Abstract
Background
HIV pretreatment drug resistance (PDR) to NNRTIs in persons initiating ART is increasing in Mexico.
Objectives
To compare HIV PDR in eight sub-regions of Mexico.
Patients and methods
A large PDR survey was implemented in Mexico (September 2017–March 2018) across eight sub-regions. All larger clinics (which provide ART to 90% of all initiators) were included, allocating sample size using the probability-proportional-to-size method. Both antiretroviral-naive and prior antiretroviral-exposed persons were included. HIV PDR levels were estimated from pol Sanger sequences obtained at a WHO-designated laboratory.
Results
A total of 2006 participants were enrolled from 74 clinics. PDR to NNRTIs was higher than to other drug classes (P < 0.0001), crossing the 10% threshold in the North-East, East, South-West and South-East. NNRTI PDR was higher in the South-West (P = 0.02), coinciding with the highest proportion of restarters in this sub-region (14%). We observed higher PDR prevalence to any drug in women compared with men (16.5% versus 12.2%, P = 0.04). After multivariable adjustment, higher NNRTI PDR remained significantly associated with previous antiretroviral exposure in the Centre-North, North-West, South-West and South-East [adjusted OR (aOR): 21, 5, 8 and 25, respectively; P < 0.05]. Genetic network analyses showed high assortativity by sub-region (P < 0.0001), with evidence of drug resistance mutation transmission within local clusters.
Conclusions
Diversification of the public health response to HIV drug resistance based on sub-regional characteristics could be considered in Mexico. Higher NNRTI PDR levels were associated with poorer regions, suggesting opportunities to strengthen local HIV programmes. Price and licensing negotiations of drug regimens containing integrase inhibitors are warranted.
Introduction
HIV-1 pretreatment drug resistance (PDR) to NNRTIs is increasing at a considerable rate in low and middle income countries (LMICs).1,2 Several LMICs that have implemented nationally representative surveys report NNRTI PDR levels >10%,2 which is worrisome given that PDR may compromise HIV control in persons initiating or re-initiating ART regimens containing NNRTIs.2–5 The rise of NNRTI PDR is a real threat to the UNAIDS 90–90–90 targets, particularly in regards to the ‘third 90’ referring to viral load suppression among individuals on ART.6 Modelling for South Africa suggests that with PDR levels >10% and no action taken, resistant virus alone could result in 890 000 deaths due to AIDS and 450 000 new infections from 2016 to 2030.7 Thus, WHO has implemented an aggressive global plan with a 5 year framework to face and tackle the problem of HIV drug resistance, which strongly recommends a switch to first-line ART regimens that do not include NNRTIs when national NNRTI PDR levels reach 10%.8 Modelling has identified the switch to generic, low-cost dolutegravir-based first-line regimens as the most cost-effective option in the Sub-Saharan Africa setting,9 with some LMIC already transitioning to first-line regimens containing this drug.10
Mexico has initiated a response to the WHO call to act upon the problem of rising HIV drug resistance.10,11 A nationally representative survey to assess the PDR level was implemented in 2015, finding an overall PDR prevalence of 13.5%, with 9.2% resistance to efavirenz/nevirapine.2,3 Moreover, we recently identified significant increasing temporal trends of NNRTI drug resistance in ART-naive individuals since 2008 in three geographical regions of Mexico.12 The Mexican HIV Programme has analysed different policy options to respond to the rising NNRTI HIV drug resistance levels observed. Although complicated by patent laws and international treaties,13 price negotiations are taking place to prepare a switch to first-line integrase strand transfer inhibitor (INSTI)-based regimens, as national ART guidelines still recommend efavirenz-containing regimens as the preferred option.14 In addition, research-funded baseline genotyping is being performed for all ART starters in a few large and key clinics, although the volume of annual initiators has not allowed the implementation of baseline HIV sequencing at national level.15 As Mexico is a large and complex country, with state programmes varying widely in size and quality,16 the National HIV Programme, in collaboration with a WHO-accredited reference laboratory in Mexico City, implemented a new PDR survey to assess PDR levels across eight sub-regions of the country to better adapt a programmatic response. We present results of this 2017–18 representative survey assessing the PDR level in persons starting or restarting first-line ART in eight regions of Mexico, following a WHO-recommended survey design.
Materials and methods
Study population
We designed PDR surveys for eight sub-regions within Mexico according to the WHO concept note on surveillance of HIV drug resistance in adults initiating ART.17 The Mexican sub-regions were previously defined by the National Institute of Statistics, Geography and Informatics, using a combination of natural, socio-economic and cultural factors, grouping states with similar characteristics (Figure S1, available as Supplementary data at JAC Online).18 All larger Ministry of Health clinics (which provide ART to 90% of all initiators within the Ministry of Health clinics) were included (74 in total, excluding one clinic in the state of Veracruz that did not respond to the enrolment call), allocating sample size per clinic according to the probability-proportional-to-size method, using the WHO PDR sample size calculator.19 Only Ministry of Health clinics were considered for this study, and social security clinics were not included. Ministry of Health clinics provide ART for 70% of all ART initiators in the country.20 We aimed for 5% precision in the HIV drug resistance outcome, with a 10% predicted NNRTI PDR level, 90% genotyping success rate, a 7%–17% predicted prevalence of restarters and 80%–90% of people initiating NNRTI-based regimens, depending on the sub-region. Sample size calculations were based on the number of ART initiators and re-initiators recorded in the national HIV database (SALVAR) in 2016.
Enrolment took place from September 2017 to March 2018. All consecutive adults (age ≥18 years) arriving at the sampled clinics about to initiate first-line ART were invited to participate. Both ART-naive persons and persons with prior antiretroviral exposure, restarting first-line ART were included.
Ethics
The study was approved by the Ethics Review Board of the National Institute of Respiratory Diseases (project code: E08-17), the institution accommodating the WHO-designated laboratory where HIV genotyping took place. After written informed consent, participants donated a single blood sample for HIV sequencing, and HIV viral load and CD4 T cell count assessment. Demographic data were collected through a questionnaire applied by a counsellor or technician at the time of blood sample donation.
HIV sequencing and drug resistance assessment
The HIV drug resistance assessment was performed at a WHO-designated laboratory in Mexico City, according to the WHO/HIV ResNet Laboratory Operational Framework.21 Briefly, HIV RNA was extracted from 1 mL of plasma (QIAamp Viral RNA Kit; QIAGEN, Valencia, CA, USA). HIV protease (PR), RT and integrase (IN) were amplified using previously described in-house-validated protocols (HXB2 nucleotides 16–297, 1–753, 1–864 for PR, RT and IN respectively).12,22 Sequences were obtained using the BigDye 3.1 chemistry on a 3730xl Genetic Analyser (ThermoFisher, Waltham, MA, USA) and assembled using the online automated base calling program Web ReCall (British Columbia Centre for Excellence in HIV/AIDS, BCCfE).23,24 HIV drug resistance was assessed using the Stanford HIVdb tool v8.6.1.25,26 Post-testing quality assurance of HIV drug resistance genotyping was carried out using the WHO BCCfE HIV drug resistance quality control tool for each sequencing batch and for each sub-regional survey at the end of enrolment.27 All sequence pairs with genetic distance <0.5% were repeated and confirmed. Sequences with PDR were defined as those with a Stanford score of ≥15 to the following antiretroviral drugs: nevirapine, efavirenz, any NRTI, darunavir/ritonavir, lopinavir/ritonavir, atazanavir/ritonavir, raltegravir, elvitegravir, dolutegravir or bictegravir.17
Network analyses
Genetic network inference was performed using HIV-TRACE (HIV-TRAnsmission Cluster Engine, UCSD),28 as previously described.29–37 Briefly, HIV PR-RT and IN sequences were concatenated and aligned to HXB2 reference. Codons associated with major drug resistance mutations (as defined by the Stanford HIV Drug Resistance Database) were removed, and pairwise Tamura Nei 93 (TN93) genetic distances were estimated between all sequences. Putative transmission linkage was inferred when genetic distance between two sequences was <1.5%. This threshold was selected based on previous work showing that within a mono-infected person, subtype B pol sequences typically do not diverge >1% over a decade.38 Shared drug resistance mutations (considering all mutations included in the Stanford HIV Drug Resistance Database) were defined when genetically linked sequences (i.e. genetic distance <1.5%) harboured the same mutation. Newman’s assortativity coefficients (i.e. tendency for nodes that share the same attribute to be linked) were measured as previously described,29 and their significance was assessed using the R package igraph.39,40
Statistical analysis
Statistical analyses were performed in GraphPad Prism 7 (San Diego, CA, USA) and STATA 15.0 (College Station, TX, USA). Comparisons among sub-regions were performed using Fisher’s exact or χ2 tests for categorical variables and Student’s t, Kruskal–Wallis or Mann–Whitney tests for continuous numerical variables, using the Centre-South sub-region as a reference. A multivariable logistic regression model to explore associations between overall, NNRTI, NRTI, efavirenz and efavirenz + tenofovir + emtricitabine PDR with demographic and clinical variables was applied to each sub-region. The variables included in the model were age, sex, log plasma viral load, CD4 count, HIV transmission risk factor, marital status, level of education, employment and presence of AIDS-associated clinical symptoms. Results were reported as adjusted OR (aOR) with 95% CI.
Results
Demographic and clinical characteristics of participants
From a total of 2445 individuals enrolled in 74 clinics, 2006 participants were included in the survey (excluding 32 because of PR-RT genotyping failure, 31 because of IN genotyping failure, 15 duplicates and 361 successful sequences exceeding the sampling goal) (Figure S2). The goal number of participants was achieved in the Centre-South, North-West, West, South-West and South-East, and reached 84%, 93% and 89% in the Centre-North, North-East and East, respectively (Figure S2). The majority of participants were young males, mainly MSM, with low to medium education level; although differences were observed between regions (Table 1). It was particularly interesting that the North-West, including the border city of Tijuana; the South-West, including Guerrero, Oaxaca and Chiapas, the poorest states in the country; and the East, including Veracruz and Puebla, with high rates of HIV infection, showed a higher proportion of females (21%, 22% and 19% respectively) and lower education (persons with degree: 21%, 27% and 20%). The South-East, including Tabasco, showed lower education (persons with degree: 22%) and higher unemployment rates (41%). As expected, the North-West included the highest proportion of persons who inject drugs (9%). The East showed the lowest median CD4 count (197 cells/mm3) (Table 1). In summary, these observations suggest significant sub-regional differences in the HIV epidemic that affect patterns of transmission, including HIV-resistant strains, which justify a sub-regional surveillance approach.
Table 1.
Summary of sociodemographic and clinical characteristics at genotyping by sub-region
| Demographic/clinical variable | Centre-South | Centre-North | North-West | North-East | West | East | South-West | South-East |
|---|---|---|---|---|---|---|---|---|
| Number of individuals | 294 | 190 | 269 | 239 | 280 | 244 | 236 | 254 |
| Median age, years (IQR) | 28 (24–35) | 31 (25–39)* | 32 (26–40)**** | 29 (24–36) | 31 (26–40)*** | 30 (24–39) | 30 (24–37) | 27 (24–36) |
| Proportion of females, n (%) | 24 (8.2) | 27 (14.2)* | 58 (21.6)**** | 38 (15.9)** | 42 (15.0)* | 47 (19.3)*** | 51 (21.6)**** | 41 (16.1)** |
| Median viral load, log copies/mL (IQR) | 5.0 (4.4–5.4) | 4.9 (4.4–5.4) | 4.7 (4.1–5.4)* | 4.8 (4.2–5.4) | 5.0 (4.4–5.5) | 5.0 (4.4–5.6) | 4.8 (4.3–5.4) | 4.9 (4.4–5.4) |
| Median CD4+ T counts, cells/mm3 (IQR) | 258 (104–406) | 257 (63–467) | 268 (96–470) | 241 (97–456) | 212 (66–412) | 176 (59–330)** | 210 (91–345) | 235 (87–420) |
| Median % CD4+ T counts, % (IQR) | 13 (7–20) | 15 (7.7–22) | 17 (8.5–26)*** | 14 (8–24) | 15 (7–22) | 12 (5–18) | 13 (7.2–19) | 14 (7–19) |
| Prior antiretroviral exposure, n (%) | 9 (3.1) | 12 (6.3) | 30 (11.2)*** | 12 (5.0) | 26 (9.3)** | 23 (9.4)** | 32 (13.6)**** | 14 (5.5) |
| Marital status, n (%) | ||||||||
| Single | 245 (83.3) | 145 (76.3) | 179 (66.5)**** | 173 (72.4)** | 213 (76.1)* | 161 (66.0)**** | 168 (71.2)** | 187 (73.6)** |
| Married | 11 (3.7) | 14 (7.4) | 26 (9.7)** | 11 (4.6) | 25 (8.9)* | 15 (6.1) | 23 (9.7)** | 25 (9.8)** |
| domestic partnership | 31 (10.5) | 23 (12.1) | 34 (12.6) | 52 (21.8)*** | 36 (12.9) | 46 (18.9)** | 40 (16.9)* | 38 (15.0) |
| unknown | 7 (2.4) | 8 (4.2) | 30 (11.2)**** | 3 (1.3) | 6 (2.1) | 22 (9.0)*** | 5 (2.1) | 4 (1.6) |
| Education, n (%) | ||||||||
| elementary | 19 (6.5) | 25 (13.2)* | 38 (14.1)** | 28 (11.7)* | 51 (18.2)**** | 49 (20.1)**** | 58 (24.6)**** | 36 (14.2)** |
| high school | 139 (47.3) | 91 (47.9) | 137 (50.9) | 132 (55.2) | 123 (43.9) | 122 (50.0) | 104 (44.1) | 153 (60.2)** |
| degree/technician | 128 (43.5) | 57 (30.0)** | 57 (21.2)**** | 72 (30.1)** | 90 (32.1)** | 48 (19.7)**** | 63 (26.7)**** | 57 (22.4)**** |
| postgraduate | 5 (1.7) | 8 (4.2)* | 3 (1.1)** | 2 (0.8)** | 6 (2.1)* | 1 (0.4) | 1 (0.4)*** | 0 (0.0)** |
| none | 0 (0.0) | 4 (2.1)* | 4 (1.5) | 4 (1.7)* | 3 (1.1) | 7 (2.9)** | 8 (3.4)** | 2 (0.8) |
| unknown | 3 (1.0) | 5 (2.6) | 30 (11.2)**** | 1 (0.4) | 7 (2.5) | 17 (7.0)*** | 2 (0.8) | 6 (2.4) |
| Employment, n (%) | ||||||||
| employed | 163 (55.4) | 102 (53.7) | 104 (38.7)**** | 130 (54.4) | 147 (52.5) | 104 (42.6)** | 126 (53.4) | 122 (48.0) |
| unemployed | 78 (26.5) | 65 (34.5) | 109 (40.5)*** | 91 (38.1)** | 98 (35.0)* | 82 (33.6) | 89 (37.7)** | 105 (41.3)*** |
| student | 45 (15.3) | 16 (8.4) | 19 (7.1) | 16 (6.7) | 26 (9.3) | 34 (13.9) | 14 (5.9) | 20 (7.9) |
| unknown | 8 (2.7) | 7 (3.7) | 37 (13.8)**** | 2 (0.8) | 9 (3.2) | 24 (9.8)*** | 7 (3.0) | 7 (2.8) |
| HIV transmission risk factor, n (%) | ||||||||
| heterosexual | 63 (21.4) | 59 (31.1)* | 86 (32.0)** | 62 (25.9) | 92 (32.9)** | 88 (36.1)*** | 108 (45.8)**** | 85 (33.5)** |
| MSM | 157 (53.4) | 113 (59.5) | 111 (41.3)** | 129 (54.0) | 146 (52.1) | 115 (47.1) | 110 (46.6) | 138 (54.3) |
| bisexual | 10 (3.4) | 8 (4.2) | 8 (3.0) | 27 (11.3)*** | 21 (7.5)* | 17 (7.0) | 13 (5.5) | 13 (5.1) |
| PWID | 5 (1.7) | 4 (2.1) | 23 (8.6)*** | 4 (1.7) | 10 (3.6) | 2 (0.8) | 0 (0.0) | 5 (2.0) |
| unknown | 59 (20.1) | 6 (3.2)**** | 41 (15.2) | 17 (7.1)**** | 11 (3.9)**** | 22 (9.0)*** | 5 (2.1)**** | 13 (5.1)**** |
| Clinical symptoms, n (%) | ||||||||
| asymptomatic | 138 (46.9) | 63 (33.2)** | 42 (15.6)**** | 96 (40.2) | 110 (39.3) | 88 (36.1)* | 96 (40.7) | 77 (30.3)**** |
| symptomatic non-AIDS | 37 (12.6) | 19 (10.0) | 14 (5.2)** | 45 (18.8) | 33 (11.8) | 32 (13.1) | 36 (15.3) | 14 (5.5)** |
| symptomatic AIDS | 49 (16.7) | 105 (55.3)**** | 171 (63.6)**** | 75 (31.4)**** | 119 (42.5)**** | 78 (32.0)**** | 78 (33.1)**** | 141 (55.5)**** |
| unknown | 70 (23.8) | 3 (1.6)**** | 42 (15.6)* | 23 (9.6)**** | 18 (6.4)**** | 46 (18.9) | 26 (11.0)*** | 22 (8.7)**** |
| Subtype, n (%) | ||||||||
| B | 292 (99.3) | 185 (97.4) | 269 (100.0) | 238 (99.6) | 274 (97.9) | 242 (99.2) | 232 (98.3) | 251 (98.8) |
| non-B | 2 (0.7) | 5 (2.6) | 0 (0.0) | 1 (0.4) | 6 (2.1) | 2 (0.8) | 4 (1.7) | 3 (1.2) |
Centre-South was used as a reference for comparisons.
P < 0.05,
P < 0.01,
P < 0.001,
P < 0.0001 by Fisher exact or Mann–Whitney test. PWID, people who inject drugs.
PDR levels in eight sub-regions of Mexico
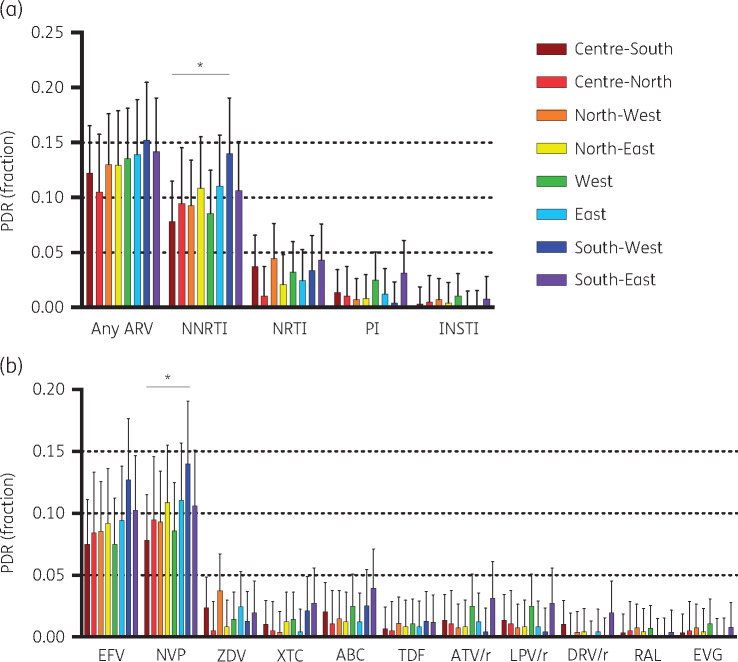
PDR to NNRTIs in all ART initiators was higher than to the other drug classes in all eight sub-regions (P < 0.0001), crossing the 10% threshold in the North-East, East, South-West and South-East (Figure 1a and Table 2). PDR to all other drug classes remained <5% in all sub-regions, being particularly low (≤1%) to INSTIs (Figure 1a). In relation to the currently preferred first-line option (efavirenz + tenofovir + emtricitabine), PDR to efavirenz in all ART initiators ranged from 7.5% in the Centre-South and West to 12.7% in the South-West; while PDR to emtricitabine and tenofovir remained low in all regions (<3% and 1.5% respectively) (Figure 1b). No PDR to dolutegravir/bictegravir was observed.
Figure 1.
HIV PDR in eight sub-regions of Mexico. (a) PDR prevalence by drug class. (b) PDR prevalence by antiretroviral drug. Only relevant drugs in the Mexican setting are shown. No PDR to dolutegravir was observed in any of the sub-regions. *P < 0.05 Fisher’s exact test. EFV, efavirenz; NVP, nevirapine; ZDV, zidovudine; XTC, lamivudine/emtricitabine; ABC, abacavir; TDR, tenofovir; ATV/r, boosted atazanavir; LPV/r, boosted lopinavir; DRV/r, boosted darunavir; RAL, raltegravir; EVG, elvitegravir. This figure appears in colour in the online version of JAC and in black and white in the printed version of JAC.
Table 2.
PDR levels in persons starting or restarting first-line ART in eight regions of Mexico
| HIV drug resistance, % (95% CI) |
|||||
|---|---|---|---|---|---|
| Any ARV | NRTI | NNRTI | TDF+FTC+EFV | EFV | |
| Centre-South | |||||
| All ART initiators (N = 294) | 12.2 (9.0–16.5) | 3.7 (2.1–6.6) | 7.8 (5.2–11.5) | 9.2 (6.4–13.1) | 7.5 (5.0–11.1) |
| women (N = 24) | 16.7 (6.3–37.5) | 4.2 (0.6–25.3) | 12.5 (4.0–33.1) | 12.5 (4.0–33.1) | 8.3 (2.0–2.9) |
| men (N = 270) | 11.9 (8.5–16.3) | 3.7 (2.0–6.8) | 7.4 (4.8–11.2) | 8.9 (6.0–12.9) | 7.4 (4.8–11.2) |
| ART-naive (N = 285) | 11.9 (8.6–16.2) | 3.9 (2.1–6.8) | 7.7 (5.1–11.5) | 9.1 (6.3–13.1) | 7.4 (4.8–11.0) |
| women (N = 22) | 13.6 (4.3–35.5) | 4.5 (0.6–27.1) | 9.1 (2.2–30.7) | 9.1 (2.2–30.7) | 4.5 (0.1–27.1) |
| men (N = 263) | 11.8 (8.4–16.3) | 3.8 (2.1–6.9) | 7.6 (5.0–11.5) | 9.1 (6.2–13.3) | 7.6 (5.0–11.5) |
| ART-exposed (N = 9) | 22.2 (5.1–60.2) | 0 | 11.1 (1.3–53.6) | 11.1 (1.3–53.6) | 11.1 (1.3–53.6) |
| women (N = 2) | 50.0 (1.9–98.1) | 0 | 50.0 (1.9–98.1) | 50.0 (1.9–98.1) | 50.0 (1.9–98.1) |
| men (N = 7) | 14.3 (1.7–62.2) | 0 | 0 | 0 | 0 |
| Centre-North | |||||
| All ART initiators (N = 190) | 10.5 (6.9–15.8) | 1.1 (0.3–4.1) | 9.5 (6.0–14.6) | 8.4 (5.2–13.3) | 8.4 (5.2–13.3) |
| women (N = 27) | 7.4 (1.8–25.8) | 0 | 7.4 (1.8–25.9) | 7.4 (1.8–25.9) | 7.4 (1.8–25.9) |
| men (N = 163) | 11.0 (7.1–16.9) | 1.2 (0.3–4.8) | 9.8 (6.1–15.4) | 8.6 (5.1–14.0) | 8.6 (5.1–14.0) |
| ART-naive (N = 178) | 8.4 (5.1–13.5) | 0.6 (0.1–3.9) | 7.9 (4.7–12.9) | 6.7 (3.9–11.5) | 6.7 (3.9–11.5) |
| women (N = 25) | 8.0 (1.9–27.6) | 0 | 8.0 (1.9–27.6) | 8.0 (1.9–27.6) | 8.0 (1.9–27.6) |
| men (N = 153) | 8.5 (5.0–14.1) | 0.7 (0.1–4.5) | 7.8 (4.5–13.3) | 6.5 (3.5–11.7) | 6.5 (3.5–11.7) |
| ART-exposed (N = 12) | 41.7 (17.7–70.3) | 8.3 (1.0–44.0) | 33.3 (12.4–63.9) | 33.33 (12.4–63.9) | 33.3 (12.4–63.9) |
| women (N = 2) | 0 | 0 | 0 | 0 | 0 |
| men (N = 10) | 50.0 (21.1–78.9) | 10.0 (1.2–49.9) | 40.0 (14.8–71.9) | 40.0 (14.8–71.9) | 40.0 (14.8–71.9) |
| North-West | |||||
| All ART initiators (N = 269) | 13.0 (9.5–17.6) | 4.5 (2.5–7.7) | 9.3 (6.4–13.4) | 10.0 (7.0–14.3) | 8.6 (5.7–12.5) |
| women (N = 58) | 17.2 (9.5–29.3) | 6.9 (2.6–17.2) | 15.5 (8.2–27.4) | 17.2 (9.5–29.3) | 15.5 (8.2–27.4) |
| men (N = 211) | 11.8 (8.1–17.0) | 3.8 (1.9–7.4) | 7.6 (4.7–12.0) | 8.1 (5.1–12.6) | 6.6 (4.0–10.9) |
| ART-naive (N = 239) | 10.0 (6.8–14.6) | 2.9 (1.4–6.0) | 7.1 (4.5–11.2) | 7.1 (4.5–11.1) | 6.3 (3.8–10.2) |
| women (N = 49) | 14.3 (6.9–27.2) | 6.1 (2.0–17.5) | 12.2 (5.6–24.9) | 14.3 (6.9–27.2) | 12.2 (5.6–24.9) |
| men (N = 190) | 8.9 (5.6–13.9) | 2.1 (0.8–5.5) | 5.8 (3.2–10.2) | 5.3 (2.8–9.5) | 4.7 (2.5–8.9) |
| ART-exposed (N = 30) | 36.7 (21.4–55.2) | 16.7 (7.0–34.9) | 26.7 (13.7–45.5) | 33.3 (18.7–52.1) | 26.7 (13.7–45.5) |
| women (N = 9) | 33.3 (10.3–68.5) | 11.1 (1.3–53.6) | 33.3 (10.2–68.7) | 33.3 (10.2–68.7) | 33.3 (10.2–68.7) |
| men (N = 21) | 38.1 (20.0–60.3) | 19.0 (7.1–42.0) | 23.8 (10.0–46.8) | 33.3 (16.4–56.1) | 23.8 (10.0–46.8) |
| North-East | |||||
| All ART initiators (N = 239) | 13.0 (9.3–17.9) | 2.1 (0.9–4.9) | 10.9 (7.5–15.5) | 10.0 (6.8–14.6) | 9.2 (6.1–13.6) |
| women (N = 38) | 15.8 (7.2–31.2) | 2.6 (0.4–16.9) | 13.2 (5.5–28.3) | 15.8 (7.2–31.3) | 13.2 (5.5–28.3) |
| men (N = 201) | 12.4 (8.5–17.8) | 2.0 (0.7–5.2) | 10.4 (6.9–15.5) | 9.0 (5.7–13.8) | 8.5 (5.3–13.2) |
| ART-naive (N = 227) | 11.9 (8.3–16.8) | 1.3 (0.4–4.0) | 10.1 (6.8–14.8) | 8.8 (5.7–13.2) | 8.4 (5.4–12.8) |
| women (N = 31) | 19.4 (8.8–37.3) | 3.2 (0.4–20.2) | 16.1 (6.8–33.7) | 19.4 (8.8–37.3) | 16.1 (6.8–33.7) |
| men (N = 196) | 10.7 (7.1–15.9) | 1.0 (0.3–4.0) | 9.2 (5.9–14.1) | 7.1 (4.3–11.7) | 7.1 (4.3–11.7) |
| ART-exposed (N = 12) | 33.3 (12.5–63.7) | 16.7 (3.9–49.7) | 25.0 (7.8–56.9) | 33.3 (12.4–63.9) | 25.0 (7.8–56.9) |
| women (N = 7) | 0 | 0 | 0 | 0 | 0 |
| men (N = 5) | 80.0 (25.6–97.9) | 40.0 (8.2–83.3) | 60.0 (16.7–91.8) | 80.0 (25.3–97.9) | 60.0 (16.7–91.8) |
| West | |||||
| All ART initiators (N = 280) | 13.6 (10.0–18.1) | 3.2 (1.7–6.1) | 8.6 (5.8–12.5) | 9.6 (6.7–13.7) | 7.5 (4.9–11.2) |
| women (N = 42) | 21.4 (11.4–36.5) | 7.1 (2.3–20.2) | 9.5 (3.6–23.1) | 14.3 (6.5–28.6) | 7.1 (2.3–20.2) |
| men (N = 238) | 12.2 (8.6–17.0) | 2.5 (1.1–5.5) | 8.4 (5.5–12.7) | 8.8 (5.8–13.2) | 7.6 (4.8–11.7) |
| ART-naive (N = 254) | 11.8 (8.4–16.4) | 1.6 (0.6–4.1) | 7.9 (5.1–11.9) | 7.5 (4.8–11.4) | 6.7 (4.2–10.5) |
| women (N = 35) | 14.3 (6.0–30.4) | 0 | 8.6 (2.7–23.8) | 5.7 (1.4–20.5) | 5.7 (1.4–20.5) |
| men (N = 219) | 11.4 (7.8–16.4) | 1.8 (0.7–4.8) | 7.8 (4.9–12.1) | 7.8 (4.9–12.1) | 6.8 (4.2–11.1) |
| ART-exposed (N = 26) | 30.8 (16.0–51.0) | 19.2 (8.0–39.3) | 15.4 (5.7–35.2) | 30.8 (15.9–51.1) | 15.4 (5.7–35.2) |
| women (N = 7) | 57.1 (20.9–87.1) | 42.9 (12.8–79.3) | 14.3 (1.6–62.5) | 57.1 (20.7–87.2) | 14.3 (1.6–62.5) |
| men (N = 19) | 21.1 (7.9–45.3) | 10.5 (2.5–34.9) | 15.8 (5.0–40.2) | 21.1 (7.8–45.5) | 15.8 (5.0–40.2) |
| East | |||||
| All ART initiators (N = 244) | 13.9 (10.1–18.9) | 2.5 (1.1–5.4) | 11.1 (7.7–15.7) | 10.2 (7.0–14.7) | 9.4 (6.3–13.8) |
| women (N = 47) | 12.8 (5.8–25.8) | 2.1 (0.3–14.0) | 8.5 (3.2–20.8) | 6.4 (2.0–18.3) | 6.4 (2.0–18.3) |
| men (N = 197) | 14.2 (10.0–19.8) | 2.5 (1.1–6.0) | 11.7 (7.9–17.0) | 11.2 (7.5–16.4) | 10.2 (6.6–15.2) |
| ART-naive (N = 221) | 12.7 (8.9–17.8) | 1.8 (0.7–4.7) | 10.0 (6.6–14.7) | 9.0 (5.9–13.6) | 8.1 (5.2–12.6) |
| women (N = 38) | 13.2 (5.5–28.2) | 2.6 (0.4–16.8) | 7.9 (2.5–22.1) | 5.3 (1.3–19.1) | 5.3 (1.3–19.1) |
| men (N = 183) | 12.6 (8.5–18.2) | 1.6 (0.5–5.0) | 10.4 (6.7–15.7) | 9.8 (6.3–15.1) | 8.7 (5.4–13.8) |
| ART-exposed (N = 23) | 26.1 (12.0–47.8) | 8.7 (2.1–29.8) | 21.7 (9.1–43.5) | 21.7 (9.1–43.5) | 21.7 (9.1–43.5) |
| women (N = 9) | 11.1 (1.4–53.2) | 0 | 11.1 (1.3–53.6) | 11.1 (1.3–53.6) | 11.1 (1.3–53.6) |
| men (N = 14) | 35.7 (15.1–63.4) | 14.2 (3.4–44.4) | 28.6 (10.6–57.4) | 28.6 (10.6–57.4) | 28.6 (10.6–57.4) |
| South-West | |||||
| All ART initiators (N = 236) | 15.3 (11.2–20.4) | 3.4 (1.7–6.6) | 14.0 (10.1–19.0) | 12.7 (9.0–17.6) | 12.7 (9.0–17.6) |
| women (N = 51) | 25.5 (15.3–39.3) | 5.9 (1.9–16.9) | 21.6 (12.3–35.1) | 19.6 (10.8–33.0) | 19.6 (10.8–33.0) |
| men (N = 185) | 12.4 (8.4–18.0) | 2.7 (1.1–6.3) | 11.9 (7.9–17.4) | 10.8 (7.1–16.2) | 10.8 (7.1–16.2) |
| ART-naive (N = 204) | 10.8 (7.2–15.9) | 1.5 (0.5–4.5) | 9.3 (6.0–14.2) | 7.8 (4.9–12.4) | 7.8 (4-9–12.4) |
| women (N = 38) | 15.8 (7.2–31.2) | 2.6 (0.4–16.8) | 10.5 (4.0–25.2) | 7.9 (2.5–22.1) | 7.9 (2.5–22-1) |
| men (N = 166) | 9.6 (6.0–15.2) | 1.2 (0.3–4.7) | 9.0 (5.5–14.5) | 7.8 (4.6–13.0) | 7.8 (4.6–13.0) |
| ART-exposed (N = 32) | 43.8 (27.7–61.3) | 15.6 (6.5–33.0) | 43.8 (27.6–61.4) | 43.8 (27.6–61.4) | 43.8 (27.6–61.4) |
| women (N = 13) | 53.8 (27.3–78.4) | 15.4 (3.6–46.9) | 53.8 (27.1–78.5) | 53.8 (27.1–78.5) | 53.8 (27.1–78.5) |
| men (N = 19) | 36.8 (18.3–60.3) | 15.8 (5.0–40.2) | 36.8 (18.2–60.5) | 36.8 (18.2–60.5) | 36.8 (18.2–60.5) |
| South-East | |||||
| All ART initiators (N = 254) | 14.2 (10.4–19.0) | 4.3 (2.4–7.7) | 10.6 (7.4–15.1) | 11.4 (8.0–16.0) | 10.2 (7.1–14.6) |
| women (N = 41) | 12.2 (5.1–26.4) | 7.3 (2.3–20.7) | 9.8 (3.7–23.6) | 12.2 (5.1–26.4) | 9.8 (3.7–23.6) |
| men (N = 213) | 14.6 (10.4–20.0) | 3.8 (1.9–7.3) | 10.8 (7.3–15.7) | 11.3 (7.7–16.3) | 10.3 (6.9–15.2) |
| ART-naive (N = 240) | 12.5 (8.9–17.3) | 2.5 (1.1–5.5) | 9.2 (6.1–13.5) | 10.0 (6.8–14.5) | 9.2 (6.1–13.5) |
| women (N = 35) | 5.7 (1.4–20.5) | 0 | 5.7 (1.4–20.5) | 5.7 (1.4–20.5) | 5.7 (1.4–20.5) |
| men (N = 205) | 13.7 (9.6–19.1) | 2.9 (1.3–6.4) | 9.8 (6.4–14.7) | 10.7 (7.2–15.8) | 9.8 (6.4–14.7) |
| ART-exposed (N = 14) | 42.9 (20.0–69.2) | 35.7 (15.0–63.5) | 35.7 (15.0–63.5) | 35.7 (15.0–63.5) | 28.6 (10.6–57.4) |
| women (N = 6) | 50.0 (14.8–85.2) | 50.0 (14.6–85.4) | 33.3 (7.1–76.5) | 50.0 (14.6–85.4) | 33.3 (7.1–76.5) |
| men (N = 8) | 37.5 (11.5–73.5) | 25.0 (5.6–65.1) | 37.5 (11.4–73.7) | 25.0 (56.1–65.1) | 25.0 (5.6–65.1) |
| All sub-regions together | |||||
| All ART initiators (N = 2006) | 13.3 (11.8–14.8) | 3.2 (2.5–4.1) | 10.1 (8.9–11.5) | 10.2 (9.0–11.6) | 9.1 (7.9–10.5) |
| women (N = 328) | 16.8 (13.1–21.2) | 4.9 (3.0–7.8) | 12.8 (9.6–16.9) | 13.7 (10.4–17.9) | 11.6 (8.5–15.5) |
| men (N = 1678) | 12.6 (11.1–14.3) | 2.9 (2.2–3.8) | 9.6 (8.3–11.1) | 9.5 (8.2–11.0) | 8.6 (7.4–10.1) |
| ART-naive (N = 1848) | 11.4 (10.0–12.9) | 2.1 (1.5–2.9) | 8.6 (7.4–10.0) | 8.3 (7.2–9.7) | 7.6 (6.5–8.9) |
| women (N = 273) | 13.2 (9.7–17.8) | 2.6 (1.2–5.3) | 9.9 (6.9–14.0) | 9.5 (6.6–13.6) | 8.4 (5.7–12.4) |
| men (N = 1575) | 11.0 (9.6–12.7) | 2.0 (1.4–2.9) | 8.4 (7.1–9.9) | 8.1 (6.9–9.6) | 7.4 (6.2–8.8) |
| ART-exposed (N = 158) | 35.4 (28.4–43.2) | 15.8 (10.9–22.4) | 27.8 (21.4–35.4) | 32.3 (25.4–40.0) | 27.2 (20.8–34.7) |
| women (N = 55) | 34.5 (23.1–48.1) | 16.4 (8.7–28.7) | 27.3 (17.1–40.6) | 34.5 (23.1–48.1) | 27.3 (17.1–40.6) |
| men (N = 103) | 35.9 (27.2–45.7) | 15.5 (9.7–23.9) | 28.2 (20.3–37.6) | 31.1 (22.9–40.7) | 27.2 (19.4–36.6) |
TDF, tenofovir; FTC, emtricitabine; EFV, efavirenz; ARV, antiretroviral.
As expected, the PDR level (mainly to NNRTIs and NRTIs) was significantly higher in re-initiators compared with ART-naive individuals in most of the regions, even with wide CIs due to small numbers of re-initiators in each sub-region (Table 2 and Figure S3). Considering all sub-regions together, ART-exposed individuals showed PDR levels to any antiretroviral three times higher than ART-naive persons: 35.4% (95% CI: 28.4%–43.2%) versus 11.4% (95% CI: 10.0%–12.9%), P < 0.0001 (Table S1). The NNRTI PDR reached 44% in the South-West, and NRTI PDR reached 36% in the South-East in re-initiators (Figure S3a and b). No differences in PI or INSTI PDR were observed in re-initiators between sub-regions (Figure S3c and d). On the other hand, no differences were observed in the sub-regional PDR level to NNRTIs (ranging from 7.1% to 10.1%; 8.6% overall), or any other drug class, when considering only ART-naive individuals, except for a slightly higher NRTI PDR prevalence in the Centre-South compared with the Centre-North (3.9% versus 0.6%, P = 0.03) (Table 2).
Considering all ART initiators, we observed a higher level of NNRTI PDR in the South-West, including the three poorest states of Mexico, compared with the Centre-South (P = 0.02) (Figure S4a). The proportion of persons re-initiating ART varied significantly by region (from 3% to 14%) (Figure S4b). Although, overall, the proportion of re-initiators did not correlate with the NNRTI PDR level by region (r = 0.3, P = 0.5), the higher prevalence of NNRTI PDR in the South-West coincided with the highest proportion of re-initiators in this sub-region (Figure S4).
We observed a higher prevalence of PDR to any drug in all women included in the study compared with men (16.8% versus 12.6%, P = 0.05), which was particularly marked in the South-West (P = 0.03) (Table S1, Table 2 and Figure S5). The PDR to the currently preferred first-line option (efavirenz + tenofovir + emtricitabine) was also higher in women than in men (13.7% versus 9.5%, P = 0.03) (Table S1). Interestingly, compared with 15% of all ART-naive persons being female, the proportion of re-initiators that were female reached 35% (P < 0.0001) (ranging from 17% to 58% in the sub-regions); and similarly, the overall proportion of re-initiators among females was 17% (ranging from 7% to 25% in the sub-regions), contrasting with 6% in males (P < 0.0001). Among all males, MSM showed lower NNRTI PDR than heterosexual men (7.9% versus 12.1%; OR: 0.64, 95% CI 0.46–0.89, P < 0.008).
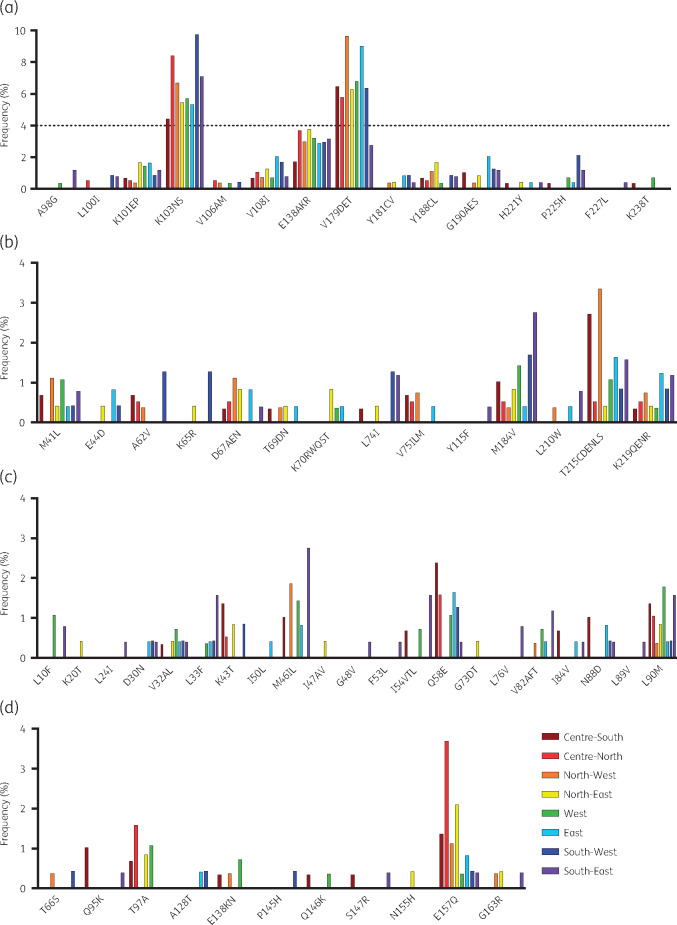
The most common surveillance drug resistance mutation was K103N, ranging in frequency in all ART initiators from 4.4% in the Centre-South to 9.7% in the South-West (Figure 2). Sixty-three per cent (128 of 203) of NNRTI PDR cases were associated with K103N. The prevalence of K103N was significantly higher in the South-West compared with the Centre-South (P = 0.02), coinciding with a trend toward higher efavirenz PDR in this region (P = 0.06) (Figure S6a and b). Additionally, re-initiators in the Centre-North, North-West and South-West showed significantly higher K103N prevalence than ART-naive persons (P < 0.01 in all cases) (Figure S6c). Considering other surveillance NNRTI mutations, only K101EP and V108I were found in all sub-regions (frequency ≤2%). Among NRTI surveillance resistance mutations, M184V was observed in all sub-regions and ranged in frequency from 0.4% in the North-West and West to 1.7% in the South-West. K65R was only observed in the North-East (0.4%) and South-West (1.3%) (Figure 2).
Figure 2.
Frequency of drug resistance mutations in eight sub-regions of Mexico. Mutations are divided by drug class: (a) NNRTIs, (b) NRTIs, (c) PIs, (d) INSTIs. All mutations considered in the Stanford HIVdb program were analysed. Mutations not observed in any of the regions are not shown. Scale for (a) is different from the rest. This figure appears in colour in the online version of JAC and in black and white in the printed version of JAC.
Taken together, our observations confirm NNRTI PDR levels reaching the 10% threshold in Mexico, and suggest sub-regional differences in HIV PDR, possibly associated with poverty, risk of HIV transmission and characteristics of local HIV programmes resulting in varying quality of viral load follow-up, retention in care and viral suppression, which may be associated with HIV drug resistance.
Associations between PDR level and demographic/clinical variables
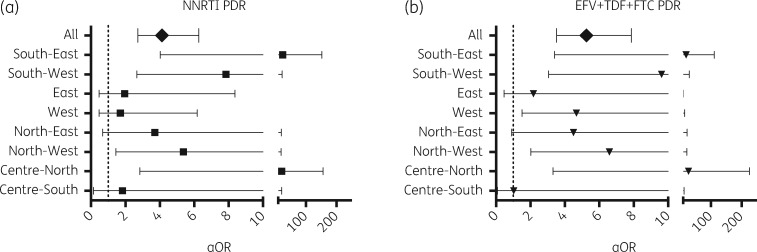
Considering all participants, persons with PDR to any drug included a higher proportion of females (20.7% versus 15.7%; P = 0.04), a higher proportion of restarters (21.1% versus 5.9%; P < 0.0001) and a lower proportion of MSM (42.9% versus 52.0%; P = 0.01) (Table S2). Using a multivariable logistic regression model including all demographic and clinical variables (see Materials and methods), the higher NNRTI PDR remained significantly associated with previous antiretroviral exposure in the Centre-North, North-West, South-West and South-East (aOR: 21, 5, 8 and 25 respectively; P < 0.05) (Figure 3; Tables S3–S10). The higher NNRTI PDR was associated with higher education in the North-West (postgraduate level aOR: 93, P = 0.01) and East (degree level aOR: 6; P = 0.04), with unemployment in the North-West (aOR: 13; P = 0.005) and being a student in the West (aOR: 6; P = 0.014). Lower NNRTI PDR was associated with MSM in the North-East (aOR: 0.2; P = 0.02) (Tables S3–S10). PDR to the preferred antiretroviral regimen, efavirenz + tenofovir + emtricitabine, was also significantly associated with previous antiretroviral exposure in the Centre-North, North-West, West, South-West and South-East (aOR: 27, 7, 5, 10 and 20 respectively; P < 0.01) (Figure 3; Tables S3–S10). Considering all participants together, only previous exposure to antiretrovirals remained significantly associated to higher PDR to any drug, NNRTI, NRTI, efavirenz + tenofovir + emtricitabine or efavirenz alone (aOR: 5, 4, 9, 5 and 5, respectively; P < 0.0001 in all cases), while using injectable drugs was associated with higher NNRTI PDR (aOR: 2, P = 0.03) (Table S11).
Figure 3.
PDR association with previous antiretroviral exposure in a multivariable model. A multivariable logistic regression model to explore associations between PDR to (a) NNRTIs and to (b) the preferred first-line ART option (efavirenz + tenofovir + emtricitabine) with demographic and clinical variables was applied to each sub-region. Variables considered included: age, sex, log plasma viral load, CD4 count, HIV transmission risk factor, marital status, level of education, employment and presence of AIDS-associated clinical symptoms. Associations with prior exposure to antiretroviral drugs are shown as aOR with 95% CI. EFV, efavirenz; TDF, tenofovir; FTC, emtricitabine.
These observations confirm the higher risk of re-initiators for HIV drug resistance and suggest specific sub-regional scenarios associated with the demographic and behavioural characteristics of the local population.
HIV drug resistance transmission network
A total of 486 (24.2%) sequences were linked forming transmission clusters ranging in size from 2 to 13 individuals (167 dyads, 44 clusters with 3 or more individuals) (Figure 4a and b). Forty-five individuals in 21 clusters (17 dyads, 4 clusters of three individuals) shared drug resistance mutations, mostly to NNRTIs (19 of 21 clusters) (Figure 4c). The most frequently putatively transmitted mutations were K103N (15 individuals in 7 clusters) and V179D/E (17 individuals in 8 clusters). Individuals in clusters were significantly younger (31 versus 33 years, P = 0.0003), arrived earlier to clinical care (CD4 count 302 versus 274 cells/mm3, P = 0.02), included a higher proportion of students (20.4% versus 13.2%; P < 0.0001), a lower proportion of restarters (2.5% versus 9.6%, P < 0.0001) and had a lower PDR to any drug (9.9% versus 14.3%, P = 0.01), NRTIs (1.2% versus 3.8%, P = 0.004) or NNRTIs (7.6% versus 10.9%, P = 0.04). No significant differences were observed in the proportion of clustering sequences by sub-region (range 20.0%–28.4%; P = 0.2), nor in the proportion of clustering sequences sharing drug resistance mutations by sub-region (range 0.8%–3.4%; P = 0.4) (Figure S7). Of note, even when clustering individuals showed a lower proportion of restarters than non-clustering individuals, within clustering individuals, no difference was observed in the proportion of restarters among individuals sharing and not sharing drug resistance mutations (4.4% versus 2.3%, P = 0.3).
Figure 4.
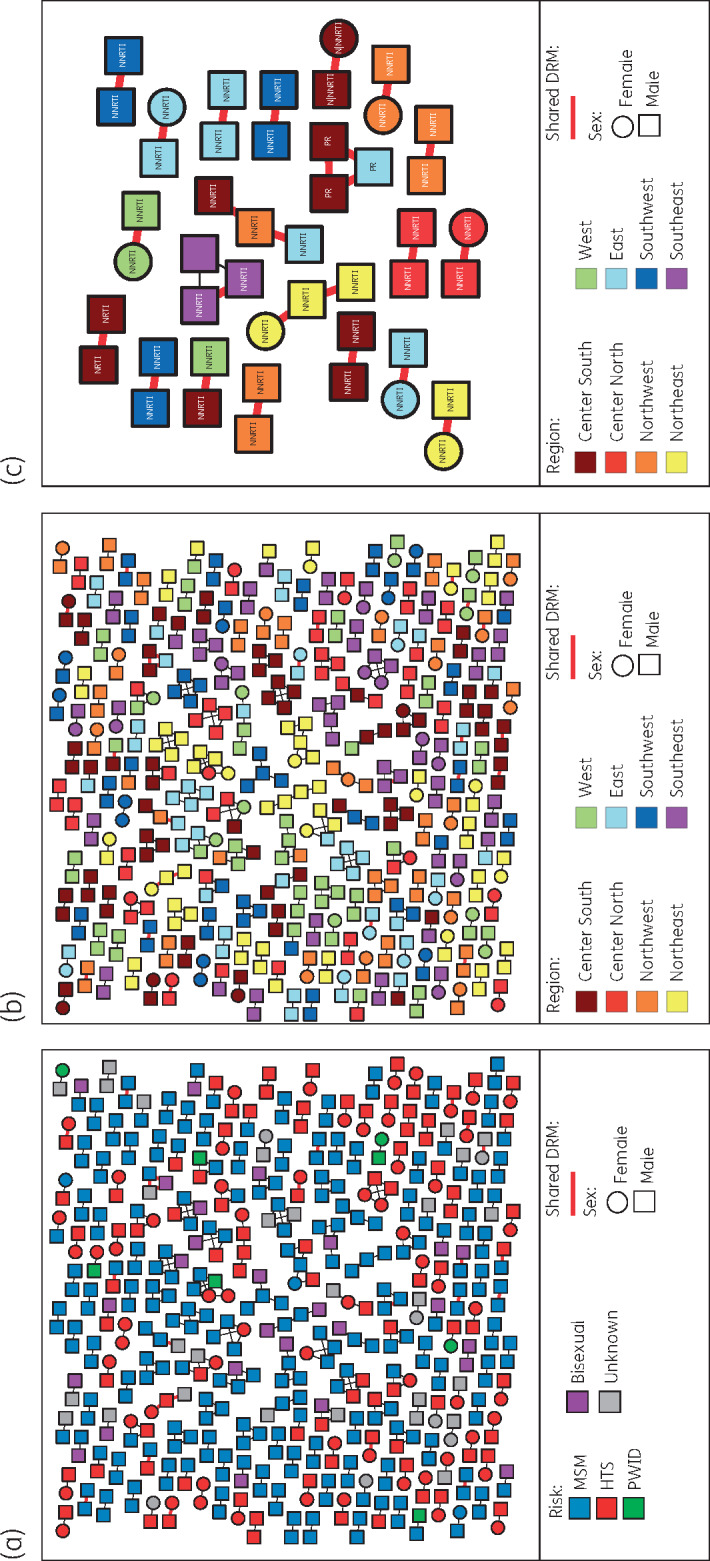
HIV transmission network across Mexico. Nodes represent clustering sequences at a TN93 genetic distance threshold of <1.5%. Shape of nodes denotes gender. Nodes are coloured by (a) risk factor for HIV transmission; (b) and (c) geographical sub-region (see Figure S1). Only clusters of sequences that shared DRMs are shown in (c). Edges in bold red indicate sequences with shared DRMs. HTS, heterosexual; PWID, persons who inject drugs; DRM, drug resistance mutation. This figure appears in colour in the online version of JAC and in black and white in the printed version of JAC.
Interestingly, the genetic network showed high assortativity by location (sub-region and city, P < 0.0001) and risk factor for HIV transmission (P = 0.02). Nevertheless, no assortativity was observed by gender (P = 0.4) or age (P = 0.2) (Figure S8). Geospatial simulation showed that genetically linked individuals resided in significantly closer proximity (P < 0.001) compared with a random distribution (median distance considering city midpoints: 0 km versus 838 km), with 226 of a total of 329 links (69%) inferred between sequences from the same city.
Taken together, these analyses confirm high compartmentalization of the epidemic in Mexico by geographic location and risk of HIV transmission, and demonstrate evidence of HIV drug resistance mutation transmission within local clusters with a limited role of re-initiators.
Participant follow-up after the survey
Follow-up information was obtained from the national HIV database, SALVAR, 5 months after closing the survey (August 2018). Registries for 1484 of the 2006 (74%) participants were found in the database. All the registered participants had already started ART, but information on the current regimen was only available for 609: 68% NNRTI-based, 20% INSTI-based, 11% PI-based and 1% NRTI only. From the registered participants, 1146 (77%) had an HIV viral load result at least 3 months after the date of enrolment, with 83.6% and 69.2% under 200 and 40 copies/mL respectively (Table S12). No differences in viral suppression by sub-region were observed (P = 0.2). Persons with NNRTI PDR were significantly less suppressed compared with persons without NNRTI PDR (viral load <200 copies/mL: 75.4% versus 84.5%; P = 0.01), were on NNRTI-based regimens less frequently (32.0% versus 73.2%) and on INSTI-based (41.3% versus 17.4%) or PI-based (24.0% versus 8.6%) regimens more frequently (all cases P < 0.0001).
Discussion
The present sub-national survey confirms previous observations of our 2015 national survey, with NNRTI PDR level approaching the 10% threshold in Mexico, ranging between sub-regions from 7.1% to 10.1% in ART-naive persons (9.2% in the national survey).2 Although the NNRTI PDR prevalence was similar in the different sub-regions when considering only antiretroviral-naive persons, differences were found when including persons with prior antiretroviral exposure (ranging from 7.8% to 14.0%), with higher PDR in the South-West, a sub-region including the poorest states (>60% of the population with income under the cost of the basic food basket).41 Even when the WHO methodology is not powered to generate HIV drug resistance prevalence in re-initiators due to small numbers resulting in wide CIs for each sub-regional survey, multivariable analyses considering all the participants identified previous exposure to antiretrovirals as the only significant variable associated with higher PDR to NNRTIs (aOR: 4.1, 95% CI: 2.7–6.3; P < 0.0001) and NRTIs (aOR 8.8, 4.9–16.0; P < 0.0001), with prevalence estimates of 27.8% (95% CI: 21.4%–35.4%) and 15.8% (95% CI: 10.9–22.4), respectively (Table S1). Our study thus agrees with previous observations that prior exposure to antiretroviral drugs is a significant risk factor for PDR,10 with 3 in 10 persons restarting ART showing NNRTI PDR (overall in this study: 8.6% in ART-naive versus 27.8% in prior-exposed; P < 0.0001). The fact that this association was only significant for some sub-regions (i.e. Centre-North, North-West, South-West and South-East; Tables S3–S10), possibly suggests distinct epidemiological scenarios in different parts of the country.
An important observation of our study was an overall higher PDR level in women, compared with men (P = 0.04), mainly in the South-West (although this association was lost after multivariable analysis). This is a common observation in other LMIC, with women being overall twice as likely as men to harbour resistant viruses.2,10 Significant differences have been previously identified in the socio-economic and behavioural profiles between women and men living with HIV in Mexico.42 In agreement with these observations, our survey suggests higher vulnerability of women compared with men, associated with lower socio-economic and education level. Indeed, the women in our survey had significantly lower literacy (elementary or lower: 41% versus 12%, P < 0.0001; degree or higher: 4% versus 35%, %, P < 0.0001) and lower employment rate (29% versus 54%, P < 0.0001). Women also presented to clinical care later than men (mean age: 34 versus 32 years, P < 0.0001; mean CD4 count: 244 versus 288 cells/mm3, P = 0.002) and, importantly, the prevalence of restarters among women was significantly higher than in men (17% versus 6%, P < 0.0001).
Network analyses suggested high geographical compartmentalization of HIV transmission within each sub-region (Figure 4 and Figure S8) and of drug resistance transmission (only 2 of 21 clusters sharing drug resistance mutations included individuals from more than one sub-region) (Figure 4), with different sub-epidemics characterized by distinct demographic, behavioural and clinical characteristics (Table 1). Differences in PDR levels, strongly associated with the proportion of groups with a higher prevalence of PDR such as re-initiators and persons with lower socio-economic status with heterosexual risk of transmission in different sub-regions, suggest that both demographic characteristics of the population and heterogeneous quality of local HIV programmes (based on viral load follow-up, retention in care, average viral suppression, mortality, as proxy)16 could be associated with increasing local HIV PDR. Thus, it is not surprising that the poorest sub-region, characterized by low education level, high proportion of women among persons living with HIV, higher rates of heterosexual transmission, as well as the highest rate of ART defaulting (13.6% restarters) (Table 1), was associated with higher NNRTI PDR.
Our study has limitations that need to be acknowledged. First, sampling was designed to obtain an HIV PDR prevalence outcome with 5% precision, which could be insufficient to find differences between sub-regions. Second, our study was not designed to obtain nationally representative PDR estimates. Although the large number of participants allowed estimation of consolidated PDR estimates with narrow CIs, these estimates could be biased as different sub-regions could contribute with different weight to the national estimate. In addition, the sub-regions selected for the study, although carefully defined, are a subject of controversy, sometimes grouping states with important differences regarding access to and quality of HIV care. Furthermore, our study included only Ministry of Health clinics, which care for persons with no access to social security. Although Ministry of Health clinics provide ART to the majority of persons (70%) living with HIV in the country,20 exclusion of social security clinics could affect the generalizability of our observations to the whole country, introducing bias to social determinants of the participants, with possible impact on epidemiological dynamics, including emergence and transmission of HIV drug resistance. Epidemiological and social studies directed to specific populations, such as women, restarters and heterosexual men are warranted to complement the observations of the present survey. In addition, molecular epidemiology studies in densely sampled populations could help not only to understand better the HIV drug resistance transmission in specific local contexts, but also to design and assess the effectiveness of targeted prevention interventions.
In large and complex countries such as Mexico, diversification of the public health response to HIV drug resistance based on regional prevalence could be considered. Higher NNRTI PDR levels were associated with poorer regions, suggesting opportunities to strengthen local HIV programmes. Price and licensing negotiations of drug regimens containing INSTIs are warranted.
During the time of revision of this manuscript, and after presentation of these results to the Mexican ART Guidelines Steering Committee, an updated version of the National ART Guidelines was published, featuring the introduction of INSTI-based single-tablet regimens as part of the preferred first-line ART options. Baseline HIV drug resistance tests are also recommended when choosing efavirenz-based regimens as first line.43
Supplementary Material
Acknowledgements
We thank Ramón Hernández-Juan, Edna H. Rodríguez-Aguirre, Carolina Demeneghi, Nelly E. López de Jesús, Maribel Soto-Nava and Humberto Valenzuela-Ponce, from the National Institute of Respiratory Diseases, for their assistance with sample processing, HIV viral load and CD4 T cell count tests.
Members of the HIVDR MexNet Group
The Mexico HIVDR Surveillance Network is composed of: Jorge A. Gamboa-Marroquín, Alan F. Espinoza-Fernández, Mario Lam-Enríquez, Oscar A. Castillo-Soria, Samuel Navarro-Álvarez, Noemí Varela-Lara, Rogelio Ortiz-Batanero, Andrés Flores-Gómez, Luis Velasco-Robledo, Arturo Alatorre-Manjarrez, Rita E. Gutiérrez-Zúñiga, Jesús Peña-Gutiérrez, Alejandro Rivera-Marroquín, Berenice Robles, Maribel Gálvez-Martínez, Raúl Hernández-Gutiérrez, David Solís-Grajales, María G. Mora-Castellanos, Manuel Vidal-López, Maribel A. González-Pacheco, Carmen Salazar-Pérez, Juan C. A. Padilla-Acosta, Andrea González-Rodríguez, Florentino Badial-Hernández, José C. Tecalero-Hernández, Patricia G. García-Martínez, Arturo Cendejas-Hernández, Juan L. Mosqueda-Gómez, José M. Benítez-Carrasco, José L. Sánchez Bello, Benjamín Fierro-Teliz, Jazmín Arellano-Torreblanca, Ramiro Manríquez-Gómez, Elizabeth Zaragoza-Zapata, Saúl O. Ruiz-Torrez, Luz A. González-Hernández, Raúl Soria-Rodríguez, Nora P. Quintero-Pérez, Gerardo Amaya-Tapia, Juana Arredondo-Fuentes, Javier Santiesteban-Garay, María S. Mendoza-García, Gustavo Durán-Arias, Margarita M. Rosas-Dossetti, Juana C. Chacón-Sánchez, Angélica Uribe-Iturbide, Pedro Castro-Melchor, Luis G. Castillo-Reyna, Lesvia M. Rivera-Abarca, Jorge E. de la Cruz-Castillo, Elizabeth Papaqui-Limón, María P. Velázquez-Esqueda, Alexandra S. Domínguez-Sánchez, Zoila Magdaleno-Sandoval, Jorge Silva-Herrera, Mario A. Esparza-Pérez, Alejandro Muñoz-Doana, Gabriela Aldapa, Fernando Arrollo-Romero, Sergio A. Salazar-Arriola, Elva C. Vásquez-Bañuelos, Mario J. Hernández-Morales, Daniel Prado-Rosas, Mercedes Santos-Villegas, Esteban Sánchez-Hernández, Adonay Jiménez-Jiménez, Alejandro Cárdenas-Anzures, Juan F. Ortiz-Brisuela, Juana Díaz-García, Carlos A. Carrillo-Garza, Juan Beltrán-Saldaña, Santos Sánchez-Rivas, Lizbeth Domínguez-Ramírez, Jorge M. de la Roca-Chiapas, Hilda Basilio-Badillo, Uri Torruco-García, Juan A. Pérez-Alonso, Luis E. Arias-Tlacuilo and Omar Palacios-Lara.
Funding
This work was supported by grants from the Mexican Government (Comisión de Equidad y Género de las Legislaturas LX-LXI y Comisión de Igualdad de Género de la Legislatura LXII de la H. Cámara de Diputados de la República Mexicana), received by G. R. T.; the National HIV Programme, CENSIDA (Convocatoria Pública 2017; Proy2da-2017–0002), received by G. R. T. and S. A. R., and Consejo Nacional de Ciencia y Tecnología (CONACyT SALUD-2017–01-289725), received by S. A. R. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Transparency declarations
None to declare.
Contributor Information
HIVDR MexNet Group:
Jorge A Gamboa-Marroquín, Alan F Espinoza-Fernández, Mario Lam-Enríquez, Oscar A Castillo-Soria, Samuel Navarro-Álvarez, Noemí Varela-Lara, Rogelio Ortiz-Batanero, Andrés Flores-Gómez, Luis Velasco-Robledo, Arturo Alatorre-Manjarrez, Rita E Gutiérrez-Zúñiga, Jesús Peña-Gutiérrez, Alejandro Rivera-Marroquín, Berenice Robles, Maribel Gálvez-Martínez, Raúl Hernández-Gutiérrez, David Solís-Grajales, María G Mora-Castellanos, Manuel Vidal-López, Maribel A González-Pacheco, Carmen Salazar-Pérez, Juan C A Padilla-Acosta, Andrea González-Rodríguez, Florentino Badial-Hernández, José C Tecalero-Hernández, Patricia G García-Martínez, Arturo Cendejas-Hernández, Juan L Mosqueda-Gómez, José M Benítez-Carrasco, José L Sánchez Bello, Benjamín Fierro-Teliz, Jazmín Arellano-Torreblanca, Ramiro Manríquez-Gómez, Elizabeth Zaragoza-Zapata, Saúl O Ruiz-Torrez, Luz A González-Hernández, Raúl Soria-Rodríguez, Nora P Quintero-Pérez, Gerardo Amaya-Tapia, Juana Arredondo-Fuentes, Javier Santiesteban-Garay, María S Mendoza-García, Gustavo Durán-Arias, Margarita M Rosas-Dossetti, Juana C Chacón-Sánchez, Angélica Uribe-Iturbide, Pedro Castro-Melchor, Luis G Castillo-Reyna, Lesvia M Rivera-Abarca, Jorge E de la Cruz-Castillo, Elizabeth Papaqui-Limón, María P Velázquez-Esqueda, Alexandra S Domínguez-Sánchez, Zoila Magdaleno-Sandoval, Jorge Silva-Herrera, Mario A Esparza-Pérez, Alejandro Muñoz-Doana, Gabriela Aldapa, Fernando Arrollo-Romero, Sergio A Salazar-Arriola, Elva C Vásquez-Bañuelos, Mario J Hernández-Morales, Daniel Prado-Rosas, Mercedes Santos-Villegas, Esteban Sánchez-Hernández, Adonay Jiménez-Jiménez, Alejandro Cárdenas-Anzures, Juan F Ortiz-Brisuela, Juana Díaz-García, Carlos A Carrillo-Garza, Juan Beltrán-Saldaña, Santos Sánchez-Rivas, Lizbeth Domínguez-Ramírez, Jorge M de la Roca-Chiapas, Hilda Basilio-Badillo, Uri Torruco-García, Juan A Pérez-Alonso, Luis E Arias-Tlacuilo, and Omar Palacios-Lara
References
- 1. Gupta RK, Gregson J, Parkin N et al. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: a systematic review and meta-regression analysis. Lancet Infect Dis 2018; 18: 346–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. HIV Drug Resistance Report2017. http://www.who.int/hiv/pub/drugresistance/hivdr-report-2017/en/.
- 3. Ávila-Ríos S, García-Morales C, Matías-Florentino M et al. Pretreatment HIV-drug resistance in Mexico and its impact on the effectiveness of first-line antiretroviral therapy: a nationally representative 2015 WHO survey. Lancet HIV 2016; 3: e579–91. [DOI] [PubMed] [Google Scholar]
- 4. Hamers RL, Schuurman R, Sigaloff KC et al. Effect of pretreatment HIV-1 drug resistance on immunological, virological, and drug-resistance outcomes of first-line antiretroviral treatment in sub-Saharan Africa: a multicentre cohort study. Lancet Infect Dis 2012; 12: 307–17. [DOI] [PubMed] [Google Scholar]
- 5. Kantor R, Smeaton L, Vardhanabhuti S et al. Pretreatment HIV drug resistance and HIV-1 subtype C are independently associated with virologic failure: results from the multinational PEARLS (ACTG A5175) clinical trial. Clin Infect Dis 2015; 60: 1541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UNAIDS. 90-90-90: An Ambitious Treatment Target to Help End the AIDS Epidemic. http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf.
- 7. Phillips AN, Stover J, Cambiano V et al. Impact of HIV drug resistance on HIV/AIDS-associated mortality, new infections, and antiretroviral therapy program costs in Sub-Saharan Africa. J Infect Dis 2017; 215: 1362–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Global Action Plan on HIV Drug Resistance 2017-2021. http://www.who.int/hiv/pub/drugresistance/hivdr-action-plan-2017-2021/en/.
- 9. Phillips AN, Cambiano V, Nakagawa F et al. Cost-effectiveness of public-health policy options in the presence of pretreatment NNRTI drug resistance in sub-Saharan Africa: a modelling study. Lancet HIV 2018; 5: e146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. Global Action Plan on HIV Drug Resistance 2017-2021: 2018 Progress Report. 2018. http://apps.who.int/iris/bitstream/handle/10665/273049/WHO-CDS-HIV-18.12-eng.pdf?ua=1.
- 11.WHO. Guidelines on the Public Health Response to Pretreatment HIV Drug Resistance. 2017. http://www.who.int/hiv/pub/guidelines/hivdr-guidelines-2017/en/.
- 12. García-Morales C, Tapia-Trejo D, Quiroz-Morales VS et al. HIV pretreatment drug resistance trends in three geographic areas of Mexico. J Antimicrob Chemother 2017; 72: 3149–58. [DOI] [PubMed] [Google Scholar]
- 13. Chaumont C, Bautista-Arredondo S, Calva JJ et al. Antiretroviral purchasing and prescription practices in Mexico: constraints, challenges and opportunities. Salud Publica Mex 2015; 57 Suppl 2: s171–82. [DOI] [PubMed] [Google Scholar]
- 14.CENSIDA. Guía de Manejo Antirretroviral de las Personas con VIH. Octava Edición. México: CENSIDA/Secretaría de Salud, 2018. https://www.gob.mx/cms/uploads/attachment/file/326631/Gu_a_ARV_2018.pdf.
- 15.UNAIDS. Country: Mexico. http://www.unaids.org/en/regionscountries/countries/mexico.
- 16.CENSIDA. Boletín de Atención Integral de Personas con VIH/CENSIDA. 2018. https://www.gob.mx/censida/articulos/boletin-de-diagnostico-y-tratamiento-antirretroviral-censida.
- 17.WHO. Surveillance of HIV Drug Resistance in Adults Initiating Antiretroviral Therapy (Pre-Treatment HIV Drug Resistance). Concept note. 2014. http://www.who.int/hiv/pub/drugresistance/pretreatment_drugresistance/en/.
- 18. Regiones de México. https://es.wikipedia.org/wiki/Regiones_de_Mexico.
- 19.WHO. Sample Size Calculators to Design PDR Surveys. http://www.who.int/hiv/topics/drugresistance/protocols/en/.
- 20.CENSIDA. Informe nacional del monitoreo de compromisos y objetivos ampliados para poner fin al sida (Informe GAM). Mexico, 2018. https://www.gob.mx/censida/documentos/informe-nacional-del-monitoreo-de-compromisos-y-objetivos-ampliados-para-poner-fin-al-sida-informe-gam-mexico-2018?idiom=es.
- 21.WHO. WHO/HIV ResNet HIV Drug Resistance Laboratory Operational Framework. 2017. http://www.who.int/hiv/pub/drugresistance/hivdr-laboratory-framework-2017/en/.
- 22. Van Laethem K, Schrooten Y, Covens K et al. A genotypic assay for the amplification and sequencing of integrase from diverse HIV-1 group M subtypes. J Virol Methods 2008; 153: 176–81. [DOI] [PubMed] [Google Scholar]
- 23. Woods CK, Brumme CJ, Liu TF et al. Automating HIV drug resistance genotyping with RECall, a freely accessible sequence analysis tool. J Clin Microbiol 2012; 50: 1936–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Web ReCall. British Columbia Centre for Excellence in HIV/AIDS. http://pssm.cfenet.ubc.ca/.
- 25. Liu TF, Shafer RW. Web resources for HIV type 1 genotypic-resistance test interpretation. Clin Infect Dis 2006; 42: 1608–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.HIVdb Program. HIV Drug Resistance Database. Stanford University. https://hivdb.stanford.edu/hivdb/by-mutations/.
- 27.WHO BCCfE HIVDR QC Tool. http://pssm.cfenet.ubc.ca/who_qc/.
- 28. Kosakovsky Pond SL, Weaver S, Leigh Brown AJ et al. HIV-TRACE (Transmission Cluster Engine): a tool for large scale molecular epidemiology of HIV-1 and other rapidly evolving pathogens. Mol Biol Evol 2018; 35: 1812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chaillon A, Avila-Ríos S, Wertheim JO et al. Identification of major routes of HIV transmission throughout Mesoamerica. Infect Genet Evol 2017; 54: 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mehta SR, Wertheim JO, Brouwer KC et al. HIV Transmission Networks in the San Diego-Tijuana Border Region. EBioMedicine 2015; 2: 1456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Little SJ, Kosakovsky Pond SL, Anderson CM et al. Using HIV networks to inform real time prevention interventions. PLoS One 2014; 9: e98443.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang X, Wu Y, Mao L et al. Targeting HIV prevention based on molecular epidemiology among deeply sampled subnetworks of men who have sex with men. Clin Infect Dis 2015; 61: 1462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wertheim JO, Kosakovsky Pond SL, Forgione LA et al. Social and genetic networks of HIV-1 transmission in New York City. PLoS Pathog 2017; 13: e1006000.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wertheim JO, Leigh Brown AJ, Hepler NL et al. The global transmission network of HIV-1. J Infect Dis 2014; 209: 304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wertheim JO, Oster AM, Hernandez AL et al. The international dimension of the U.S. HIV transmission network and onward transmission of HIV recently imported into the United States. AIDS Res Hum Retroviruses 2016; 32: 1046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wertheim JO, Oster AM, Johnson JA et al. Transmission fitness of drug-resistant HIV revealed in a surveillance system transmission network. Virus Evol 2017; 3: vex008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Whiteside YO, Song R, Wertheim JO et al. Molecular analysis allows inference into HIV transmission among young men who have sex with men in the United States. AIDS 2015; 29: 2517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hightower GK, May SJ, Pérez-Santiago J et al. HIV-1 clade B pol evolution following primary infection. PLoS One 2013; 8: e68188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Csardi G, Nepusz T. The igraph software package for complex network research. InterJournal Complex Syst 2006; Complex Systems: 1695. [Google Scholar]
- 40. igraph—The network analysis package. http://igraph.org/.
- 41.CONEVAL. [Poverty Measurment in Mexico]. Consejo Nacional de Evaluación de la Política de Desarrollo Social. https://www.coneval.org.mx/Paginas/principal.aspx.
- 42. Bautista-Arredondo S, Servan-Mori E, Beynon F et al. A tale of two epidemics: gender differences in socio-demographic characteristics and sexual behaviors among HIV positive individuals in Mexico City. Int J Equity Health 2015; 14: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guía de manejo antirretroviral de las personas con VIH. Novena Edición. México: CENSIDA/Secretaría de Salud, 2018. https://www.gob.mx/censida/articulos/guia-de-manejo-antirretroviral-de-las-personas-con-vih-89591?idiom=es.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.