Abstract
Background & Aims:
Nonalcoholic fatty liver disease is common in HIV, but there are no approved therapies. The aim of this open-label proof-of-concept study was to determine the effect of the mineralocorticoid receptor antagonist eplerenone on hepatic fat in HIV-infected patients with hepatic fat ≥ 5% by magnetic resonance spectroscopy.
Methods:
Five subjects received eplerenone (25 mg daily × 1 week followed by 50 mg daily × 23 weeks). Laboratory tests were done at each visit, and the primary endpoint, change in hepatic fat content, was determined by MRI spectroscopy at baseline and week 24.
Results:
The study was stopped early after observing unexpected significant increases in hepatic fat at week 24 (mean increase 13.0±7.3%, p=0.02). The increases in steatosis were accompanied by a tendency for transaminase values to decrease (ALT mean change −14 ± 16 IU/L, p=0.14). There were no consistent changes in other metabolic parameters or blood pressure. Repeat assessment of hepatic steatosis 1–2 months after stopping study medication revealed improvements in steatosis towards baseline values.
Conclusions:
The unexpected observation of increased hepatic steatosis with administration of eplerenone led to early termination of the investigation. While limited due to the small number of participants and the open-label design, the present study provides data to suggest that mineralocorticoid receptor antagonism with eplerenone may not be an effective approach to treat hepatic steatosis in HIV or the general population. Additional research is needed to determine the pathophysiologic mechanism behind these unanticipated observations.
Keywords: NAFLD, HIV, MR antagonist, hepatic steatosis
Introduction
Metabolic disturbances including insulin resistance, diabetes, and dyslipidemia are increased among persons living with HIV infection and confer an increased risk of cardiovascular disease. In particular, visceral adipose tissue (VAT) is increased in HIV-infected patients compared to non-infected controls1 and is associated with cardiac risk factors such as decreased insulin sensitivity2,3 and increased systemic inflammatory markers.4–6 Further, excess VAT stores are closely associated with both cardiac and hepatic steatosis in HIV-infected individuals, thus supporting a pathophysiologic role of central adiposity in the metabolic disturbances observed in HIV infection.7,8
In non–HIV-infected individuals, data have shown that aldosterone, a known mineralocorticoid receptor (MR) agonist, is increased in association with increased VAT and decreased insulin sensitivity.9,10 The role of aldosterone and MR activation linked to excess VAT could offer a novel treatment strategy to target metabolic disturbances, including hepatic steatosis, observed in individuals with visceral fat accumulation.
Promising animal models demonstrate marked reductions in fasting glucose, insulin, hepatic steatosis, and inflammation with MR antagonist targeting the aldosterone axis. Using a murine model of metabolic syndrome and non-alcoholic steatohepatitis, Wada and colleagues showed that MR blockade with eplerenone resulted in improvements in hepatic triglyceride content, liver transaminase levels, circulating triglycerides, and measures of insulin resistance.11 Additional murine studies have shown decreased hepatic fat, reduced inflammation, or improved insulin resistance after treatment using an MR antagonist to block aldosterone binding.12–14
While rodent models suggest a physiologic role for MR blockade with eplerenone to improve metabolic parameters and hepatic steatosis, the benefits of this approach in humans remain to be determined. We therefore examined the effects of the MR antagonist eplerenone on hepatic and cardiac steatosis in HIV-infected adults with hepatic steatosis and abdominal fat accumulation.
Methods:
The aim of this open-label, proof-of-concept study (ClinicalTrials.gov NCT02629094) was to determine the effect of the MR antagonist eplerenone (50 mg/day) on hepatic and cardiac steatosis in HIV-infected participants. Subjects were required to have hepatic steatosis at baseline, defined as hepatic fat ≥5% by magnetic resonance spectroscopy (MRS). Further, participants were required to have a waist circumference >102 cm for men or >88 cm for women, and to be on a stable suppressive antiretroviral regimen for at least three months. Subjects were excluded for uncontrolled hypertension or diabetes, current or recent steroid use, and medications with possible drug interactions with eplerenone or known to cause hyperkalemia, non-stable dose testosterone, estrogen, or progesterone, and growth hormone or growth hormone-releasing hormone. Subjects with history of substance abuse, including excessive alcohol consumption, were excluded. Finally, subjects were excluded for co-infection with Hepatitis C virus, other serious infections, history of a serious cardiovascular event, or pregnancy. All subjects provided written informed consent and the protocol was approved by the National Institute of Allergy and Infectious Diseases’ Institutional Review Board.
Subjects were evaluated at outpatient visits at baseline and 1, 2, 4, 8, 12, 18, and 24 weeks. The original primary endpoints, change in hepatic fat content and cardiac steatosis, were determined by MRS7 at baseline and week 24. All MRS scans were performed in the same scanner and interpreted by the same radiologist. Participants were instructed to fast for six hours before each appointment, and the majority of scans were completed before noon. Baseline and week 24 visits included anthropometric measurements, a whole body DEXA scan, transient elastography with FibroScan (Echosens, Paris, France), and an optional liver biopsy. VAT was calculated by DEXA scan at baseline and week 24.
Five subjects received eplerenone at 25 mg daily for one week, followed by an increase to 50 mg daily for 23 weeks. The study was terminated early, after enrollment of only five subjects, due to unexpected observations in hepatic fat content. Participants were invited to return 1–2 months following discontinuation of eplerenone for a follow-up MRS off eplerenone therapy. Stored samples for every time point were used for determinations of aldosterone, free fatty acids, renin, and inflammatory markers.
Paired t-tests were performed for comparisons, and all analyses were completed using JMP software (Version 13.0, SAS Inc., Cary, NC) and Microsoft Excel (Version 15.39, Microsoft Corporation, Redmond, WA).
Results:
The baseline characteristics of the five enrolled subjects are presented in Table 1. Participants had a median age of 60 years (range 37 – 62) and two subjects were women. The group was overweight with a median baseline BMI of 32.3 kg/m2 (range 27.8 – 42.8) and median waist circumference of 116.4 cm (range 104.5 – 131). The median CD4 T-cell count was 860 cells/mL (range 422 – 1341). All subjects tolerated the initial 25 mg/day dose of eplerenone and increased to 50 mg/day after one week.
Table 1.
Individual Demographic Characteristics with Baseline and Week 24 Clinical Measurements
| Baseline Characteristics | Change in Clinical Characteristics | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Age (y) | Sex | CD4 (cells/ mL) | ARVs | BMI (kg/m2) | Heart Fat (%) | Liver Fat (%) | BP (mmHg) | Aldosterone (ng/dL) | ALT (U/L) | AST (U/L) | |||||||
| BSL | Wk 24 | BSL | Wk 24 | BSL | Wk 24 | BSL | Wk 24 | BSL | Wk 24 | BSL | Wk 24 | BSL | Wk 24 | |||||
| 1 | 61 | M | 860 | ABC/3TC/ DTG | 30.6 | 30.3 | 0.6 | 0 | 8 | 20 | 133/69 | 122/79 | 10.1 | 20.4 | 26 | 25 | 27 | 24 |
| 2 | 37 | F | 1305 | ABC/3TC/ DTG | 34.8 | 34.4 | 8.1 | 0.9 | 35 | 45 | 128/80 | 113/71 | 10.1 | 10.4 | 64 | 21 | 55 | 16 |
| 3 | 62 | M | 422 | ABC/3TC/ DTG | 27.8 | 27.7 | 0.7 | 4.5 | 9 | 10 | 123/74 | 132/85 | 10.4 | 13.7 | 39 | 26 | 33 | 27 |
| 4 | 50 | F | 1341 | ABC/FTC/ TDF | 42.8 | 43.4 | 1.5 | 4.2 | 5 | 24 | 149/76 | 110/74 | 9.9 | 106 | 41 | 27 | 39 | 31 |
| 5 | 60 | M | 759 | ABC/3TC/ DTG | 32.3 | 33.4 | 1.5 | 1.1 | 10 | 32 | 144/86 | 144/89 | 6.4 | 7.5 | 45 | 45 | 43 | 41 |
| Total, Median | 60 | - | 860 | - | 32.3 | 33.4 | 1.5 | 1.1 | 8.6 | 24 | 133/76 | 122/79 | 10.1 | 13.7 | 41 | 26 | 39 | 27 |
BSL = Baseline, ABC= abacavir, 3TC = lamivudine, DTG = dolutegravir, FTC = emtricitabine, TDF=tenofovir
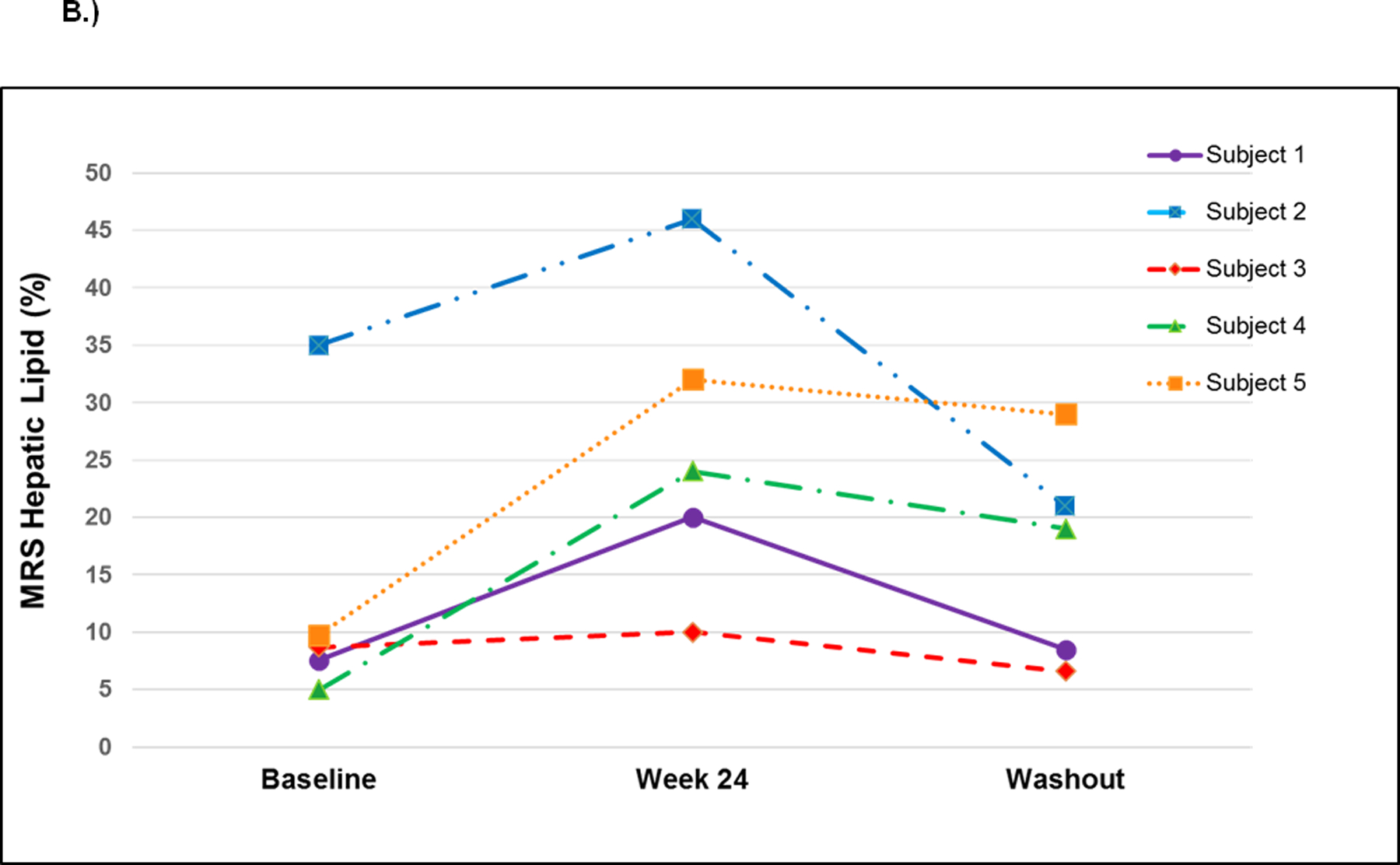
We observed unexpected significant increases in hepatic fat at week 24 for all participants (mean increase 13.0 ± 7.3%, p=0.02) (Figure 1). In the two participants with pre- and post-eplerenone liver biopsies, steatosis grade increased, confirming the observations quantifying hepatic fat made with MR spectroscopy. Both participants had grade 1 steatosis at baseline, and increased to grade 2 and 3, respectively, at week 24. Surprisingly, the increases in steatosis were accompanied by a tendency for transaminase values to decrease: ALT mean change was −14 ± 16 IU/L (p=0.14) and AST mean change was −12 ± 14 IU/L (p=0.17). One participant’s liver biopsy fibrosis score decreased from 1 to 0; the second subject had no change in fibrosis (0 at both time points). All participants had transient elastography at baseline and week 24: there was no significant change in liver stiffness (mean change 0.46 ± 2.6 kPa, p=0.74). We observed an initial rise in aldosterone from baseline to week 4 for all participants; however, serum aldosterone levels were not increased significantly at week 24 compared to baseline (mean increase 22.2 ± 37.1 ng/dL, p=0.30). One subject had an extreme increase in aldosterone, from 9.9 to 106 ng/dL at the week 24 visit on eplerenone, however, this subject did receive several doses of progesterone (10mg) from an outside provider at week 14, which may have affected aldosterone levels. Renin values tended to increase from baseline to week 24, but the change was not statistically significant (mean increase 4.10 ± 6.98 ng/mL/hr, p=0.45). Systolic blood pressure tended to decrease (mean change −11.2 ± 16.3 mmHg, p=0.24), with minimal change in diastolic blood pressure (mean change 2.6 ± 7.5 mmHg, p=0.53).
Figure 1.
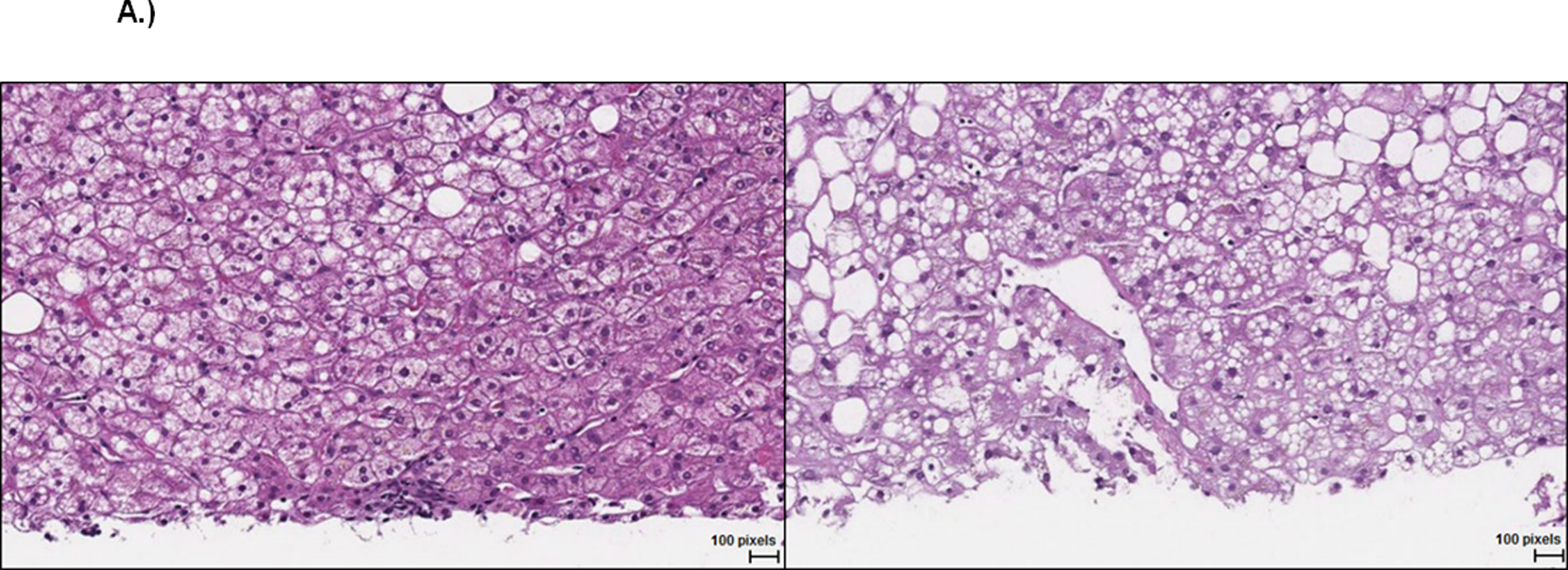
A.) Representative liver histopathology (H&E stain) for subject 2 at baseline (left, steatosis grade 1) and week 24 on eplerenone (right, steatosis grade 3).
B.) Hepatic lipid content changes by MR spectroscopy in each participant at baseline, after 24 weeks on eplerenone (paired t-test between baseline and week 24, p=0.02) and washout (1–2 months after termination of study drug - paired t-test between week 24 and washout, p=0.08).
There was a non-significant increase in VAT as estimated by DEXA, with mean change of 101 ± 153 mL (p=0.26). One participant had a decrease of −163 mL VAT at week 24 and this participant had the smallest increase in hepatic fat; all other participants demonstrated increases in visceral fat. Waist to hip ratio did not change significantly at week 24 (mean change −0.02 ± 0.02, p=0.15), although participants had significant decreases in waist circumferences (mean change −3.14 ± 1.51 cm, p=0.01). We observed a mean decrease in sex hormone binding globulin (SHBG) at week 24, though the change was not statistically significant (mean −14.2 ± 12.0 nmol/L, p=0.08). There were no significant alterations in potassium, creatinine, HbA1c, fasting glucose, total cholesterol, triglycerides, free fatty acids, or CD4 count at week 24.
Hepatic fat content tended to return to baseline levels during the ‘washout’ period (mean change from week 24 to follow-up −9.4 ± 7.9%, p=0.08). One participant had a liver biopsy only at baseline and at the washout period, both of which showed grade 1 steatosis. There was a non-significant decrease in aldosterone at the washout (mean −20.9 ± 38.4, p=0.4), which may have been skewed by the participant who had an extremely high week 24 aldosterone returning to a normal aldosterone level.
Discussion:
It is well established that HIV-infected individuals are at higher risk for developing metabolic, cardiovascular, and body composition abnormalities; however, there is currently no standard of care to address many of these conditions. Mounting evidence from animal models suggests that the use of MR antagonists, such as eplerenone, may represent a novel treatment approach for hepatic steatosis and other related metabolic comorbidities.
The current study presents evidence to reconsider MR antagonists for attenuation of non-alcoholic fatty liver disease (NAFLD) in HIV-infected patients or the general population. The unexpected observation of increased hepatic steatosis with administration of eplerenone led to early termination of the investigation. While our study was small with only five participants in an open-label design, the results were surprising and clinically significant after only 24 weeks of drug administration. The potential harmful effect of eplerenone on hepatic steatosis occurred without associated increases in liver transaminases, suggesting that patients currently taking eplerenone for clinical indications (i.e. hypertension and congestive heart failure in patients with prior myocardial infarction) may be inducing increases in hepatic fat content without corresponding changes in AST or ALT.
We observed improvements in liver transaminase levels with eplerenone, similar to observations by Wada and colleagues using a murine model of non-alcoholic steatohepatitis (NASH) with eplerenone.11 This model also demonstrated decreased levels of triglycerides and glucose, whereas our study showed no improvements in either parameter. Pizarro et al. showed significantly lower steatosis and fibrosis scores in mice treated with eplerenone compared to controls.13 In contrast, the two participants in our study who had liver biopsies demonstrated worsening steatosis scores and no change in fibrosis after 24 weeks on eplerenone. Of note, compared to other therapies that intervene more proximally in the renin-angiotensin-aldosterone system, MR blockade with eplerenone may increase circulating renin, angiotensin II, and aldosterone. Effects of these hormones that are independent of the MR may be mechanisms by which eplerenone increased liver fat content. A recent large cohort study showed an association between increased aldosterone and fatty liver in black women, demonstrating that perturbations in the renin-angiotensin-aldosterone system likely affect hepatic steatosis.15 Regardless of mechanism, this pilot study provides preliminary data suggesting that the pathophysiologic benefits of MR antagonism seen in animal models may not translate directly into humans.
Increased waist circumference and VAT have both been shown to correlate with a higher prevalence of hepatic steatosis in HIV.8,16 Though not statistically significant, VAT increased with eplerenone and may therefore explain in part the increases observed in hepatic steatosis. Further, the only laboratory test changes which seemed to signal the increases in hepatic steatosis were the decreases in SHBG levels in four of the five participants. Low levels of SHBG are associated with fatty liver disease17 and may serve as a serologic marker representative of changes in hepatic fat in this context.
In a recent clinical trial evaluating the effects of spironolactone on fatty liver, Polyzos et al. found decreased NAFLD liver fat scores in participants who received spironolactone plus vitamin E compared to vitamin E alone.18 This study did not measure change in hepatic fat content by MRS or biopsy, but used NAFLD liver fat score developed by Kotronen and colleagues19 which uses weighted values for presence of metabolic syndrome, type 2 diabetes, fasting insulin, fasting AST, and AST/ALT ratio to predict presence of NAFLD. The combination of spironolactone and Vitamin E resulted in significant reductions in weight, BMI, and blood pressure and therefore may have reduced NAFLD liver fat score without impacting liver fat content. Further, in a recent study assessing the accuracy of non-invasive indices to quantify longitudinal changes in hepatic fat content, Keating and colleagues found that changes in NAFLD liver fat score were not associated with changes in hepatic fat measured by MRS.20 In contrast, our study used MRS data to directly observe changes in fatty liver for participants with NAFLD over time while taking eplerenone and during a washout period following eplerenone exposure.
In summary, the unexpected observation of increased hepatic steatosis with administration of eplerenone led to early termination of the investigation. While this study is limited due to the small number of participants and the open-label design, we provide data to suggest that MR antagonism with eplerenone may not be a reasonable approach to treat NAFLD in HIV or in the general population. Additional research is needed to determine the pathophysiologic mechanism behind these unanticipated observations.
Key Points.
Rodent models suggest a role for mineralocorticoid receptor blockade to improve metabolic parameters and hepatic steatosis; the benefits of this approach in humans is undetermined.
We aimed to determine effects of 24 weeks of MR antagonist eplerenone on hepatic fat in HIV patients measured by MRI spectroscopy.
The study was stopped early after unexpected significant increases in hepatic fat at week-24 without associated laboratory value changes.
While data show spironolactone may be an effective MR antagonist, this study provides compelling data suggesting MR antagonist eplerenone is not a reasonable approach to treat fatty liver in HIV or the general population.
Financial Support:
This work was supported by the Intramural Research program of the National Institute of Allergy and Infectious Diseases.
Abbreviations:
- HIV
human immunodeficiency virus
- NAFLD
non-alcoholic fatty liver disease
- MR
mineralocorticoid receptor
- MRS
magnetic resonance spectroscopy
- VAT
visceral adipose tissue
- ALT
alanine amino transferase
- AST
aspartate aminotransferase
- BMI
body mass index
- NASH
non-alcoholic steatohepatitis
- SHBG
sex hormone binding globulin
Footnotes
Conflicts of Interest: No authors have any conflicts of interest to disclose.
Clinical Trial: NCT02629094
References:
- 1.Joy T, Keogh HM, Hadigan C, et al. Relation of body composition to body mass index in HIV-infected patients with metabolic abnormalities. J Acquir Immune Defic Syndr. 2008;47(2):174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grunfeld C, Rimland D, Gibert CL, et al. Association of upper trunk and visceral adipose tissue volume with insulin resistance in control and HIV-infected subjects in the FRAM study. J Acquir Immune Defic Syndr. 2007;46(3):283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He Q, Engelson ES, Ionescu G, Glesby MJ, Albu JB, Kotler DP. Insulin resistance, hepatic lipid and adipose tissue distribution in HIV-infected men. Antivir Ther. 2008;13(3):423–428. [PMC free article] [PubMed] [Google Scholar]
- 4.Dolan SE, Hadigan C, Killilea KM, et al. Increased cardiovascular disease risk indices in HIV-infected women. Journal of acquired immune deficiency syndromes. 2005;39(1):44–54. [DOI] [PubMed] [Google Scholar]
- 5.Samaras K, Gan SK, Peake PW, Carr A, Campbell LV. Proinflammatory markers, insulin sensitivity, and cardiometabolic risk factors in treated HIV infection. Obesity. 2009;17(1):53–59. [DOI] [PubMed] [Google Scholar]
- 6.Reingold J, Wanke C, Kotler D, et al. Association of HIV infection and HIV/HCV coinfection with C-reactive protein levels: the fat redistribution and metabolic change in HIV infection (FRAM) study. Journal of acquired immune deficiency syndromes. 2008;48(2):142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thiara DK, Liu CY, Raman F, et al. Abnormal Myocardial Function Is Related to Myocardial Steatosis and Diffuse Myocardial Fibrosis in HIV-Infected Adults. J Infect Dis. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price JC, Seaberg EC, Latanich R, et al. Risk factors for fatty liver in the Multicenter AIDS Cohort Study. Am J Gastroenterol. 2014;109(5):695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg R, Hurwitz S, Williams GH, Hopkins PN, Adler GK. Aldosterone production and insulin resistance in healthy adults. The Journal of clinical endocrinology and metabolism. 2010;95(4):1986–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodfriend TL, Kelley DE, Goodpaster BH, Winters SJ. Visceral obesity and insulin resistance are associated with plasma aldosterone levels in women. Obes Res. 1999;7(4):355–362. [DOI] [PubMed] [Google Scholar]
- 11.Wada T, Miyashita Y, Sasaki M, et al. Eplerenone ameliorates the phenotypes of metabolic syndrome with NASH in liver-specific SREBP-1c Tg mice fed high-fat and high-fructose diet. Am J Physiol Endocrinol Metab. 2013;305(11):E1415–1425. [DOI] [PubMed] [Google Scholar]
- 12.Wada T, Kenmochi H, Miyashita Y, et al. Spironolactone improves glucose and lipid metabolism by ameliorating hepatic steatosis and inflammation and suppressing enhanced gluconeogenesis induced by high-fat and high-fructose diet. Endocrinology. 2010;151(5):2040–2049. [DOI] [PubMed] [Google Scholar]
- 13.Pizarro M, Solis N, Quintero P, et al. Beneficial effects of mineralocorticoid receptor blockade in experimental non-alcoholic steatohepatitis. Liver Int. 2015;35(9):2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gamliel-Lazarovich A, Raz-Pasteur A, Coleman R, Keidar S. The effects of aldosterone on diet-induced fatty liver formation in male C57BL/6 mice: comparison of adrenalectomy and mineralocorticoid receptor blocker. Eur J Gastroenterol Hepatol. 2013;25(9):1086–1092. [DOI] [PubMed] [Google Scholar]
- 15.Kumar A, Blackshear C, Subauste JS, Esfandiari NH, Oral EA, Subauste AR. Fatty Liver Disease, Women, and Aldosterone: Finding a Link in the Jackson Heart Study. J Endocr Soc. 2017;1(5):460–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guaraldi G, Squillace N, Stentarelli C, et al. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin Infect Dis. 2008;47(2):250–257. [DOI] [PubMed] [Google Scholar]
- 17.Flechtner-Mors M, Schick A, Oeztuerk S, et al. Associations of fatty liver disease and other factors affecting serum SHBG concentrations: a population based study on 1657 subjects. Horm Metab Res. 2014;46(4):287–293. [DOI] [PubMed] [Google Scholar]
- 18.Polyzos SA, Kountouras J, Mantzoros CS, Polymerou V, Katsinelos P. Effects of combined low-dose spironolactone plus vitamin E vs vitamin E monotherapy on insulin resistance, non-invasive indices of steatosis and fibrosis, and adipokine levels in non-alcoholic fatty liver disease: a randomized controlled trial. Diabetes Obes Metab. 2017;19(12):1805–1809. [DOI] [PubMed] [Google Scholar]
- 19.Kotronen A, Peltonen M, Hakkarainen A, et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137(3):865–872. [DOI] [PubMed] [Google Scholar]
- 20.Keating SE, Parker HM, Hickman IJ, et al. NAFLD in clinical practice: Can simple blood and anthropometric markers be used to detect change in liver fat measured by (1) H-MRS? Liver Int. 2017;37(12):1907–1915. [DOI] [PubMed] [Google Scholar]


