Abstract
Objectives
Fentanyl is a potent analgesic that accounts for an increasing number of overdose deaths in the United States. This study tested whether altered pharmacokinetics plays a pivotal role in the increased fentanyl dose requirements in patients receiving the enzyme-inducing anticonvulsant, carbamazepine.
Methods
Neurosurgical patients receiving carbamazepine for >6 weeks (N = 11) or no carbamazepine (N = 6, controls) received a single bolus dose of fentanyl (200 μg) intravenously. Plasma was collected before and for up to 9 h after the bolus. Fentanyl concentrations were measured using liquid chromatography–mass spectrometry. Pharmacokinetic variables were derived from plasma concentration–time curves best fitted to a two-compartment model.
Key findings
Fentanyl clearance was significantly higher in the carbamazepine group compared to controls (mean ± SD: 20.1 ± 6.8 vs 13.2 ± 4.8 ml/min per kg, P < 0.05), and area under the plasma concentration curve (AUC) was significantly lower (150 ± 65 vs 233 ± 70 ng/ml × min, P < 0.02). Volume of distribution was larger in the carbamazepine group, but the difference was not statistically significant (5.4 ± 3.1 vs 3.6 ± 1.2 l/kg, P > 0.15). The terminal elimination half-life did not differ between the two groups.
Conclusions
Chronic carbamazepine therapy leads to increased fentanyl clearance and decreased AUC, which may result in decreased duration of therapeutic plasma concentrations of fentanyl and an increased dose requirement. Assuming that carbamazepine does not change fentanyl pharmacodynamics, patients on chronic carbamazepine therapy may require more frequent or higher fentanyl doses to maintain therapeutic plasma concentrations.
Keywords: carbamazepine, enzyme induction, fentanyl, pharmacokinetics
Introduction
Fentanyl is a potent opioid agonist with well-established analgesic efficacy and minimal cardiovascular side effects, and is among the more common opioid analgesic drugs administered during the perioperative period.[1] Fentanyl use is also increasing in the palliative care of patients with chronic pain since the introduction of its oral transmucosal (Actiq lollipop and Fentora buccal tablet) and conventional or iontophoretic transdermal preparations (Duragesic or fentanyl patch). However, there is concern that the rapid and unpredictable respiratory depressant effects of fentanyl have contributed to its role as a principal cause of morbidity and mortality in the current opioid overdose epidemic in the United States.[2]
Patients treated chronically with certain antiepileptic drugs (AEDs) such as carbamazepine have been noted to require higher fentanyl doses to achieve adequate analgesic efficacy.[3] The mechanism of the higher dose requirements in these patients has not been fully established, but may result from increased hepatic clearance due to induction of the enzymes responsible for fentanyl clearance.[4] Better understanding of the pharmacokinetics of fentanyl during AED therapy should provide a basis to guide clinicians in predicting dose adjustments to avoid supratherapeutic or suboptimal dosing, and the associated respiratory depressant effects or inadequate analgesia. This study tested the hypothesis that carbamazepine-induced alterations in fentanyl pharmacokinetics may result in subtherapeutic plasma concentrations, and play a pivotal role in its increased dose requirements. The study evaluated neurosurgical patients on chronic carbamazepine therapy, along with control patients who were not taking carbamazepine or other known enzyme inducers or inhibitors.
Methods
The study was reviewed and approved by the Institutional Review Board of the Massachusetts General Hospital. Written informed consent was obtained from all study participants after explanation of the study design in lay terms. Subjects were patients scheduled to undergo elective craniotomy that required placement of an arterial and peripheral venous catheter for perioperative care, which could be used for blood sample collection. Nineteen adult subjects with American Society of Anesthesiologists (ASA) physical status classification 1–3 were enrolled. Using the fentanyl exposure data reported for the interaction between ketoconazole and fentanyl,[5] it was estimated that a 35% difference of carbamazepine on fentanyl exposure could be shown with 10 subjects with an α-error of 5% and a power of 80%. Nineteen patients were enrolled, 11 of whom were receiving chronic treatment with carbamazepine for greater than 6 weeks duration (the AED or carbamazepine group). The remainder eight patients served as controls. Exclusion criteria included age younger than 18 years, ASA physical status 4 or greater, chronic opioid treatment, history of hepatic or renal dysfunction (laboratory values >2 times normal limits), treatment with other medications that induce or inhibit drug-metabolizing enzymes (e.g. cimetidine, azole antifungals, antiretroviral agents and rifampin), a medical contraindication to administration of fentanyl, or pregnancy.
All patients received a single intravenous bolus dose of 200 μg of fentanyl by their anaesthetist at induction of anaesthesia, after which no additional doses of fentanyl were given during or after surgery. Alternative analgesics other than fentanyl were used, and included morphine, hydromorphone, remifentanil or sufentanil, administered, as indicated, at the discretion of the primary anaesthetic or surgical teams. Induction and maintenance of anaesthesia were conducted according to the standard routine for all craniotomies at the institution. Induction of anaesthesia was with propofol, followed by the use of a nondepolarizing neuromuscular blocking drug to facilitate endotracheal intubation. Anaesthesia was maintained with either total intravenous or volatile anaesthetics, as determined by the primary anaesthesia team.
A baseline blood sample was obtained before fentanyl administration, and subsequent blood samples were collected at 1, 5, 15, 30 and 60 min, and then hourly for up to 9 h after the fentanyl bolus dose. Blood samples were collected in EDTA tubes, centrifuged within 30 min of sampling, and the plasma was stored in aliquots at −70° until later assay.
Analysis of fentanyl in plasma
Plasma fentanyl concentrations were determined by liquid chromatography–tandem mass spectrometry (LC-MS/MS). To each (0.2 ml) plasma sample and appropriate calibration standards, fentanyl-D5 was added as internal standard. Samples were extracted with methyl-t-butyl ether. The extract was separated, evaporated to dryness, and reconstituted with mobile phase for analysis. The analytic instrument was an AB Sciex API 5000 triple quadrupole mass spectrometer equipped with a QJet ion guide and accelerated by a LINAC collision cell (AB Sciex, Foster City, CA, USA) with an atmospheric pressure chemical ionization probe in a Turbo Vion source, interfaced with a Waters Corporation (Milford, MA, USA) Acquity ultra pressure liquid chromatograph. Analyst software 1.6.2 (AB Sciex, Foster City, CA, USA) was used for system control and data processing. The LC system was equipped with an Acquity UPLC HSS T3 1.8 μm, 2.1 × 50 mm HPLC column, and an Acquity UPLC HSS T3 1.8 μm VanGuard precolumn (Milford, MA, USA). The mobile phase consisted of a mixture of 0.1% formic acid in water (solvent A) and 0.1% formic acid in acetonitrile (solvent B), with a flow rate of 0.5 ml/min and run time of 2 min. Solvents A and B were combined in a gradient: 0–1 min: 85% A; 1–1.5 min: 50% A; 1.6–2 min: return to initial conditions and hold for 3 min. The electrospray source was operated in the positive ionization mode, using collision gas (CAD) 12, curtain gas (CUR) 20, ion source gas 40, and ion spray voltage 5500 V with temperature 500 °C. The instrument was operated in the multiple reaction monitoring (MRM) mode. The following MRM transitions of precursor ions to product ions were selected: fentanyl, m/z 337.2 → 188.2 (collision energy, CE 24 V); fentanyl-D5, m/z 342.2 → 188.2 (collision energy, CE 35 V). The scan time was 100 ms for all analyses. The concentration range in calibration standards was 0.01–20 ng/ml of fentanyl. The limit of sensitivity was 0.05 ng/ml of plasma using a 0.2-ml sample. The within- and between-day variability did not exceed 10%.
Pharmacokinetic and statistical analyses
A linear sum of either two or three exponential terms, consistent with a two- or three-compartment model,[6,7] was fitted to data points by nonlinear regression using GraphPad Prism 7. Residual errors were weighted by the reciprocal of the measured concentration. Coefficients and exponents from the fitted functions were used to calculate the following kinetic variables for fentanyl: volume of the central compartment (V1), total volume of distribution using the area method (Vd), elimination half-life in the terminal ‘beta’ phase (T1/2), total area under the plasma concentration curve (AUC), and total clearance.
Differences between control and carbamazepine groups in patient characteristics and fentanyl kinetic variables were compared statistically using Student's t-test for independent groups, or by an analogous nonparametric test.
Results
Two subjects in the control group had a sampling duration of only 3 h, and could not be included in the pharmacokinetic analysis due to the short sampling duration. Consequently, the final sample sizes were N = 6 in the control group and N = 11 in the carbamazepine group.
The control and carbamazepine groups were comparable in demographic characteristics and baseline laboratory values (Table 1). The mean duration of surgery was longer in the control patients. Both groups received other opioids intraoperatively.
Table 1.
Patient characteristics, surgery type and other intraoperative opioidsa
| Carbamazepine (N = 11) | Control (N = 6) | P value | |
|---|---|---|---|
| Characteristics | |||
| Female, n (%) | 5 (45) | 3 (50) | 0.72 |
| Age (years) | 49.5 ± 16.5 | 53 ± 17.6 | 0.67 |
| Weight (kg) | 72.6 ± 12.1 | 80.1 ± 4.2 | 0.43 |
| Height (cm) | 170 ± 9 | 172 ± 12 | 0.63 |
| BMI (kg/m2) | 24.7 ± 2.7 | 27.2 ± 8.1 | 0.41 |
| Creatinine (mg/100 ml) | 0.87 ± 0.11 | 0.73 ± 0.20 | 0.10 |
| eGFR (ml/min) | 107 ± 23 | 94 ± 15 | 0.57 |
| Albumin (g/100 ml) | 4.4 ± 0.2 | 4.3 ± 0.2 | 0.36 |
| Surgery type | |||
| Craniotomy for tumour | 4 (50) | ||
| Acoustic neuroma resection | 2 (25) | ||
| Microvascular decompression | 10 (91) | ||
| Other neurosurgical procedures | 1 (9) | 2 (25) | |
| Duration of surgery (min) | 257 ± 58 | 407 ± 163 | 0.01 |
| Intraoperative opioids | |||
| Alfentanil (mg) | 0.13 | ||
| Hydromorphone (mg) | 0.09 ± 0.3 | 0.26 ± 0.39 | |
| Meperidine (mg) | 2.27 | ||
| Morphine (mg) | 1.54 ± 3.5 | 1.25 ± 3.5 | |
| Remifentanil (mg) | 0.51 ± 0.87 | 0.36 ± 0.51 | |
| Sufentanil (mcg) | 17.5 ± 27.6 | 68.1 ± 57.2 | |
P values are from Student's t-test for independent groups. aValues are mean ± standard deviation, or number and per cent of total, as appropriate.
Figure 1 shows mean plasma fentanyl concentrations in the two groups, along with pharmacokinetic functions consistent with the aggregated data points. An extensive phase of drug distribution is evident during the first 30 min after the fentanyl injection. At 5 and 15 min after injection, generally corresponding to the time of maximum analgesic effect, concentrations were lower in the carbamazepine recipients than in the control patients.
Figure 1.
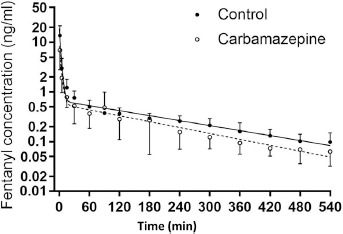
Mean (±standard error) plasma fentanyl concentrations at corresponding times in the control patients and in the carbamazepine-treated patients. Lines represent the pharmacokinetic functions determined by nonlinear regression analysis.
Kinetic parameters are provided in Table 2. Central compartment volumes and total volumes of distribution, with or without normalization for body weight, were larger in carbamazepine-treated patients than in controls, though the differences were not statistically significant. Total area under the plasma concentration curve was significantly lower in the carbamazepine group than in controls, and total clearance of fentanyl was correspondingly increased (Table 2). Elimination half-life of fentanyl did not differ between groups.
Table 2.
Pharmacokinetic variables for fentanyl
| Mean (±SD) value for | P valuea | ||
|---|---|---|---|
| Control (N = 6) | With carbamazepine (N = 11) | ||
| Central compartment volume | |||
| Litres | 35 (±33) | 42 (±42) | >0.5 |
| Litres/kg | 0.49 (±0.47) | 0.51 (±0.40) | >0.5 |
| Total volume of distribution | |||
| Litres | 258 (±91) | 422 (±270) | >0.15 |
| Litres/kg | 3.6 (±1.2) | 5.4 (±3.1) | >0.15 |
| Total AUC (ng/ml × min) | 233 (±70) | 150 (±65) | <0.02 |
| Clearance | |||
| ml/min | 919 (±242) | 1554 (±624) | <0.02 |
| ml/min per kg | 13.2 (±4.8) | 20.1 (±6.8) | <0.05 |
| Elimination half-life (h) | 3.28 (±0.89) | 3.09 (±1.34) | >0.5 |
| Distribution half-life (min) | 6.6 (±5.1) | 2.7 (±1.4) | <0.03 |
Bold numbers indicate statistically significant differences (P < 0.05)
Based on parametric or nonparametric test.
Discussion
This study demonstrates that chronic carbamazepine therapy significantly influences the pharmacokinetics of an intravenously-administered single bolus dose of fentanyl, increasing its plasma clearance and reducing the total AUC. The trend for larger total Vd of fentanyl among carbamazepine recipients did not reach statistical significance. It is notable, however, that during the distribution phase, mean concentrations of fentanyl were lower at those times when the peak analgesic effect of fentanyl is anticipated (5–15 min after injection). Despite the enhanced clearance observed with concomitant carbamazepine therapy, the elimination half-life was not different between groups. The relationship between T1/2, Vd and clearance is given by the following equation[8,9]:
We observed that carbamazepine treatment was associated with an increase in both Vd and clearance. As such, T1/2 was not different between groups. Nonetheless, the increased CL and Vd were associated with lower mean concentrations of fentanyl at the clinically important time of its peak analgesic onset, that is at 5 and 15 min after administration. These findings might explain the increased dose requirement of fentanyl reported in patients receiving carbamazepine therapy for prolonged periods. This is a novel finding with important perioperative implications. Fentanyl is the most common intraoperative opioid, used globally for many types of surgeries including neurological surgery and craniotomies. Carbamazepine, on the other hand, is an important antiepileptic agent and is also commonly administered for other conditions such as trigeminal neuralgia. Co-administration of these agents is hence a common clinical scenario, yet the absence of pharmacokinetic data had limited the clinician's ability to optimize the perioperative pain control. An insufficient dose of fentanyl in this scenario can lead to significant pain upon emergence from anaesthesia, while a larger than required dose can lead to excessive respiratory depression and delayed extubation. The current study elucidates the altered plasma fentanyl concentration in patients with this AED, guiding the clinician to optimize its dose and frequency for effective pain control without excessive respiratory suppression.
It should be noted, however, that the pharmacodynamic consequences of the pharmacokinetic changes were not directly assessed in this study. The therapeutic window for fentanyl is defined as the range between the minimally effective analgesic dose and that associated with respiratory depression. A concentration of 0.6 ng/ml results in slight but measurable analgesia.[10,11] At a serum concentration of 1.4 ng/ml, a 50% decrease in pain intensity is reported with 12% reduction in minute ventilation, and at 3 ng/ml a 23% reduction. Thus, the therapeutic window for fentanyl providing analgesia without clinically significant respiratory depression in awake patients is reported between 0.6 and 2 ng/ml. During the intraoperative period, however, the pharmacodynamic effects of opioids need to be considered in conjunction with other analgesic and anaesthetic agents, as well as the significance and magnitude of the surgical stimuli, the need for airway control (e.g. intubation of the patient), and mechanical ventilation.
Carbamazepine itself has no opioid receptor activity.[12] However, AED drugs, including carbamazepine, are commonly used for treating neuropathic pain conditions and to potentiate opioid analgesic effects.[13] Although AEDs have no direct interaction with opioid receptor activity, carbamazepine has inhibitory actions on the sodium channel, explaining the opioid potentiation in neuropathic conditions. Whether chronic carbamazepine actions are different from acute effects remains unclear. However, the pharmacodynamic effects and pharmacological interactions of these drugs can be different when administered acutely as compared to chronic and long-term administration. This feature is well exemplified by many drugs including opioids and other centrally acting agents. For example, while opioids have profound analgesic effects when administered for short periods, their prolonged administration can lead to increased nociceptive behaviours (opioid-induced hyperalgesia). Similarly, it is well established that midazolam acutely potentiates opioid effects. However, prolonged co-administration of midazolam with morphine induces greater opioid tolerance than morphine alone.[14] Experimental studies suggest that this phenomenon is not explained by a pharmacokinetic interaction.[15] Whether chronic carbamazepine can similarly have divergently opposite effects on analgesia during acute vs chronic administration needs further study. It is notable, however, that in another study of AED interaction with a different type of analgesic, dexmedetomidine, no changes in pharmacodynamics or other definitive interactions were observed.[16]
The mechanism of enhanced clearance of fentanyl in carbamazepine-treated patients is not established. Fentanyl is a high-clearance drug after intravenous administration, with mean total clearance values among control patients in the range of 900 to 1000 ml/min. This is approximately two-thirds of estimated hepatic blood flow (HBF) in healthy individuals (about 1500 ml/min), and as such clearance of intravenous fentanyl is partly dependent on HBF, and can be influenced by changes in HBF.[17,18] We previously reported significant increases in fentanyl clearance among patients under treatment following burn injury, presumably due to the high cardiac output and increased HBF in patients with burn injury.[7] The burned patients also had an increase in fentanyl Vd compared to nonburned controls.
The remaining component of fentanyl clearance is explained by hepatic metabolism, with the principal pathway being transformation by cytochrome P450-3A (CYP3A) enzymes[5,19,20] to norfentanyl, hydroxyproprionyl-fentanyl and hydroxyproprionyl-norfentanyl, all with minimal pharmacological activity.[21] Clearance of intravenous fentanyl is impaired by co-administration of the CYP3A inhibitor ketoconazole,[5] and transmucosal fentanyl clearance is similarly impaired by the CYP3A inhibitor troleandomycin.[22] Enzyme-inducing agents have the opposite effect on fentanyl clearance. Reduced analgesic efficacy of fentanyl is reported in patients receiving the inducer rifampin.[23,24] In a pharmacokinetic study, co-administration of rifampin greatly reduced AUC and increased clearance of oral transmucosal fentanyl.[22] The increased clearance of fentanyl in this study is likely explained by some combination of increased liver size or HBF associated with the inducer carbamazepine, together with the well-established capacity of carbamazepine to induce CYP3A metabolic activity. It is unlikely that fentanyl clearance was significantly influenced by differences in renal excretion of the intact drug, as there were no differences in the renal function (creatinine levels and estimated glomerular filtration rates) between carbamazepine and control groups. In addition, renal clearance is minimal for fentanyl, with less than 10% of the intact drug excreted by the kidneys.[25]
In addition to the above drug–drug interaction and the increased fentanyl clearance, patients with chronic carbamazepine treatment are also reported to be resistant to other important drugs that are commonly used during the intraoperative period or in the intensive care setting. These medications include a common neuromuscular blocking agent vecuronium,[26] as well as the α-2 agonist dexmedetomidine.[16] The suggested mechanism is similar to that of fentanyl, and appears to include the enzyme induction by this anticonvulsant. It is of great importance that the clinicians consider these interactions in all patients on chronic carbamazepine treatment.
The relatively small sample size is a limitation of this study. However, this limitation needs to be considered in the context of the difficulty to enroll patients with significant pain or seizure disorders requiring surgery under general anaesthesia with an arterial catheter, for whom the needs of a research protocol cannot override the many clinical perioperative considerations. We believe, nevertheless, that the availability of a dense sampling scheme for each subject is a strong point of the design, enabling full characterization of the pharmacokinetics in each subject individually. Also, the current study was not designed to elucidate the pharmacodynamic consequences of the reported pharmacokinetic changes. Hence, despite clear differences in fentanyl clearance, the exact analgesic differences and other hemodynamic and respiratory consequences of these changes are not explored. Acute administration of carbamazepine is commonly a part of the therapeutic regimen of multimodal analgesia to potentiate opioid drugs.[27] As discussed above, whether chronic carbamazepine, similar to chronic opioids, antagonizes opioid effects needs further study.
In conclusion, chronic treatment with carbamazepine results in decreased fentanyl plasma concentrations after a single intravenous dose, due to increased fentanyl clearance. This effect may contribute to the decreased sensitivity to the analgesic effects of fentanyl and to the increased perioperative or nonoperative dose requirements.
Declaration
Funding
This work was partially supported by grants from NIH RO1 GM 11847 and from Shriners Hospital Research Philanthropy Grant # 85124 (to JAJM).
References
- Lunn JK et al. High dose fentanyl anesthesia for coronary artery surgery: plasma fentanyl concentrations and influence of nitrous oxide on cardiovascular responses. Anest Analg 1979; 58: 390–395. [PubMed] [Google Scholar]
- Greenblatt DJ. Opioid prescribing: what are the numbers? Clin Pharmacol Drug Dev 2018; 7: 6–8. [DOI] [PubMed] [Google Scholar]
- Tempelhoff R et al. Anticonvulsant therapy increases fentanyl requirements during anaesthesia for craniotomy. Can J Anaesth 1990; 37: 327–332. [DOI] [PubMed] [Google Scholar]
- Spina E et al. Clinically significant pharmacokinetic drug interactions with carbamazepine. An update. Clin Pharmacokinet 1996; 31: 198–214. [DOI] [PubMed] [Google Scholar]
- Ziesenitz VC et al. Pharmacokinetic interaction of intravenous fentanyl with ketoconazole. J Clin Pharmacol 2015; 55: 708–717. [DOI] [PubMed] [Google Scholar]
- Greenblatt DJ, Abourjaily PN. Pharmacokinetics and pharmacodynamics for medical students: a proposed course outline. J Clin Pharmacol 2016; 56: 1180–1195. [DOI] [PubMed] [Google Scholar]
- Han T et al. Fentanyl clearance and volume of distribution are increased in patients with major burns. J Clin Pharmacol 2007; 47: 674–680. [DOI] [PubMed] [Google Scholar]
- Abernethy DR et al. Alterations in drug distribution and clearance due to obesity. J Pharmacol Exp Ther 1981; 217: 681–685. [PubMed] [Google Scholar]
- Hanley MJ et al. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet 2010; 49: 71–87. [DOI] [PubMed] [Google Scholar]
- Hill HF et al. Steady-state infusions of opioids in human. II. Concentration-effect relationships and therapeutic margins. Pain 1990; 43: 69–79. [DOI] [PubMed] [Google Scholar]
- Nimmo WS, Todd JG. Fentanyl by constant rate i.v. infusion for postoperative analgesia. Br J Anaesth 1985; 57: 250–254. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Schmidt J. The electron microscopic autoradiographic localization of alpha-bungarotoxin binding sites within the central nervous system of the rat. Brain Res 1978; 142: 152–159. [DOI] [PubMed] [Google Scholar]
- Siniscalchi A et al. Antiepileptic drugs for central post-stroke pain management. Pharmacol Res 2012; 65: 171–175. [DOI] [PubMed] [Google Scholar]
- Song L et al. Midazolam exacerbates morphine tolerance and morphine-induced hyperactive behaviors in young rats with burn injury. Brain Res 2014; 1564: 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller SJ et al. Pharmacokinetics cannot explain the increased effective dose requirement for morphine and midazolam in rats during their extended administration alone or in combination. J Pharm Pharmacol 2017; 69: 82–88. [DOI] [PubMed] [Google Scholar]
- Flexman AM et al. Enzyme-inducing anticonvulsants increase plasma clearance of dexmedetomidine: a pharmacokinetic and pharmacodynamic study. Anesthesiology 2014; 120: 1118–1125. [DOI] [PubMed] [Google Scholar]
- Greenblatt DJ. Presystemic extraction: mechanisms and consequences. J Clin Pharmacol 1993; 33: 650– 656. [DOI] [PubMed] [Google Scholar]
- Tsunoda SM et al. Differentiation of intestinal and hepatic cytochrome P450 3A activity with use of midazolam as an in vivo probe: effect of ketoconazole. Clin Pharmacol Ther 1999; 66: 461–471. [DOI] [PubMed] [Google Scholar]
- Feierman DE, Lasker JM. Metabolism of fentanyl, a synthetic opioid analgesic, by human liver microsomes. Role of CYP3A4. Drug Metab Dispos 1996; 24: 932–939. [PubMed] [Google Scholar]
- Labroo RB et al. Fentanyl metabolism by human hepatic and intestinal cytochrome P450 3A4: implications for interindividual variability in disposition, efficacy, and drug interactions. Drug Metab Dispos 1997; 25: 1072–1080. [PubMed] [Google Scholar]
- Mather LE. Clinical pharmacokinetics of fentanyl and its newer derivatives. Clin Pharmacokinet 1983; 8: 422– 446. [DOI] [PubMed] [Google Scholar]
- Kharasch ED et al. Influence of hepatic and intestinal cytochrome P4503A activity on the acute disposition and effects of oral transmucosal fentanyl citrate. Anesthesiology 2004; 101: 729–737. [DOI] [PubMed] [Google Scholar]
- Morii H et al. Failure of pain control using transdermal fentanyl during rifampicin treatment. J Pain Symptom Manage 2007; 33: 5–6. [DOI] [PubMed] [Google Scholar]
- Takane H et al. Rifampin reduces the analgesic effect of transdermal fentanyl. Ann Pharmacother 2005; 39: 2139–2140. [DOI] [PubMed] [Google Scholar]
- McClain DA, Hug CC Jr. Intravenous fentanyl kinetics. Clin Pharmacol Ther 1980; 28: 106–114. [DOI] [PubMed] [Google Scholar]
- Soriano SG et al. Pharmacokinetics and pharmacodynamics of vecuronium in children receiving phenytoin or carbamazepine for chronic anticonvulsant therapy. Br J Anaesth 2001; 86: 223–229. [DOI] [PubMed] [Google Scholar]
- Martyn JAJ et al. Critical illness induced opioid tolerance. NEJM 2019; 380: 365–378. [DOI] [PMC free article] [PubMed] [Google Scholar]


