Abstract
Current technologies and available scaffold materials do not support long-term cell viability, differentiation and maintenance of podocytes, the ultra-specialized kidney resident cells that are responsible for the filtration of the blood. We developed a new platform which imitates the native kidney microenvironment by decellularizing fibroblasts grown on surfaces with macromolecular crowding. Human immortalized podocytes cultured on this platform displayed superior viability and metabolic activity up to 28 days compared to podocytes cultured on tissue culture plastic surfaces. The new platform displayed a softer surface and an abundance of growth factors and associated molecules. More importantly it enabled podocytes to display molecules responsible for their structure and function and a superior development of intercellular connections/interdigitations, consistent with maturation. The new platform can be used to study podocyte biology, test drug toxicity and determine whether sera from patients with podocytopathies are involved in the expression of glomerular pathology.
Keywords: Podocytes, cell derived matrix, decellularization, extracellular matrix, kidney engineering
Graphical Abstract
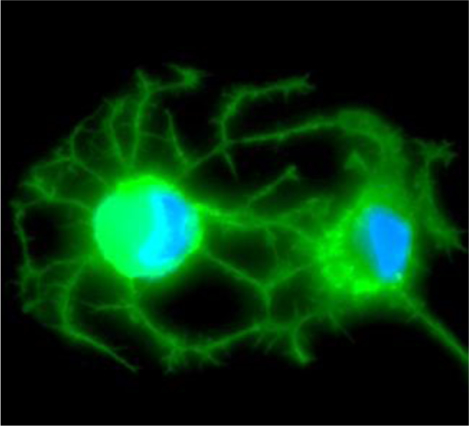
A new culture system based on cell-derived decellularized matrix allows enhanced survival and differentiation of podocytes in vitro. Specifically, fibroblasts are grown in the presence of macromolecular crowding on plates to form monolayers and subsequently they are decellularized. Podocytes survive and differentiate on plates coated with decellularized matrices significantly better than on non-coated plates.
1. Introduction
Biomaterials serve as a scaffolding matrix to construct an in vitro environment and offer a unique niche that best fits cell-specific requirements. Cells sentience, intercommunicate, and react to signals from surrounding matrix and reveal appropriate adhesion, propagation, differentiation, and subsequently gene expression [1]. Stiff two-dimensional (2D) cell culture environments dearth features of the in vivo surroundings, whereas soft and three-dimensional (3D) platforms, such as hydrogel, electrospun membranes, Matrigel® and extracellular matrix (ECM) molecules (collagen, fibrin gels) [2], do not match natural conditions of cell growth and tissue repair [3]. Shortfalls include immunogenicity of the used material, toxicity of degradation products, induction of inflammatory responses, fibrous tissue formation, and mismatch between degradation and new tissue formation [4].
Natural scaffolds constitute of ECM resulted from decellularized tissues or organs and seeded with stem cells in order to regenerate a functional tissue or organ have been utilized to repair skin [5], bladder [6], heart valve [7] and small intestinal submucosa [8]. FDA has approved several commercialized decellularized scaffolds for use in humans [9]. Although decellularized tissue scaffold lacks immunogenic components the process of harsh decellularization may result in denaturation and disturbance of molecular interactions. Conversely, the use of decellularized single cell type culture may yield a fibrillar matrix with predominance of fibronectin and collagen [10]. However, a decellularized ECM has discrete effects on matrix assembly, cell migration, proliferation, signaling, and cellular feedbacks compared to planar protein-coated surfaces [11].
Although various decellularized prototypes have delivered significant insights into matrix directed properties of cells in 3D culture, they do not reiterate the physiology and morphology of many cells, including kidney glomerular cells. Additionally, they lack the complexity of the in vivo basement membrane, in that the ‘matrisome’ of ECM is well-known to be comprised of numerous different proteins and proteoglycans [12]. Moreover, cells in a culture dish require prolonged time to produce sufficient ECM that may jeopardize clinical translation and commercialization. In vivo, both the intracellular environment and the extracellular space are closely filled with numerous biological macromolecules, which create an enormously crowded environment [13]. Segregation of volume by solutes leads to the reduction of the entropy of the crowded milieu and generates an increase in the thermodynamic action of solutes [14]. MMC has been revealed to affect protein folding, shape, structure, conformational strength, enzymatic-activity and protein-protein interaction [15]. Cells are cultured in vitro supplemented with big volumes of diluted aqueous media which do not deliver an adequately crowded physiological atmosphere. MMC, that is the inclusion of polydispersed and inactive macromolecules in culture media [e.g. dextran sulphate (glucose) 500 kDa; carrageenan (galactose-based) 550 kDa (estimated); Ficoll™ (sucrose) cocktail of 70 and 400 kDa], has exerted a positive effect on biological processes and to enhance several-fold ECM deposition in many cell culture systems without changing the cellular physiology [13, 16].
The glomerular epithelial cells, known as podocytes [17], are responsible for the filtration of the blood [18]. They are also frequently the target of injurious insults and the cell origin of various kidney diseases [17]. Therefore, systems which allow the study of their physiology and contribution to disease are needed. Isolation of podocytes from fresh tissues is difficult because their foot processes attach to the glomerular basement membrane (GBM) and hence their study relies on the use of immortalized cells using various cell culture platforms [19]. An ideal culture system should offer macromolecular structures similar to those provided by the GBM so that the cells can express molecules like nephrin, podocin, synaptopodin, catenins and P-cadherin, which determine proper structure and function [20] along with sufficient development of interdigitations between processes similar to those existing in a normal glomerulus [21].
Available 2D and 3D culture systems utilize primarily collagen-based substrates that are unable to mimic native in vivo microenvironment and physiology of podocytes [22] and also the development of cell processes that interdigitate with each other [23]. The use of functional materials as biologic scaffolds comprised of native ECM, derived by methods that encompass the decellularization of cells, tissues or organs, offers new opportunities for kidney tissue engineering research [24].
In this communication, we present a new platform (Figure 1) that involves the combination of MMC and decellularization of seeded fibroblasts to provide a near natural environment for the culture of podocytes. We demonstrate that podocytes cultured using the new method display superior ability to proliferate, differentiate and maintain the native phenotype and physiology.
Figure 1: Culture of podocytes.
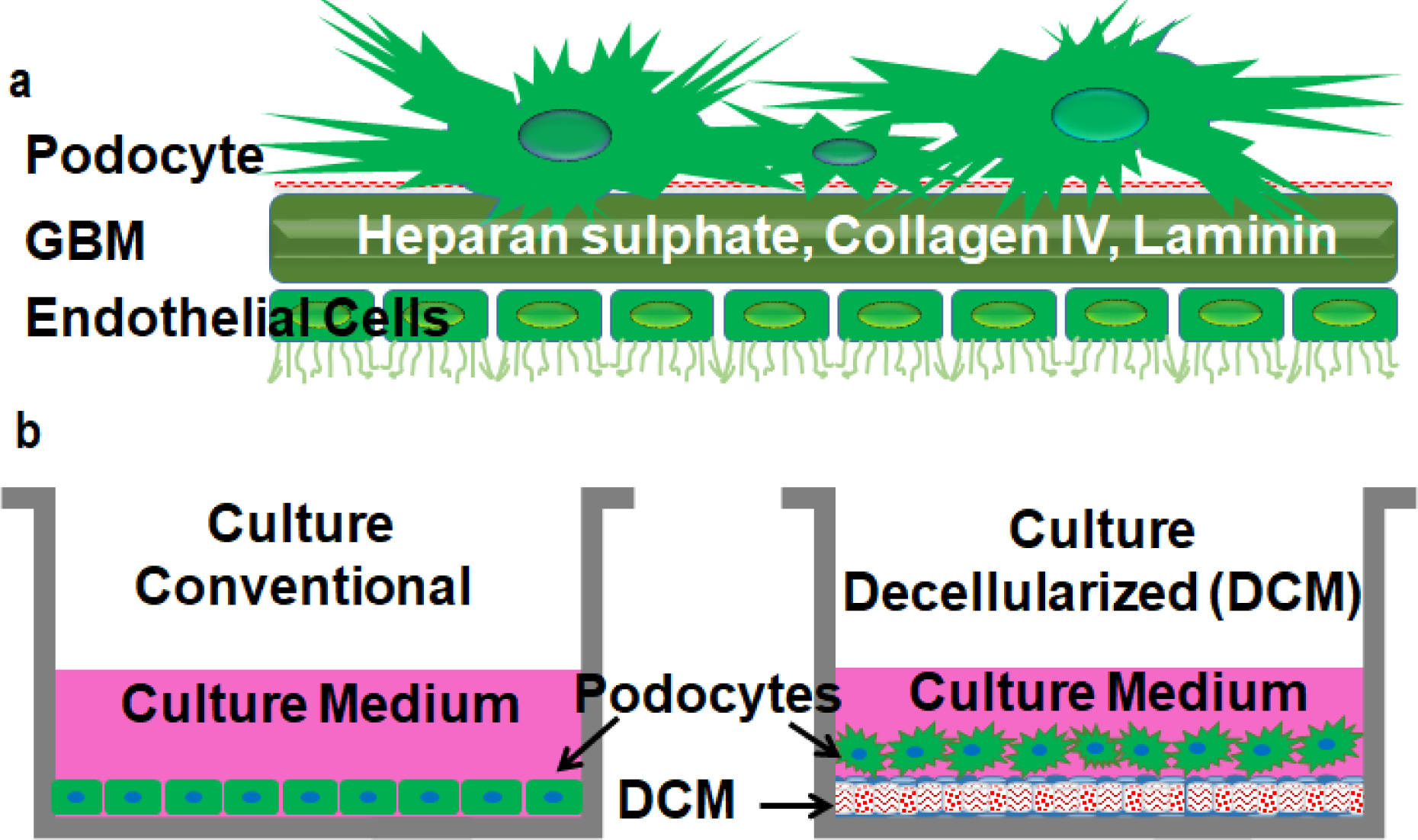
In conventional podocyte culture systems the morphology and physiology of cells are far from physiological conditions (a). Podocytes cultured on cell-derived decellularized matrix (DCM) display interdigitating foot processes and imitate the in vivo environment (b). The DCM contains dense array of extracellular matrix deposited by human fibroblasts.
2. Results
2.1. MMC enhances ECM deposition from fibroblasts
To acquire maximum ECM deposition from human fibroblasts, macromolecular crowders (37.5 mg/mL Ficoll 70 and 25 mg/mL Ficoll 400) were added in the culture media. SDS-PAGE (Figure 2a, densitometric analysis Figure 2b) confirmed that maximum deposition (p<0.0001) of collagen type I was achieved using mix (mixture of 37.5 mg/mL Ficoll 70 and 25 mg/mL Ficoll 400) MMC conditions (supplementary figure S1). alamarBlue® analysis demonstrated that MMC did not affect cell metabolic activity and viability of fibroblasts (Figure 2c and supplementary figure S2a, b).
Figure 2: Podocytes cultured on DCM display better viability.
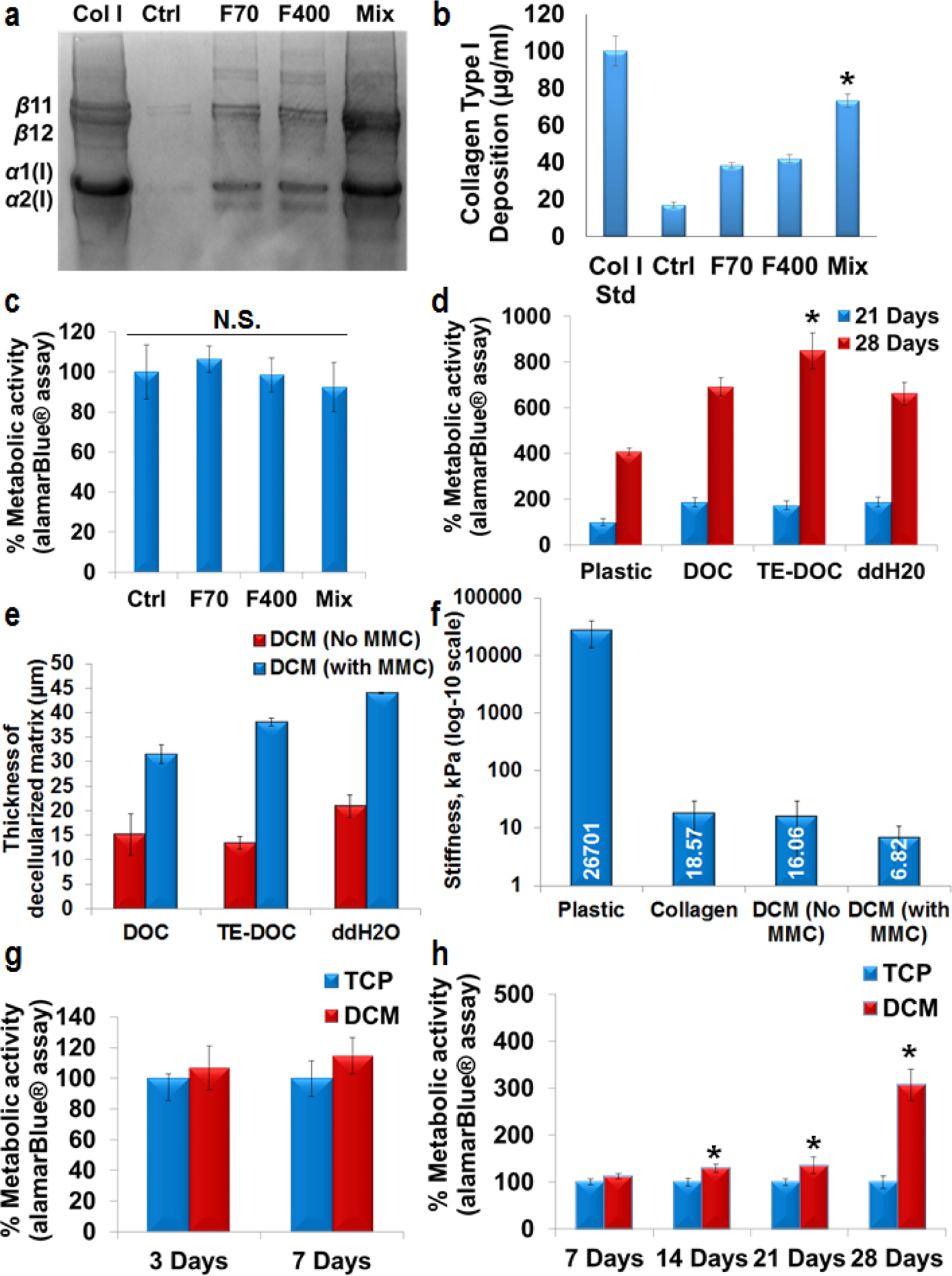
SDS-PAGE (a) and densitometry analysis (n=3) (b) of human skin fibroblasts cultured for 7 days. Maximum (*p<0.0001) collagen type I deposition was achieved under mix [Ficoll (F) 70 + F400] MMC conditions. Col I std – 100 μg/ml collagen type I from rat tail. alamar-Blue® analysis showed that there was no change (N.S. – no significance, p>0.05) in metabolic activity of fibroblasts cultured under MMC conditions (c). Significant increase (p<0.0001) in metabolic activity was observed in all the decellularization conditions in comparison with plastic for 21 days and 28 days. Decellularization solution with TE-DOC supported significantly higher metabolic activity (*p<0.0001, n=3) of podocytes up to 28 days (d). Different decellularization methods did not change the thickness of decellularized matrix. However, MMC significantly enhanced (p<0.0001, n=3) the thickness of decellularized matrix (e, thickness measured by SEM). AFM confirmed the higher stiffness of tissue culture plastic surface compared to collagen or DCM coated surfaces (f). Significant increase (*p<0.0001, n=3) in metabolic activity of podocytes was observed at various culture time (no difference in proliferation at 33° C, g) for differentiation at 37° C (h) and up to 28 days. All of the alamar-Blue® metabolic activity data were normalized for cell number. Ctrl – Control (no crowder added). SEM and AFM were done after 21 days of podocyte culture.
2.2. Cell-derived decellularized matrix increases viability of podocytes
We used alamarBlue® and DAPI stained nucleus counting to asses viability/proliferation of podocytes cultured on decellularized matrices, which were derived using three different protocols: (i) DOC - 0.5% Sodium deoxycholate, (ii) TE-DOC - 0.5% Sodium deoxycholate with Triton X-100 and EDTA and (iii) double distilled water. We found that decellularization using TE-DOC supported significantly higher metabolic activity and viability (p<0.0001) of podocytes up to 28 days compared to the use of the other two protocols (Figure 2d and supplementary figure S3a, b). Measurement of thickness of decellularized matrix using scanning electron microscopy (SEM) confirmed that various decellularization methods (DOC, TE-DOC or ddH2O) did not change the thickness of decellularized matrix. However, MMC significantly enhanced (p<0.0001, n=4) the thickness of decellularized matrix (Figure 2e and supplementary figure S4). Atomic force microscopy (AFM) confirmed the higher stiffness of tissue culture plastic surface compared to collagen (approximately 1400 times softer than plastic) or DCM without MMC (approximately 1650 times softer than plastic) and DCM with MMC (approximately 3900 times softer than plastic) surfaces (Figure 2f). A significant increase (p<0.0001) in metabolic activity and viability of podocytes was observed when cultured under differentiation (37 °C) conditions up to 28 days (Figure 2g, h and supplementary figure S5a, b and S6a, b). In subsequent experiments we used TE-DOC solution to decellularize seeded fibroblasts.
2.3. Decellularized matrix contains major ECM molecules and enables cultured podocytes to display features resembling those in observed in vivo
We evaluated the composition of the deposited ECM after decellularization by immunofluorescence and demonstrated higher deposition of collagen type I, IV, fibronectin and laminin when fibroblasts were seeded under MMC conditions compared to all other conditions (Figure 3a). SEM and AFM revealed abundant fibrillar ECM in decellularized fibroblasts seeded under MMC conditions. Podocytes demonstrated the presence of interdigitations and foot-like processes attaching to the matrix only when they were cultured on DCM developed using MMC but not when cultured on DCM developed without MMC, or on collagen type I coated plates, or on plain non-coated plates (Figure 3b, supplementary figure S7 and S8). SDS-PAGE studies confirmed higher deposition of collagen type I in DCM well plates prepared using MMC compared to those prepared without MMC (Figure 3c). When podocytes were cultured in DCM plates prepared using MMC conditions, their foot processes interdigitated with the neighboring cells and resembled in vivo podocytes (Figure 3d, supplementary figure S8). Moreover, the number of interdigits between podocytes, as evaluated by immunofluorescence, were significantly higher in the DCM plates prepared with MMC conditions compared to those cultured under other conditions (Figure 3 e).
Figure 3. Podocytes maturating on ECM produced by fibroblasts cultured in MMC conditions develop interdigitating foot processes.
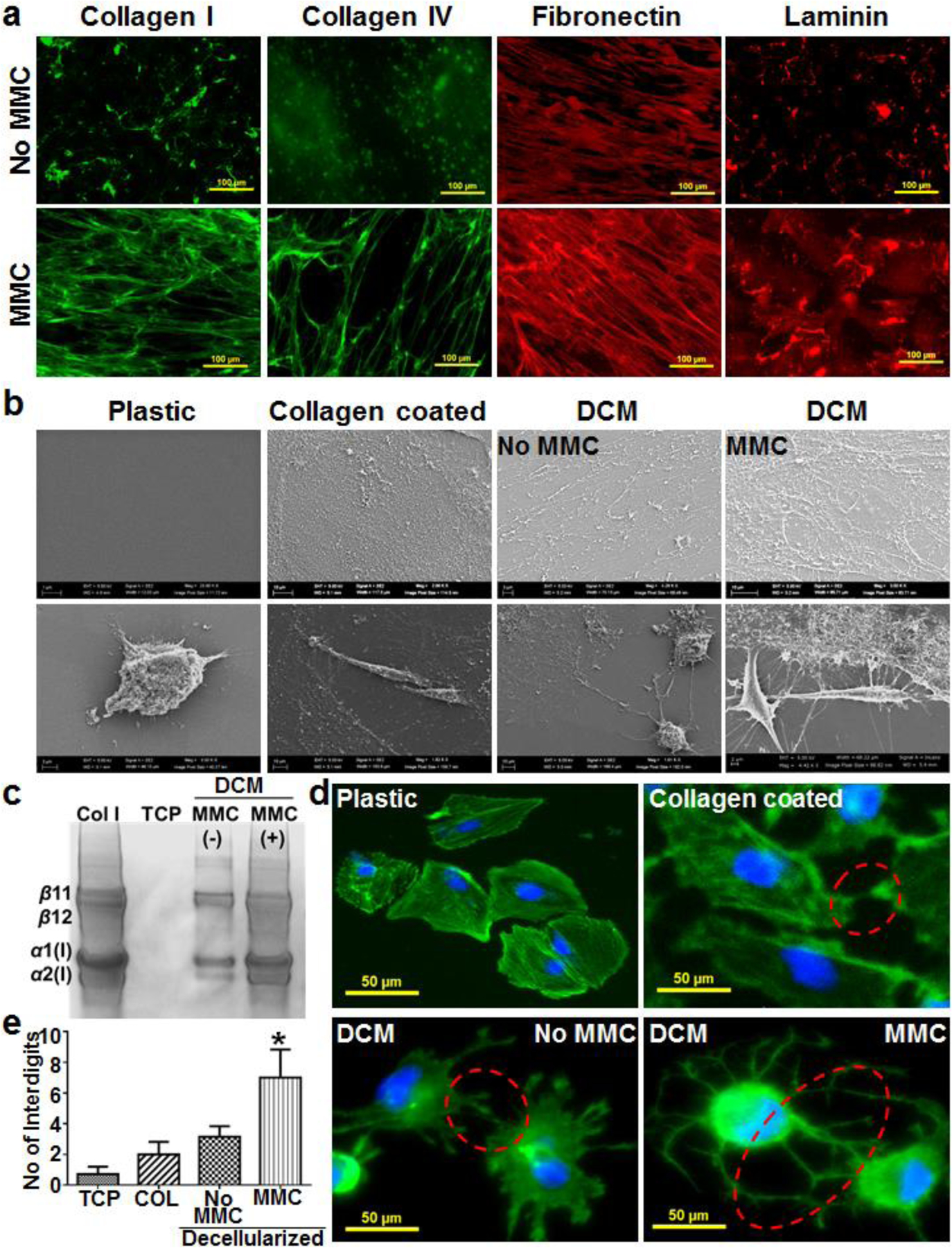
Immunofluorescence analysis of the tissue culture well plates with DCM confirmed higher deposition of collagen type I, IV, fibronectin and laminin under MMC conditions (a). Scanning electron microscopy of tissue culture plastic, collagen type I coated and cell derived decellularized matrix (DCM, with and without MMC) plates revealed the abundant deposition of fibrillar ECM in the DCM plates (b, upper panel) that helped podocytes to connect with matrix through their foot processes (b, lower panel). The collagen type I coated plastic plates did not show the presence of fibrillar matrix and assemblage between podocytes and matrix (b). Collagen type I was isolated using pepsin treatment and run on SDS-PAGE that further confirmed the higher deposition of collagen type I in DCM plates with MMC (c). Podocytes cultured (21 days) in DCM plates developed interdigitating foot processes among neighboring cells (d) and the number of interdigits between podocytes was significantly higher (*p<0.0001, n=15) in DCM plates with MMC (e), 15 images (3 images from 5 different culture wells were analyzed to count interdigits. TCP – Tissue culture plastic plates. DCM – Cell derived decellularized matrix well plates. COL – Tissue culture plastic plates coated with collagen type I. Col I std – 100 μg/ml collagen type I from rat tail.
2.4. The secretome of podocytes cultured on cell-derived decellularized matrix contains enhanced levels of growth factors and associated molecules.
To find the mechanisms governing podocyte morphology, proliferation and differentiation on decellularized matrix, the secretome of podocytes were examined for relative expression of 41 different growth factors, binding proteins and their receptors using membrane based antibody array (Fig. 4 and supplementary figure S9, S10 and S11). Densitometry analysis confirmed higher expression of many growth factors (Figure 4a, b; bFGF, TGF-α, TGF-β, TGF-β2, TGF-β3, IGF-I, IGF-II, AR, HBEGF, VEGF-A, SCF and PIGF), biding proteins (Figure 4c; IGFBP-1, IGFBP-2, IGFBP-3, IGFBP-4 and IGFBP-6) and receptors (Figure 4d; EGFR, IGF-I sR, MCSF-R, PDGFRα, PDGFRβ, SCFR, VEGFR2 and VEGFR3) in podocytes cultured on decellularized matrix with MMC in comparison with other culture conditions (plastic, collagen coated, DCM without MMC).
Figure 4. Podocytes maturating on ECM produced by fibroblasts cultured in MMC conditions display enriched growth factor profile.
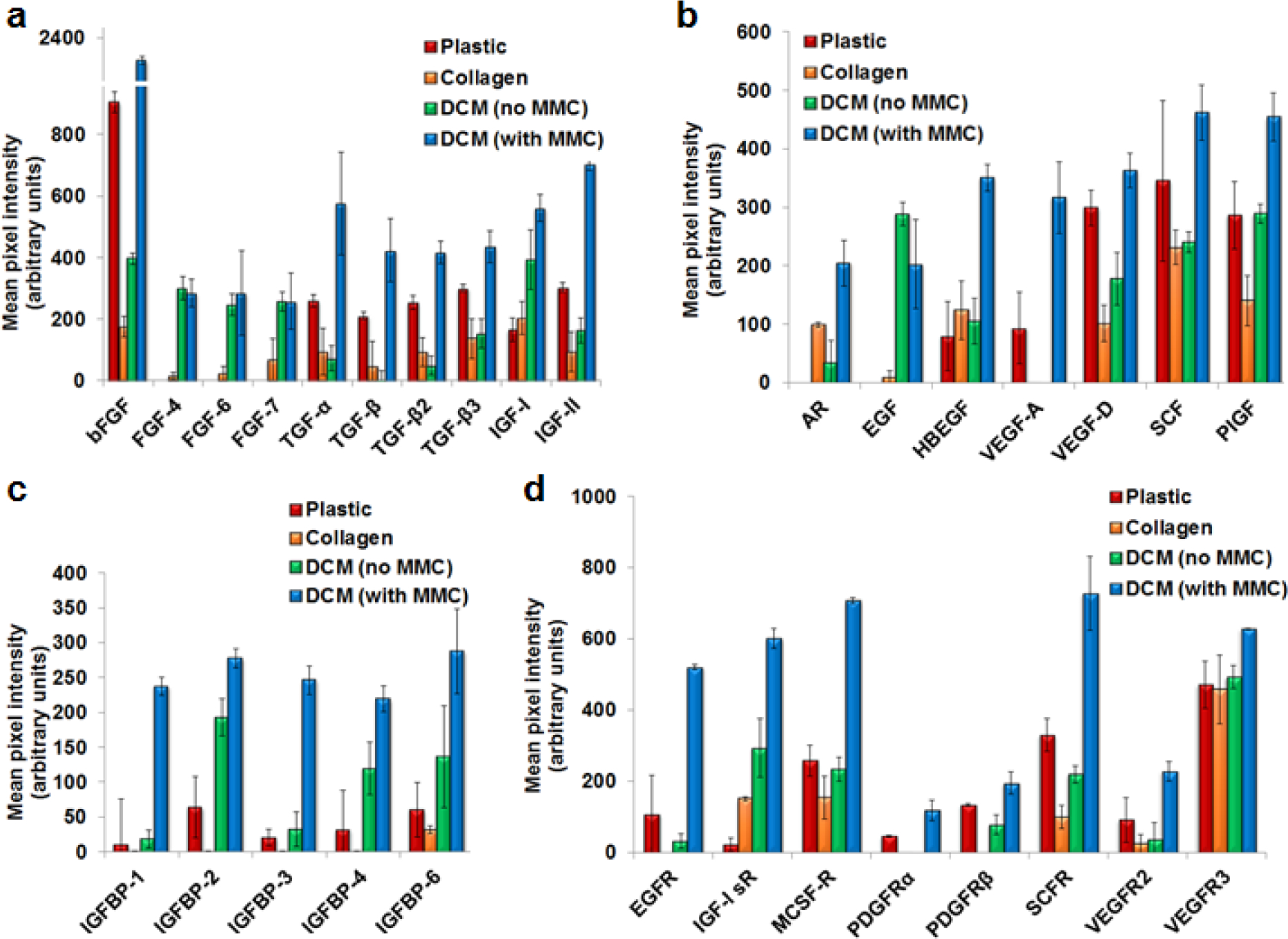
The densitometry analysis of multiplex growth factors membrane antibody array confirmed higher expression of many growth factors (Figure 4a, b; bFGF, TGF-α, TGF-β, TGF-β2, TGF-β3, IGF-I, IGF-II, AR, HBEGF, VEGF-A, SCF and PIGF), biding proteins (Figure 4c; IGFBP-1, IGFBP-2, IGFBP-3, IGFBP-4 and IGFBP-6) and receptors (Figure 4d; EGFR, IGF-I sR, MCSF-R, PDGFRα, PDGFRβ, SCFR, VEGFR2 and VEGFR3) in podocytes cultured (21 days) on decellularized matrix with MMC in comparison with other culture conditions (plastic, collagen coated, DCM without MMC).
2.4. Cell-derived DCM supports podocyte morphology and the expression of podocyte-specific molecules
Human podocytes were cultured on plastic plates or plates coated with decellularized ECM up to 21 days under differentiation (37 °C) conditions and the expression of molecules was evaluated by immunofluorescence. As seen in Figure 5a, synaptopodin, an actin-associated protein of differentiated podocytes, colocalized with the actin cytoskeletal network. Western blotting revealed that the levels of synaptopodin and nephrin were higher in lysates of podocytes harvested after culture (37 °C, differentiation conditions) on ECM-rich coated plates compared to those harvested from non-coated plates (Figure 5b, densitometric analysis Figure 5c). Similarly, immunofluorescence staining confirmed higher expression of synaptopodin and nephrin in podocytes cultured on ECM-coated plates under differentiation conditions compared to those cultured on non-coated plates (Figure 5d, e and supplementary figure S12).
Figure 5. Podocytes maturating on ECM produced by fibroblasts cultured in MMC conditions express high levels of specific molecules.
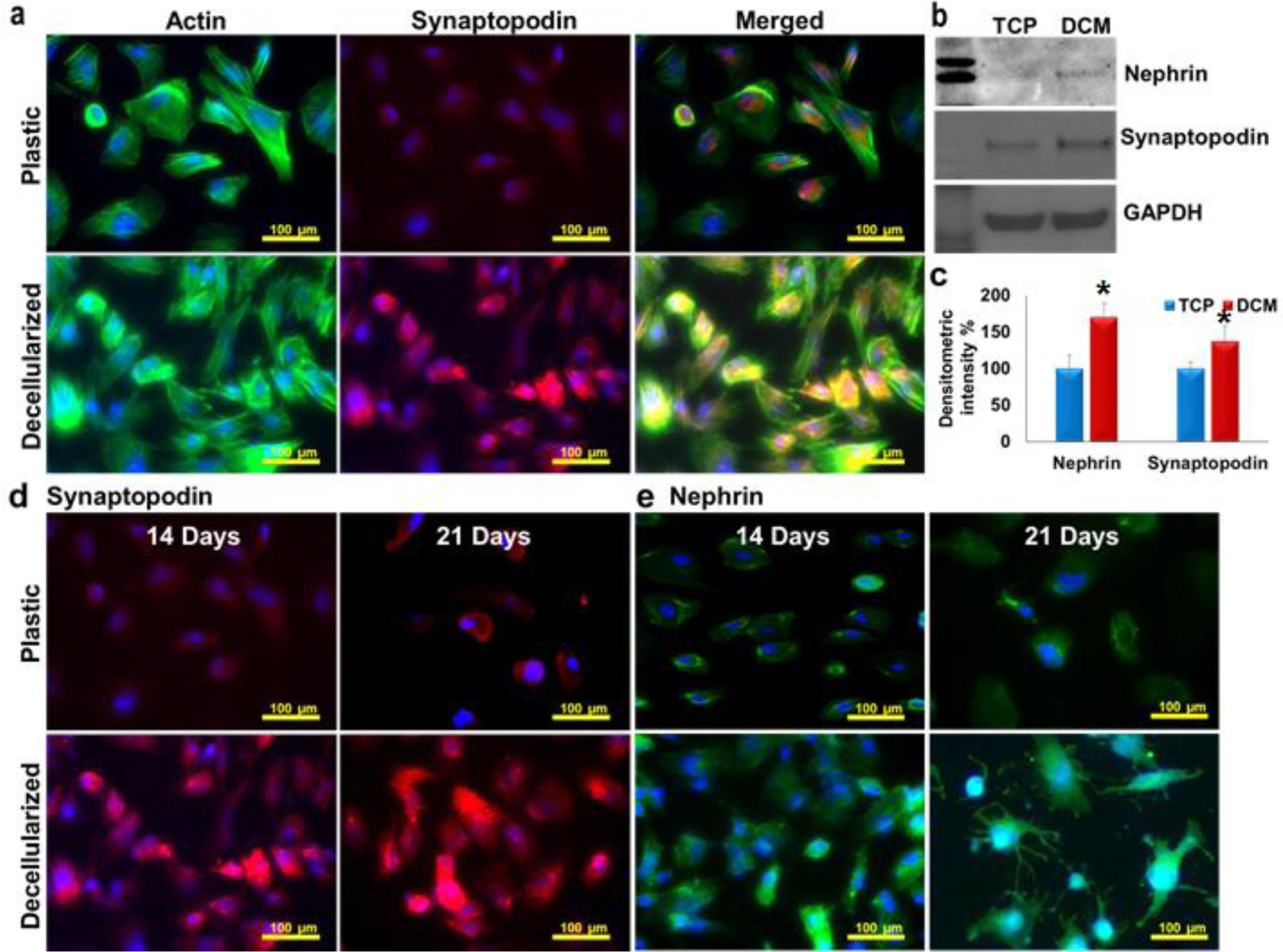
Podocytes cultured (14 days) on DCM showed native morphology and co localization of synaptopodin with actin cytoskeletal network (a). Western blotting (b) (densitometric readings c, n=3,) confirmed higher expression of nephrin (*p<0.0001) and synaptopodin (*p<0.002) on decellularized ECM rich substrate under differentiation condition (14 days). The immunofluorescence staining of podocytes cultured in ECM produced by fibroblasts cultured under MMC conditions confirmed high expression of synaptopodin (d) and nephrin (e).
2.5. Podocytes cultured on Cell-derived DCM conditions produced many extracellular matrix molecules.
The presence of major ECM components in the podocyte cultures was evaluated by using immunofluorescence staining for collagen type I, collagen type IV, fibronectin and laminin. Human podocytes were cultured on plastic or decellularized ECM rich well plates for up 21 days under differentiation conditions (37 °C). Podocytes cultured on DCM showed higher levels of collagen type I as compared to tissue culture plastic well plates (Figure 6a). To further confirm the source of deposited collagen type I, we isolated collagen type I from both plastic and decellularized plates of cultured podocytes using pepsin digestion [16a, 16b]. Coomassie brilliant blue-stained SDS-PAGE gels confirmed the absence of collagen type I in samples isolated from tissue culture plastic plates. However, well plates with DCM showed higher presence of collagen type I (Supplementary figure S13). These findings demonstrate that collagen type I was deposited by fibroblasts in the decellularized well plates and podocytes are not major producers of collagen type I. On the contrary, the deposition of other major ECM components, collagen type IV (Figure 6b), fibronectin (Figure 6c) and laminin (Figure 6d), was detected in plastic well plates with cultured podocytes, which confirmed that podocytes can produce collagen type IV, fibronectin and laminin in culture. However, the presence of these ECM molecules was higher when podocytes were cultured in plates with DCM as compared to plastic well plates (Figure 6b–d). The ECM from the decellularized well plates combined with podocyte deposited ECM created a better microenvironment for the culture of podocytes.
Figure 6. Podocytes cultured on decellularized ECM produced by fibroblasts cultured in MMC conditions produced many extracellular matrix molecules.
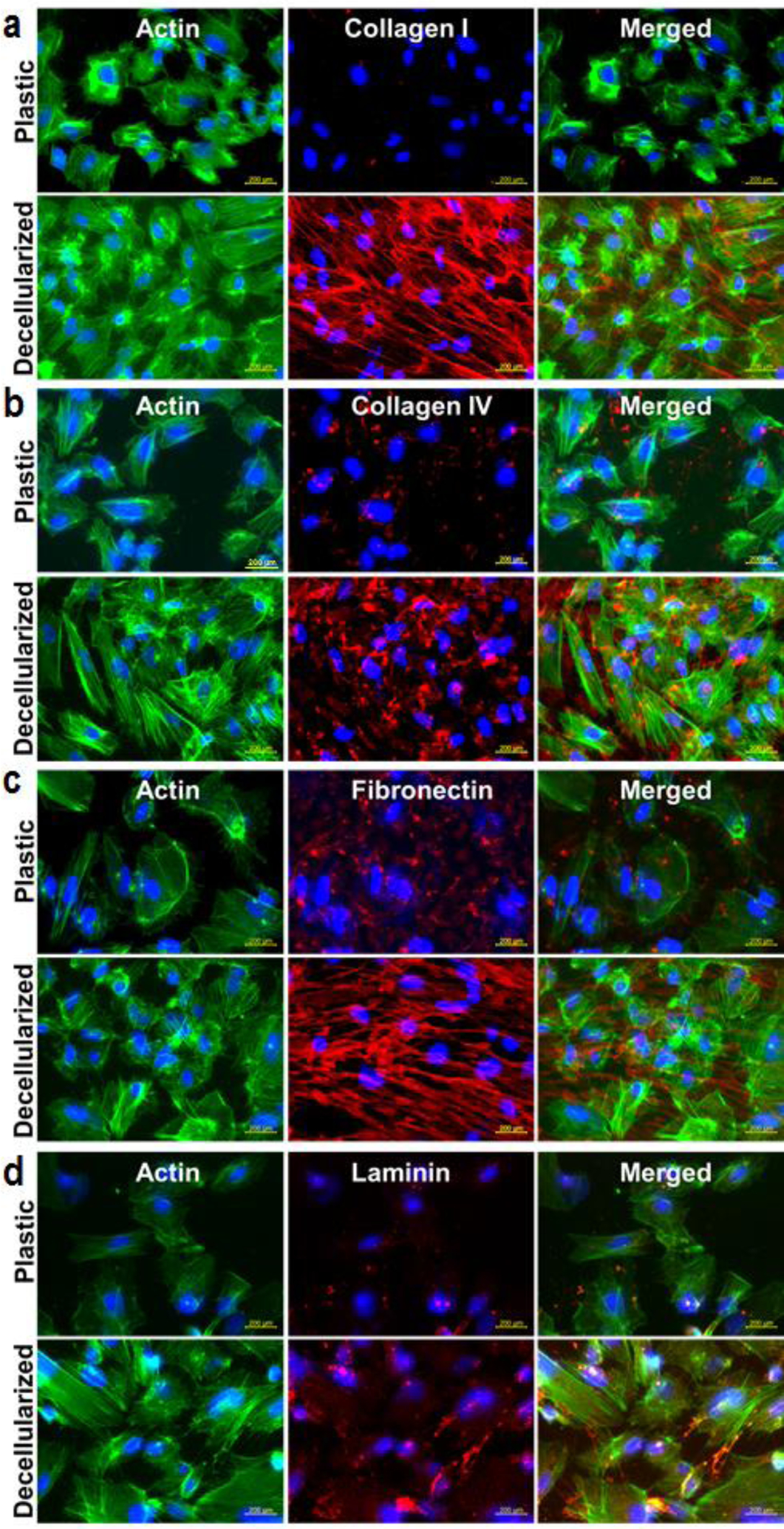
Human podocytes were cultured in plastic or decellularized ECM rich well plates for up 21 days under differentiation condition (37 °C) (n=3). Podocytes cultured on decellularized matrix showed higher level of collagen type I (a), collagen type IV* (b), fibronectin (c) and laminin (d). Actin staining was performed to locate podocytes with EMC in the culture. *Minor cross reactivity of collagen type IV antibody was observed with collagen type I.
2.6. Podocytes cultured on cell-derived decellularized matrix display better metabolism
Oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) are important indicators of mitochondrial respiration and glycolysis and provide a systems level view of cellular metabolic function in cultured cells. Podocytes cultured on plates coated with DCM displayed higher OCR, ECAR and PPR (proton production rates) compared to podocytes cultured on plastic plates (Figure 7) suggesting that the new culture platform offers metabolic advantages to podocytes.
Figure 7. Podocytes maturating on ECM produced by fibroblasts cultured in MMC conditions display improved metabolic profile.
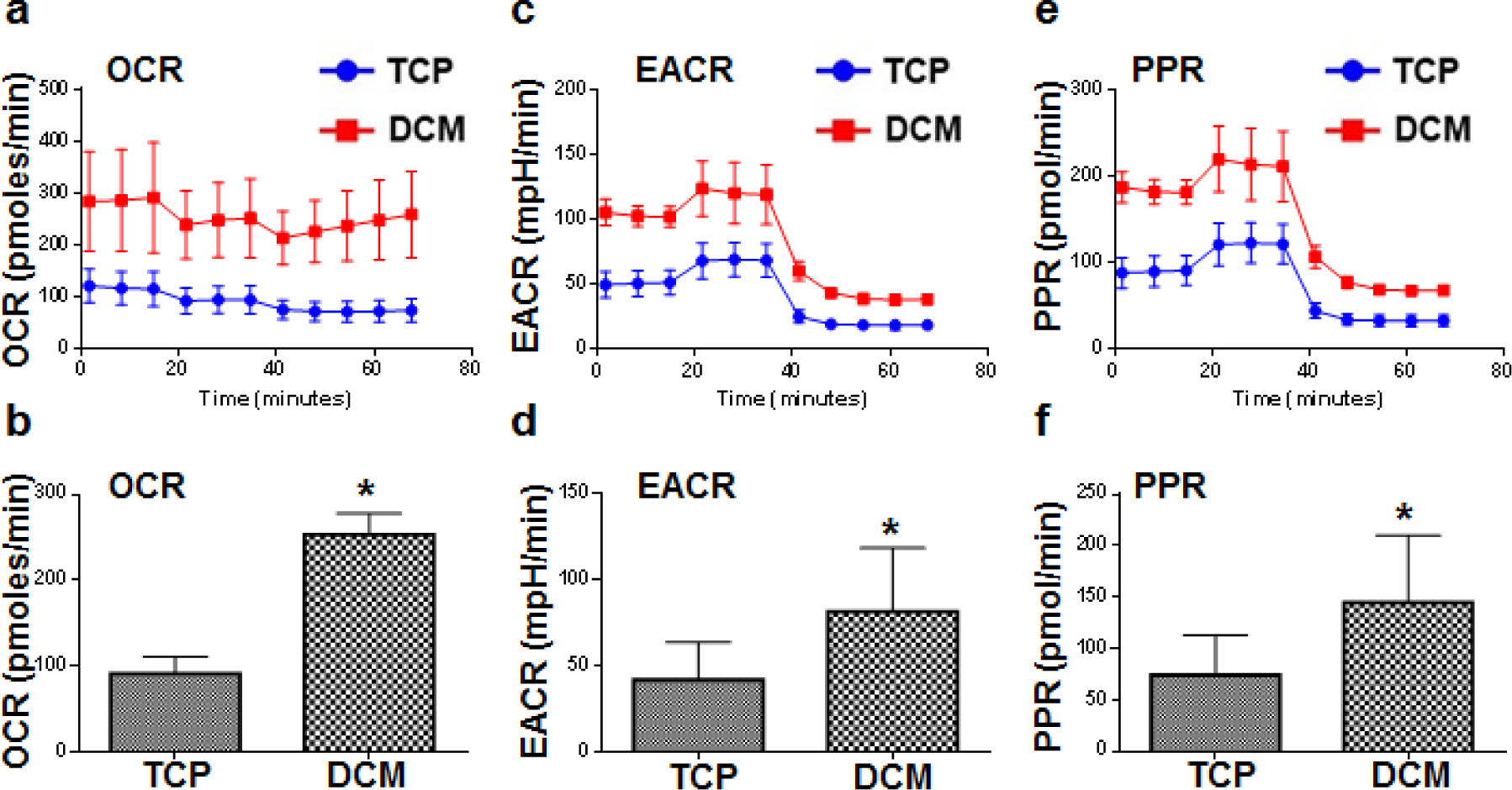
A Seahorse XF analyzer was used to study the metabolic profile of podocytes which matured for 21 days on ECM produced by fibroblasts cultured in MMC conditions (DCM). Podocytes cultured on DCM displayed higher oxygen consumption rate (OCR, *p<0.0001) (a, b) and extracellular acidification rate (ECAR, *p<0.03) (c, d) and proton production rates (PPR, *p<0.03) (e, f) compared to podocytes cultured on plastic (TCP). (n=3)
3. Discussion and Conclusion
In this communication we present a new strategy for designing a material derived from ECM rich, cell-derived decellularized matrices which provide sustenance to podocytes. We demonstrate that MMC enhances the deposition of ECM from fibroblasts and that podocytes cultured on ECM-generated from decellularized fibroblasts display superior interdigitation between the foot process, expression of specific structural and functional molecules as well as metabolic profile.
Cell behaviour and response to the extracellular surroundings is controlled by a progressively multifaceted set of micro-environmental cues derived from cells, comprising the ECM [25]. The cellular interactions with neighbouring ECM regulate nearly all aspect of tissue homeostasis by providing essential cues for survival, growth and differentiation to cells within tissues [26]. The lack of an appropriate in vitro method represents a primary hurdle in describing the exact role of ECM that recapitulates complex extracellular microenvironments. Apart from MMC, tightly anchored tissue-mimetic MSC-derived ECM has been proposed for stem cell microenvironments, that support consistent anchorage of inherent cell secreted ECM for differentiation and growth of bone-marrow stem cells [27]. Tissue composition in vivo is the outcome of molecules produced by many different cell types. However, the main stromal ECM-producing cells are tissue fibroblasts and the assortment of proteins and other molecules secreted and deposited by these cells is expected to represent a close imitation of the complex in vivo protein composition [28]. Thus, we decided to use fibroblast-generated ECM that proved to be a promising alternative to synthetic or natural 3D scaffolds for in vitro culture of glomerular epithelial cells. The cell derived ECM produced with the help of MMC gave stable support and anchorage to podocytes when cultured up to 28 days.
Animal models of renal disease do not always fully replicate their human counterpart and therefore the use of human cells grown in vitro becomes mandatory [21]. Traditional podocyte culture protocols fail to emulate the inherent tissue function, causing loss of native physiology, growth arrest, cellular senescence and phenotypic drift [21, 29]. The feet of podocytes adhere to the ECM of the GBM which is composed of several proteins assembled into an intricate arrangement [30]. The ECM network is believed to deliver the essential tensile strength to endure filtration forces [31]. The assembly of ECM molecules influences the physiology of podocytes through an orchestrated intracellular signaling initiated by many ECM molecules [30]. Cell-derived decellularized matrices generated from ECM-producing cells (e.g. fibroblasts) can provide a microenvironment to study specific biological questions pertaining to the structure and function of glomerular cells. In addition, the stiffness of cell derived decellularized matrices has been established to closely imitate the in vivo range, despite being produced on stiff plastic or glass surfaces [32].
Podocytes recognize a specific geometric pattern, promoting the idea that defined 3D micro-architectures imitating the in vivo microenvironment may deliver better conditions for in vitro investigations. Nanoporous substrates constructed with grooves shows a better network of the actin cytoskeleton and focal adhesions of podocytes [33]. Podocytes sense the stiffness of substrate that regulates proliferation, migration and spreading of podocyte. A marked stiffness sensitivity of podocytes has been revealed on gelatin microbial transglutaminase cell culture platform [34]. The morphology of podocytes is inherently fragile and transient. Any biochemical imbalances may lead to permanent morphological alterations accompanied with pathophysiological changes [35]. Careful design of substrate nanotopography, stiffness, and coating materials may help in maintaining podocytes in vitro. ECM modulates early kidney development in embryonic organ cultures [36]. Magno et. al. reported that MMC will be useful for tailoring kidney-derived fibrillated ECM. Here ECM was obtained from decellularized and grounded porcine kidneys [16d]. Combination of MMC with metabolic stimuli was shown to result in tissue specific, vastly organized ECM capable of retaining growth factors and glycosaminoglycans [37]. These systems may be useful to culture kidney specific cells.
The unique features of the podocyte culture platform presented herein enables the development of interconnected foot processes, increased cell viability, higher and early expression of all podocyte-specific molecules, including nephrin and synaptopodin, and better metabolic performance. Thus its use will serve a number of significant goals: (1) Facilitate the study of physiology, biochemistry and cell biology of podocytes. (2) Advance the study of pathophysiology in diseases in which the injury of podocyte is central including systemic lupus erythematosus, anti-neutrophil cytoplasm antibody-associated glomeruloneprhritis, minimal change nephrotic syndrome and focal segmental glomerulosclerosis. (3) Provide a method to screen sera of patients for the presence of antibodies [38] or other molecules able to cause podocyte injury (cell-based assay). (5) Enable screening of drugs under development for possible podocytotoxicity.
In summary, we have provided evidence that cell-derived DCM produced by human fibroblasts supports podocyte differentiation and function. We propose that the new approach to culture podocytes in vitro will facilitate the study of podocyte biology, enable discovery and provide a cell-based assay to screen for factors in the sera of patients or drugs which may cause podocyte injury.
4. Experimental Section
Cell culture and macromolecular crowding:
Human neonatal foreskin fibroblasts were cultured in Dulbecco’s modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (PS) at 37°C in a humidified atmosphere of 5% CO2. Fibroblasts were seeded at 25,000 cells/cm2 in 24-well or 12-well plates and were allowed to attach for 24 hours. After 24 hours the medium was changed with medium containing macromolecular crowders (37.5 mg/mL Ficoll 70 and 25 mg/mL Ficoll 400) to enhance ECM deposition and 100 μM L-ascorbic acid phosphate to induce collagen synthesis [16b]. Immortalized human podocyte cell line were cultured in Roswell Park Memorial Institute (RPMI) – 1640 medium with 10% fetal bovine serum (FBS), 1% Insulin-transferrin-sodium selenite and 1% penicillin-streptomycin at 33°C in a humidified atmosphere of 5% CO2. Podocytes were moved to 37°C after reaching 80% confluency.
Construction of cell derived decellularized matrix:
After 7 days of culture of human skin fibroblasts, medium was removed and cells were washed twice with PBS. Fibroblasts were decellularized using double distilled water or 0.5% Sodium deoxycholate with or without Triton X-100 and EDTA. Decellularized well plates were washed with phosphate buffer saline (PBS) and sterilized under ultraviolet light for 2 hours.
Cell metabolic activity and viability:
alamarBlue® (Invitrogen, US) cell metabolic activity assay was performed to quantify cell viability of the podocytes. Briefly, at the end of culture time points, cells were washed with Hanks’ Balanced Salt solution (HBSS, Sigma, US) and then diluted alamarBlue® was added. After 4 hours of incubation at 37°C, absorbance was measured at 550 and 595nm with help of Synergy™ HT multi-mode microplate reader (BioTek Instruments, US). Cell metabolic activity was expressed in terms of reduction percentage of alamarBlue® and normalized with cell number. For cell number (viability) calculation the DAPI stained nuclei counting was carried out using ImageJ 1.48v (NIH, USA) software.
Quantification of deposited collagen in decellularized matrix:
Decellularized fibroblast matrix was digested with porcine gastric mucosa pepsin (Sigma, US) in a final concentration of 1mg/ml in 0.5M acetic acid (Sigma, US). Samples were incubated at 37°C for 2 hours with gentle shaking followed by neutralization with 0.1N sodium hydroxide (Sigma, US). The extracted collagen samples were analyzed by SDS-PAGE (Sodium dodecyl sulphate-polyacrylamide gel electrophoresis) under non-reducing conditions. 100–500μg/ml of rat tail collagen type I (Sigma, US) was used as standards with every gel. Protein bands were stained with the coomassie blue or silver stain. Densitometric analysis of gels was performed using ImageJ 1.48v (NIH, USA) software. Collagen bands were quantified by defining each band with the rectangular tool with background subtraction.
Western blotting:
The total protein from human podocytes was extracted using radio immunoprecipitation assay (RIPA) buffer. Protein concentrations were normalized for all samples and separated on NuPAGE Novex 4–12% Bis-Tris gels (ThermoFisher Scientific). Subsequently, the proteins were transferred to polyvinylidene difluoride membranes (ThermoFisher Scientific), blocked with 5% skimmed milk in tris-buffered saline Tween (TBST), and incubated overnight with guinea pig anti– nephrin antibody (Progen Biotechnik, Germany) at a dilution 1:500, goat anti– synaptopodin antibody (Santa Cruz Biotechnology) at a dilution 1:1000, and mouse anti- glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (BioLegend) at dilution a 1:5000 in TBST containing 5% skimmed milk. After three washes with TBST the membranes were incubated with appropriate horseradish peroxidase–conjugated secondary antibodies (Santa Cruz Biotechnology). Protein bands were visualized using a chemiluminescent ECL™ detection kit (GE Healthcare).
Immunocytochemistry:
Human skin fibroblasts were seeded on 4 or 8-well Lab-Tek™ II (Nunc, Denmark) chamber slides at 25,000 cells/cm2 and were decellularized. Human podocytes were seeded on 4 or 8-well Lab-Tek™ II chamber slides with decellularized matrix. Human podocytes were also seeded on chamber slides without decellularized matrix as a control group. At the end of cell culture time points, medium was removed and cell layers were washed with HBSS and fixed with 4% paraformaldehyde (Sigma, US) at room temperature for 15min. After several washes in phosphate-buffered saline (PBS, Sigma, US), nonspecific sites were blocked with 3% bovine serum albumin (Sigma, US) in PBS for 30 minutes. The cells were incubated for 90 minutes at room temperature or overnight at 4°C simultaneously with the primary antibody of collagen type I (EMD Millipore, dilution 1:200), IV (Developmental Studies Hybridoma Bank, dilution 1:50), laminin (Abcam, dilution 1:200), fibronectin (Sigma, dilution 1:200), α-smooth muscle actin/phalloidin (Thermo Fisher, dilution 1:400), nephrin (Progen Biotechnik, dilution 1:200) and synaptopodin (Santa Cruz Biotechnology, dilution 1:100). Bound antibodies were visualised using AlexaFluor®488 chicken anti-rabbit (Invitrogen, USA) and AlexaFluor®555 goat anti-mouse (Invitrogen, USA) 1:400 in PBS for 30 minutes. Post-fixation was done with 2% PFA for 15 minutes. Cell nuclei were counterstained with Hoechst stains (bisBenzimide H 33342 trihydrochloride; Invitrogen, USA) and slides were mounted with Vectashield® mounting media (Vector Lab, US). Images were captured and fluorescence intensity measurements were performed with a Zeis Axio Observer.A1 inverted fluorescence microscope (ZEISS, Germany) or a Nikon eclipse Ti-E confocal laser scanning microscopy (Nikon, Japan).
Growth factor membrane Array:
Growth factor profile of podocytes cultured on plastic, collagen coated, DCM without MMC and DCM with MMC culture well plates, were analyzed by semi-quantitative Western spot blot analyses using human growth factor membrane antibody array (ab134002, Abcam) according to the manufacturer’s instruction. Podocytes were cultured in RPMI – 1640 medium with FBS, ITS and PS in 12-well plates at a density of at 25,000 cells/cm2 for 17 days at 37° C. After 14 days medium was changed twice at the interval of 48 hours with serum free condition (without FBS, ITS and PS) and then podocytes were harvested. For quantification of growth factors, membranes were sequentially incubated with supernatants (overnight at 4 °C), biotin-conjugated anti-growth factor antibodies, HRP-conjugated streptavidin, and ECL detection system. Signals were captured by ChemiDoc XRS (Bio-Rad Laboratories). Data were quantified via densitometry using the Image Lab software with normalization to blanks and negative controls.
Scanning Electron Microscopy (SEM):
Human skin fibroblasts were seeded on Thermanox coverslips (Thermo Scientific) at 25,000 cells/cm2 and were decellularized. Human podocytes were seeded on coverslip with decellularized matrix and on normal coverslip. At the end of cell culture time points, medium was removed and cell layers were washed with HBSS and fixed with 4% paraformaldehyde (Sigma, US) and 2.5% glutaraldehyde for at room temperature for 1 hour. Post-fixation was done using 1.5% of osmium tetra oxide. Subsequently, the podocytes on coverslip were washed three times with PBS and serially dehydrated with 30%, 50%, 70%, 90% and 100% ethanol. The dehydrated podocytes on coverslip were placed on adhesive carbon tabs and mounted on SEM specimen stubs (Ted Pella). The dried specimens were subsequently coated with gold using an Emitech K550 coating system. SEM images [from 3 different slides (n=3)] were obtained using a Hitachi S-4700 field emission microscope operating with a beam voltage of 15kV.
Atomic force microscopy (AFM):
AFM was performed using the MFP-3D and Cypher AFM systems with an open fluid droplet containing de-ionized water (Asylum Research, Santa Barbara, CA). The commercial plastic slides were fixed to glass slides and stainless-steel discs using carbon tape (Ted Pella, Redding, CA). All modulus measurements for hydrated hydrogels were collected in contact mode with soft, gold coated silicon nitride bio-levers and a trigger force of 0.5 to 1.0 nN (Olympus TR400PB, Asylum Research Probe Store, Santa Barbara, CA). The cantilevers were calibrated in air as well as DI water using the Sader thermal method built into the instrument software. Force maps were collected in 3×3um areas with at least a 12×12 array of force distance curves (FDCs) from 3 or more sites for each sample in DI water. The scan rate (1.0 Hz) and distance traveled (2 um) was kept constant for each FDC, which were analyzed using the Johnson-Kendall-Roberts (JKR) model built into the instrument software to estimate the elastic modulus of the samples. AFM imaging of the fixed cells was also performed in air using AC/tapping mode with a soft tapping mode tip (2 N/m) (Olympus AC240, Asylum Research Probe Store, Santa Barbara, CA).
Metabolism Assays:
Oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were measured using 8-well XFp Extracellular Flux Analyzer. Assay buffer was made of XF base medium (without Gln) with 10 mM glucose and 1.0 mM sodium pyruvate. OCR and ECAR were measured before and after exposure of the assay buffer with or without 2.0 mM Gln. All other procedures were performed according to the manufacturer’s instructions.
Statistical analyses:
Numerical data is expressed as mean ± SD. Analysis was performed using statistical software (Graphpad Prism, USA). Comparisons among groups were performed by Mann–Whitney U test or one-way ANOVA, followed by Bonferroni’s multiple comparison test. One-way ANOVA was employed after confirming the following assumptions: (a) the distribution of the sample mean was normal (Anderson-Darling normality test); and (b) the variances of the population of the samples were equal to one another (Bartlett’s and Levene’s tests for homogeneity of variance). Kruskal-Wallis test, followed by Dunn’s multiple comparison test, was used when either or both of the above assumptions were violated. Statistical significance was accepted at p<0.05. Experiments were performed in triplicate or quadruplicate.
Supplementary Material
Acknowledgements:
Atomic force microscopy and Scanning Electron Microscopy was performed in part at the Center for Nanoscale Systems (CNS, Harvard University), a member of the National Nanotechnology Coordinated Infrastructure Network (NNCI), which is supported by the National Science Foundation under NSF award no. 1541959. Authors would like to thank Mr. Timothy Cavanaugh, CNS, for assistance in Scanning Electron Microscopy.
Footnotes
Supporting Information: Supporting Information is available online from the Wiley Online Library or from the author.
Conflict of Interest: The authors declare no conflict of interest.
Contributor Information
Jason S. Tresback, Center for Nanoscale Systems, Laboratory for Integrated Science and Engineering, Harvard University, Cambridge, MA, 02138, United States
Dimitrios I. Zeugolis, Regenerative, Modular & Developmental Engineering Laboratory (REMODEL), Centre for Research in Medical Devices (CURAM), Biomedical Sciences Building, National University of Ireland Galway (NUI Galway), Galway, Ireland
George C. Tsokos, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, 02215, United States
References
- [1].a) Lim JY, Dreiss AD, Zhou Z, Hansen JC, Siedlecki CA, Hengstebeck RW, Cheng J, Winograd N, Donahue HJ, Biomaterials 2007, 28, 1787; [DOI] [PubMed] [Google Scholar]; b) Yim EKF, Pang SW, Leong KW, Experimental Cell Research 2007, 313, 1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tuffin J, Burke M, Richardson T, Johnson T, Saleem MA, Satchell S, Welsh GI, Perriman A, Advanced Healthcare Materials 2019, 0, 1900698. [DOI] [PubMed] [Google Scholar]
- [3].Hollister SJ, Nature Materials 2005, 4, 518. [DOI] [PubMed] [Google Scholar]
- [4].Williams DF, Biomaterials 2008, 29, 2941. [DOI] [PubMed] [Google Scholar]
- [5].Livesey SA, Herndon DN, Hollyoak MA, Atkinson YH, Nag A, Transplantation 1995, 60, 1. [PubMed] [Google Scholar]
- [6].Sutherland RS, Baskin LS, Hayward SW, Cunha GR, The Journal of Urology 1996, 156, 571. [DOI] [PubMed] [Google Scholar]
- [7].Dohmen PM, Lembcke A, Holinski S, Pruss A, Konertz W, The Annals of Thoracic Surgery 2011, 92, 1308. [DOI] [PubMed] [Google Scholar]
- [8].Badylak SF, Taylor D, Uygun K, Annual Review of Biomedical Engineering 2011, 13, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].a) Gilbert TW, Sellaro TL, Badylak SF, Biomaterials 2006, 27, 3675; [DOI] [PubMed] [Google Scholar]; b) Yang Q, Peng J, Guo Q, Huang J, Zhang L, Yao J, Yang F, Wang S, Xu W, Wang A, Lu S, Biomaterials 2008, 29, 2378. [DOI] [PubMed] [Google Scholar]
- [10].Singh S, Bandini SB, Donnelly PE, Schwartz J, Schwarzbauer JE, Journal of Materials Chemistry B 2014, 2, 1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].a) Cukierman E, Pankov R, Stevens DR, Yamada KM, Science 2001, 294, 1708; [DOI] [PubMed] [Google Scholar]; b) Harris GM, Madigan NN, Lancaster KZ, Enquist LW, Windebank AJ, Schwartz J, Schwarzbauer JE, Matrix Biology 2017, 60–61, 176; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Hellewell AL, Rosini S, Adams JC, JoVE 2017, DOI: doi: 10.3791/55051e55051; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Mao Y, Schwarzbauer JE, Journal of Cell Science 2005, 118, 4427; [DOI] [PubMed] [Google Scholar]; e) Vlodavsky I, Current Protocols in Cell Biology 1999, 1, 10.4.1. [DOI] [PubMed] [Google Scholar]
- [12].Hynes RO, Naba A, Cold Spring Harbor Perspectives in Biology 2012, 4, a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dewavrin J-Y, Abdurrahiem M, Blocki A, Musib M, Piazza F, Raghunath M, The Journal of Physical Chemistry B 2015, 119, 4350. [DOI] [PubMed] [Google Scholar]
- [14].Kuznetsova IM, Zaslavsky BY, Breydo L, Turoverov KK, Uversky VN, Molecules 2015, 20, 1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kuznetsova IM, Turoverov KK, Uversky VN, International Journal of Molecular Sciences 2014, 15, 23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].a) Satyam A, Kumar P, Cigognini D, Pandit A, Zeugolis DI, Acta Biomaterialia 2016, 44, 221; [DOI] [PubMed] [Google Scholar]; b) Satyam A, Kumar P, Fan X, Gorelov A, Rochev Y, Joshi L, Peinado H, Lyden D, Thomas B, Rodriguez B, Raghunath M, Pandit A, Zeugolis D, Advanced Materials 2014, 26, 3024; [DOI] [PubMed] [Google Scholar]; c) Cigognini D, Gaspar D, Kumar P, Satyam A, Alagesan S, Sanz-Nogués C, Griffin M, O’Brien T, Pandit A, Zeugolis DI, Scientific reports 2016, 6, 30746; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Magno V, Friedrichs J, Weber HM, Prewitz MC, Tsurkan MV, Werner C, Acta Biomaterialia 2017, 55, 109. [DOI] [PubMed] [Google Scholar]
- [17].a) Mathieson PW, Current Opinion in Nephrology and Hypertension 2009, 18, 206; [DOI] [PubMed] [Google Scholar]; b) Patrakka J, Tryggvason K, Nature Reviews Nephrology 2009, 5, 463. [DOI] [PubMed] [Google Scholar]
- [18].a) Kriz W, Gretz N, Lemley KV, Kidney International 1998, 54, 687; [DOI] [PubMed] [Google Scholar]; b) Pavenstadt H, Kriz W, Kretzler M, Physiological Reviews 2003, 83, 253. [DOI] [PubMed] [Google Scholar]
- [19].a) Da Sacco S, Lemley KV, Sedrakyan S, Zanusso I, Petrosyan A, Peti-Peterdi J, Burford J, De Filippo RE, Perin L, Plos One 2013, 8, e81812; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) O’Hare MJ, Bond J, Clarke C, Takeuchi Y, Atherton AJ, Berry C, Moody J, Silver AR, Davies DC, Alsop AE, Neville AM, Jat PS, Proceedings of the National Academy of Sciences of the United States of America 2001, 98, 646; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P, Journal of the American Society of Nephrology 2002, 13, 630; [DOI] [PubMed] [Google Scholar]; d) Stamps AC, Davies SC, Burman J, O’Hare MJ, International Journal of Cancer 1994, 57, 865. [DOI] [PubMed] [Google Scholar]
- [20].Saleem MA, Ni L, Witherden I, Tryggvason K, Ruotsalainen V, Mundel P, Mathieson PW, The American Journal of Pathology 2002, 161, 1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shankland SJ, Pippin JW, Reiser J, Mundel P, Kidney International 2007, 72, 26. [DOI] [PubMed] [Google Scholar]
- [22].Chittiprol S, Chen P, Petrovic-Djergovic D, Eichler T, Ransom RF, American Journal of Physiology. Renal physiology 2011, 301, F660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ichimura K, Miyazaki N, Sadayama S, Murata K, Koike M, Nakamura K, Ohta K, Sakai T, Scientific Reports 2015, 5, 8993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].a) Jakus AE, Laronda MM, Rashedi AS, Robinson CM, Lee C, Jordan SW, Orwig KE, Woodruff TK, Shah RN, Advanced Functional Materials 2017, 27, 1700992; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Krishtul S, Baruch L, Machluf M, Advanced Functional Materials 2019, 0, 1900386; [Google Scholar]; c) Du C, Narayanan K, Leong MF, Ibrahim MS, Chua YP, Khoo VMH, Wan ACA, Advanced Healthcare Materials 2016, 5, 2080. [DOI] [PubMed] [Google Scholar]
- [25].Rozario T, DeSimone DW, Developmental Biology 2010, 341, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Muncie JM, Weaver VM, in Current Topics in Developmental Biology, Vol. 130 (Eds: Litscher ES, Wassarman PM), Academic Press; 2018, p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Prewitz MC, Seib FP, von Bonin M, Friedrichs J, Stißel A, Niehage C, Müller K, Anastassiadis K, Waskow C, Hoflack B, Bornhäuser M, Werner C, Nature Methods 2013, 10, 788. [DOI] [PubMed] [Google Scholar]
- [28].a) Tracy LE, Minasian RA, Caterson EJ, Advances in Wound Care 2014, 5, 119; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Amatangelo MD, Bassi DE, Klein-Szanto AJP, Cukierman E, The American Journal of Pathology 2005, 167, 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lan N, Moin S, W MP, Nephrology 2012, 17, 525.22591222 [Google Scholar]
- [30].Byron A, Randles MJ, Humphries JD, Mironov A, Hamidi H, Harris S, Mathieson PW, Saleem MA, Satchell SS, Zent R, Humphries MJ, Lennon R, Journal of the American Society of Nephrology 2014, 25, 953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].a) Gunwar S, Ballester F, Noelken ME, Sado Y, Ninomiya Y, Hudson BG, Journal of Biological Chemistry 1998, 273, 8767; [DOI] [PubMed] [Google Scholar]; b) Kalluri R, Shield CF, Todd P, Hudson BG, Neilson EG, The Journal of Clinical Investigation 1997, 99, 2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].a) Ahlfors J-EW, Billiar KL, Biomaterials 2007, 28, 2183; [DOI] [PubMed] [Google Scholar]; b) Soucy PA, Werbin J, Heinz W, Hoh JH, Romer LH, Acta Biomaterialia 2011, 7, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zennaro C, Rastaldi MP, Bakeine GJ, Delfino R, Tonon F, Farra R, Grassi G, Artero M, Tormen M, Carraro M, International Journal of Nanomedicine 2016, 11, 4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hu M, Azeloglu EU, Ron A, Tran-Ba K-H, Calizo RC, Tavassoly I, Bhattacharya S, Jayaraman G, Chen Y, Rabinovich V, Iyengar R, Hone JC, He JC, Kaufman LJ, Scientific Reports 2017, 7, 43934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Falkenberg CV, Azeloglu EU, Stothers M, Deerinck TJ, Chen Y, He JC, Ellisman MH, Hone JC, Iyengar R, Loew LM, PLOS Computational Biology 2017, 13, e1005433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sebinger DDR, Ofenbauer A, Gruber P, Malik S, Werner C, Biomaterials 2013, 34, 6670. [DOI] [PubMed] [Google Scholar]
- [37].Prewitz MC, Stißel A, Friedrichs J, Träber N, Vogler S, Bornhäuser M, Werner C, Biomaterials 2015, 73, 60. [DOI] [PubMed] [Google Scholar]
- [38].Ichinose K, Ushigusa T, Nishino A, Nakashima Y, Suzuki T, Horai Y, Koga T, Kawashiri S.-y., Iwamoto N, Tamai M, Arima K, Nakamura H, Obata Y, Yamamoto K, Origuchi T, Nishino T, Kawakami A, Tsokos GC, Arthritis & Rheumatology 2016, 68, 944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


