Abstract
We report a 64-year-old caucasian woman diagnosed with membranous nephropathy secondary to alpha-1 antitrypsin deficiency (AATD). AATD is a rare autosomal codominant genetic disorder. Its clinical manifestations are mostly observed in the lungs, with early-onset emphysema. Nephropathy due to AATD is still very rare and only a few cohort studies have been reported. It has been recognised that alpha-1 antitrypsin has a protective role in the kidneys which enhances the possibility of development of kidney failure, such as nephrotic syndrome, in cases of AATD. Further clinical investigation is needed to understand the relationship between the development of nephropathy, namely membranous nephropathy, and AATD.
Keywords: pulmonary emphysema, nephrotic syndrome, genetics
Background
Alpha-1 antitrypsin deficiency (AATD) is a rare autosomal codominant genetic disorder with clinical manifestations mostly observed in the lungs with early-onset emphysema.1 2 Nephrotic syndrome (NS) and nephropathy as a consequence of AATD is unusual. The first case report with this association was described by Miller and Kuschner3 and since then some case reports and small case series have been published.4–9 Although its physiopathology is not totally established yet, it has been recognised that alpha-1 antitrypsin (AAT) has a protective role in the kidneys10 11 acting as an anti-inflammatory and immune-modulator protein.12–14 In AATD cases, this might promote the development of nephropathy.
This clinical case establishes a relationship between AATD and NS, namely membranous nephropathy (MN).
Case presentation
We present a 64-year-old, non-smoker, non-alcoholic drinker, caucasian woman who worked as an accountant. She has a clinical history of asthma and recurrent chest infections in childhood, AATD (Pi*ZZ genotype) with bronchiectasis, hypertension and gall stones. No history of liver disease or connective tissue disease is known. She takes ramipril 10 mg once daily (od), salbutamol inhaler as needed (prn), lercanidipine 10 mg od, carbocisteine 750 mg two times a day and atorvastatin 10 mg od.
She was diagnosed with AATD at 46-years-old (AAT level 0.4 g/L (N: 0.8–1.9 g/L); genotype Pi*ZZ; cylindrical bronchiectasis without emphysema on chest CT scan), with her lung function remaining within normal range from diagnosis (FEV1 [Forced expiratory volume in 1 second] 2.60 L (105% predicted), FVC [Forced vital capacity] 3.10 L (108% predicted), FEV1/FVC 82% predicted, TLCO [Transfer factor for carbon monoxide] 8.50 mmol/min/kPa, KCO [Carbon monoxide transfer coefficient] 1.85 mmol/min/kPa/L) to the present day (at 64 years old: FEV1 1.82 L (84% predicted), FVC 2.39 L (87% predicted), TLCO 6.58 mmol/min/kPa, KCO 1.56 mmol/min/kPa/L)). Liver elastography - FibroScan - (4.3 kPa) and liver function are also in the normal range. At 53 years old, she started to develop emphysema, predominantly in the right lower and middle lobe, with cylindrical and varicose bronchiectasis in all lobes, and more marked cystic bronchiectasis in the middle lobe. Two years later, she became more breathlessness (mMRC [modified Medical Research Council dyspnoea scale] 2–3), with 2–3 exacerbation/year, requiring antibiotics. She had a trial with inhaled steroids which was followed by four chest infections within 6 months. It was withdrawn for that reason.
At 64 years old, she was referred to a nephrologist for increased lower limb swelling over 4–5 weeks and worse during the day. Deep venous thrombosis was ruled out (D-dimer test: negative). She also noticed increased hand swelling overnight and generalised aches in her hands and around her thumb. She denied foamy urine or visible haematuria but reported passing urine more frequently during the night. At her first examination, blood pressure was 154/90 mmHg, pulse 86 bpm, temperature 35.6°C and weight 63 kg. Her jugular venous pulse was visible, chest and heart sounds were normal and abdomen was soft and non-tender. She had bilateral lower limb oedema. Urinalysis showed marked proteinuria with microscopic haematuria. Urine albumin-to-creatinine ratio (ACR) was also markedly elevated at 829 mg/mmol and albumin was low (22 g/L). The diagnosis of NS was made.
Investigations
To investigate the cause of the NS she underwent virology, myeloma and immunology screenings (table 1). Renal function was within normal range. Serum protein electrophoresis was normal except albumin. No anaemia or diabetes (haemoglobin A1c normal) were noted.
Table 1.
Laboratory results
| Laboratory analysis | Relevant results |
| Albumin | 22 g/L |
| Cholesterol | 7.1 mmol/L |
| Immunology | ANCA (PR3; MPO), ANA, IgA, IgM, IgG: All Negative |
| Anti-PLA2R antibody: Negative | |
| Virology screening | Negative |
| Myeloma screening | Negative |
| Renal function | Normal |
| Diabetes screening | Haemoglobin A1c: Normal |
ANA, Antinuclear antibodies; ANCA, Antineutrophil cytoplasmic antibodies; Anti-PLA2R, Anti-phospholipase A2 receptor antibodies; Ig, Immunoglobulins.
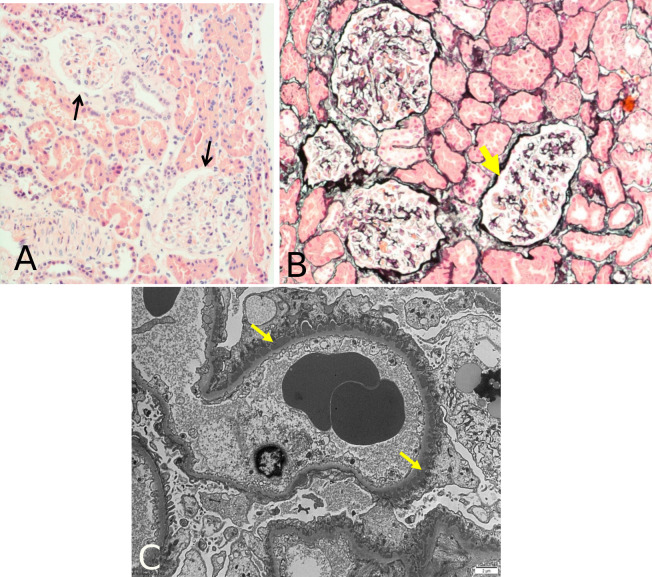
She had a previous echocardiogram without any significant change and a whole body CT scan without any signs of malignancy. A kidney ultrasound revelled normal sized kidneys, both with good cortical thickness and corticomedullary differentiation; no evidence of focal lesions or hydronephrosis. Furosemide 40 mg od was started and she underwent a kidney biopsy a week later. The first biopsy report suggested minimal change disease — no interstitial inflammation, no significant atrophy nor glomerulonephritis (GN). Immunofluorescence showed a strong diffuse granular immunoglobulin (Ig) G along the glomerular basement membrane (GBM); the glomeruli were negative for IgA, IgM, complement C3 and C1q; lambda light chains appeared stronger then kappa along the GBM but were negative in the tubular basement membrane (BM); electron microscopy showed multiple small regular size subepithelial electron dense deposits with limited new BM deposition (figure 1). This confirmed a diagnosis of early MN instead. Immunohistochemistry was negative for AAT protein.
Figure 1.
Kidney biopsy results from light microscopy and electron microscopy. Light microscopy images did not actually showed a diagnosis of MN, with H&E stain (A) showing absence of thickening of GBM (arrows), and silver stain (B) showing absence of spikes along the GBM (arrow). When electron microscopy (C) was performed, small, electron dense, subepithelial deposits were observed with a strong diffuse granular IgG along the GBM (arrows), compatible with MN. GBM, glomerular basement membrane; MN, membranous nephropathy; H&E, Hematoxylin & Eosin.
Treatment
She was treated with maximum angiotensin-converting enzyme (ACE)-inhibition in the form of ramipril 10 mg daily and her blood pressure has controlled to a target of below 130/80 mmHg. She did not require anticoagulation as her albumin level remained persistently above 20 g/L. After 2 months her lower limb oedema improved and the dose of furosemide was lowered to 20 mg od. Although, at this time, the urinalysis still showed protein (+++), her urine ACR has continued to improve (121.7 mg/mmol—not nephrotic range).
Outcome and follow-up
She is currently followed up with stable symptoms and investigations showed normal blood analysis, normal cholesterol and a good blood pressure control. She remains clinically stable.
Discussion
MN is the most common cause of NS in adults.15 16 Most patients present a slow progression of oedema (over weeks to months), proteinuria (>3.5 g of protein in the urine per day) and hypoalbuminaemia. Dyslipidaemia may be present. In cases of NS without any apparent cause, early kidney biopsy is crucial to support a reliable diagnosis and the best therapeutic approach.17 On the results of our biopsy, glomeruli showed to be normal on light microscopy but when electron microscopy was performed, small, electron dense, subepithelial deposits were observed—figure 1—and were confirmed, through immunofluorescence, to be IgG. These findings support a diagnosis of MN.15 18 The absence of serum anti-PLA2R antibodies along with presence of IgG deposits in GBM suggested a secondary cause for MN. Serum anti-PLA2R antibodies are closely related to disease activity and became a strong marker for idiopathic MN.19 They are rarely detectable in secondary MN. In our case, serum anti-PLA2R antibodies were negative (i.e. undetectable) when ACR was elevated, which did not suggest an idiopathic MN and made us believe to be in presence of a secondary MN. In this context, it was not relevant to monitor these antibodies any further. Deposits of IgG, like subclasses IgG1, IgG2 or IgG3, are more frequently found in secondary forms of MN.16 20 IgG was present in our biopsy but unfortunately the IgG subclasses were not quantified. We could speculate IgG1 would be the major subclass since it is usually the one present in early stages in MN.21 IgG1 may initially activate the complement classic pathway in early stages, with activation of the lectin or alternative pathways in later stages. As it comes to our knowledge, complement activation has been showed to be increased in AATD.22 Nevertheless, further studies are needed to clarify the interplay of different complement systems in AATD.
Secondary MN represents one third of recognised cases of MN and is usually caused by autoimmune diseases, malignancies, infections or drugs,15 which were all excluded. On the results of our biopsy, laboratory analyses, as well as clinical evidence, we believe there is a strong association between MN and AATD in our case report.
Nephropathy in AATD has been a challenging diagnosis because of its high clinical heterogeneity spectrum.4–9 The most common has been membranous proliferative glomerulonephritis (MPGN) and mesangiocapillary GN, with an abnormal accumulation of Z protein.4 7 They have been especially noted in children with chronic liver disease.4 23 24 MN has been less reported in AATD and its mechanism remains unclear. Another association is with ANCA-associated vasculitis (especially PR3-ANCA) presenting with GN and progressive renal failure.25 26 The main pathophysiological mechanism for MN in AATD patients is poorly understood, since MN has been less reported in AATD. The relationship between AATD and MN is weaker than with MPGN.4–9 Nevertheless, the main mechanism that would trigger both, MN and MPGN may be similar in AATD patients. Since both have only been seen in patients with a Z allele, Pi*SZ or Pi*ZZ, it has been elucidated that the presence of an abnormal Z protein would be essential. The polymerisation and dysfunction of Z protein could induce the formation of immune complexes, act as an antigen for in situ immune-complex,27 28 or contribute to the development of auto antibodies promoting the development of MN. How these immune complexes form has been proposed through the following hypothesis: preformed immune complexes which are entrapped in the subepithelial space16 21; circulating pathogenic antigens localised in subepithelial sites that subsequently form in situ immune-complex deposits with antibodies; or auto antibodies bound to podocyte membrane antigens and leading to subepithelial deposition of immune complex.16 21 Another hypothesis could be related to the fact that AATD might be causing an important deregulation in the immune response, with a prominent proinflammatory role created outside the kidneys,22 which justifies the absence of AAT protein isoelectric forms in our biopsy, and not from local damage caused by accumulation of Z protein polymers and its’ subsequent potential proinflammatory role.4 7
Treatment in secondary MN should be aimed to the underlying cause including conservative treatment for NS.21 29 Optimal supportive care with achievement of remission of proteinuria is the main goal,16 21 29 preventing deterioration of renal function and progression to end-stage renal disease. Prophylactic anticoagulation should be initiated if hypoalbuminaemia is severe.18 AAT augmentation therapy has been used in AATD-related lung disease30 31 and panniculitis, but it is not licensed to be used in other manifestations of AATD.32 33 All other known cases of nephropathy AATD-related were treated with the same approach as in non-AATD patients.
Learning points.
From this clinical case, we establish that membranous nephropathy (MN) is of secondary cause as illustrated by the absence of anti-PLA2R antibodies and the presence of immunoglobulin deposits at the glomerular basement membrane, thus excluding an idiopathic cause. The most probable cause would be due to alpha-1 antitrypsin deficiency (AATD) since all other causes were ruled out.
The relationship between AATD and MN is weaker than with membranous proliferative glomerulonephritis.4–9
Treatment of secondary MN aims to achieve remission of proteinuria. It remains uncertain what could be the role of AAT-augmentation therapy in these patients, although available evidence has already imputed a role to AAT as a protective layer of the kidney.10 11
Nephropathy due to AATD is rare and our patient provides further support for the association between AATD and MN. Additional clinical investigation is still needed in this field.
Acknowledgments
We thank A.J. Walker, from Pathology Department from Royal Stoke University Hospital, University Hospitals North Midlands, NHS Trust, for supplying the images and helping on their interpretation.
Footnotes
Contributors: All coauthors have actively participated in the writing, discussion and revision of the manuscript. GFS participated in the writing, conceiving, discussion and revision of the manuscript; PE and DF participated in conceiving, discussion and revising it critically for important intellectual content and AMT participated in revising it critically for important intellectual content and final approval of the version to be submitted.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Stockley RA, Turner AM. α-1-Antitrypsin deficiency: clinical variability, assessment, and treatment. Trends Mol Med 2014;20:105–15. 10.1016/j.molmed.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 2.Hobbs BD, Silverman E, Cho M. Genetics and epidemiology. : Strnad P, Brantly ML, Bals R, . α1-Antitrypsin deficiency ERS monograph sheffield. European Respiratory Society, 2019: 27–38. [Google Scholar]
- 3.Miller F, Kuschner M. Alpha1-Antitrypsin deficiency, emphysema, necrotizing angiitis and glomerulonephritis. Am J Med 1969;46:615–23. 10.1016/0002-9343(69)90080-1 [DOI] [PubMed] [Google Scholar]
- 4.Davis ID, Burke B, Freese D, et al. The pathologic spectrum of the nephropathy associated with alpha 1-antitrypsin deficiency. Hum Pathol 1992;23:57–62. 10.1016/0046-8177(92)90012-R [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Soriano J, Fidalgo I, Camarero C, et al. Juvenile cirrhosis and membranous glomerulonephritis in a child with alpha1-antitrypsin deficiency PiSZ. Acta Paediatr Scand 1978;67:793–6. 10.1111/j.1651-2227.1978.tb16263.x [DOI] [PubMed] [Google Scholar]
- 6.Stauber RE, Horina JH, Trauner M, et al. Glomerulonephritis as late manifestation of severe alpha 1-antitrypsin deficiency. Clin Investig 1994;72:404–8. 10.1007/BF00252839 [DOI] [PubMed] [Google Scholar]
- 7.Elzouki AN, Lindgren S, Nilsson S, et al. Severe alpha1-antitrypsin deficiency (PiZ homozygosity) with membranoproliferative glomerulonephritis and nephrotic syndrome, reversible after orthotopic liver transplantation. J Hepatol 1997;26:1403–7. 10.1016/S0168-8278(97)80478-3 [DOI] [PubMed] [Google Scholar]
- 8.Loreno M, Boccagni P, Rigotti P, et al. Combined liver-kidney transplantation in a 15-year-old boy with alpha1-antitrypsin deficiency. J Hepatol 2002;36:565–8. 10.1016/S0168-8278(02)00012-0 [DOI] [PubMed] [Google Scholar]
- 9.Lewis M, Kallenbach J, Zaltzman M, et al. Severe deficiency of alpha 1-antitrypsin associated with cutaneous vasculitis, rapidly progressive glomerulonephritis, and colitis. Am J Med 1985;79:489–94. 10.1016/0002-9343(85)90036-1 [DOI] [PubMed] [Google Scholar]
- 10.Jeong K-H, Lim J-H, Lee K-H, et al. Protective effect of alpha 1-antitrypsin on renal ischemia-reperfusion injury. Transplant Proc 2019;51:2814–22. 10.1016/j.transproceed.2019.04.084 [DOI] [PubMed] [Google Scholar]
- 11.Maicas N, van der Vlag J, Bublitz J, et al. Human alpha-1-antitrypsin (hAAT) therapy reduces renal dysfunction and acute tubular necrosis in a murine model of bilateral kidney ischemia-reperfusion injury. PLoS One 2017;12:e0168981–18. 10.1371/journal.pone.0168981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janciauskiene SM, Bals R, Koczulla R, et al. The discovery of α1-antitrypsin and its role in health and disease. Respir Med 2011;105:1129–39. 10.1016/j.rmed.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 13.Greulich T, Nell C, Hohmann D, et al. The prevalence of diagnosed α1-antitrypsin deficiency and its comorbidities: results from a large population-based database. Eur Respir J 2017;49:1600154–9. 10.1183/13993003.00154-2016 [DOI] [PubMed] [Google Scholar]
- 14.Stockley RA. Alpha 1-antitrypsin: more than just deficiency. Thorax 2004;59:363–4. 10.1136/thx.2004.023572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fervenza FC, Sethi S, Specks U. Idiopathic membranous nephropathy: diagnosis and treatment. Clin J Am Soc Nephrol 2008;3:3:905–19. 10.2215/CJN.04321007 [DOI] [PubMed] [Google Scholar]
- 16.Lai WL, Yeh TH, Chen PM, Ling W, Hao T, et al. Membranous nephropathy: a review on the pathogenesis, diagnosis, and treatment. J Formos Med Assoc 2015;114:102–11. 10.1016/j.jfma.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 17.Waldman M, Crew RJ, Valeri A, et al. Adult minimal-change disease: clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol 2007;2:445–53. 10.2215/CJN.03531006 [DOI] [PubMed] [Google Scholar]
- 18.Ponticelli C, Glassock RJ. Glomerular diseases: membranous nephropathy--a modern view. Clin J Am Soc Nephrol 2014;9:9:609–16. 10.2215/CJN.04160413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X, Liu L, Guo Y, et al. Clinical value of a serum anti-PLA2R antibody in the diagnosis and monitoring of primary membranous nephropathy in adults. Int J Nephrol Renovasc Dis 2018;11:241–7. 10.2147/IJNRD.S176665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qu Z, Liu G, Li J, et al. Absence of glomerular IgG4 deposition in patients with membranous nephropathy may indicate malignancy. Nephrol Dial Transplant 2012;27:1931–7. 10.1093/ndt/gfr534 [DOI] [PubMed] [Google Scholar]
- 21.Trujillo H, Praga M, Department of Nephrology, University Hospital 12 de Octubre, Madrid, Spain . Membranous nephropathy: an update. Pjnh 2019;33:19–27. 10.32932/pjnh.2019.04.006 [DOI] [Google Scholar]
- 22.O'Brien ME, Fee L, Browne N, et al. Activation of complement component 3 is associated with airways disease and pulmonary emphysema in alpha-1 antitrypsin deficiency. Thorax 2020;75:1–10. 10.1136/thoraxjnl-2019-214076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moroz SP, Cutz E, Balfe JW, et al. Membranoproliferative glomerulonephritis in childhood cirrhosis associated with alpha1-antitrypsin deficiency. Pediatrics 1976;57:232–8. [PubMed] [Google Scholar]
- 24.Strife CF, Hug G, Chuck G, et al. Membranoproliferative glomerulonephritis and alpha 1-antitrypsin deficiency in children. Pediatrics 1983;71:88–92. [PubMed] [Google Scholar]
- 25.Jolobe OMP. Alpha-1 antitrypsin deficiency. Am J Med 2008:9343. 10.1016/j.amjmed.2008.02.027 [DOI] [PubMed] [Google Scholar]
- 26.ERS monograph . Alpha1-Antitrypsin deficiency, 2019. [Google Scholar]
- 27.Morse JO, James O, Morse MD. Alpha1-Antitrypsin deficiency (first of two parts). N Engl J Med 1978;299:1045–8. 10.1056/NEJM197811092991905 [DOI] [PubMed] [Google Scholar]
- 28.Morse JO, James O, Morse MD. Alpha1-Antitrypsin deficiency (second of two parts). N Engl J Med 1978;299:1099–105. 10.1056/NEJM197811162992003 [DOI] [PubMed] [Google Scholar]
- 29.Bomback AS, Fervenza FC. Membranous nephropathy: approaches to treatment. Am J Nephrol 2018;47 Suppl 1:30–42. 10.1159/000481635 [DOI] [PubMed] [Google Scholar]
- 30.Marciniuk DD, Hernandez P, Balter M, et al. Alpha-1 antitrypsin deficiency targeted testing and augmentation therapy: a Canadian thoracic Society clinical practice guideline. Can Respir J 2012;19:109–16. 10.1155/2012/920918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellis P, Dirksen A, Turner AM. Treatment of lung disease. : Strnad P, Brantly ML, Bals R, . α1-Antitrypsin deficiency ERS monograph sheffield. European Respiratory Society, 2019: 78–92. [Google Scholar]
- 32.Lopes AP, Mineiro MA, Costa F, et al. Portuguese consensus document for the management of alpha-1-antitrypsin deficiency. Pulmonology 2018;24 Suppl 1:1–21. 10.1016/j.pulmoe.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 33.Sandhaus RA, Turino G, Brantly ML, Committee W, et al. The diagnosis and management of alpha-1 antitrypsin deficiency in the adult. Chronic Obstr Pulm Dis 2016;3:668–82. 10.15326/jcopdf.3.3.2015.0182 [DOI] [PMC free article] [PubMed] [Google Scholar]