [Available from: http://www.healthypeople.gov/sites/default/files/PhaseI_0.pdf. [Google Scholar]
- 4.Canino G, McQuaid EL, Rand CS. Addressing asthma health disparities: a multilevel challenge. J Allergy Clin Immunol. 2009;123(6):1209–17; quiz 18–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akinbami LJ, Simon AE, Rossen LM. Changing Trends in Asthma Prevalence Among Children. Pediatrics. 2016;137(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim Y, Blomberg M, Rifas-Shiman SL, Camargo CA, Gold DR, Thyssen JP, et al. Racial/Ethnic Differences in Incidence and Persistence of Childhood Atopic Dermatitis. J Invest Dermatol. 2019;139(4):827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131(1):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sicherer SH, Muñoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol. 2003;112(6):1203–7. [DOI] [PubMed] [Google Scholar]
- 9.Sicherer SH, Muñoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125(6):1322–6. [DOI] [PubMed] [Google Scholar]
- 10.Sicherer SH, Muñoz-Furlong A, Sampson HA. Prevalence of seafood allergy in the United States determined by a random telephone survey. J Allergy Clin Immunol. 2004;114(1):159–65. [DOI] [PubMed] [Google Scholar]
- 11.Gupta RS, Warren CM, Smith BM, Blumenstock JA, Jiang J, Davis MM, et al. The Public Health Impact of Parent-Reported Childhood Food Allergies in the United States. Pediatrics. 2018;142(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta RS, Warren CM, Smith BM, Jiang J, Blumenstock JA, Davis MM, et al. Prevalence and Severity of Food Allergies Among US Adults. JAMA Netw Open. 2019;2(1):e185630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128(1):e9–17. [DOI] [PubMed] [Google Scholar]
- 14.Johns CB, Savage JH. Access to health care and food in children with food allergy. J Allergy Clin Immunol. 2014;133(2):582–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keet CA, Savage JH, Seopaul S, Peng RD, Wood RA, Matsui EC. Temporal trends and racial/ethnic disparity in self-reported pediatric food allergy in the United States. Ann Allergy Asthma Immunol. 2014;112(3):222–9.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox AT, Kaymakcalan H, Perkin M, du Toit G, Lack G. Changes in peanut allergy prevalence in different ethnic groups in 2 time periods. J Allergy Clin Immunol. 2015;135(2):580–2. [DOI] [PubMed] [Google Scholar]
- 17.Botha M, Basera W, Facey-Thomas HE, Gaunt B, Gray CL, Ramjith J, et al. Rural and urban food allergy prevalence from the South African Food Allergy (SAFFA) study. J Allergy Clin Immunol. 2019;143(2):662–8.e2. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Hu Y, Allen KJ, Ho MH, Li H. The prevalence of food allergy in infants in Chongqing, China. Pediatr Allergy Immunol. 2011;22(4):356–60. [DOI] [PubMed] [Google Scholar]
- 19.Gupta RS, Springston EE, Smith B, Warrier MR, Pongracic J, Holl JL. Geographic variability of childhood food allergy in the United States. Clin Pediatr (Phila). 2012;51(9):856–61. [DOI] [PubMed] [Google Scholar]
- 20.Koplin JJ, Peters RL, Ponsonby AL, Gurrin LC, Hill D, Tang ML, et al. Increased risk of peanut allergy in infants of Asian-born parents compared to those of Australian-born parents. Allergy. 2014;69(12):1639–47. [DOI] [PubMed] [Google Scholar]
- 21.Panjari M, Koplin JJ, Dharmage SC, Peters RL, Gurrin LC, Sawyer SM, et al. Nut allergy prevalence and differences between Asian-born children and Australian-born children of Asian descent: a state-wide survey of children at primary school entry in Victoria, Australia. Clin Exp Allergy. 2016;46(4):602–9. [DOI] [PubMed] [Google Scholar]
- 22.Umasunthar T, Leonardi-Bee J, Hodes M, Turner PJ, Gore C, Habibi P, et al. Incidence of fatal food anaphylaxis in people with food allergy: a systematic review and meta-analysis. Clin Exp Allergy. 2013;43(12):1333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner PJ, Campbell DE, Motosue MS, Campbell RL. Global Trends in Anaphylaxis Epidemiology and Clinical Implications. J Allergy Clin Immunol Pract. 2020;8(4):1169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner PJ, Baumert JL, Beyer K, Boyle RJ, Chan CH, Clark AT, et al. Can we identify patients at risk of life-threatening allergic reactions to food? Allergy. 2016;71(9):1241–55. [DOI] [PubMed] [Google Scholar]
- 25.Turner PJ, Worm M, Ansotegui IJ, El-Gamal Y, Rivas MF, Fineman S, et al. Time to revisit the definition and clinical criteria for anaphylaxis? World Allergy Organ J. 2019;12(10):100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jerschow E, Lin RY, Scaperotti MM, McGinn AP. Fatal anaphylaxis in the United States, 1999–2010: temporal patterns and demographic associations. J Allergy Clin Immunol. 2014;134(6):1318–28.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buka RJ, Crossman RJ, Melchior CL, Huissoon AP, Hackett S, Dorrian S, et al. Anaphylaxis and ethnicity: higher incidence in British South Asians. Allergy. 2015;70(12):1580–7. [DOI] [PubMed] [Google Scholar]
- 28.Mahdavinia M, Fox SR, Smith BM, James C, Palmisano EL, Mohammed A, et al. Racial Differences in Food Allergy Phenotype and Health Care Utilization among US Children. J Allergy Clin Immunol Pract. 2017;5(2):352–7.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bozen A, Zaslavsky JM, Cohn D, Samady W, Lombard L, Nadeau K, et al. Barriers to food allergy management among Americans with low income. Ann Allergy Asthma Immunol. 2020. [DOI] [PubMed] [Google Scholar]
- 30.Nurmatov U, Dhami S, Arasi S, Pajno GB, Fernandez-Rivas M, Muraro A, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72(8):1133–47. [DOI] [PubMed] [Google Scholar]
- 31.Begin P, Chinthrajah RS, Nadeau KC. Oral immunotherapy for the treatment of food allergy. Hum Vaccin Immunother. 2014;10(8):2295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wechsler ME, Szefler SJ, Ortega VE, Pongracic JA, Chinchilli V, Lima JJ, et al. Step-Up Therapy in Black Children and Adults with Poorly Controlled Asthma. N Engl J Med. 2019;381(13):1227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perkin MR, Bahnson HT, Logan K, Marrs T, Radulovic S, Knibb R, et al. Factors influencing adherence in a trial of early introduction of allergenic food. J Allergy Clin Immunol. 2019;144(6):1595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DunnGalvin A, Koman E, Raver E, Frome H, Adams M, Keena A, et al. An Examination of the Food Allergy Quality of Life Questionnaire Performance in a Countrywide American Sample of Children: Cross-Cultural Differences in Age and Impact in the United States and Europe. J Allergy Clin Immunol Pract. 2017;5(2):363–8.e2. [DOI] [PubMed] [Google Scholar]
- 35.Widge AT, Flory E, Sharma H, Herbert LJ. Food Allergy Perceptions and Health-Related Quality of Life in a Racially Diverse Sample. Children (Basel). 2018;5(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joseph CL, Zoratti EM, Ownby DR, Havstad S, Nicholas C, Nageotte C, et al. Exploring racial differences in IgE-mediated food allergy in the WHEALS birth cohort. Ann Allergy Asthma Immunol. 2016;116(3):219–24.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGowan EC, Matsui EC, Peng R, Salo PM, Zeldin DC, Keet CA. Racial/ethnic and socioeconomic differences in self-reported food allergy among food-sensitized children in National Health and Nutrition Examination Survey III. Ann Allergy Asthma Immunol. 2016;117(5):570–2.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sitarik A, Havstad S, Kim H, Zoratti EM, Ownby D, Johnson CC, et al. Racial disparities in allergic outcomes persist to age 10 years in black and white children. Ann Allergy Asthma Immunol. 2020;124(4):342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang J, Warren C, J F, O N, A C, A B, et al. Early Introduction of Peanut, Egg, and Milk Among Black and White Food-Allergic Children in the FORWARD Study.. Journal of Allergy and Clinical Immunology 2020;145(2). [Google Scholar]
- 40.Wong L, Huang CH, Lee BW. Shellfish and House Dust Mite Allergies: Is the Link Tropomyosin? Allergy Asthma Immunol Res. 2016;8(2):101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arruda LK, Vailes LD, Ferriani VP, Santos AB, Pomés A, Chapman MD. Cockroach allergens and asthma. J Allergy Clin Immunol. 2001;107(3):419–28. [DOI] [PubMed] [Google Scholar]
- 42.Sarpong SB, Hamilton RG, Eggleston PA, Adkinson NF. Socioeconomic status and race as risk factors for cockroach allergen exposure and sensitization in children with asthma. J Allergy Clin Immunol. 1996;97(6):1393–401. [DOI] [PubMed] [Google Scholar]
- 43.Williams DR, Sternthal M, Wright RJ. Social determinants: taking the social context of asthma seriously. Pediatrics. 2009;123 Suppl 3:S174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burbank AJ, Sood AK, Kesic MJ, Peden DB, Hernandez ML. Environmental determinants of allergy and asthma in early life. J Allergy Clin Immunol. 2017;140(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Artiga S, Hinton E. Beyond Health Care: The Role of Social Determinants in Promoting Health and Health Equity, Washington DC: Kaiser Family Foundation; 2018. [Available from: https://www.kff.org/disparities-policy/issue-brief/beyond-health-care-the-role-of-social-determinants-in-promoting-health-and-health-equity/. [Google Scholar]
- 46.Marmot M, Wilkinson R. Social determinants of health. Oxford, UK: Oxford University Press; 2005. [Google Scholar]
- 47.Soller L, Ben-Shoshan M, Harrington DW, Knoll M, Fragapane J, Joseph L, et al. Prevalence and predictors of food allergy in Canada: a focus on vulnerable populations. J Allergy Clin Immunol Pract. 2015;3(1):42–9. [DOI] [PubMed] [Google Scholar]
- 48.Mobile Fact Sheet: Pew Research Center; 2019. [Available from: https://www.pewresearch.org/internet/fact-sheet/mobile/.
- 49.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. [DOI] [PubMed] [Google Scholar]
- 50.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372(9):803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perkin MR, Logan K, Tseng A, Raji B, Ayis S, Peacock J, et al. Randomized Trial of Introduction of Allergenic Foods in Breast-Fed Infants. N Engl J Med. 2016;374(18):1733–43. [DOI] [PubMed] [Google Scholar]
- 52.Du Toit G, Foong RX, Lack G. Prevention of food allergy - Early dietary interventions. Allergol Int. 2016;65(4):370–7. [DOI] [PubMed] [Google Scholar]
- 53.Venter C, Maslin K, Holloway JW, Silveira LJ, Fleischer DM, Dean T, et al. Different Measures of Diet Diversity During Infancy and the Association with Childhood Food Allergy in a UK Birth Cohort Study. J Allergy Clin Immunol Pract. 2020;8(6):2017–26. [DOI] [PubMed] [Google Scholar]
- 54.Nwaru BI, Takkinen HM, Niemelä O, Kaila M, Erkkola M, Ahonen S, et al. Introduction of complementary foods in infancy and atopic sensitization at the age of 5 years: timing and food diversity in a Finnish birth cohort. Allergy. 2013;68(4):507–16. [DOI] [PubMed] [Google Scholar]
- 55.Roduit C, Frei R, Depner M, Schaub B, Loss G, Genuneit J, et al. Increased food diversity in the first year of life is inversely associated with allergic diseases. J Allergy Clin Immunol. 2014;133(4):1056–64. [DOI] [PubMed] [Google Scholar]
- 56.Shim JS, Oh K, Kim HC. Dietary assessment methods in epidemiologic studies. Epidemiol Health. 2014;36:e2014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chmurzynska A, Mlodzik-Czyzewska MA, Malinowska AM, Czarnocinska J, Wiebe D. Use of a Smartphone Application Can Improve Assessment of High-Fat Food Consumption in Overweight Individuals. Nutrients. 2018;10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin CK, Correa JB, Han H, Allen HR, Rood JC, Champagne CM, et al. Validity of the Remote Food Photography Method (RFPM) for estimating energy and nutrient intake in near real-time. Obesity (Silver Spring). 2012;20(4):891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maugeri A, Barchitta M. A Systematic Review of Ecological Momentary Assessment of Diet: Implications and Perspectives for Nutritional Epidemiology. Nutrients. 2019;11(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Connelly K, Stein KF, Chaudry B, Trabold N. Development of an Ecological Momentary Assessment Mobile App for a Low-Literacy, Mexican American Population to Collect Disordered Eating Behaviors. JMIR Public Health Surveill. 2016;2(2):e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin CK, Han H, Coulon SM, Allen HR, Champagne CM, Anton SD. A novel method to remotely measure food intake of free-living individuals in real time: the remote food photography method. Br J Nutr. 2009;101(3):446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nicklas T, Islam NG, Saab R, Schulin R, Liu Y, Butte NF, et al. Validity of a Digital Diet Estimation Method for Use with Preschool Children. J Acad Nutr Diet. 2018;118(2):252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.The National Children’s Study research plan: A review.. National Academies Press: National Research Council.; 2008. August 16. [PubMed] [Google Scholar]
- 64.Hedeker D, Mermelstein RJ, Demirtas H. Modeling between-subject and within-subject variances in ecological momentary assessment data using mixed-effects location scale models. Stat Med. 2012;31(27):3328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Warren CM, Otto AK, Walkner MM, Gupta RS. Quality of Life Among Food Allergic Patients and Their Caregivers. Curr Allergy Asthma Rep. 2016;16(5):38. [DOI] [PubMed] [Google Scholar]
- 66.Weiss D, Marsac ML. Coping and posttraumatic stress symptoms in children with food allergies. Ann Allergy Asthma Immunol. 2016;117(5):561–2. [DOI] [PubMed] [Google Scholar]
- 67.Marsac ML, Kassam-Adams N, Delahanty DL, Widaman KF, Barakat LP. Posttraumatic stress following acute medical trauma in children: a proposed model of bio-psycho-social processes during the peri-trauma period. Clin Child Fam Psychol Rev. 2014;17(4):399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heron KE, Smyth JM. Ecological momentary interventions: incorporating mobile technology into psychosocial and health behaviour treatments. Br J Health Psychol. 2010;15(Pt 1):1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baptist AP, Islam N, Joseph CL. Technology-Based Interventions for Asthma-Can They Help Decrease Health Disparities? J Allergy Clin Immunol Pract. 2016;4(6):1135–42. [DOI] [PubMed] [Google Scholar]
- 70.Bakken S, Marden S, Arteaga SS, Grossman L, Keselman A, Le PT, et al. Behavioral Interventions Using Consumer Information Technology as Tools to Advance Health Equity. Am J Public Health. 2019;109(S1):S79–S85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spasoff R Epidemiologic Methods for Health Policy. New York, NY: Oxford University Press; 1999 [Google Scholar]
- 72.Gupta RS, Bilaver LA, Johnson JL, Hu JW, Jiang J, Bozen A, et al. Assessment of Pediatrician Awareness and Implementation of the Addendum Guidelines for the Prevention of Peanut Allergy in the United States. JAMA Netw Open. 2020;3(7):e2010511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Turner PJ, Campbell DE, Boyle RJ, Levin ME. Primary Prevention of Food Allergy: Translating Evidence from Clinical Trials to Population-Based Recommendations. J Allergy Clin Immunol Pract. 2018;6(2):367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]


