Abstract
Assessing the ‘value’ of potential cures can be challenging, as some have suggested that cures may offer distinctive benefits from noncurative treatments. We explore what these – previously unspecified – additional benefits may be. We suggest that three new elements of value seem distinctive to cures: liberation from the identity of being diseased, liberation from the stigma associated with the disease and liberation from the burden of ongoing therapy. However, including additional elements of value in health technology assessment may result in double counting and requires consideration of potential opportunity costs. We suggest health technology assessment should explore the relevance of these three elements of value and may have good reasons to – judiciously – integrate them through the deliberative process.
Keywords: : cost–effectiveness analysis, health technology assessment, methodology
A wave of potential cures, including a large number of novel cell and gene therapies, is working its way through the development pipeline toward likely regulatory approval over the coming decade [1–5]. These new potential cures will offer important opportunities to relieve suffering but will also pose challenges to traditional health technology assessment (HTA) methods. The most frequently considered challenge of potential cures, especially those delivered as single or short-term therapies, involves navigating significant uncertainty about their long-term effects and their high upfront cost [1–4,6–14].
Another challenge for HTA lies in determining whether the ‘value’ of potential cures includes special elements of benefit – to patients, families or society – that are distinctive from the elements of value measured for other treatments [2,4,7,11,13,14]. It has long been noted that health gain is not the only type of benefit that decision makers wish to consider when making decisions to prioritize health resources [15,16]. Work by many researchers and policy groups, including the Value Framework Task Force of the International Society for Pharmacoeconomics Research (ISPOR), has identified elements of value that some believe should be routinely included in any assessment of value [16–19]. There are strong arguments for and against inclusion of these additional elements of value alongside traditional measures of health gain, and the purpose of this paper is not to revisit those long-standing debates. Instead, we seek to evaluate whether there is something ‘special’ about potential cures that should lead HTA to consider more regular inclusion of the previously proposed additional elements of value or whether there are elements of value distinctive to cures alone that lie beyond even the expanded list presented by the ISPOR Task Force.
Defining potential cures
For the purposes of selecting which interventions may have distinctive elements of value and thereby raise the possibility of adapted HTA methods, a clear definition of potential cures is needed [1–3,20]. Given that previous efforts to define potential cures (Table 1) have not resulted in consensus [2,4,14,20–23] we propose the following working definition: “Treatments with significant potential to eradicate a disease or condition, thereby providing for sustained health benefits extending throughout patients’ lifetimes”.
Table 1. . Proposed definitions of cures.
| Ref. | |
|---|---|
| “An innovative one-time (or short-term) treatment, delivered via an irreversible process (or procedure or drug), and followed by a significant (multiyear) disease-free interval (i.e., long-term durable effect)” | [2] |
| A subgroup of “single or short-term transformative therapies: therapies delivered through a single intervention or a short-term course of treatment that demonstrate a significant potential for substantial and sustained health benefits extending throughout patients’ lifetimes”; cures “can eradicate a disease or condition” | [4] |
| “Future interventions that aim to truly correct the underlying cause of disease …. These include regenerative therapies that are intended ‘to restore or establish normal function’ to organs, tissues, cells and genes (11).Truly curative therapies would presumably be given once, without the need for ongoing monitoring or treatment (e.g., immunosuppressant therapy) with the recipients enjoying the same length and quality of life as everyone else.” | [14] |
| “Leads to the absence of disease or condition following the completion of treatment and restores the health of the individual to the same as that of an individual without the disease or condition.” | [24] |
| A technology that does not confer disability group identify on the user | [20] |
| Restores, at least partially, both the mode and level of function to what is statistically normal for members of the human species | [25, as paraphrased by 20] |
Several elements of this definition help define a limited set of interventions that would be distinguished as potential cures. First, our definition requires that treatments have “significant potential to …”. Since many new and existing treatments offer a potential cure to a very small fraction of patients treated, counting as a cure any treatment which offers the slightest hope of being curative would mischaracterize the nature (and value) of these therapies. While more quantitative guidance on the threshold for a ‘significant potential’ might be helpful, any specific number would be arbitrary and would lack needed flexibility. For example, recognizing a treatment as a potential cure might be reasonable for a treatment that offers a 5% chance of a cure for a chronic disease that is disabling despite the standard of care, but makes less sense for a treatment for a disease for which effective cures are already available.
Eradicating the disease or condition is at the core of the difference between a curative and a noncurative therapy [2,4,14]. We believe that treatments that relieve or suppress symptoms or halt disease progress – without addressing the underlying disease – should not be considered true cures [4,14]. If patients need to continue to undergo therapy to maintain the anticipated therapeutic effect, this therapy is not a cure (as the need for continued therapy implies that the disease has not been eradicated); cures thus involve administration of limited duration. As an example, antibiotics can be a cure for bacterial pneumonia, but antiretroviral therapy is not a cure for HIV.
At last, providing ‘sustained health benefits’ follows from the absence of diseases [2]. We suggest that patients whose infectious diseases have been eliminated may be considered cured, even if people could get reinfected later in life. In our view, while cures should eradicate the primary disease, they do not need to return the person to perfect health. Complications of the disease or side effects of the cure may persist, causing the individual to remain in a less good health state (e.g., a cure for hepatitis C virus [HCV] may eradicate the disease but some irreversible liver damage may remain).
Elements of value
To assess the value of new therapies, health economists and HTA agencies routinely evaluate whether treatments produce gains in length of life and/or health-related quality of life (QoL). When performing cost–effectiveness analyses these elements are often combined into a single measure, the quality-adjusted life year (QALY) [17].
Potential cures, especially single or short-term treatments, have distinctive considerations related to their prospective risks and benefits. Heightened uncertainty about the long-term duration of benefit is frequently one issue. Long-term risks may also be highly uncertain. Single treatments that involve implantable devices and/or irreversible changes may pose greater risks compared with treatments given chronically that can be stopped immediately if significant side effects are noted.
But neither specific clinical benefits and harms evaluated in clinical trials nor the summary measure of the QALY easily convey contextual considerations about uncertainty in the evidence, nor do they always capture all the elements of value that will be important to patients, families and decision makers. An ISPOR Taskforce recently summarized a broader set of value elements which they suggest could either be included in HTA generally or in specific cases [17]. We will briefly explore the relevance of these previously identified elements of value in assessments of potential cures. We will then introduce and analyze new, additional elements that we believe may be uniquely salient when evaluating potential cures. Figure 1 displays all these elements of value as part of the ISPOR ‘value flower’, highlighting those elements we believe are more salient for some potential cures and the additional elements of value we propose.
Figure 1. . Elements of value.
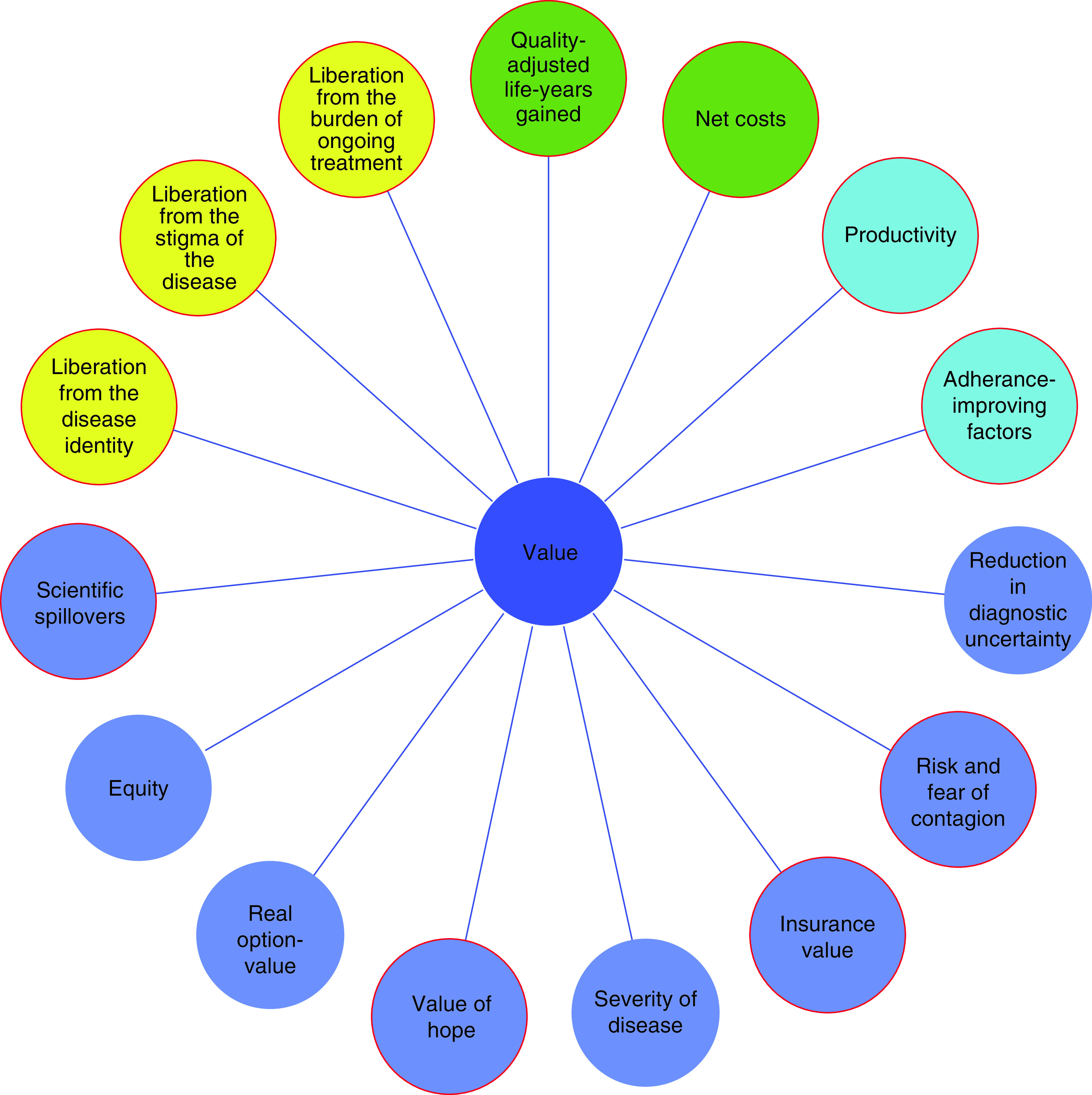
Green circles: core elements of value [17]; light blue circles: common but inconsistently used elements of value [17], dark blue circles: potential novel elements of value proposed by the Value Framework Task Force of ISPOR [17], yellow circles: potential additional elements of value for cures proposed in this article. Red outline: element of value may be salient for (some) potential cures.
Adapted from [17].
Of note, this discussion focuses on the special added value that potential cures may provide, whether when comparing a potential cure to a noncurative treatment or when comparing two or more potential cures to each other.
Previously proposed additional elements of value
Potential cures do not inherently provide higher value than other treatments when considering the additional elements of value described by ISPOR [17]. However, this section will briefly discuss for which elements of value some potential cures may offer added value over chronic treatments with similar health gains.
Adherence
Cures set out to lead to the absence of disease; if patients need to continue to undergo therapy to maintain the anticipated therapeutic effect, this therapy is not a cure [14]. Cures thus involve administration of limited duration, which likely improves adherence compared with chronic therapies, thereby improving the clinical value of treatment [26].
Productivity
Similarly, cures, which by definition involve administration of limited duration, may require less medical follow-up than chronic treatments [12]. Cures that require less follow-up will reduce productive time lost to clinician appointments and other medical requirements.
Insurance value
Healthy individuals run the risk of developing a medical condition in the future; having a treatment available for this condition thus offers a physical and financial risk protection for healthy individuals (i.e., insurance value) [17]. Those who favor consideration of insurance value would argue that it is particularly salient for cures of serious conditions since it is to have just this kind of intervention for which individuals seek insurance. However, this added insurance value appears to be driven largely by disease severity and health gains, rather than the particular value of a cure versus a noncurative treatment.
Scientific spillovers
Newly-developed potential cures are often based on new mechanisms of action. As such, many potential cures would be likely to have a higher chance of beneficial scientific spillover through which treatments for other conditions might be developed in the future [4,12].
Value of hope
Potential cures, even those that may not be cures for a very high percentage of treated patients, may provide a ‘value of hope’ beyond their usually measured health gains [4,11]. This value would be most salient when overall health gain might be similar between a potential cure with high short-term risks and existing treatments that are safe but offer very little or no hope of long-term recovery. In such cases patients may prefer to take the risk of poor short-term outcomes for the potential gain of a long-term cure. The value of hope may be particularly important to patients in end-of-life contexts [16,17].
Risk of contagion
Cures of infectious diseases are likely to provide more value to society by reducing the risk of contagion than treatments that reduce transmissibility but require adherence or may become ineffective over time. Permanently eliminating the risk of transmission to others was also identified as a key desired feature of a cure by patients themselves [27–31]. Conversely, in cases when behavior substantially affects transmission risk, the availability of a cure may decrease incentives to prevent infection, such that having a cure may increase the total number of infections [32,33]. Because of the substantial uncertainty regarding the impact of cures on future transmission rates, some HTA assessments have considered reduced infection rates as a contextual consideration and not included it as part of the base–case cost–effectiveness analysis [34,35].
The remaining novel elements of value as described by ISPOR [17] do not seem to vary in relation to whether a treatment is a cure or not. We see little reason to think that cures will offer more value than noncures in terms of equity. The value of reducing uncertainty due to a new diagnostic is specific to diagnostics. Real option value refers to the extension of life which can create opportunities for patients to benefit from other future advances in medicine, a scenario that is made irrelevant with a cure. Finally, disease severity refers to a premium value some think should be assigned to health gains for individuals with a poorer state of health [17]. While some cures will be for very serious or fatal conditions, there is nothing inherent about cures that differentiate them from chronic treatments in terms of the severity of the targeted disease.
To conclude, while this section noted that some cures would produce more value than noncurative treatments through consideration of the nontraditional elements of value summarized by ISPOR, all these elements of value are also relevant for noncurative treatments. As such, none of these elements of value seem good candidates to account for the potential ‘special, distinctive value’ of cures to which several commentators in the literature have alluded [2,4,7,11,13,14].
Distinctive elements of value of cures
In the discussion below, we present arguments for novel, additional elements of value that present a way in which cures can offer value that noncurative therapies would not (i.e., these elements of value are unique to cures, although not all cures will offer this value). While cures may provide these additional elements of value, this does not imply that these novel elements of value are the most important way that cures provide value. In many cases, including, for example, in end-of-life contexts, health gains may be more important, and these novel elements of value merely present a way in which cures may offer additional value as compared with noncurative treatments.
An inherent feature of cures is that they eradicate the disease or condition. The resulting freedom of no longer having a disease may have powerful psychological and social meaning and benefits, especially for diseases that are not themselves self-limiting. While this has not yet been brought forward clearly in other conceptual HTA literature we are familiar with, several authors have hinted at largely undefined psychological and social benefits of cures, including ‘a psychological sense of well-being’ [7], ‘a host of psychological factors’ [14], ‘intrinsic psychological benefits that are not captured in the QALY of feeling “cured” of a lifelong illness’ [4] and ‘an important psychological, social and emotional distinction … between curing HIV and controlling it via therapy’ [36]. Patients refer to the term ‘freedom’ in qualitative research exploring the value of cures [27,28,37,38].
We suggest freedom of no longer having a disease could encompass three elements of value. These include liberation from the identity of being diseased or disabled, liberation from the stigma associated with the disease or disability and liberation from the burden of ongoing therapy. The analysis below provides support for these to be perceived benefits for cures.
Liberation from disease identity
In a recent article, Stramondo argued that a change in narrative identity is the defining difference between cures and noncures [20]; repressing the disease/symptoms, even if that would lead to the same health state, would not have this effect on disease identity. Correspondingly, several studies investigating why patients value cures reveal that they anticipate that being cured would affect their identity as having the disease and allow them to return to being ‘normal’ [27,28,33,37,39,40], which would make them ‘very happy’ [39,40] and ‘feel better about themselves’ [27–29,41]. Some patients even anticipate that being cured may not just relieve them from the psychological burden of anticipating the deterioration of their health, but may also motivate them to adopt healthier lifestyles or pursue getting a job or education [27,30,31,33,42]. Studies exploring experiences of cured chronic HCV patients indeed describe ‘transformative nonclinical outcomes’, including increased self-worth, changes in identity and lifestyle changes such as reduced substance misuse [28–31,43]. The negative effect of identifying as having a disease depends on the disease (e.g., flu vs HIV), making the value of no longer identifying with a disease especially salient for conditions that are psychologically laden (which may be culturally dependent). For example, patients suggested that their ‘whole lives’ would change (HIV, sickle cell disease) or has changed (HCV) upon being freed from the disease [27,42,43]. However, other patients expressed skepticism about whether a cure would fully dissociate individuals from the disease identity or about the impact of this change [29,33,39]. The particular effects of a potential cure and its distinct context may be important in assessing the impact of this new element of value. For example, being liberated from a disease identity likely has more limited value for cures that leave patients with significant tissue damage already caused by the disease (e.g., a cure for HCV which leaves patients with previously obtained liver damage). Such considerations can be part of a critical assessment – grounded in evidence – of the extent to which liberation of a disease – identity may provide value for a specific cure.
Of note, for some individuals, this change in identity could be distressing and would require a period of adjustment [27,29,42]. Furthermore, some people consider themselves better off with a certain disability identity and thus without a cure [20]. For example, this is the case for part of the Deaf community [44]. On a policy level, it may be important to identify disorders/cures in which such views are prevalent, such that whether to assign (positive) value to this element of cures can be considered.
Liberation from stigma
Stigma toward individuals with certain conditions involves medically unwarranted negative attitudes and behaviors which may be embedded into social structures; alleviating this stigma thus requires social changes [45]. However, for individuals, being cured from a stigmatized condition may mean that this stigma is no longer directed against them [27,33,37,46]. The magnitude of this benefit for specific cures depends on the extent to which a disease or disability is stigmatized in a certain sociocultural context. This particular advantage of cures has been frequently referred to for HIV and HCV cures; patients described the stigma associated with these diseases as resulting in being ‘unable to live freely’ [37] and relief of this stigma was one of the main anticipated advantages of a cure [27,29,31,33,38,47]. For example, patients hope that relief from HIV or HCV stigma may increase their opportunities for partnerships/marriage, having children, strengthening relationships, finding employment and regaining social mobility [27,29,30,33,43]. Relief of stigma may not only affect the patient but could also affect their families [27,29]. Indeed, some cured HCV patients report a relief from stigma and enhanced social connections [29,31]. However, while being cured of a disease may decrease the level of stigma, some residual stigma can remain after a cure [31,33,43]. This might be especially relevant for cures of diseases that cause irreversible tissue damage (e.g., gene therapy for a neurodegenerative disease that restores the causative mutation and halts disease progress but is unable to restore some previously damaged tissue and function). A critical assessment of the extent to which the liberation of stigma may provide value for a specific cure, taking into account the relevant context, is therefore warranted.
Additionally, some have suggested that stigma associated with certain conditions relates to the incurability of these diseases, such that the availability of a cure may change social attitudes toward the disease and reduce the stigma toward affected individuals [33]. As a result, the existence of a cure may, for example, motivate individuals currently unwilling to get tested and/or receive treatment out of concerns relating to stigma to pursue medical care [33].
Finally, there may be diseases where stigma is associated with visible symptoms. In such cases, cures may sustainably reduce stigma but noncurative treatments that remove visible symptoms may also provide some of this value.
Liberation from the burden of ongoing treatment
Another element of value that some cures may offer over noncures is a complete liberation from the physical, social, financial and psychological burdens of ongoing treatment. This includes improvements in the quality of or process of care that are not captured by measures of improved outcomes (e.g., invasiveness of interventions, number of medications, time spent on organizing, receiving and monitoring treatment [48]). It may also include subjective aspects, such as the fear of medication supply running out [48]. The importance of treatment burden to patients has been established in several areas [48], ranging from infertile patients [41,49], to seriously ill patients [50]. High treatment burden may negatively affect patients’ (and their family and caregivers’) well-being and may lead them to prefer treatments with inferior effectiveness [48]. The extent of the burden of ongoing treatments depends on the disease/treatment, the individual and the context. For example, taking (daily) medications may be a distressing reminder of having the disease [39,51], may create stress or worry [51] and may reveal disease status to third parties, which is especially important in stigmatized conditions [40]. Furthermore, ongoing therapy and medical care may put restraints, on, for example, the ability to travel and live in places where medications are not available [27].
Some authors have proposed that treatment burden is sufficiently meaningful to patients that it should be considered explicitly in some HTA analyses [12,52,53]. Treatment burden can be incorporated in health-related QoL [54,55], but it is unclear how well or consistently this is done in HTA evaluations [56–58]. Of note, deriving value from reduced treatment burden may be relevant to noncures; however, cures could offer a complete liberation of the burden of ongoing treatment – whether this is different in degree or in kind may be context dependent. While treatment burden may also affect adherence, it is already captured separately in HTA.
Cures would by our definition entail a limited duration of treatment, which in many cases would reduce the overall burden of treatment and the time spent on acquiring (follow-up) care as compared with alternative (chronic) treatment [13]. Several empirical studies suggest that being relieved of the burden of treatment is (one of) the main reason(s) patients would want to pursue a cure [27,37,38,40]. However, especially during the early introduction of a new cure, the reduction in treatment burden from the cure may be offset by the burdens of additional checkups or procedures for research or long-term follow-up. Additionally, the treatment modality of cures may result in a (short-term) treatment burden that is very high, which may be difficult to weigh against a long-term lower treatment burden. As such, inclusion of treatment burden as a separate element of value would add the most value for cures that replace high-burden treatments and cures that do not require extensive follow-up due to their experimental nature. As with the other new elements of value, the extent to which the liberation of the burden of ongoing treatment may provide value for a specific cure should be critically assessed, taking into account the relevant context.
Challenges in consideration of new elements of value
While cures may offer certain important value beyond what is captured by traditional HTA, incorporating new elements of value raises three key issues that should be taken seriously.
First, in order to consider these new elements of value in HTA in a manner that is consistent and is grounded in the actual features of the assessed cures, there is a need to objectively assess the new elements of value and quantify them if appropriate tools are available [4,18,59]. A failure to develop and implement such accurate assessments runs the risk of over or understating the value of therapeutics. However, to the best of our knowledge, no measures exist for the three newly proposed elements of value. Even treatment burden cannot yet be fully measured [48,52]. Until quantitative analyses are available, these additional elements of value could be included in the assessment of potential cures in a qualitative manner [4].
Second, considering additional elements of value may lead to unfairly favorable value assessments if (part of) these additional elements of value are also reflected in other considered elements of value (i.e., double counting [59]). For example, part of the psychosocial benefits from being liberated from a disease may also be counted in the utility weights for health states that factor into the QALY.
Third, adding additional elements of value that are specific to cures without some concurrent reduction in valuation for other services would raise the question of whether the opportunity cost should trigger some change to the operative cost–effectiveness threshold [4,12,16,60,61]. If the number of potential cures given extra value is small this is unlikely to pose a major problem, but as the number of potential cures rises, and with it the cumulative budget impact, questions about the opportunity cost of attributing higher value to these treatments will intensify. If including these additional elements of value in HTA assessment would require a reduction or elimination of other services that would have provided greater health benefits, this, in our view, requires greater evidentiary standards for the relative importance of these additional elements of value.
Given these concerns, several authors have argued for assessing the value society and patients attach to the nonhealth related elements of the value of cures [11–14].
Some sources of empirical evidence relating to people’s willingness to accept trade-offs for treatments that are potential cures are already available. The clearest evidence of patients valuing a cure even when the alternative provides greater overall ‘success’ comes from reproductive medicine. Surveyed infertile patients prefer a treatment that cured infertility over treatments leading to a single pregnancy with a 20% higher pregnancy rate per cycle (with 20% being an average derived from this discrete choice experiment) [62]. Whereas the main goal of treatment is to achieve a pregnancy, liberation from the condition and the need for further treatments appear to hold significant value to these patients. In another study, treatment decision making of surveyed chronic HCV patients is affected more by whether treatment results in a viral cure, than by long-term survival or side effects [63]. There remains a need for much additional research to tease out whether the preferences for some patients for ‘risky cures’ or for ‘less effective cures’ are related to specific elements of value not captured by traditional utility measures.
Despite these challenges, we think there are good reasons to incorporate liberation from the identity of being diseased, liberation from the stigma associated with the disease and liberation from the burden of ongoing therapy in a qualitative fashion as part of the deliberative process of appraising cures. These elements of value reflect meaningful ways in which cures may provide value and consideration of these elements of value would be more consistent and transparent than ad hoc reference to undefined psychological and social benefits of cures. However, such deliberations should also consider double counting and opportunity costs. More research is needed about the relative value of the sense of liberation from a disease in order to determine how best to assess it – or choose not to – in HTA in the future. Until data suggests otherwise, we think the weight attached to these new elements of value in HTA should be limited, compared with the weight of QALYs, as the basis for requiring significant opportunity costs is not yet justified.
Conclusion
This paper explored whether there is something ‘special’ about potential cures that should lead HTA to assign them additional value beyond core elements of value captured in traditional cost–effectiveness analysis. We argue that potential cures may provide additional value beyond that traditionally captured by QoL gains. Although the additional elements of value described by the ISPOR Task Force are not unique to potential cures, certain cures are likely to provide some of these additional elements of value, such as the value of hope. Beyond these previously described elements of value we identify three new elements of value that are distinctive to cures. These encompass liberation from the identity of being diseased or disabled, liberation from the stigma associated with the disease or disability and liberation from the burden of ongoing therapy. There is some evidence that stakeholders attach importance to these additional elements of value.
However, we currently lack robust methods to quantify these new elements of value. Furthermore, inclusion of additional elements of value in HTA assessment comes with drawbacks, including the risk of double counting and assumed opportunity costs. Further research will be needed on methods to quantify these additional elements of value and their importance relative to QoL gains.
A lack of means to quantify these elements need not imply that HTA cannot explore their relevance and judiciously integrate these considerations through the deliberative process. The latter would entail carefully considering the potential drawbacks of including these elements of value, and, until data suggests that significant opportunity costs are justified, ensuring that the weight attached to these new elements of value in HTA is limited compared with the weight of QALYs. With these caveats, considering the new elements of value in HTA would ensure capturing meaningful ways in which cures may provide value and be more consistent and transparent than ad hoc reference to undefined psychological and social benefits of cures. With the rising number of potential cures entering clinical use around the world, HTA processes should adapt to make sure that they are fit for purpose moving forward in helping health systems weigh the many different aspects of value these potential cures can offer.
Future perspective
We hope that in the future, HTA agencies will make decisions on whether to include these additional elements of value in their assessments based on a substantive body of data on the relative value of cures including the acceptability of opportunity costs. If their inclusion is justified, we hope tools will be available to quantitatively assess these additional elements of value and avoid double counting.
Executive summary.
Some commentators have suggested that cures can offer distinctive benefits from noncurative treatments. We explore what these – previously unspecified – additional benefits may be.
Although the additional elements of value described by the International Society for Pharmacoeconomics Research Task Force are not unique to potential cures, we argue that some are particularly salient for certain potential cures (e.g., the value of hope).
We identify three new elements of value that are distinctive of potential cures: liberation from the identity of being diseased or disabled, liberation from the stigma associated with the disease or disability and liberation from the burden of ongoing therapy.
We suggest that health technology assessment should explore the relevance of these three elements of value and that there are good reasons to – judiciously – integrate these considerations in value assessment through the deliberative process.
Doing so could help health systems fully consider the differential value potential cures can offer.
Acknowledgments
The authors thank J Millum for helpful feedback on an earlier draft of this paper. The views expressed are the authors’ own and do not necessarily reflect those of the National Institutes of Health, the Department of Health and Human Services or the US government.
Footnotes
Author contributions
Both the authors contributed to the conceptual analysis and critical discussion. S Hendriks contributed to manuscript drafting. SD Pearson contributed to manuscript editing.
Financial & competing interests disclosure
The authors were supported by the National Institutes of Health Intramural Research Program. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest
- 1.Thomas SK. Regenerative therapies: are we ready for a cure? Key value and policy considerations to facilitate access. Value Outcomes Spotlight January/February 18–20 (2017). www.ispor.org/docs/default-source/publications/value-outcomes-spotlight/january-february-2017/vos-regenerative-therapies.pdf?sfvrsn=9c7a0ed7_2 [Google Scholar]
- 2.Tapestry Networks. ViewPoints: building a sustainable health system for curative therapies (2016). www.tapestrynetworks.com/initiatives/healthcare/upload/Curative-Therapies-ViewPoints-Building-a-sustainable-health-system-for-curative-therapies-May-2016.pdf
- 3.Faulkner E, Werner MJ, Slocomb T, Han D. Ensuring patient access to regenerative and advanced therapies in managed care: how do we get there?. JMCM ARM Monograph 3–18 (2018). https://alliancerm.org/wp-content/uploads/2018/05/JMCMArm.pdf [Google Scholar]
- 4.Chapman R, Kumar V, Samur S, Zaim R, Segel C, Pearson SD. Value assessment methods and pricing recommendations for potential cures: a technical brief (2019). https://icer-review.org/Valuing-a-Cure-Technical-Brief ; • Outlines some of the challenges in assessing the value of cures.
- 5.Massachusetts Institute of Technology New drug Development ParadIGmS Initiative. MIT NEWDIGS FoCUS Project (2017) Existing gene therapy pipeline likely to yield dozens of approved products within five years (2017). https://newdigs.mit.edu/sites/default/files/FoCUS_Research_Brief_2017F211v011.pdf
- 6.Zettler PJ, Fuse Brown EC. The challenge of paying for cost-effective cures. Am. J. Manag. Care 23(1), 62–64 (2017). [PubMed] [Google Scholar]
- 7.Salzman R, Cook F, Hunt T et al. Addressing the value of gene therapy and enhancing patient access to transformative treatments. Mol. Ther. 26(12), 2717–2726 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montazerhodjat V, Weinstock DM, Lo AW. Buying cures versus renting health: financing health care with consumer loans. Science Translational Medicine 8(327), 327ps326–327ps326 (2016). https://pubmed.ncbi.nlm.nih.gov/26912902/ [DOI] [PubMed] [Google Scholar]
- 9.Basu A. Financing cures in the United States. Expert Rev. Pharmacoecon. Outcomes Res. 15(1), 1–4 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Danzon PM. Affordability challenges to value-based pricing: mass diseases, orphan diseases, and cures. Value Health 21(3), 252–257 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Marsden G, Towse A, Pearson SD, Dreitlein B, Henshall C. Gene therapy: understanding the science, assessing the evidence, and paying for value:a report from the 2016 ICER Membership Policy Summit (2017). https://icer.org/wp-content/uploads/2020/10/ICER-Gene-Therapy-White-Paper-030317.pdf
- 12.Jonsson B, Hampson G, Michaels J, Towse A, Von Der Schulenburg JG, Wong O. Advanced therapy medicinal products and health technology assessment principles and practices for value-based and sustainable healthcare. Eur. J. Health Econ. 20(3), 427–438 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hampson G, Towse A, Pearson SD, Dreitlein WB, Henshall C. Gene therapy: evidence, value and affordability in the US health care system. J. Comp. Eff. Res. 7(1), 15–28 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Husereau D. How do we value a cure? Expert Rev. Pharmacoecon. Outcomes Res. 15(4), 551–555 (2015). [DOI] [PubMed] [Google Scholar]; • Outlines some of the challenges in assessing the value of cures.
- 15.Neumann PJ, Cohen JT. Measuring the value of prescription drugs. New England Journal of Medicine 373(27), 2595–2597 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Garrison LP, Kamal-Bahl S, Towse A. Toward a broader concept of value: identifying and defining elements for an expanded cost-effectiveness analysis. Value Health 20(2), 213–216 (2017). [DOI] [PubMed] [Google Scholar]; • Describes some of the novel potential elements of value that health technology assessment may consider.
- 17.Lakdawalla DN, Doshi JA, Garrison LP, Phelps CE, Basu A, Danzon PM. Defining elements of value in health care-a health economics approach: an ISPOR Special Task Force Report [3]. Value Health 21(2), 131–139 (2018). [DOI] [PubMed] [Google Scholar]; • Describes some of the novel potential elements of value that health technology assessment may consider.
- 18.Garrison LP Jr, Zamora B, Li M, Towse A. Augmenting cost–effectiveness analysis for uncertainty: the implications for value assessment-rationale and empirical support. J. Manag. Care Spec. Pharm. 26(4), 400–406 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lakdawalla DN, Phelps CE. Health technology assessment with risk aversion in health. J. Health Econ. 72, 102346 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stramondo JA. The distinction between curative and assistive technology. Sci. Eng. Ethics 25(4), 1125–1145 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Johnson P, Greiner W, Al-Dakkak I, Wagner S. Which metrics are appropriate to describe the value of new cancer therapies? BioMed Res. Int. 2015, 865101 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chacińska W, Brzostowska M, Nojszewska M, Podlecka-Piętowska A, Jędrzejczak W, Snarski E. “Cure” for multiple sclerosis (MS) – evolving views of therapy goals in patients on different stages of the disease: a pilot study in a cohort of Polish MS patients. Brain Behav. 7, e00701 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zoulim F, Durantel D. Antiviral therapies and prospects for a cure of chronic hepatitis B. Cold Spring Harb. Perspect. Med. 5, a021501–a021501 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hampson G, Mott D, Shah K, Devlin N. Public preferences for health gains and cures: a discrete choice experiment. OHE Consulting Report (2018). www.ohe.org/publications/public-preferences-health-gains-and-cures-discrete-choice-experiment
- 25.Daniels N. Health-care needs and distributive justice. Philos. Public Aff. 10(2), 146–179 (1981). [PubMed] [Google Scholar]
- 26.Sabaté E. Adherence to long-term therapies – evidence for action (2003). www.who.int/chp/knowledge/publications/adherence_report/en/
- 27.Sylla L, Evans D, Taylor J et al. If we build it, will they come? Perceptions of HIV cure-related research by people living with HIV in four U.S. cities: a qualitative focus group study. AIDS Res. Hum. Retroviruses 34(1), 56–66 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richmond JA, Ellard J, Wallace J et al. Achieving a hepatitis C cure: a qualitative exploration of the experiences and meanings of achieving a hepatitis C cure using the direct acting antivirals in Australia. Hepatol. Med. Pol. 3, 8–8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madden A, Hopwood M, Neale J, Treloar C. Beyond cure: patient reported outcomes of hepatitis C treatment among people who inject drugs in Australia. Harm Reduction J. 15(1), 42 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Explores what outcomes patients seek from hepatitis C virus cures, including nonclinical outcomes.
- 30.Williams BE, Nelons D, Seaman A et al. Life projects: the transformative potential of direct-acting antiviral treatment for hepatitis C among people who inject drugs. Int. J. Drug Pol. 72, 138–145 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Harris M. Managing expense and expectation in a treatment revolution: problematizing prioritisation through an exploration of hepatitis C treatment ‘benefit’. Int. J. Drug Pol. 47, 161–168 (2017). [DOI] [PubMed] [Google Scholar]; • Explores what outcomes patients seek from hepatitis C virus cures, including nonclinical outcomes.
- 32.Lakdawalla DN, Malani A, Reif J. The insurance value of medical innovation. J. Public Econ. 145, 94–102 (2017). [Google Scholar]
- 33.Chu CE, Wu F, He X et al. Exploring the social meaning of curing HIV: a qualitative study of people who inject drugs in Guangzhou, China. AIDS Res. Hum. Retroviruses 31(1), 78–84 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'keefe-Markman C, Lea KD, Mccabe C, Hyshka E, Bubela T. Social values for health technology assessment in Canada: a scoping review of hepatitis C screening, diagnosis and treatment. BMC Public Health 20(1), 89–89 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tice JA, Ollendorf DA, Pearson SD. The comparative clinical effectiveness and value of simeprevir and sofosbuvir in the treatment of chronic hepatitis C infection. JAMA Intern. Med. 174(7), 1170–1171 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Sax PE, Sypek A, Berkowitz BK et al. HIV cure strategies: how good must they be to improve on current antiretroviral therapy? PLoS ONE 9(11), e113031 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moodley K, Staunton C, De Roubaix M, Cotton M. HIV cure research in South Africa: a preliminary exploration of stakeholder perspectives. AIDS Care 28(4), 524–527 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sylla L, Louella M, Evans D, Taylor J, Jefferys R, Dube K. What would an HIV cure mean to you? Ascribing meaning through an HIV cure tree. Presented at: IAS. Paris, France: (2017). http://programme.ias2017.org//PAGMaterial/eposters/2338.pdf [Google Scholar]
- 39.Ma Q, Wu F, Henderson G et al. ‘I can coexist with HIV’: a qualitative study of perceptions of HIV cure among people living with HIV in Guangzhou, China. J. Virus Erad. 2(3), 170–174 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henderson GE, Waltz M, Meagher K et al. Going off antiretroviral treatment in a closely monitored HIV “cure” trial: longitudinal assessments of acutely diagnosed trial participants and decliners. J. Int. AIDS Soc. 22(3), e25260–e25260 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hendriks S, Dancet EA, Meissner A, Van Der Veen F, Mochtar MH, Repping S. Perspectives of infertile men on future stem cell treatments for nonobstructive azoospermia. Reprod. Biomed. Online 28(5), 650–657 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Cho HL, Kim SYH, Fitzhugh C, Hsieh M, Tisdale J, Grady C. Motivations and decision-making of adult sickle cell patients in high-risk clinical research. Biol. Blood Marrow Transplant. 26(6), 1225–1232 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crowley D, Cullen W, Lambert JS, Van Hout MC. Competing priorities and second chances: a qualitative exploration of prisoners’ journeys through the Hepatitis C continuum of care. PLoS ONE 14(9), e0222186 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dennis C. Genetics: deaf by design. Nature 431(7011), 894–896 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Scambler G. Health-related stigma. Soc. Health Illness 31(3), 441–455 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Mattingly TJ II, Perfetto EM, Johnson SL. Engaging hepatitis C infected patients in cost–effectiveness analyses: a literature review. Hepatology 67(2), 774–781 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Cho H-J, Park E. Illness experience of patients with chronic hepatitis c participating in clinical trials. Osong Public Health Res. Perspect. 7(6), 394–399 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sav A, King MA, Whitty JA et al. Burden of treatment for chronic illness: a concept analysis and review of the literature. Health Expect. 18(3), 312–324 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hendriks S, Hessel M, Mochtar MH et al. Couples with non-obstructive azoospermia are interested in future treatments with artificial gametes. Hum. Reprod. 31(8), 1738–1748 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N. Engl. J. Med. 346(14), 1061–1066 (2002). [DOI] [PubMed] [Google Scholar]
- 51.Protiere C, Spire B, Mora M et al. Patterns of patient and healthcare provider viewpoints regarding participation in HIV cure-related clinical trials. Findings from a multicentre French survey using Q methodology (ANRS-APSEC). PLoS ONE 12(11), e0187489 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mair FS, May CR. Thinking about the burden of treatment. BMJ 349, g6680 (2014). [DOI] [PubMed] [Google Scholar]
- 53.Annemans L, Aymé S, Le Cam Y et al. Recommendations from the European working group for value assessment and funding processes in rare diseases (ORPH-VAL). Orphanet J. Rare Dis. 12(1), 50 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matza LS, Sapra SJ, Dillon JF et al. Health state utilities associated with attributes of treatments for hepatitis C. Eur. J. Health Econ. 16(9), 1005–1018 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cook K, Forbes SP, Adamski K, Ma JJ, Chawla A, Garrison LP. Assessing the potential cost-effectiveness of a gene therapy for the treatment of hemophilia A. J. Med. Econ. 23(5), 501–512 (2020). [DOI] [PubMed] [Google Scholar]
- 56.Solans M, Pane S, Estrada MD et al. Health-related quality of life measurement in children and adolescents: a systematic review of generic and disease-specific instruments. Value Health 11(4), 742–764 (2008). [DOI] [PubMed] [Google Scholar]
- 57.Alsadah A, Van Merode T, Alshammari R, Kleijnen J. A systematic literature review looking for the definition of treatment burden. Heliyon 6(4), e03641 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baran-Kooiker A, Czech M, Kooiker C. Multi-criteria decision analysis (MCDA) models in health technology assessment of orphan drugs – a systematic literature review. Next steps in methodology development? Front. Public Health 6, 287 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garrison LP, Mestre-Ferrandiz J, Zamora B. The value of knowing and knowing the value: improving the health technology assessment of complementary diagnostics. (2016). https://www.ohe.org/sites/default/files/WP_EpemedOHE_final.pdf
- 60.Claxton K, Longo R, Longworth L, Mccabe C, Wailoo A. The value of innovation: report by the Decision Support Unit (2009). www.ncbi.nlm.nih.gov/books/NBK425837/ [PubMed]
- 61.Danzon PM, Drummond MF, Towse A, Pauly MV. Objectives, budgets, thresholds, and opportunity costs-a health economics approach: an ISPOR Special Task Force Report [4]. Value Health 21(2), 140–145 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Hendriks S, Van Wely M, D'hooghe TM et al. The relative importance of genetic parenthood. Reprod. Biomed. Online 39(1), 103–110 (2019). [DOI] [PubMed] [Google Scholar]
- 63.Evon DM, Golin CE, Stoica T et al. What’s important to the patient? Informational needs of patients making decisions about hepatitis C treatment. Patient 10(3), 335–344 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]


