Abstract
Purpose
To quantify potential heterogeneity of treatment effect (HTE), of early sedation with dexmedetomidine (DEX) compared with usual care, and identify patients who have a high probability of lower or higher 90-day mortality according to age, and other identified clusters.
Methods
Bayesian analysis of 3904 critically ill adult patients expected to receive invasive ventilation > 24 h and enrolled in a multinational randomized controlled trial comparing early DEX with usual care sedation.
Results
HTE was assessed according to age and clusters (based on 12 baseline characteristics) using a Bayesian hierarchical models. DEX was associated with lower 90-day mortality compared to usual care in patients > 65 years (odds ratio [OR], 0.83 [95% credible interval [CrI] 0.68–1.00], with 97.7% probability of reduced mortality across broad categories of illness severity. Conversely, the probability of increased mortality in patients ≤ 65 years was 98.5% (OR 1.26 [95% CrI 1.02–1.56]. Two clusters were identified: cluster 1 (976 patients) mostly operative, and cluster 2 (2346 patients), predominantly non-operative. There was a greater probability of benefit with DEX in cluster 1 (OR 0.86 [95% CrI 0.65–1.14]) across broad categories of age, with 86.4% probability that DEX is more beneficial in cluster 1 than cluster 2.
Conclusion
In critically ill mechanically ventilated patients, early sedation with dexmedetomidine exhibited a high probability of reduced 90-day mortality in older patients regardless of operative or non-operative cluster status. Conversely, a high probability of increased 90-day mortality was observed in younger patients of non-operative status. Further studies are needed to confirm these findings.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-021-06356-8.
Keywords: Dexmedetomidine, Sedation, Mechanical ventilation, Mortality, Critically ill
Take home message
| The early use of dexmedetomidine for sedation of ventilated critically ill patients who are older than 65 years, and in those with an operative diagnosis, across broad range of age categories, has a high probability of reduced mortality. Conversely, younger patients with a non-operative diagnosis have a high probability of increased mortality. Thus, the early use of dexmedetomidine in this group of patients, outside controlled research, is not advised. |
Introduction
Clinical practice guidelines have recommended dexmedetomidine (DEX), a high-affinity alpha-2 adrenergic agonist, for sedation of critically ill ventilated patients in preference to benzodiazepines [1, 2]. The Sedation Practice in Intensive Care Evaluation (SPICE) III trial, by far the largest study in the field, compared the early use of DEX as primary sedative agent to usual care sedation in mechanically ventilated critically ill patients [3]. While the trial reported no overall difference in mortality, a pre-specified subgroup analysis according to age, dichotomized at the median of 63.7 years, observed a divergent DEX effect on mortality with a higher mortality below and a lower mortality above such median age.
There was no apparent explanation for the observed age effect on mortality. Thus, despite a large sample size, and a wide inclusion criteria in the SPICE III trial, heterogeneity of treatment effect (HTE) on mortality could not be ruled out [4]. It is also possible that HTE for mortality in response to DEX treatment may apply to other groups with different baseline characteristics beyond age.
Accordingly, we conducted a secondary Bayesian analysis of the SPICE III trial population to quantify the HTE of early sedation with DEX compared with usual care. Our aim was to identify patients who have a high probability of lower or higher 90-day mortality, according to age categories, and other specific clinical subgroups (clusters) according to clinically relevant baseline characteristics.
Methods
Study design, population, and intervention
This was a secondary analysis of the SPICE III trial. The SPICE III study design and protocol have been previously published [3, 5]. The study was an open label, multinational, randomized clinical trial conducted in 74 intensive care units (ICU) in Australia, Ireland, Italy, Malaysia, New Zealand, Saudi Arabia, Switzerland, and the United Kingdom between November 2013 and February 2018.
The SPICE III trial enrolled mechanically ventilated patients who were expected remain ventilated after the next calendar day and needed ongoing sedation. Eligible patients were randomly assigned to receive dexmedetomidine infusion started at 1 µg/kg/h, without a loading dose and titrated to effect, or usual care sedatives as determined by the treating clinician. In the present analysis, only patients with available data for the outcomes and for the characteristics considered for group formation were included.
Definitions and subgroups
Outcomes were defined as per the primary manuscript [3]. HTE was assessed according to the following subgroups:
Age groups: patients were divided in two groups according to World Health Organization conventional definitions of older age > 65 years.
Clusters according to pre-defined baseline characteristics described below.
Clustering process
Clustering is an approach, which aims to separate patients into groups with similar traits (called “clusters”) [6, 7]. This is a non-supervised approach, meaning that no information of predefined classes is used. The number of clusters and its characteristics are completely determined by the computational algorithm. For cluster detection, based on clinical relevance, the following variables were selected a priori on the basis of clinical judgment: (1) Acute Physiology and Chronic Health Evaluation (APACHE) II score, without the age component [8]. (2) Baseline partial pressure of arterial oxygen divided by percentage of inspired oxygen (PaO2/FiO2 ratio). (3) Admission diagnosis (defined in mutually exclusive categories as sepsis, respiratory, gastrointestinal, cardiovascular, trauma, neurological or other). (4) Type of admission (defined as non-operative, elective surgery or emergency surgery). (5) ICU source of admission (defined in mutually exclusive categories as emergency department, ward, transferred from other services or operating room). (6) Gender (male or female). (7) Baseline use of opioids (morphine or fentanyl, yes/no). (8) Baseline use of propofol (yes/no). (9) Baseline use of midazolam (yes/no). (10) Baseline use of ketamine (yes/no), and (11) Baseline use of DEX. Sensitivity analysis excluding missing baseline sedatives and imputation of PaO2/FiO2 by the median showed consistent clusters, Supplementary Information [e-Table 2 and eFigure 2B].
As age is a contributing component to the APACHE II score, to create a marker of patient severity that is independent of age, the age component was removed from the APACHE II score.
The K-means for mixed large data (kamila) method was used to detect the clusters [6]. The best number of clusters was defined by inspecting the prediction strength of clusters after 1000 cross-validations. More information on the clustering process is provided in the Supplementary Information eMethods section.
Outcomes
The study primary outcome was 90-day all-cause mortality. Additional secondary outcomes were the number of days alive, free from coma or delirium and the number of ventilator-free days at day 28 after randomization. We included secondary outcomes that showed a difference between DEX and usual care in the primary intention to treat analysis.
Statistical analysis
Baseline and outcome data are presented according to the subgroups assessed. Continuous data are presented as median (quartile 25%–quartile 75%) and compared with the Wilcoxon rank-sum test; while, categorical binary data are presented as number and percentage and compared with Fisher’s exact test.
HTE was assessed independently in each of the two subgroups proposed following a Bayesian hierarchical logistic model for the primary outcome and a Bayesian hierarchical linear model for the secondary outcomes [9]. All hierarchical models were modeled as a simple regression and shrinkage model. The hierarchical models partially pool the data and shrink the estimates in each subgroup towards the overall estimate, with shrinkage proportional to the size of the subgroup limiting the risk of type 1 error. For all analyses, weakly informative priors were used, aiming to encompass all plausible effect sizes. Since the sample size of the original study is large, it is expected that the likelihood will dominate the posteriors. The models used and priors’ definitions are described in detail in the Supplementary Information statistical analysis section. The effect estimate was reported as odds ratio (OR) where an OR > 1 represents harm and an OR < 1 represents benefit with early DEX use. As the recorded mortality according to age categories was different, for clarity, a 10% risk difference represents a 4% and 2% change in mortality in older and in younger patients, respectively.
For the secondary outcome, the mean difference (MD) and 95% credible interval (CrI) of the distribution are presented, as the probability of a MD < 0.00 or > 0.00 (null) and < 2 or > 2 (2-day reduction or increase).
A mixed-effect Bayesian logistic and linear regression model with centers as random effect and considering weakly informative priors was used to assess the interaction between the treatment groups and age on the continuous scale. The results are shown in marginal effect plots. Additional analyses considering pessimistic and optimistic priors were conducted as sensitivity analyses (full description in Supplementary Information).
To further understand the interaction according to age and severity of disease on HTE for DEX, we assessed the within-age association between use of DEX and 90-day mortality in a mixed-effect Bayesian logistic regression model according to APACHE II (without the age component). In this model, interactions between APACHE II groups (stratified into six groups) and allocation groups, age subgroups (≤ 65 vs. > 65 years) and allocation groups, and age subgroups and APACHE II quintiles were included. Also, to assess the within-cluster variation for the effect of DEX according to age, a new model including interactions between age groups (stratified into six groups) and allocation groups, clusters and allocation groups, and cluster and age quintiles were included. The models considered a Bernoulli distribution, with centers as random effect and with starting values randomly generated. All priors were drawn from normal distributions and were weakly informative (full definition of priors available in the statistical section in the statistical section in Supplementary Information).
All effect estimates were drawn from the median of the posterior distribution and the 95% CrI from the 95% percentiles of the distribution. Number of missing data is shown in the Supplementary Information [eTable 1]. All analyses were performed using the R (R, version 4.0.2, Core Team, Vienna, Austria, 2016) software with the beanz package and Stan through brms [10, 11].
Results
Patients
Out of a total of 3918 patients consented and randomized in the SPICE III trial, 3904 (99.6%) with known primary outcome were included. From this population, 1825 (46.7%) were older than 65 years old [Supplementary Information Study Flow Diagram, eFigure 1]. Older patients had a similar severity of illness as measured by APACHE II scores (after removing the age component) and lower PaO2/FiO2 ratios than younger patients. In addition, a higher proportion of older patients were admitted with a cardiovascular diagnosis or from the general ward, and fewer were admitted with trauma or from an emergency department. Detailed baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics of the patients according to the categories of age and the clusters identified
| Age > 65 years (n = 1825) |
Age ≤ 65 years (n = 2079) |
p value | Cluster 1 (n = 976) |
Cluster 2 (n = 2346) |
p value | |
|---|---|---|---|---|---|---|
| Age (years) | 73.6 (69–78.4) | 53.3 (42.7–59.9) | < 0.001 | 64.7 (53.2–74.1) | 63.6 (52.2–72.6) | 0.038 |
| Female gender—no (%)* | 700 (38.4) | 795 (38.2) | 0.947 | 334 (34.2) | 927 (39.5) | 0.004 |
| Weight (kg) | 80 (67–91) | 80 (67–99) | < 0.001 | 81 (70–97) | 78 (65–93) | < 0.001 |
| APACHE II | 23 (19–28) | 20 (15–25) | < 0.001 | 19 (15–24) | 23 (18–29) | < 0.001 |
| Without age* | 18 (13–23) | 18 (13–23) | 0.885 | 15 (11–20) | 19 (15–25) | < 0.001 |
| Time to randomization (h) | 4.7 (1.9–8.6) | 4.5 (1.8–8.7) | 0.739 | 5.5 (2.5–9.6) | 4.5 (2–8.5) | < 0.001 |
| Dexmedetomidine group—no (%) | 913 (50) | 1035 (49.8) | 0.898 | 488 (50) | 1165 (49.7) | 0.879 |
| Diabetes with insulin—no (%) | 185 (10.1) | 205 (9.9) | 0.789 | 61 (6.2) | 246 (10.5) | < 0.001 |
| Type of admission—no (%)* | 0.123 | < 0.001 | ||||
| Non-operative | 1292 (70.8) | 1487 (71.5) | 11 (1.1) | 2343 (99.9) | ||
| Elective surgery | 172 (9.4) | 159 (7.6) | 309 (31.7) | 0 (0) | ||
| Emergency surgery | 361 (19.8) | 433 (20.8) | 656 (67.2) | 3 (0.1) | ||
| Admission diagnosis—no (%)* | < 0.001 | < 0.001 | ||||
| Sepsis | 1174 (64.3) | 1321 (63.5) | 411 (42.1) | 1706 (72.7) | ||
| Respiratory | 203 (11.1) | 245 (11.8) | 63 (6.5) | 304 (13) | ||
| Gastrointestinal | 101 (5.5) | 105 (5.1) | 115 (11.8) | 57 (2.4) | ||
| Cardiovascular | 276 (15.1) | 212 (10.2) | 301 (30.8) | 151 (6.4) | ||
| Trauma | 33 (1.8) | 119 (5.7) | 61 (6.2) | 66 (2.8) | ||
| Neurological | 6 (0.3) | 18 (0.9) | 0 (0) | 20 (0.9) | ||
| Other | 32 (1.8) | 59 (2.8) | 25 (2.6) | 42 (1.8) | ||
| ICU source of admission—no (%)* | < 0.001 | < 0.001 | ||||
| Emergency room | 479 (26.2) | 724 (34.8) | 2 (0.2) | 1012 (43.1) | ||
| Ward | 643 (35.2) | 567 (27.3) | 3 (0.3) | 1011 (43.1) | ||
| Transferred from another hospital | 184 (10.1) | 227 (10.9) | 40 (4.1) | 323 (13.8) | ||
| Operating room | 519 (28.4) | 561 (27) | 931 (95.4) | 0 (0) | ||
| PaO2/FiO2 ratio (mmHg)* | 192 (132–272.2) | 206.3 (136.9–302.9) | < 0.001 | 249.5 (176–340) | 182.7 (125–260) | < 0.001 |
| Sedatives and opioids—no (%) | ||||||
| Opioids*,a | 1290 (74.3) | 1529 (78) | 0.009 | 715 (73.3) | 1789 (76.3) | 0.070 |
| Propofol* | 1442 (83) | 1549 (79) | 0.002 | 898 (92) | 1791 (76.3) | < 0.001 |
| Midazolam* | 472 (27.2) | 690 (35.2) | < 0.001 | 145 (14.9) | 900 (38.4) | < 0.001 |
| Ketamine* | 112 (6.4) | 123 (6.3) | 0.840 | 36 (3.7) | 180 (7.7) | < 0.001 |
| Dexmedetomidine* | 30 (1.7) | 48 (2.4) | 0.137 | 5 (0.5) | 59 (2.5) | < 0.001 |
| Clinical outcomes | ||||||
| 90-day mortality—no (%) | 700 (38.4) | 435 (20.9) | < 0.001 | 240 (24.6) | 731 (31.2) | < 0.001 |
| Coma- and delirium-free days | 22 (5–26) | 24 (17–26) | < 0.001 | 24 (15–26) | 23 (9–26) | < 0.001 |
| Ventilator-free days | 21 (0–25) | 23 (12–26) | < 0.001 | 24 (6–26) | 22 (0–25) | < 0.001 |
Data expressed as median (quartile 25%–quartile 75%) or number (%), percentages may not total 100 because of rounding
APACHE Acute Physiology and Chronic Health Evaluation [8], ICU intensive care unit, PaO2/FiO2 partial pressure of arterial oxygen/inspired oxygen concentration ratio
*Variables considered in the cluster process. Patients may have received more than one agent
aOpioids aggregate the use of morphine and/or fentanyl
Clusters identified
From patients 3322/3904 (85.1%) with a complete dataset, cluster analysis identified two distinct clusters, one with 976 (29.4%) patients (cluster 1) and another with 2346 (70.6%) patients (cluster 2) [Supplementary Information Study Flow Diagram, eFigure 1]. The optimal number of clusters was confirmed to be two by the prediction strength and the plot of Gower’s distance as shown in the Supplementary Information [eFigure 2 A–B]. While patients in cluster 1 were admitted mainly from the operating room due to cardiovascular or gastrointestinal diseases, patients in cluster 2 were predominantly admitted due to non-operative reasons and from the emergency department due to sepsis or respiratory disease (Table 1). Patients in cluster 2 also had a higher median APACHE II scores and lower PaO2/FiO2 ratios and were more often sedated at baseline with midazolam and/or ketamine, but less often sedated with propofol compared to cluster 1. The number of patients allocated to DEX or usual care sedation were similar among the groups [Supplementary Information Study Flow Diagram, eFigure 1].
Age related heterogeneity of dexmedetomidine effect on mortality
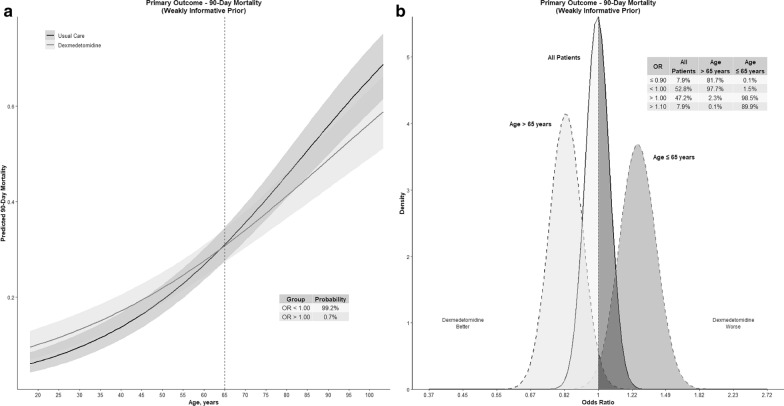
The overall 90-day mortality was higher in older patients, 700/1825 (38.4%) vs 435/2079 (20.9%) in younger patients (Supplementary Information eFigure 3). However, the probability that assignment to DEX group results in lower OR for mortality in older patients (more beneficial), compared to young patients, was 99.8% (Table 2). There was an interaction between age and the use of DEX and a 99.3% probability of lower mortality with the use of DEX with increasing age (Fig. 1a).
Table 2.
Primary and secondary outcomes—effect estimates according to age and clusters
| Weakly informative priorsa | Pessimistic priorsa | Optimistic priorsa | ||||
|---|---|---|---|---|---|---|
| Odds ratio (95% CrI) | Probability of benefit | Odds ratio (95% CrI) | Probability of benefit | Odds ratio (95% CrI) | Probability of benefit | |
| Primary outcome | ||||||
| 90-day mortality | ||||||
| Age | ||||||
| All patients | 1 (0.87–1.14) | 47.2% | 1 (0.87–1.15) | 50.5% | 0.99 (0.86–1.14) | 53.8% |
| > 65 years | 0.83 (0.68–1) | 97.7% | 0.83 (0.68–1) | 97.7% | 0.82 (0.68–1) | 97.7% |
| ≤ 65 years | 1.26 (1.02–1.56) | 1.5% | 1.26 (1.02–1.56) | 1.5% | 1.26 (1.02–1.56) | 1.5% |
| Probability of lower OR in > 65 years | 99.8% | – | 99.8% | – | 99.8% | – |
| Cluster | ||||||
| All patients | 0.99 (0.85–1.15) | 55.8% | 0.99 (0.86–1.15) | 54.2% | 0.99 (0.85–1.14) | 57.3% |
| 1 | 0.86 (0.65–1.14) | 84.8% | 0.86 (0.64–1.15) | 84.6% | 0.86 (0.64–1.15) | 84.9% |
| 2 | 1.04 (0.87–1.24) | 33.4% | 1.04 (0.87–1.24) | 33.4% | 1.04 (0.87–1.24) | 33.4% |
| Probability of lower OR in cluster 1 | 86.4% | – | 86.3% | – | 86.6% | – |
| Mean difference (95% CrI) | Probability of MD > 0 | Mean difference (95% CrI) | Probability of MD > 0 | Mean difference (95% CrI) | Probability of MD > 0 | |
|---|---|---|---|---|---|---|
| Secondary outcomes | ||||||
| Alive. Coma- and delirium-free days | ||||||
| Age | ||||||
| All patients | 0.27 (− 0.25 to 0.8) | 84.4% | 0.12 (− 0.4 to 0.65) | 67.8% | 0.41 (− 0.11 to 0.93) | 94% |
| > 65 years | 0.9 (0–1.82) | 97.4% | 0.87 (− 0.02 to 1.79) | 97.1% | 0.92 (0.03–1.83) | 97.6% |
| ≤ 65 years | − 0.01 (− 0.79 to 0.74) | 48.8% | − 0.03 (− 0.81 to 0.73) | 46.7% | 0.01 (− 0.77 to 0.76) | 50.6% |
| Probability of higher MD in > 65 years | 93.4% | – | 93.2% | – | 93.7% | – |
| Cluster | ||||||
| All patients | 0.29 (− 0.28 to 0.84) | 84.2% | 0.12 (− 0.44 to 0.68) | 66.5% | 0.45 (− 0.12 to 1.01) | 94.2% |
| 1 |
0.64 (− 0.41 to 1.7 ) |
88.5% | 0.61 (− 0.43 to 1.68) | 87.2% | 0.68 (− 0.37 to 1.75) | 89.4% |
| 2 | 0.3 (− 0.48 to 1.06) | 77.3% | 0.28 (− 0.5 to 1.05) | 76.1% | 0.31 (− 0.46 to 1.08) | 78.5% |
| Probability of higher MD cluster 1 | 69.9% | – | 69.4% | – | 70.1% | – |
| Ventilator-free days | ||||||
| Age | ||||||
| All patients | 0.1 (− 0.46 to 0.66) | 64.3% | − 0.06 (− 0.62 to 0.5) | 41.8% | 0.27 (− 0.29 to 0.82) | 82.6% |
| > 65 years | 0.84 (− 0.14 to 1.88) | 95% | 0.81 (− 0.17 to 1.85) | 94.3% | 0.87 (− 0.12 to 1.88) | 95.5% |
| ≤ 65 years | − 0.33 (− 1.18 to 0.5) | 22.1% | − 0.35 (− 1.2 to 0.49) | 20.7% | − 0.31 (− 1.16 to 0.53) | 23.7% |
| Probability of higher MD in > 65 years | 96.2% | – | 95.9% | – | 96.5% | – |
| Cluster | ||||||
| All patients | 0.11 (− 0.49 to 0.71) | 63.8% | − 0.08 (− 0.67 to 0.51) | 39.9% | 0.29 (− 0.31 to 0.90) | 83.2% |
| 1 | 0.31 (− 0.82 to 1.45) | 70.2% | 0.28 (− 0.84 to 1.43) | 68.2% | 0.34 (− 0.78 to 1.49) | 72.4% |
| 2 | 0.10 (− 0.75 to 0.94) | 58.9% | 0.07 (− 0.76 to 0.92) | 56.8% | 0.12 (− 0.71 to 0.96) | 61.1% |
| Probability of higher MD cluster 1 | 61.4% | – | 60.9% | – | 62.2% | – |
Benefit indicates reduced risk of death (OR < 1.00)
Cluster 1 is predominantly operative patients and cluster 2 is non-operative patients
OR odds ratio, MD mean difference, CrI credible interval
aDifferent priors are described in the Supp Digital Content—eMethods
Fig. 1.
Age-related heterogeneity of treatment effect—dexmedetomidine and mortality. OR odds ratio. Values less than 1 indicate lower mortality. a Marginal effect plot for the interaction between the allocation group and age, as a continuous variable, for 90-day mortality. b The posterior distribution of mortality, depicted as odds ratios. The probability of benefit (OR < 1) is 97.7% in patients > 65 years old with 98.5% probability of harm in patients ≤ 65 years old (OR > 1)
The use of DEX resulted in lower mortality compared to usual care in patients older than 65 years (OR 0.83 [95% CrI 0.68–1]), with a probability of benefit of 97.7% and of a 10% mortality benefit of 81.7% (Fig. 1b, Table 2). On the other hand, DEX led to higher mortality in patients younger than 65 years (OR 1.26 [95% CrI 1.02–1.56), with a 98.5% probability of harm and an 89.9% probability of > 10% increased mortality (Fig. 1b). The use of different priors did not materially change these findings (Table 2 and Supplementary Information eFigures 4 and 5).
Cluster related heterogeneity of dexmedetomidine effect on mortality
The overall 90-day mortality rate was higher in cluster 2 at 731/2346 (31.2%) vs 240/976 (24.6%) in cluster 1. There was a higher probability of a mortality benefit with DEX in cluster 1 patients (OR 0.86 [95% CrI 0.65–1.14], 84.8% probability of benefit, compared to cluster 2 (OR 1.04 [95% CrI 0.87–1.24], (Table 2 and Supplementary Information eFigure 6). The probability that DEX would result in lower OR of death in patients in cluster 1 compared to patients in cluster 2 was 86.4%. The use of different priors did not materially change these findings (Table 2 and Supplementary Information eFigure 7).
Interaction between age and severity of illness
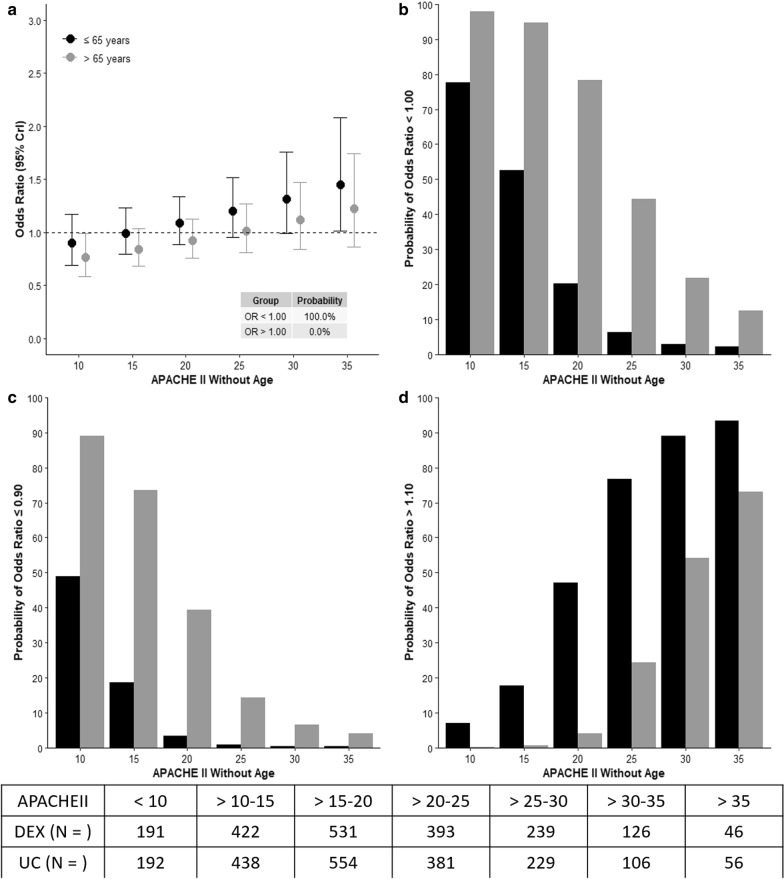
The results of the model assessing interactions between age, severity of illness and use of DEX are shown in Fig. 2a–d. In older patients, there was a high probability of lower mortality (OR < 1) with the use of DEX which declined with increasing APACHE II, panels a, b. A high probability of > 10% benefit with the use of DEX was seen in older patients with APACHE II < 20, panel c. In young patients, > 10% harm associated with the use of DEX was seen with high probability in those with an APACHE II ≥ 25, panel d.
Fig. 2.
Risk of death and interaction between age, severity of illness and dexmedetomidine treatment. APACHE II Acute Physiology and Chronic Health Evaluation II, CrI credible interval. The effect estimates, odds ratio (OR) for the interaction between dexmedetomidine allocation, age category and six different cut-offs of APACHE II are presented. OR < 1.0 represents a favorable outcome and > 1.0 represents unfavorable outcome with the use of dexmedetomidine. a Odd ratios according to age category, age group > 65 years depicts a high probability of OR < 1.0 with increased APACHEII. b Probability of benefit with the allocation to dexmedetomidine was higher in older age group. c Probability of > 10% benefit with the allocation to dexmedetomidine was higher in patients > 65 years but declined with increasing APACHEII. d Probability of > 10% harm with the allocation to dexmedetomidine was higher in patients ≤ 65 years and increased with rising APACHEII
Interaction between clusters and age
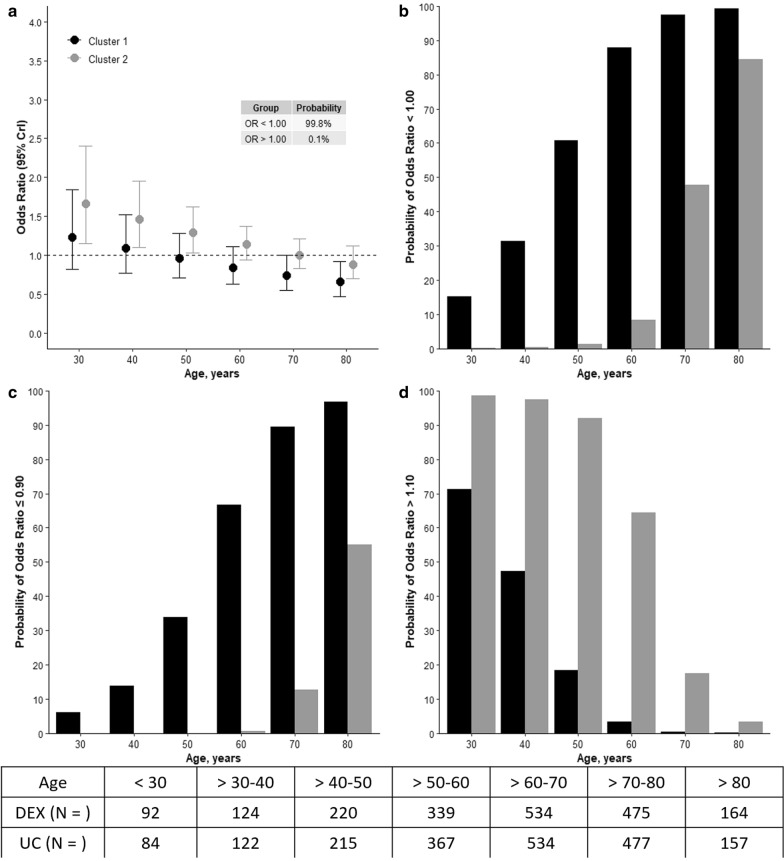
The results of the model assessing interactions between cluster, age and use of DEX are shown in Fig. 3a–d. The probability of reduced mortality (OR < 1) with DEX was high when age was greater than 50 years in patients in cluster 1 (panels a, b). In cluster 2, however, a high probability of reduced mortality with DEX was found only when age was greater than 80 years (panel b). A high probability of > 10% benefit in cluster 1 was seen with DEX with age above 60 years, panel c. In cluster 1, the probability of > 10%, harm, was low with age greater than 40 years, but in cluster 2, this probability remained high up to age of 70 years (panels c, d).
Fig. 3.
Risk of death and interaction between clusters, age and dexmedetomidine treatment. CrI credible interval. The effect estimates, odds ratio (OR) for the interaction between dexmedetomidine allocation, cluster assignment and six different cut-offs of age categories are presented. OR < 1.0 represents a favorable outcome and > 1.0 represents unfavorable outcome with the use of dexmedetomidine. a Odd ratios according to cluster, operative cluster 1 depicts a high probability of benefit with increased age. b Probability of benefit with the allocation to dexmedetomidine was higher in cluster 1 but mainly in those > 50 years old. c Probability of > 10% benefit with the allocation to dexmedetomidine was higher in cluster 1 but mainly in those > 60 years. d Probability of > 10% harm with the allocation to dexmedetomidine was higher in cluster 2, non-operative, mainly in patients younger than 50 years
Age and cluster heterogeneity of treatment effect and secondary outcomes
The probability of more days alive, and free of delirium and coma was 97.4% in patients older than 65 years, allocated to DEX, with a neutral effect in younger patients (Table 2 and Supplementary Information eFigure 8). Conversely, in clusters 1 and 2, the probability was lower, at 88.5% and 77.3%, respectively (Table 2).
A high probability of greater number of ventilator-free days with DEX treatment was seen in older patients and in those assigned to cluster 1 (Table 2 and Supplementary Information eFigure 9).
Adverse events
Adverse and serious adverse events, reported by site investigators, were distributed at comparable rates across age and cluster categories. [Supplementary Information. eTable 3].
Discussion
Key findings
In this Bayesian secondary analysis of the SPICE III trial, we assessed the HTE of early sedation with DEX according to age and two distinct clusters, based on 12 baseline characteristics. Using this approach, we found evidence of HTE on 90-day mortality and key secondary outcomes. In particular, early sedation with DEX reduced 90-day mortality in patients older than 65 years with high (97.7%) probability. In addition, early DEX was more likely to improve 90-day mortality in the mainly operative cluster. Conversely, we observed a high probability of increased 90-day mortality in younger patients and in the cluster of non-operative patients, which increased with increasing APACHE II scores. Importantly, median age in both clusters was similar, suggesting that other factors such as sedative drug interaction or neuroendocrine response, independent of age, may have accounted for HTE of DEX in these patients.
Relationship to previous studies
In the SPICE III trial, a pre-specified subgroup analysis of age, dichotomized at the cohort median, suggested a significant interaction with DEX treatment [3]. This interaction, however, was not observed within the subgroup of operative or non-operative patients [3]. In addition, there was no detailed analysis of treatment effects in any of the subgroups for the secondary outcomes or occurrence of adverse events. In this analysis, we adopted a Bayesian approach to evaluate in detail the HTE of DEX on the 90-day mortality, relevant secondary outcomes and adverse events. In addition to the age category, we identified two distinct clusters, operative and non-operative, and assessed the interaction according to different six age and six APACHEII categories.
Despite an expected age effect on the pharmacokinetics and efficacy of DEX [12, 13], no previous sedation trials have assessed age related HTE for mortality or other sedation related outcomes. This is possibly due to a significantly smaller sample size in previous randomized sedation trials compared with SPICE III [14–17]. In addition, Bayesian and/or cluster analysis was not a feature of previous sedation trials.
In the SLEAP sedation interruption trial, HTE was reported in subgroup analysis, suggesting a beneficial effect of sedative interruption for surgical/trauma patients when compared with medical non-operative patients [18]. HTE according to age, however, was not assessed. More recently, the differential effect of vasopressors in septic patients older than 65 years was investigated [19], suggesting more awareness of potential and important age related HTE in critically ill patients.
Implications of study findings
Our findings imply that the early use of DEX in ventilated critically ill patients is likely beneficial in patients older than 65 years across broad diagnostic categories and illness severity. Conversely, early DEX-based sedation in younger patients, appears likely to increase mortality, especially in non-operative critically ill patients with high severity of illness. These findings also imply that the positive effects of early DEX on delirium-, coma- and ventilator-free time are preserved in older patients irrespective of cluster assignment. Our findings also suggest a comparable distribution of adverse or serious adverse events according to age or cluster categories.
Due to the post hoc secondary nature of this analysis, no inference of causality or mediation of effect can be claimed. Thus, caution must be taken in extrapolating these findings into practice. Nonetheless, the use of early DEX as sedative in the older and operative population, appears safe and may be efficacious. Its use in younger non-operative patients, however, appears unwarranted. The mechanism for the underlying effect remains uncertain; further studies and analysis are needed to address the cause/s of the observed mortality.
Strengths and limitations
The SPICE III trial is the largest in the field of sedation, conducted in 74 ICUs in 8 countries with diverse health systems and different models of care, and virtually complete 90-day follow-up. The plan of the present analysis (including prior choices) was based on available evidence and pre-defined priors. Also, the clusters were not arbitrarily defined but rather derived from an unsupervised approach. Conventional subgroup analysis, as used in the primary trial, is at increased risk of type I and II errors. In the present paper, the use of hierarchical models according to a simple regression and shrinkage model limits the degree of type I error. Moreover, the posterior probabilities derived from the Bayesian analysis provide more power than the traditional interaction tests used for subgroup analyses [20, 21]. In addition, we assessed factors other than age as potential sources of HTE, including the interaction of age with illness severity, adding to the findings of the original trial. Finally, due to the large sample size in the original trial, the clusters assessed still had a considerable number of patients, lending statistical robustness to our observations.
The present study has some limitations. First, this is an unplanned secondary post hoc analysis. Second, the limitations of the original trial influence the present analysis. They include the lack of blinding, the inclusion of patients needing deep sedation, and the absence of a strictly protocolized strategy for managing sedation or delirium. Third, in defining the clusters, missingness in some baseline characteristics was relatively high. Nonetheless, robust sensitivity analysis revealed, exactly, the same clusters. Finally, our results are not generalizable outside the study population and should not be extrapolated to all critically ill patients. In addition, with possible selection bias in the clustering process, HTE could be present in different groups considering characteristics not assessed in this study. Due to these limitations, and because the clustering procedure is mainly descriptive, our findings can only provide the statistical underpinning and rationale for further investigations and stratification in clinical trials.
Conclusion
This secondary analysis of the SPICE III trial identified HTE of early use of DEX, vs. usual care, as primary sedative in critically ill patients needing mechanical ventilation. In particular, a high probability of reduced 90-day mortality was observed in older patients regardless of operative or non-operative cluster status. Conversely, a high probability of increased 90-day mortality was observed in younger patients of non-operative cluster status. These findings warrant confirmation in future randomized trials.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
SPICE III Study Investigators:
Site investigators (alphabetically by institution and all in Australia unless specified as New Zealand [NZ], Ireland [IR], Italy [IT], Malaysia [MY], Saudi Arabia [SA], Switzerland [CH] or United Kingdom [UK]): Albury Base Hospital, Albury, NSW, C. Mashonganyika, H. McKee, A. Tonks; Altnagelvin Area Hospital, Londonderry, UK, A. Donnelly, N. Hemmings, S. O'Kane; Auckland City Hospital CVICU, Auckland, NZ, A. Blakemore, M. Butler, K. Cowdrey, J. Dalton, E. Gilder, S. Long, L. McCarthy, S. McGuinness, R. Parke; Auckland City Hospital DCCM, Auckland, NZ, Y. Chen, C. McArthur, R. McConnochie, L. Newby; Austin Health, Melbourne, VIC, R. Bellomo, G. Eastwood, L. Peck, H. Young; Bendigo Hospital, Bendigo, VIC, C. Boschert, J. Edington, J. Fletcher, J. Smith; Blacktown Hospital, Sydney, NSW, K. Nand, A. Raza, T. Sara; Bristol Royal Infirmary, Bristol, UK, J. Bennett-Britton, J. Bewley, V. Bodenham, L. Cole, K. Driver, L. Grimmer, L. Howie, C. Searles, K. Sweet, D. Webster; Central Gippsland Health, Sale, VIC, A. van Berkel, H. Connor, J. Dennett, M. van Der Graaff; Christchurch Hospital, Christchurch, NZ, S. Henderson, J. Mehrtens, K. Miller, E. Minto, A. Morris, S. Noble, K. Parker; Dandenong Hospital, Melbourne, VIC, L. Bulfin, N. Hart, K. Shepherd, S. Vij; Derriford Hospital, Derriford, UK, S. Dickson, E. Elloway, C. Ferguson, R. Jackson, P. MacNaughton, M. Marner, R. Squire, S. Waddy, P. Wafer, J. Welbourne; Dorset County Hospital, Dorchester, UK, P. Ashcroft, D. Chambler, S. Dukes, A. Harris, S. Horton, S. Sharpe, P. Williams, S. Williams; Dunedin Hospital, Dunedin, NZ, M. Bailey, E. Blazquez, D. France, R. Hutchison, A. O'Connor; Gold Coast University Hospital, Gold Coast, QLD, G. Comadira, M. Gough, M. Tallott; Gosford Hospital, Gosford, NSW, M. Bastick, R. Cameron, S. Donovan, K. Ellis, A. Gaur, R. Gregory, J. Naumoff, E. Turner, M. White; Hornsby Ku-Ring-Gai Hospital, Sydney, NSW, KFJ. Au, J. Fratzia, S. Treloar; Hospital Pulau Pinang, Pulau Pinang, MY, CH. Lim, Maseeda.Y, AP. Tan, CL. Tang, CY. Yong; Inselspital Bern University Hospital, Bern, CH, M. Akaltan, S. Berger, D. Blaser, L. Fazlija, ML. Jong, M. Lensch, R. Ludwig, T. Merz, K. Nettelbeck, M. Roth, M. Schafer, J. Takala, A. Wehr, D. Zacharias; Institut Jantung Negara, Kuala Lumpur, MY, R. Amran, HN. Ashraf, N. Azmi, N. Basri, H. Burhanuddin, Y. Hadinata, A. Hamdan, S. Kadiman, AIYM. Rashid, IN. Sabran, S. Sulaiman, I. N. Zabidi; King Abdulaziz Medical City, King Saud Bin Abdulaziz University for Health Sciences and King Abdullah International Medical Research Center, Riyadh, SA, A. Al-Dawood, M. Aljuaid, H. Al Anizi, A. Al Saeedi, Y. Arabi, M. Dbsawy, A. Deeb, M. Hegazy, I. Magdi; Kings College Hospital, London, UK, E. Clarey, E. Corcoran, C. Finney, C. Harris, P. Hopkins, H. Noble, J. Smith, L. Thompson, T. Williams; King Saud Medical City, Riyadh, KSA, LA. Dumlao, R. Bassam, MA. Hassan, N. Naseem, MH. Al-Kurdi, AM. Al-Harthy; Knox Private Hospital, Melbourne, VIC, S. Bernard, L. Sebafundi, C. Serban; Kuala Lumpar General Hospital, Kuala Lumpur, MY, SK. Lim, N. Mazidah, N. Saidin, N. Sjamsuddin, ITA. Tan, N. Zabidi; Launceston General Hospital and Clifford Craig Medical Research Trust, Launceston, TAS, M. Brain, S. Mineall; Lyell McEwin Hospital, Adelaide, SA, M. Kanhere, N. Soar; Melaka General Hospital, Melaka, MY, N. Abd Kadir, NH. Abdullah, R. Awang, Z. Emperan, NS. Husin, NI. Ismail, SZ. Ismail, FNA. Mohd Khadzali, MF. Norddin; Middlemore Hospital, Auckland, NZ, J. Aguila, C. Bold, B. Clatworthy, A. Dias, C. Hogan, A. Kazemi, V. Lai, R. Song, A. Williams; Monash Medical Centre, Melbourne, VIC, D. Bhatia, L. Bulfin, S. Elliot, P. Galt, K. Lavrans, P. Ritchie, A. Wang; Nepean Hospital, Sydney, NSW, R. Gresham, J. Lowrey, K. Masters, P. Palejs, I. Seppelt, F. Symonds, L. Weisbrodt, C. Whitehead; Newcastle upon Tyne Hospitals (Freeman Hospital and Royal Victoria Infirmary), UK, M. Babio-Galan, V. Calder, I. Clement, A. Harrison, I. McCullagh, C. Scott, L. Thompson; North Shore Hospital, Auckland, NZ, R. Bevan, S. Caniba, D. Hacking, L. Maher; Ospedale San Raffaele, Milan, IT, ML. Azzolini, P. Beccaria, S. Colombo, G. Landoni, C. Leggieri, C. Luca, D. Mamo, E. Moizo, G. Monti, M. Mucci, A. Zangrillo; Prince of Wales, Sydney, NSW, M. Albania, S. Arora, Y. Shi; Prince Sultan Military Medical City, Riyadh, SA, A. Abudayah, G. Almekhlafi, E. Al Amodi, S. Al Samarrai, M. Badawi, R. Cubio Caba, O. Elffaki, Y. Mandourah, J. Valerio; Princess Alexandra Hospital, Brisbane, QLD, C. Joyce, J. Meyer, E. Saylor, B. Venkatesh, E. Venz, J. Walsham, K. Wetzig; Princess Royal University Hospital, London, UK, E. Clarey, C. Harris, P. Hopkins, H. Noble, L. Thompson, T. Williams; Queen Elizabeth Hospital, MY, TM. Khoo, JES. Liew, AN. Sakthi, A. Zulkurnain; Queen Elizabeth Hospital Birmingham, Birmingham, UK, A. Bamford, C. Bergin, R. Carrera, L. Cooper, L. Despy, K. Ellis, S. Harkett, L. Mee, E. Reeves, C. Snelson, E. Spruce; Queen Elizabeth Hospital Kings Lynn, UK, G. Cooper, R. Hodgson, D. Pearson, M. Rosbergen; Raja Perempuan Zainab II Hospital, Kota Bharu, MY, MN. Ali, NI. Bahar, A. Ismail, WNW. Ismail, NM. Samat, NSM. Piah, R. Abd Rahman; Redcliffe Hospital, Brisbane, QLD, M. Duroux, M. Ratcliffe, T. Warhurst; Rotorua Hospital, Rotorua, NZ, U. Buehner, E. Williams; Royal Berkshire Hospital, Reading, UK, N. Jacques, L. Keating, S. Macgill, KL. Tamang, N. Tolan, A. Walden; Royal Bournemouth Hospital, Bournemouth, UK, R. Bower, J. Cranshaw, K. Molloy, S. Pitts; Royal Brisbane and Women’s Hospital, Brisbane, QLD, J. Butler, R. Dunlop, C. Fourie, P. Jarrett, M. Lassig-Smith, A. Livermore, S. O'Donoghue, M. Reade, T. Starr, J. Stuart; Royal Darwin Hospital, Darwin, NT, L. Campbell, M. Phillips, D. Stephens, J. Thomas; Royal Hobart Hospital, Hobart, TAS, D. Cooper, R. McAllister; Royal Infirmary of Edinburgh, Scotland, UK, G. Andrew, L. Barclay, H. Dawson, DM. Griffith, D. Hope, G. Wojcik, C. McCulloch, R. Paterson; Royal Liverpool Hospital, Liverpool, UK, L. Ascough, C. Paisley, J. Patrick-Heselton, D. Shaw, V. Waugh, K. Williams, I. Welters; Royal Melbourne Hospital, Melbourne, VIC, D. Barge, A. Jordan, C. MacIsaac, T. Rechnitzer; Royal North Shore Hospital, Sydney, NSW, F. Bass, J. Gatward, N. Hammond, P. Janin, A. O'Connor, W. Stedman, E. Yarad; Sarawak General Hospital, Sarawak, MY, NA. Razak, N. Dzulkipli, SL. Jong, K. Asen, WL. Voon, S. Liew; St George's Hospital London, UK, J. Ball, V. Barnes, C. Dalton, S. Farnell-Ward, H. Farrah, K. Maher, J. Mellinghoff, C. Ryan, P. Shirley; St James University Hospital, Dublin, IR, L. Conlon, A. Glover, I. Martin-Loeches, E. O'Toole; St John of God Hospital Subiaco, Subiaco, WA, J. Ewan, J. Ferrier, E. Litton, SA. Webb; St Thomas Hospital, London, UK, , W. Berry, U. Blanco Alonso, A. Bociek, S. Campos, S. Jawara, F. Hanks, A. Kelly, K. Lei, C. McKenzie, M. Ostermann, R. Wan, St Vincent's Hospital, Sydney, NSW, S. Al-Soufi, S. Leow, K. McCann, C. Reynolds; St Vincent's University Hospital, Dublin, IR, K. Brickell, C. Fahey, L. Hays, N. Hyde, A. Nichol, D. Ryan; Sunshine Coast University Hospital and Nambour Hospital, Sunshine Coast, QLD, J. Brailsford, A. Buckley, L. Forbes, T. Maguire, J. Moore, L. Murray; The Northern Hospital, Melbourne, VIC, A. Ghosh, M. Park, S. Said; Toowoomba Hospital, Toowoomba, QLD, J. Smith, A. Visser; Universiti Sains Malaysia Hospital, MY, HZ. Abidin, S. Ali, MH. Hassan, SC. Omar, WFW. Shukeri; University College Hospital London, UK, D. Brealey, G. Bercades, E. Blackburn, N. Macallum, A. Macklin, JH. Ryu, K. Tam, D. Smyth; University Hospital of Coventry and Warwick, Coventry, UK, A. Arif, C. Bassford, C. Morgan, C. Swann, G. Ward, L. Wild; University Hospital Geelong, Geelong, VIC, A. Bone, T. Elderkin, D. Green, D. Sach, T. Salerno, N. Simpson; University Hospital of North Tees, Stockton-on-Tees, UK, F. Brohi, M. Clark, L. Williams; University Hospital of Wales, Cardiff, UK, J. Brooks, E. Cocks, J. Cole, J. Curtin, R. Davies, H. Hill, M. Morgan, N. Palmer, C. Whitton, M. Wise; University Malaya Medical Center, MY, P. Baskaran, MS. Hasan, LY. Tham; Wellington Regional Hospital, Wellington, NZ, R. Sol Cruz, D. Dinsdale, S. Edney, C. Firkin, F. FitzJohn, G. Hill, A. Hunt, S. Hurford, G. Jones, H. Judd, C. Latimer-Bell, C. Lawrence, E. Lesona, L. Navarra, Y. Robertson, H. Smellie, AM. Vucago, P. Young; Western General Hospital Scotland, Edinburgh, UK, H. Dawson, DM. Griffith, R. Paterson; Westmead Hospital, Sydney, NSW, P. Clark, J. Kong, J. Ho, V. Nayyar, C. Skelly.
Author contributions
All authors approved the version submitted. YS: Study concept and design, data interpretation, manuscript drafting and submission; ASN: Concept, Data analysis and interpretation, manuscript drafting and review; RB: Study concept and design, data interpretation, manuscript review; MB: Study concept and design, statistical analyses, manuscript review; YS, RB, MB, ASN: Accept responsibility for the integrity and the accuracy of all the data; BDH: Study concept, data interpretation, manuscript review; All authors: Acquisition and interpretation of data, manuscript review and critique. All authors approved the final submitted manuscript.
Funding
This manuscript received no funding from third parties. The parent SPICE studies, however, were supported by an unrestricted research Grant from Pfizer (Hospira Inc. IL)—USA Grant-in-Aide Program.
Compliance with ethical standards
Conflicts of interest
YS declares unrestricted research and educational Grant support from Pfizer (Hospira Inc IL) USA, Orion Pharma—Helsinki Finland in support of the SPICE Program. Speaker honorarium and travel reimbursements for educational symposia from Pfizer and Orion. MCR declares unrestricted research and educational grant support from Pfizer (Hospira Inc.—Melbourne) Australia. All authors filed in the COI ICMJE form.
Availability of data and material (data transparency)
On request.
Code availability (software application or custom code)
On request.
Footnotes
The names of all participating centers and associated investigators are listed in the Acknowledgements section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yahya Shehabi, Email: Yahya.shehabi@monashhealth.org, Email: y.shehabi@unsw.edu.au.
The SPICE III Study Investigators:
C. Mashonganyika, H. McKee, A. Tonks, A. Donnelly, N. Hemmings, S. O’Kane, A. Blakemore, M. Butler, K. Cowdrey, J. Dalton, E. Gilder, S. Long, L. McCarthy, S. McGuinness, R. Parke, Y. Chen, C. McArthur, R. McConnochie, L. Newby, R. Bellomo, G. Eastwood, L. Peck, H. Young, C. Boschert, J. Edington, J. Fletcher, J. Smith, K. Nand, A. Raza, T. Sara, J. Bennett-Britton, J. Bewley, V. Bodenham, L. Cole, K. Driver, L. Grimmer, L. Howie, C. Searles, K. Sweet, D. Webster, A. van Berkel, H. Connor, J. Dennett, M. van Der Graaff, S. Henderson, J. Mehrtens, K. Miller, E. Minto, A. Morris, S. Noble, K. Parker, L. Bulfin, N. Hart, K. Shepherd, S. Vij, S. Dickson, E. Elloway, C. Ferguson, R. Jackson, P. MacNaughton, M. Marner, R. Squire, S. Waddy, P. Wafer, J. Welbourne, P. Ashcroft, D. Chambler, S. Dukes, A. Harris, S. Horton, S. Sharpe, P. Williams, S. Williams, M. Bailey, E. Blazquez, D. France, R. Hutchison, A. O’Connor, G. Comadira, M. Gough, M. Tallott, M. Bastick, R. Cameron, S. Donovan, K. Ellis, A. Gaur, R. Gregory, J. Naumoff, E. Turner, M. White, K. F. J. Au, J. Fratzia, S. Treloar, C. H. Lim, Y. Maseeda, A. P. Tan, C. L. Tang, C. Y. Yong, M. Akaltan, S. Berger, D. Blaser, L. Fazlija, M. L. Jong, M. Lensch, R. Ludwig, T. Merz, K. Nettelbeck, M. Roth, M. Schafer, J. Takala, A. Wehr, D. Zacharias, R. Amran, H. N. Ashraf, N. Azmi, N. Basri, H. Burhanuddin, Y. Hadinata, A. Hamdan, S. Kadiman, A. I. Y. M. Rashid, I. N. Sabran, S. Sulaiman, I. N. Zabidi, A. Al-Dawood, M. Aljuaid, H. Al Anizi, A. Al Saeedi, Y. Arabi, M. Dbsawy, A. Deeb, M. Hegazy, I. Magdi, E. Clarey, E. Corcoran, C. Finney, C. Harris, P. Hopkins, H. Noble, J. Smith, L. Thompson, T. Williams, L. A. Dumlao, R. Bassam, M. A. Hassan, N. Naseem, M. H. Al-Kurdi, A. M. Al-Harthy, S. Bernard, L. Sebafundi, C. Serban, S. K. Lim, N. Mazidah, N. Saidin, N. Sjamsuddin, I. T. A. Tan, N. Zabidi, M. Brain, S. Mineall, M. Kanhere, N. Soar, N. Abd Kadir, N. H. Abdullah, R. Awang, Z. Emperan, N. S. Husin, N. I. Ismail, S. Z. Ismail, F. N. A. Mohd Khadzali, M. F. Norddin, J. Aguila, C. Bold, B. Clatworthy, A. Dias, C. Hogan, A. Kazemi, V. Lai, R. Song, A. Williams, D. Bhatia, L. Bulfin, S. Elliot, P. Galt, K. Lavrans, P. Ritchie, A. Wang, R. Gresham, J. Lowrey, K. Masters, P. Palejs, I. Seppelt, F. Symonds, L. Weisbrodt, C. Whitehead, M. Babio-Galan, V. Calder, I. Clement, A. Harrison, I. McCullagh, C. Scott, L. Thompson, R. Bevan, S. Caniba, D. Hacking, L. Maher, M. L. Azzolini, P. Beccaria, S. Colombo, G. Landoni, C. Leggieri, C. Luca, D. Mamo, E. Moizo, G. Monti, M. Mucci, A. Zangrillo, M. Albania, S. Arora, Y. Shi, A. Abudayah, G. Almekhlafi, E. Al Amodi, S. Al Samarrai, M. Badawi, R. Cubio Caba, O. Elffaki, Y. Mandourah, J. Valerio, C. Joyce, J. Meyer, E. Saylor, B. Venkatesh, E. Venz, J. Walsham, K. Wetzig, E. Clarey, C. Harris, P. Hopkins, H. Noble, L. Thompson, T. Williams, T. M. Khoo, J. E. S. Liew, A. N. Sakthi, A. Zulkurnain, A. Bamford, C. Bergin, R. Carrera, L. Cooper, L. Despy, K. Ellis, S. Harkett, L. Mee, E. Reeves, C. Snelson, E. Spruce, G. Cooper, R. Hodgson, D. Pearson, M. Rosbergen, M. N. Ali, N. I. Bahar, A. Ismail, W. N. W. Ismail, N. M. Samat, N. S. M. Piah, R. Abd Rahman, M. Duroux, M. Ratcliffe, T. Warhurst, U. Buehner, E. Williams, N. Jacques, L. Keating, S. Macgill, K. L. Tamang, N. Tolan, A. Walden, R. Bower, J. Cranshaw, K. Molloy, S. Pitts, J. Butler, R. Dunlop, C. Fourie, P. Jarrett, M. Lassig-Smith, A. Livermore, S. O’Donoghue, M. Reade, T. Starr, J. Stuart, L. Campbell, M. Phillips, D. Stephens, J. Thomas, D. Cooper, R. McAllister, G. Andrew, L. Barclay, H. Dawson, D. M. Griffith, D. Hope, G. Wojcik, C. McCulloch, R. Paterson, L. Ascough, C. Paisley, J. Patrick-Heselton, D. Shaw, V. Waugh, K. Williams, I. Welters, D. Barge, A. Jordan, C. MacIsaac, T. Rechnitzer, F. Bass, J. Gatward, N. Hammond, P. Janin, A. O’Connor, W. Stedman, E. Yarad, N. A. Razak, N. Dzulkipli, S. L. Jong, K. Asen, W. L. Voon, S. Liew, J. Ball, V. Barnes, C. Dalton, S. Farnell-Ward, H. Farrah, K. Maher, J. Mellinghoff, C. Ryan, P. Shirley, L. Conlon, A. Glover, I. Martin-Loeches, E. O’Toole, J. Ewan, J. Ferrier, E. Litton, S. A. Webb, W. Berry, U. Blanco Alonso, A. Bociek, S. Campos, S. Jawara, F. Hanks, A. Kelly, K. Lei, C. McKenzie, M. Ostermann, R. Wan, S. Al-Soufi, S. Leow, K. McCann, C. Reynolds, K. Brickell, C. Fahey, L. Hays, N. Hyde, A. Nichol, D. Ryan, J. Brailsford, A. Buckley, L. Forbes, T. Maguire, J. Moore, L. Murray, A. Ghosh, M. Park, S. Said, J. Smith, A. Visser, H. Z. Abidin, S. Ali, M. H. Hassan, S. C. Omar, W. F. W. Shukeri, D. Brealey, G. Bercades, E. Blackburn, N. Macallum, A. Macklin, J. H. Ryu, K. Tam, D. Smyth, A. Arif, C. Bassford, C. Morgan, C. Swann, G. Ward, L. Wild, A. Bone, T. Elderkin, D. Green, D. Sach, T. Salerno, N. Simpson, F. Brohi, M. Clark, L. Williams, J. Brooks, E. Cocks, J. Cole, J. Curtin, R. Davies, H. Hill, M. Morgan, N. Palmer, C. Whitton, M. Wise, P. Baskaran, M. S. Hasan, L. Y. Tham, R. Sol Cruz, D. Dinsdale, S. Edney, C. Firkin, F. FitzJohn, G. Hill, A. Hunt, S. Hurford, G. Jones, H. Judd, C. Latimer-Bell, C. Lawrence, E. Lesona, L. Navarra, Y. Robertson, H. Smellie, A. M. Vucago, P. Young, H. Dawson, D. M. Griffith, R. Paterson, P. Clark, J. Kong, J. Ho, V. Nayyar, and C. Skelly
References
- 1.Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJ, Pandharipande PP, Watson PL, Weinhouse GL, Nunnally ME, Rochwerg B. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 2.Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 3.Shehabi Y, Howe B, Bellomo R, Arabi YM, Bailey M, Bass FE, Bin Kadiman S, McArthur CJ, Murray L, Reade MC, et al. Early sedation with dexmedetomidine in critically ill patients. N Engl J Med. 2019;380(26):2506–2517. doi: 10.1056/NEJMoa1904710. [DOI] [PubMed] [Google Scholar]
- 4.Kravitz RL, Duan N, Braslow J. Evidence-based medicine, heterogeneity of treatment effects, and the trouble with averages. Milbank Q. 2004;82(4):661–687. doi: 10.1111/j.0887-378X.2004.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shehabi Y, Forbes AB, Arabi Y, Bass F, Bellomo R, Kadiman S, Howe BD, McArthur C, Reade MC, Seppelt I, et al. The SPICE III study protocol and analysis plan: a randomised trial of early goal-directed sedation compared with standard care in mechanically ventilated patients. Crit Care Resusc. 2017;19(4):318. [PubMed] [Google Scholar]
- 6.Foss AH, Markatou M. kamila: clustering mixed-type data in R and Hadoop. J Stat Softw. 2018;83(1):1–44. [Google Scholar]
- 7.Foss A, Markatou M, Ray B. Distance metrics and clustering methods for mixed-type data. Int Stat Rev. 2018 doi: 10.1111/insr.12274. [DOI] [Google Scholar]
- 8.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Bürkner P. An R package for Bayesian multilevel models using Stan. J Stat Softw. 2017 doi: 10.18637/jss.v080.i01. [DOI] [Google Scholar]
- 10.Wang C, Louis TA, Henderson NC, Weiss CO, Varadhan R. beanz: an R package for Bayesian analysis of heterogeneous treatment effects with a graphical user interface. J Stat Softw. 2018;7:1–31. [Google Scholar]
- 11.Bürkner P-C (2017) Advanced Bayesian multilevel modeling with the R package BRMS. arXiv preprint arXiv:170511123
- 12.Weerink MA, Struys MM, Hannivoort LN, Barends CR, Absalom AR, Colin P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin pharmacokinet. 2017;56(8):893–913. doi: 10.1007/s40262-017-0507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu B, Li Z, Zhou D, Li L, Li P, Huang H. The influence of age on sensitivity to dexmedetomidine sedation during spinal anesthesia in lower limb orthopedic surgery. Anesth Analg. 2017;125(6):1907–1910. doi: 10.1213/ANE.0000000000002531. [DOI] [PubMed] [Google Scholar]
- 14.Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, Whitten P, Margolis BD, Byrne DW, Ely EW, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients a randomized trial. JAMA. 2009;301(5):489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 15.Jakob SM, Ruokonen E, Grounds RM, Sarapohja T, Garratt C, Pocock SJ, Bratty JR, Takala J, DfL-TS I. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA. 2012;307(11):1151–1160. doi: 10.1001/jama.2012.304. [DOI] [PubMed] [Google Scholar]
- 16.Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, Shintani AK, Thompson JL, Jackson JC, Deppen SA. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients. JAMA. 2007;298(22):2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 17.Olsen HT, Nedergaard HK, Strøm T, Oxlund J, Wian K-A, Ytrebø LM, Kroken BA, Chew M, Korkmaz S, Lauridsen JT. Nonsedation or light sedation in critically ill, mechanically ventilated patients. N Engl J Med. 2020;382(12):1103–1111. doi: 10.1056/NEJMoa1906759. [DOI] [PubMed] [Google Scholar]
- 18.Mehta S, Burry L, Cook D, Fergusson D, Steinberg M, Granton J, Herridge M, Ferguson N, Devlin J, Tanios M. Daily sedation interruption in mechanically ventilated critically ill patients cared for with a sedation protocol. A randomized controlled trial daily sedation interruption in ventilated patients. JAMA. 2012;308(19):1985–1992. doi: 10.1001/jama.2012.13872. [DOI] [PubMed] [Google Scholar]
- 19.Lamontagne F, Richards-Belle A, Thomas K, Harrison DA, Sadique MZ, Grieve RD, Camsooksai J, Darnell R, Gordon AC, Henry D. Effect of reduced exposure to vasopressors on 90-day mortality in older critically ill patients with vasodilatory hypotension: a randomized clinical trial. JAMA. 2020;323(10):938–949. doi: 10.1001/jama.2020.0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kent DM, Steyerberg E, van Klaveren D (2018) Personalized evidence based medicine: predictive approaches to heterogeneous treatment effects. BMJ 363 [DOI] [PMC free article] [PubMed]
- 21.Henderson NC, Louis TA, Wang C, Varadhan R. Bayesian analysis of heterogeneous treatment effects for patient-centered outcomes research. Health Serv Outcomes Res Method. 2016;16(4):213–233. doi: 10.1007/s10742-016-0159-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.