Abstract
Objectives
To increase effectiveness of the cervical cancer screening program, self-sampling can be an option. Both self-collected vaginal samples (SCV) and urine samples may be useful alternatives to clinician-taken cervical samples (CS).
Design
Cross-sectional study.
Setting
Colposcopy clinic.
Participants
Women (n=305) referred to colposcopy after abnormal cervical screening result or conditions like postcoital bleeding.
Intervention
All women self-collected a urine and a vaginal sample prior to colposcopy, where a CS and biopsies were taken. All samples were tested for high-risk human papillomavirus (HPV) using the Cobas HPV assay. The gold standard was histology diagnoses (CIN2+/CIN3+) from biopsies obtained at the same examination.
Primary outcome
Absolute and relative sensitivity and specificity of HPV testing on SCV and urine to detect CIN2+/CIN3+ compared with the CS.
Secondary outcome
The acceptability by women of self-sampling.
Results
Both the vaginal and urine sample were comparable to the CS in identifying severe intraepithelial neoplasia (CIN2+/CIN3+). Absolute sensitivity ranged from 93% for urine samples to 96% for SCV for detecting CIN2+, which is comparable to the sensitivity of CS (overlapping 95% CI).
The relative sensitivity for detecting CIN2+ was 1.00 (95% CI 0.96 to 1.04) for SCV and 0.96 (95% CI 0.91 to 1.03) for urine samples. At CIN3+, the relative sensitivity was 1.00 (95% CI 0.96 to 1.08) and 0.97 (95% CI 0.89 to 1.07) for SCV and urine samples, respectively. There were no statistical differences between the self-collected samples and the CS (McNemar’s test >0.05). The relative specificity was also similar (1.03 (95% CI 0.95 to 1.12) for SCV and 0.98 (95% CI 0.89 to 1.09) for urine samples) (McNemar’s test >0.05).
The acceptability of self-sampling was evaluated by questionnaire. The women found the instructions on sample collection easy to understand and were positive about self-sampling with a preference for the urine sample.
Conclusion
Self-sampling by SCV and urine is a clinically safe alternative to CS with a high degree of acceptability.
Keywords: pathology, molecular diagnostics, gynaecological oncology, public health
Strengths and limitation of this study.
All samples were collected at the same visit, including the biopsies.
Only women with all samples available for human papillomavirus (HPV) testing and biopsies were included in the study.
The HPV status was unknown to the pathologist on diagnoses.
The women’s preference towards self-sampling was examined.
The study was performed in colposcopy clinics enrolling primarily women referred with abnormal screening results and not in a screening population.
Introduction
Denmark has a tradition of >50 years for screening for cervical cancer, which has effectively decreased the incidence and mortality of the disease.1 2 However, the incidence of cervical cancer is still relatively high compared with other high-income countries.2 3 One major issue is that only 73% of the eligible women attend the screening programme, and half of the cervical cancers are diagnosed among non-attendees.4 5 Some of the barriers against attending the screening programme have been reported to be discomfort during the gynaecological examination, embarrassment, forgetting to make an appointment, fear of pain, fear of cancer, feeling healthy and not being in risk of developing cervical cancer.6–8 Some of these barriers may be reduced by offering the possibility of self-sampling.9–13
Accordingly, several studies have investigated the clinical performance of self-collected vaginal samples (SCV) compared with clinician-taken cervical samples (CS) in order to detect cervical cancer or precancer. A meta-analysis, including 36 studies, concluded that the sensitivity and specificity of an SCV were non-inferior to that of a clinician-taken CS when a clinically validated PCR-based human papillomavirus (HPV) DNA assay was used. However, it is still recommended to do feasibility studies assessing clinical accuracy, population compliance, logistics and for evaluation of costs before more widespread implementation.14 15
An alternative specimen to a vaginal sample is urine. Urine is desirable as sample material as the collection is non-invasive, and the procedure is well accepted by women.16–18 However, conflicting data have been published on the sensitivity of urine samples. Some papers have shown that urine has a lower clinical sensitivity compared with SCV and clinician-obtained samples,19 20 which may be caused by a lower viral load in the urine sample.21 In contrast, other studies have shown high concordance between urine samples and clinician-taken vaginal samples.22 23 These differences may be explained by variations in collection and processing procedures for urine samples. It has also been discussed whether first-void, mid-stream or morning urine is better for detection of HPV, but no clear conclusion can be made.17 23–26 Preservation, storage and processing methods for urine samples have been studied, and data have indicated that it was favourable to use preservation of fresh urine samples to avoid degradation of HPV DNA and to prevent inhibition of the subsequent HPV detection.27 28 Overall, there is still a need for further studies to uncover urine as test material.
In Denmark, SCV for cervical cancer screening have routinely been offered to non-attendees in one out of five regions since 2018.29 A study carried out in the Central Denmark Region has shown promising results in increasing participation by employing a mailing strategy, where a test kit was send directly to the woman, as an alternative to a standard second reminder to attend screening.30 This is in line with the data from a meta-analysis, where efficiency to reach underscreened women was higher when offering self-sampling compared with receiving a reminder.31
A nationwide offer to non-attendees on self-sampling for cervical cancer screening is currently being planned by the Danish National Steering Committee for cervical cancer screening for expected implementation in 2021. However, before implementing self-sampling as a part of the Danish screening programme, it is very important to evaluate the performance of a given self-sampling set-up, that is, the combination of specific HPV assay, sampling device and material.1 14
Accordingly, the aim of the present study was to examine the clinical performance of urine samples, SCV and clinician-taken CS to detect severe intraepithelial lesions (CIN2+/CIN3+). All materials were analysed by HPV testing and the study was carried out in a Danish colposcopy setting. We also investigated the women’s preference towards self-collected vaginal and urine samples using a questionnaire.
Materials and methods
Study design
Women referred for colposcopy at the gynaecological departments at Lillebaelt Hospital (Kolding) and Odense University Hospital (Odense and Svendborg Hospitals) in Denmark were offered to participate in this study. The women were referred either because of an abnormal screening sample or because of symptoms like postcoital bleeding.
Before arriving at the hospital, all women received written information about the study. At the hospital, after filling out the informed consent, an Evalyn Brush (Rovers Medical Devices, Oss, the Netherlands) for the CS collection and a 50 mL conical tube for the urine sample were handed out together with written instructions on how to collect the two samples. The participants were also asked to fill out a short questionnaire about self-sampling. The women collected the samples in privacy before the scheduled examination. During the colposcopy, the gynaecologist routinely collected a CS in ThinPrep Media (Hologic, Marlborough, Massachusetts, USA) and cervical biopsies, when clinically relevant. In this present study, only women with cervical biopsies were included.
Patient and public involvement
There were no patient involved in the design or outcome measures of the study. But all participants were given the opportunity to see the final results at the end of the study.
Study population
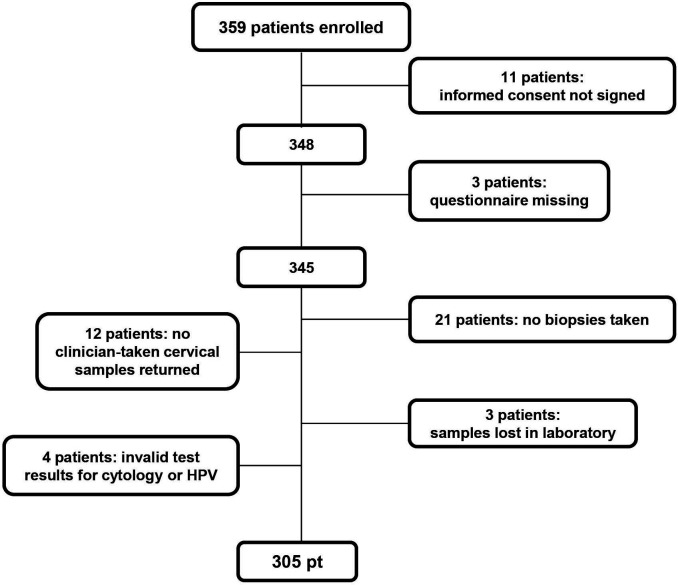
A total of 359 women were enrolled over a period of 20 months from December 2016 to August 2018. Eleven women were subsequently excluded due to missing informed consent formulas and two due to missing questionnaire. As outlined in the Consolidated Standards of Reporting Trials flow diagram in figure 1, we did not receive all four sample specimens from all women (urine sample, SCV, CS and biopsies). In addition, three samples were found to be inadequate for cytology examination during testing in the laboratory; one sample had an invalid HPV test result and three samples were lost in the laboratory. Consequently, the results in this study are based on 305 women with all four sample specimens and valid test results (figure 1). The median age of the 305 women was 34 years ranging from 17 to 85 years.
Figure 1.
Consolidated Standards of Reporting Trials flow diagram. HPV, human papillomavirus.
Sampling procedures
The first sample collected by the woman was the urine sample. Earlier studies have discussed the need for processing of urine to improve sensitivity of HPV testing,17 27 32 and therefore a small pilot study was conducted evaluating the impact of temperature, centrifugation, collection time (first void or full-voided) and preservation (data not shown). Consequently, the following instruction for urine sampling and handling was prepared: a minimum of 8 mL full-voided urine was poured into a 50 mL tube containing 8 mL EDTA (0.5 M, pH 8). Within 5 days, the urine sample was shipped to the laboratory. The sample was centrifuged for 10 min at 1500 rpm, and the pellet was re-suspended in 2 mL PreservCyt solution (Hologic). The sample was then stored at room temperature until HPV testing.
The second sample to be collected by the woman was the vaginal sample using the Evalyn Brush. At the Pathology Department, the material on the Evalyn Brush was suspended in a ThinPrep vail (Hologic), and then stored at room temperature until HPV testing.
Afterwards, the questionnaire on the use of self-sampling was filled out.
Sample processing
At the Pathology Department at Lillebaelt Hospital, Vejle both the HPV test and cytology was performed. The cervical biopsies were examined at the Pathology Department at either Odense University Hospital or Lillebaelt Hospital. The CS were analysed routinely by cytology before HPV testing was performed on the residual material. The cytology slides were processed on the ThinPrep5000 Autoloader Instrument, Hologic according to the manufacturer’s instructions and stained with ThinPrep Stain. The slides were scanned by the Thin Prep Imaging System with selection of 22 fields of view, which were reviewed by a cytotechnologist in a review scope. Specimens were examined for adequacy and abnormal cells, and the threshold for an abnormal/positive sample was set as atypical squamous cells of unknown significance or more (ASCUS+). If abnormal cells were detected, a pathologist made the final diagnosis. All cytology was classified using the Bethesda Nomenclature.33 34 The histology was evaluated as part of the routine and reported using the CIN (cervical intraepithelial neoplasia) classification. The cytology and histology results were subsequently extracted from ‘Patobank’, a nationwide pathology register containing all cytological and histological diagnoses in Denmark. The histology result was used as the gold standard for evaluation of the ability of the different samples to detect severe lesions on the cervix (CIN2+/CIN3+).
Detection of high-risk HPV DNA
Detection of high-risk HPV was performed using the Cobas HPV assay automated on the Cobas 4800 instrument (Roche, Heidelberg, Germany). The Cobas system integrates sample preparation (including DNA extraction and set-up of PCR) and real-time PCR for detection of 12 HR-HPV types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68) as a pooled result, whereas HPV16 and HPV18 are reported individually. Β-globin is used as internal control to access sample adequacy and possible inhibition of the real-time PCR.
Statistical analysis
The performance of the self-collected samples were evaluated by calculating the absolute and relative sensitivity and specificity including the 95% CI. For assessing the significance of the relative sensitivity and specificity, McNemar’s test was used with a significance threshold of 0.05.
Concordance between the three samples (CS, SCV and urine) was calculated using Cohen’s kappa statistics.
The percentage of HPV-positive samples in the CS (control) group was estimated to be 80%, whereas the percentage of positive results in the test groups (Evalyn Brush and urine sample) was expected to be lower. With a power of 80% (significance level of 0.05) and the ability to show a difference in HPV-positive samples of 10% between control and test group, a minimum of 300 women were to be enrolled in the study.
Results
Detection of high-risk HPV
In table 1, the HPV test results from the SCV, the urine samples and the CS are presented together with the cytology results according to the histological diagnosis made based on the concurrent biopsies.
Table 1.
HPV tests and cytology results in relation to the histological results in the different sample specimens
| Histology | No (%) | HPV | Cytology | ||
| Self-collected vaginal sample | Urine sample | Clinician-taken cervical sample | Clinician-taken cervical sample | ||
| HPV pos no. (%) | HPV pos no. (%) | HPV pos no. (%) | ASCUS+ no. (%) | ||
| Normal | 191 (62.7) | 92 (48) | 90 (47) | 86 (45) | 35 (18) |
| CIN1 | 45 (14.8) | 42 (93) | 38 (84) | 44 (98) | 29 (64) |
| CIN2 | 30 (9.8) | 28 (93) | 27 (90) | 28 (93) | 24 (80) |
| CIN3 | 36 (11.8) | 35 (97) | 34 (94) | 35 (97) | 32 (89) |
| AIS | 2 (0.6) | 2 (100) | 2 (100) | 2 (100) | 2 (100) |
| Carcinoma | 1 (0.3) | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| Total | 305 (100) | ||||
AIS, adenocarcinoma in situ; ASCUS+, atypical squamous cells of unknown significance or more; HPV, human papillomavirus.
The overall HPV positivity rate was similar for SCV, the urine samples and CS, showing positive percentages of 65.6%, 63.0% and 64.3%, respectively. One squamous cell carcinoma and two adenocarcinoma in situ were detected and the corresponding SCV, urine and CS were all positive for high-risk HPV. Thirty-six samples were diagnosed as CIN3 and 35 (97%) of these samples were HPV positive in the SCV and CS, while in urine 34 (94%) samples were HPV positive. For CIN2 at total of 30 cases were detected and 28 (93%) of the SCV and CS were HPV positive, whereas the HPV positivity was marginally lower (27 HPV-positive samples) in the urine samples.
The vast majority of the samples detected with CIN1 was HPV positive, but variation was seen between the three specimens; the highest HPV positivity rate was found in the CS (98%), followed by the SCV (93%). In the urine samples, the positivity rate was only 84%. Women with normal histology results presented a HPV positivity rate quite similar in all three sample specimens (48% for SCV, 47% for urine samples and 45% for the CS).
Looking at the cytology results, ASCUS or more was detected in 40% of the 305 samples. This is significantly lower (p<0.0001) than the HPV positivity rates detected for CIN2 or more for the SCV, urine and CS, where 66%, 63% and 64% were HPV positive, respectively. The woman diagnosed with carcinoma had an abnormal cytology result.
Detection of CIN2+/CIN3+
In table 2A, the absolute sensitivity and specificity are shown. For detecting CIN2+, 66 of the SCV and CS samples were true positive while 3 samples were false negative, whereas for urine 64 samples were true positive and 5 samples were false negative. For detecting CIN3+, 38 SCV and CS were true positives and 1 false negative, whereas for urine 37 samples were true positives and 2 false negative. No differences in sensitivity were observed between the three sample specimens indicated by the overlapping CIs. Our results showed that both self-collected sample specimens were non-inferior compared with the clinician-taken CS in identifying CIN2+ and CIN3+.
Table 2A.
Absolute sensitivity and specificity for detecting CIN2+ and CIN3+ for the different samples
| CIN2+ | CIN3+ | ||||
| Sensitivity (95% CI) | Specificity (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | ||
| Self-collected vaginal sample | HPV | 96 (88 to 99) | 43 (37 to 50) | 97 (87 to 100) | 39 (33 to 45) |
| Urine sample | 93 (84 to 97) | 46 (40 to 52) | 95 (83 to 99) | 42 (36 to 48) | |
| Clinician-taken cervical sample | 96 (88 to 99) | 45 (39 to 51) | 97 (87 to 100) | 41 (35 to 47) | |
| Clinician-taken cervical sample (ASCUS+) | Cytology | 86 (75 to 92) | 73 (67 to 78) | 90 (76 to 96) | 67 (61 to 72) |
Table 2B.
Relative sensitivity and specificity for the self-collected sample versus the clinician-taken cervical sample
| Relative sensitivity (95% CI) | Relative specificity (95% CI) | Pmcn | |
| Self-collected vaginal samples versus clinician-taken cervical samples | |||
| CIN2+ | 1 (0.96 to 1.04) | 1 | |
| CIN3+ | 1 (0.93 to 1.08) | 1 | |
| ≤CIN1 | 1.03 (0.95 to 1.12) | 0.585 | |
| Urine samples versus clinician-taken cervical samples | |||
| CIN2+ | 0.96 (0.91 to 1.03) | 0.625 | |
| CIN3+ | 0.97 (0.89 to 1.07) | 0.564 | |
| ≤CIN1 | 0.98 (0.89 to 1.09) | 0.885 | |
ASCUS+, atypical squamous cells of unknown significance or more; HPV, human papillomavirus.
For cytology, the sensitivity for identifying CIN2+ and CIN3+ was markedly lower than that of HPV testing for all three types of samples. For CIN2+, 59 samples had ASCUS or more, while 10 were false negative. For CIN3+, 35 samples were ASCUS+ and 4 were false negative. However, the specificity of cytology was significantly better for detection of both CIN2+ and CIN3+ compared with the HPV-tested specimens.
The relative sensitivity and specificity for SCV and urine samples compared with the CS are shown in table 2B. For CIN2+ and CIN3+, the sensitivity of urine and SCV are comparable to the CS with relative specificity of 1 or very close to 1 and all with CIs overlapping 1. The p values for the McNemar’s test were all above 0.05. Likewise, no difference was seen at <CIN1, where the relative specificity for SCV was 1.03 (95% CI 0.95 to 1.12) and for urine samples it was 0.98 (95% CI 0.89 to 1.09).
Concordance between the sample specimens
The concordance of HPV test results are shown in table 3. The observed concordance between the SCV and the two other sample specimens was 90%, with a kappa value of 0.77 (273 out of 305 samples had the same test result). The observed concordance between urine samples and CS samples was slightly lower, 83% with a kappa value of 0.66, but the difference was not significant (table 3).
Table 3.
Concordance between self-collected vaginal samples, urine samples and clinician-taken cervical samples
| HPV pos (no.) | HPV neg (no.) | Observed concordance (%) | Kappa | 95% CI | ||
| Urine sample | ||||||
| Self-collected vaginal sample | Pos | 180 | 20 | 90 | 0.77 | 0.70 to 0.85 |
| Neg | 12 | 93 | ||||
| Clinician-taken cervical sample | ||||||
| Self-collected vaginal sample | Pos | 182 | 18 | 90 | 0.77 | 0.70 to 0.85 |
| Neg | 14 | 91 | ||||
| Urine sample | ||||||
| Clinician-taken cervical sample | Pos | 168 | 28 | 83 | 0.66 | 0.57 to 0.74 |
| Neg | 24 | 85 | ||||
HPV, human papillomavirus; neg, negative; pos, positive.
We also examined the concordance at the genotype level for the HPV-positive samples (online supplemental table 1A). One hundred sixty-four samples were HPV positive in all three sample specimens, and in 141 of those (86%), identical HPV genotype results were found. When calculating the concordance, the highest concordance was present between SCV and urine samples (95%), and lowest concordance was between urine and CS (87%).
bmjopen-2020-041512supp001.pdf (168.3KB, pdf)
In regard to the 23 discrepant samples, results with more than one HPV genotype detected were found in 17 of the urine samples, 12 in the SCV and only 7 in the CS (online supplemental table 1B).
Questionnaire
Three hundred forty-six women filled out a questionnaire with five questions regarding the use of self-sampling (table 4). A very high percentage of the women (99% within urine sampling and 98% within Evalyn Brush for vaginal sampling) found the instructions for sample collection very easy or fairly easy to understand and the majority (96 %) also found the samples suitable for self-collection.
Table 4.
Results of questionnaire
| No. | Question | No. | Very easy (%) | Fairly easy (%) | A little difficult (%) | Difficult (%) | Do not know (%) |
| 1 | Was the instruction on the urine sample easy to understand? | 339 | 90 | 9 | 1 | 0.3 | 1 |
| 2 | Was the instruction on the Evalyn Brush easy to understand? | 345 | 83 | 15 | 2 | 0 | 1 |
| Urine sample (%) | Evalyn Brush (%) | No preference (%) | |||||
| 3 | Which method would you prefer, if they were equally precise? | 344 | 33 | 17 | 50 | ||
| 4 | Which method do you think women, who do not participate in the screening programme, would prefer? | 323 | 64 | 14 | 23 | ||
| No (%) | Yes (%) | Evalyn Brush (%) | Urine (%) | Not indicated (%) | |||
| 5 | Do you think any of the tests are unsuitable for self-collection? | 323 | 96 | 4 | 2,5 | 1,2 | 1,2 |
No, number of women who have answered the questionnaire.
Seventeen per cent of the women preferred the Evalyn Brush, 33% the urine sample, whereas 50% had no preference for either of the two self-sampling methods.
However, when asked about the expected preference for women, who were not attending the screening programme, the majority (64%) of the women responded that a urine sample was expected to be the preferred method.
Discussion
In the search for strategies to reach non-attendees in the screening programme for cervical cancer, several studies indicate that the use of self-collected samples may be a desirable option to increase participation.30 31 35
In the present study, we have compared the absolute and relative sensitivity and specificity of two self-collected specimens and a clinician-taken CS to detect high-grade cervical intraepithelial lesions (CIN2+/CIN3+). The results show that both urine and vaginal self-collected sampling are non-inferior to the clinician-taken CS in order to identify CIN2+/CIN3+. These results are very promising and are in alignment with other studies, where the Cobas HPV assay has been used for HPV testing.20 36 37 Our results derive from a cross-sectional diagnostic test accuracy study where women were enrolled from three colposcopy clinics in the Region of Southern Denmark. The advantage of enrolling a referral population is that the samples, including cervical biopsies, are collected at the same visit. A meta-analysis by Arbyn et al conclude that the relative accuracy of HR-HPV testing is not affected by the clinical setting,14 31 38 and the referral setting is quite efficient given the great power to assess relative sensitivity questions, the high prevalence of CIN2+ in a referral population and the absence of partial verification bias.
In the present study, the majority of women identified urine as the sampling method of choice, especially when asked about the expected preference for women not attending the screening programme. The preference of the women towards urine as the method of choice was also reported in the study by Sellors et al.16 Therefore, urine is an attractive option on self-sampling, but it still holds several challenges in standardisations as test material. One of the major issues is the need for preservation to prevent invalid quantitative PCR results. Different preservation solutions and collection methods have been tested to solve this issue.21 23 39 In our study, preservation has been optimised by adding 8 mL of EDTA to the collection tube before adding up to 40 mL of urine (one full collection), all kept at room temperature. This is a cheap and easy solution, which is different from a number of other studies, where for instance first-void urine was collected.25 26 In this present study, we had no invalid urine samples. However, before wider use of urine samples for HPV testing and screening it is necessary to standardise the preservation liquid, and to make the tubes suitable for transport (avoid spill).
Even though there is no statistical difference between the three sample specimens, minor differences appeared. The concordance between the SCV, urine sample and clinician-taken CS were comparable and high, whereas the concordance between the urine sample and clinician-taken CS was slightly lower. The difference was most evident in women with normal and CIN1 histology. In other studies, similar concordance between urine and clinician-taken CS has been reported, ranging from 84% to 88%.37 40 41 Still, this observation should be considered and evaluated in a screening population, as this may affect the specificity of the test.
We found more infections with more than one HPV genotype in the self-collected vaginal and urine samples compared with CS (17% and 33% more infections, respectively). This is an interesting observation, which may be caused by the fact that the samples are collected from different anatomic locations.
A limitation of this study was that the women did not collect the samples at home. However, the women did collect the samples by themselves in privacy after receiving only written information. Also, according to the questionnaire, the vast majority of the women found the manual for sampling to be very clear, indicating that the sampling procedure was not difficult for them. Therefore, it seems reasonable to anticipate that in a screening setting the women would also be able to collect the samples at home.
The high sensitivity of self-collected specimens for HPV testing in our and other studies supports initiatives to implement self-sampling as an option to reach non-attendees in the screening population. It also holds promise for a safe alternative to a clinician-taken CS for all women attending the screening programme for cervical cancer. Studies have been published showing that self-sampling is a more effective alternative for recruiting non-attendees in current screening programmes than the routine invitations to have cervical specimen taken.10 31 42 Self-sampling may also prove to be cost-effective in terms of reduced morbidity and mortality among the non-attendees.43 However, increased participation and willingness to attend follow-up at the general practitioner or by a gynaecologist after an HPV-positive self-sample, rely on further education about cervical cancer and its precursors (CIN).44 45 This is demonstrated by the fact that the incidence of CIN2 or worse is reported to be higher among non-attendees than in the screening population.42 46
As this study was a feasibility and safety study on the combination of test method and sample specimen, cost-effectiveness was not examined. Still, it is an important factor to clarify in a screening setting. It is concurrently necessary to evaluate the potential impact of different self-sampling strategies (opt-in, opt-out, mail, etc),47–49 as this may affect the participation rate and thereby the overall cost-effectiveness.30 31
Conclusion
In conclusion, this study shows that SCV as well as urine samples have high sensitivities, comparable specificities and they are non-inferior to clinician-taken CS for HPV testing using the Cobas HPV assay in detection of CIN2+/CIN3+. Accordingly, both sample specimens are considered safe alternatives and options in recruitment of non-attendees in the screening programme. Even though most women prefer urine sampling, this method needs to be standardised and made suitable for transportation and therefore further studies are needed.
Supplementary Material
Acknowledgments
The authors would like to thank all the women, who participated in the study. The authors would also like to thank the technicians involved in the laboratory work, and the Region of Southern Denmark for funding the project.
Footnotes
Contributors: DØ performed HPV testing, data interpretation and first draft of the manuscript. MW was the project manager and performed data interpretation. KJ, PHS, IMG and AWL were responsible for inclusion of the women and the questionnaire. All authors participated in the study design and reviewed the last version of the manuscript.
Funding: This project was supported by the Region of Southern Denmark.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the Ethical Committee of the Region of Southern Denmark (S-20160127) and the Danish Data Protection Agency (18/33083). All of the invited women received written information about the study before visiting the hospital, and only women with a signed informed consent were enrolled in the study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. Data are available and will be shared on reasonable request.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Lynge E. Self-collected versus clinician-collected samples for HPV testing. Lancet Oncol 2019;20:170–1. 10.1016/S1470-2045(18)30934-3 [DOI] [PubMed] [Google Scholar]
- 2.Vaccarella S, Franceschi S, Engholm G, et al. 50 years of screening in the Nordic countries: quantifying the effects on cervical cancer incidence. Br J Cancer 2014;111:965–9. 10.1038/bjc.2014.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kjær SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ASCUS/LSIL, HSIL, or cervical cancer: what is the potential for prevention?. Cancer Causes Control 2014;25:179–89. 10.1007/s10552-013-0320-z [DOI] [PubMed] [Google Scholar]
- 4.Dugué P-A, Lynge E, Bjerregaard B, et al. Non-participation in screening: the case of cervical cancer in Denmark. Prev Med 2012;54:266–9. 10.1016/j.ypmed.2012.01.012 [DOI] [PubMed] [Google Scholar]
- 5.Danish Quality Database for Cervical Cancer Screening (DKLS) . Danish quality database for cervical cancer screening, annual report 2017. 201. Aarhus: DKLS, 2017. [Google Scholar]
- 6.Espersen MM, Holten IW. [Barriers in screening for cervical cancer]. Ugeskr Laeger 2005;167:4371–4. [PubMed] [Google Scholar]
- 7.Oscarsson MG, Benzein EG, Wijma BE. Reasons for non-attendance at cervical screening as reported by non-attendees in Sweden. J Psychosom Obstet Gynaecol 2008;29:23–31. 10.1080/01674820701504619 [DOI] [PubMed] [Google Scholar]
- 8.Waller J, Bartoszek M, Marlow L, et al. Barriers to cervical cancer screening attendance in England: a population-based survey. J Med Screen 2009;16:199–204. 10.1258/jms.2009.009073 [DOI] [PubMed] [Google Scholar]
- 9.Bosgraaf RP, Ketelaars PJW, Verhoef VMJ, et al. Reasons for non-attendance to cervical screening and preferences for HPV self-sampling in Dutch women. Prev Med 2014;64:108–13. 10.1016/j.ypmed.2014.04.011 [DOI] [PubMed] [Google Scholar]
- 10.Enerly E, Bonde J, Schee K, et al. Self-Sampling for human papillomavirus testing among non-attenders increases attendance to the Norwegian cervical cancer screening programme. PLoS One 2016;11:e0151978. 10.1371/journal.pone.0151978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Virtanen A, Nieminen P, Luostarinen T, et al. Self-sample HPV tests as an intervention for nonattendees of cervical cancer screening in Finland: a randomized trial. Cancer Epidemiol Biomarkers Prev 2011;20:1960–9. 10.1158/1055-9965.EPI-11-0307 [DOI] [PubMed] [Google Scholar]
- 12.Nelson EJ, Maynard BR, Loux T, et al. The acceptability of self-sampled screening for HPV DNA: a systematic review and meta-analysis. Sex Transm Infect 2017;93:56–61. 10.1136/sextrans-2016-052609 [DOI] [PubMed] [Google Scholar]
- 13.Gupta S, Palmer C, Bik EM, et al. Self-Sampling for human papillomavirus testing: increased cervical cancer screening participation and incorporation in international screening programs. Front Public Health 2018;6:77. 10.3389/fpubh.2018.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arbyn M, Verdoodt F, Snijders PJF, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol 2014;15:172–83. 10.1016/S1470-2045(13)70570-9 [DOI] [PubMed] [Google Scholar]
- 15.Arbyn M, Castle PE. Offering self-sampling kits for HPV testing to reach women who do not attend in the regular cervical cancer screening program. Cancer Epidemiol Biomarkers Prev 2015;24:769–72. 10.1158/1055-9965.EPI-14-1417 [DOI] [PubMed] [Google Scholar]
- 16.Sellors JW, Lorincz AT, Mahony JB, et al. Comparison of self-collected vaginal, vulvar and urine samples with physician-collected cervical samples for human papillomavirus testing to detect high-grade squamous intraepithelial lesions. CMAJ 2000;163:513–8. [PMC free article] [PubMed] [Google Scholar]
- 17.Senkomago V, Des Marais AC, Rahangdale L, et al. Comparison of urine specimen collection times and testing fractions for the detection of high-risk human papillomavirus and high-grade cervical precancer. J Clin Virol 2016;74:26–31. 10.1016/j.jcv.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 18.Shin HY, Lee B, Hwang SH, et al. Evaluation of satisfaction with three different cervical cancer screening modalities: clinician-collected Pap test vs. HPV test by self-sampling vs. HPV test by urine sampling. J Gynecol Oncol 2019;30:e76. 10.3802/jgo.2019.30.e76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asciutto KC, Henningsson AJ, Borgfeldt H, et al. Vaginal and urine self-sampling compared to cervical sampling for HPV-testing with the COBAS 4800 HPV test. Anticancer Res 2017;37:4183–7. 10.21873/anticanres.11807 [DOI] [PubMed] [Google Scholar]
- 20.Stanczuk G, Baxter G, Currie H, et al. Clinical validation of hrHPV testing on vaginal and urine self-samples in primary cervical screening (cross-sectional results from the papillomavirus Dumfries and Galloway-PaVDaG study). BMJ Open 2016;6:e010660. 10.1136/bmjopen-2015-010660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vorsters A, Van Keer S, Biesmans S, et al. Long-term follow-up of HPV infection using urine and cervical quantitative HPV DNA testing. Int J Mol Sci 2016;17 10.3390/ijms17050750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahasrabuddhe VV, Gravitt PE, Dunn ST, et al. Evaluation of clinical performance of a novel urine-based HPV detection assay among women attending a colposcopy clinic. J Clin Virol 2014;60:414–7. 10.1016/j.jcv.2014.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leeman A, Del Pino M, Molijn A, et al. HPV testing in first-void urine provides sensitivity for CIN2+ detection comparable with a smear taken by a clinician or a brush-based self-sample: cross-sectional data from a triage population. BJOG 2017;124:1356–63. 10.1111/1471-0528.14682 [DOI] [PubMed] [Google Scholar]
- 24.Hagihara M, Yamagishi Y, Izumi K, et al. Comparison of initial stream urine samples and cervical samples for detection of human papillomavirus. J Infect Chemother 2016;22:559–62. 10.1016/j.jiac.2016.05.009 [DOI] [PubMed] [Google Scholar]
- 25.Van Keer S, Tjalma WAA, Pattyn J, et al. Human papillomavirus genotype and viral load agreement between paired first-void urine and clinician-collected cervical samples. Eur J Clin Microbiol Infect Dis 2018;37:859–69. 10.1007/s10096-017-3179-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pattyn J, Van Keer S, Biesmans S, et al. Human papillomavirus detection in urine: effect of a first-void urine collection device and timing of collection. J Virol Methods 2019;264:23–30. 10.1016/j.jviromet.2018.11.008 [DOI] [PubMed] [Google Scholar]
- 27.Sargent A, Fletcher S, Bray K, et al. Cross-sectional study of HPV testing in self-sampled urine and comparison with matched vaginal and cervical samples in women attending colposcopy for the management of abnormal cervical screening. BMJ Open 2019;9:e025388. 10.1136/bmjopen-2018-025388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vorsters A, Van den Bergh J, Micalessi I, et al. Optimization of HPV DNA detection in urine by improving collection, storage, and extraction. Eur J Clin Microbiol Infect Dis 2014;33:2005–14. 10.1007/s10096-014-2147-2 [DOI] [PubMed] [Google Scholar]
- 29.Ejegod DM, Pedersen H, Alzua GP, et al. Time and temperature dependent analytical stability of dry-collected Evalyn HPV self-sampling brush for cervical cancer screening. Papillomavirus Res 2018;5:192–200. 10.1016/j.pvr.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tranberg M, Bech BH, Blaakær J, et al. HPV self-sampling in cervical cancer screening: the effect of different invitation strategies in various socioeconomic groups - a randomized controlled trial. Clin Epidemiol 2018;10:1027–36. 10.2147/CLEP.S164826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arbyn M, Smith SB, Temin S, et al. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ 2018;363:k4823. 10.1136/bmj.k4823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vorsters A, Micalessi I, Bilcke J, et al. Detection of human papillomavirus DNA in urine. A review of the literature. Eur J Clin Microbiol Infect Dis 2012;31:627–40. 10.1007/s10096-011-1358-z [DOI] [PubMed] [Google Scholar]
- 33.Nayar R, Wilbur DC. The Pap Test and Bethesda 2014: "The reports of my demise have been greatly exaggerated." (after a quotation from Mark Twain). J Am Soc Cytopathol 2015;4:170–80. 10.1016/j.jasc.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 34.Apgar BS, Zoschnick L, Wright TC. The 2001 Bethesda system terminology. Am Fam Physician 2003;68:1992–8. [PubMed] [Google Scholar]
- 35.Elfström KM, Sundström K, Andersson S, et al. Increasing participation in cervical screening by targeting long-term nonattenders: randomized health services study. Int J Cancer 2019;145:3033–9. 10.1002/ijc.32374 [DOI] [PubMed] [Google Scholar]
- 36.Ketelaars PJW, Bosgraaf RP, Siebers AG, et al. High-risk human papillomavirus detection in self-sampling compared to physician-taken smear in a responder population of the Dutch cervical screening: results of the vera study. Prev Med 2017;101:96–101. 10.1016/j.ypmed.2017.05.021 [DOI] [PubMed] [Google Scholar]
- 37.Bernal S, Palomares JC, Artura A, et al. Comparison of urine and cervical samples for detecting human papillomavirus (HPV) with the COBAS 4800 HPV test. J Clin Virol 2014;61:548–52. 10.1016/j.jcv.2014.10.001 [DOI] [PubMed] [Google Scholar]
- 38.Arbyn M, Peeters E, Benoy I, et al. VALHUDES: a protocol for validation of human papillomavirus assays and collection devices for HPV testing on self-samples and urine samples. J Clin Virol 2018;107:52–6. 10.1016/j.jcv.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 39.Cuzick J, Cadman L, Ahmad AS, et al. Performance and diagnostic accuracy of a urine-based human papillomavirus assay in a referral population. Cancer Epidemiol Biomarkers Prev 2017;26:1053–9. 10.1158/1055-9965.EPI-16-0960 [DOI] [PubMed] [Google Scholar]
- 40.Burroni E, Bonanni P, Sani C, et al. Human papillomavirus prevalence in paired urine and cervical samples in women invited for cervical cancer screening. J Med Virol 2015;87:508–15. 10.1002/jmv.24085 [DOI] [PubMed] [Google Scholar]
- 41.Stanczuk GA, Currie H, Baxter G, et al. Cobas 4800 HPV detection in the cervical, vaginal and urine samples of women with high-grade CIN before and after treatment. J Clin Pathol 2015;68:567–70. 10.1136/jclinpath-2014-202851 [DOI] [PubMed] [Google Scholar]
- 42.Lam JUH, Elfström KM, Ejegod DM, et al. High-grade cervical intraepithelial neoplasia in human papillomavirus self-sampling of screening non-attenders. Br J Cancer 2018;118:138–44. 10.1038/bjc.2017.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vassilakos P, Poncet A, Catarino R, et al. Cost-effectiveness evaluation of HPV self-testing offered to non-attendees in cervical cancer screening in Switzerland. Gynecol Oncol 2019;153:92–9. 10.1016/j.ygyno.2019.01.021 [DOI] [PubMed] [Google Scholar]
- 44.Musa J, Achenbach CJ, O'Dwyer LC, et al. Effect of cervical cancer education and provider recommendation for screening on screening rates: a systematic review and meta-analysis. PLoS One 2017;12:e0183924. 10.1371/journal.pone.0183924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andersson S, et al. Acceptance of self-sampling among long-term cervical screening non-attenders with HPV-positive results: promising opportunity for specific cancer education. J Cancer Educ 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gök M, Heideman DAM, van Kemenade FJ, et al. Offering self-sampling for human papillomavirus testing to non-attendees of the cervical screening programme: characteristics of the responders. Eur J Cancer 2012;48:1799–808. 10.1016/j.ejca.2011.11.022 [DOI] [PubMed] [Google Scholar]
- 47.Broberg G, Gyrd-Hansen D, Miao Jonasson J, et al. Increasing participation in cervical cancer screening: offering a HPV self-test to long-term non-attendees as part of RACOMIP, a Swedish randomized controlled trial. Int J Cancer 2014;134:2223–30. 10.1002/ijc.28545 [DOI] [PubMed] [Google Scholar]
- 48.Giorgi Rossi P, Fortunato C, Barbarino P, et al. Self-sampling to increase participation in cervical cancer screening: an RCT comparing home mailing, distribution in pharmacies, and recall letter. Br J Cancer 2015;112:667–75. 10.1038/bjc.2015.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verdoodt F, Jentschke M, Hillemanns P, et al. Reaching women who do not participate in the regular cervical cancer screening programme by offering self-sampling kits: a systematic review and meta-analysis of randomised trials. Eur J Cancer 2015;51:2375–85. 10.1016/j.ejca.2015.07.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-041512supp001.pdf (168.3KB, pdf)