Abstract
Objective:
We sought to identify and investigate the functional role of the major endothelial cell (EC)-derived factors that control pericyte recruitment to EC tubes and pericyte-induced tube maturation during capillary network formation.
Approach and Results:
We identify PDGF-BB, PDGF-DD, endothelin (ET-1), TGFβs, and HB-EGF, as the key individual and combined regulators of pericyte assembly around EC tubes. Using novel pericyte only assays, we demonstrate that PDGF-BB, PDGF-DD, and ET-1 are the primary direct drivers of pericyte invasion. Their addition to pericytes induces invasion as if ECs were present. In contrast, TGFβs and HB-EGF, have minimal ability to directly stimulate pericyte invasion. In contrast, TGFβ1 can act as an upstream pericyte primer to stimulate invasion in response to PDGFs and ET-1. HB-EGF stimulates pericyte proliferation along with PDGFs and ET-1. Using EC-pericyte co-cultures, individual or combined blockade of these EC-derived factors, or their pericyte receptors, using neutralizing antibodies or chemical inhibitors, respectively, interferes with pericyte recruitment and proliferation. As individual factors, PDGF-BB and ET-1 have the strongest impact on these events. However, when the blocking reagents are combined to interfere with each of the above factors or their receptors, more dramatic and profound blockade of pericyte recruitment, proliferation and pericyte-induced basement membrane deposition occurs. Under these conditions, ECs form tubes that become much wider and less elongated as if pericytes were absent.
Conclusions:
Overall, these new studies define and characterize a functional role for key EC-derived factors controlling pericyte recruitment, proliferation, and pericyte-induced basement membrane deposition during capillary network assembly.
Keywords: Capillary assembly, pericytes, endothelial cells, PDGF-BB, PDGF-DD, endothelin-1, TGFβ, HB-EGF, pericyte recruitment
Subject Code: Basic Research, Vascular Biology, Growth Factors/ Cytokines, Angiogenesis
Graphical Abstract

INTRODUCTION
Capillaries, the smallest and by far the most abundant vessels, are primarily composed of endothelial cell (EC) tube networks and associated pericytes1–8. These cells act in concert with one another to create a functional vascular plexus, capable of supporting tissue development and maintenance via communications with adjacent tissues to promote tissue health4, 5, 7. It is essential to understand the detailed molecular and cellular biology of capillary network assembly in health and in disease states where capillary dysfunction plays an important pathogenic role. An understanding of the critical functional roles of both ECs and pericytes during these processes as well as the factors and signals that control their behaviors to regulate capillary assembly is necessary to elucidate what becomes dysfunctional in specific diseases. Capillary dysfunction and/or regression is clearly associated with key human diseases including diabetes, ischemia/ infarction, hypertension, heart failure, infectious diseases (e.g. sepsis), neurodegenerative diseases, and malignant cancers5, 9–22. The nature of the dysfunction from either an EC or pericyte perspective in these diseases is not well understood. In addition, this knowledge is central toward our ability to create functional capillaries as well as arteries and veins to properly vascularize bioengineered tissues and organs23, 24.
Considerable progress has occurred over the past decade in our understanding of pericyte development and their functional roles, including their fundamental importance in capillary assembly, capillary basement membrane deposition, and blood brain barrier function2, 3, 25–30. Pericyte lineage tracing markers such as PDGFRβ and NG2 proteoglycan have been utilized to demonstrate their primary anatomic position in capillary beds31–34, but also have implicated them as being one of the developmental precursors of vascular smooth muscle cells32. Genetic disruption of either PDGF-BB or its receptor, PDGFRβ in mice leads to a strong decrease in the accumulation of pericytes within capillary beds resulting in abnormalities that were compared to those visualized in diabetes35–40. Although reducing the levels of PDGF-BB or its receptor, caused strong phenotypes and abnormalities, it did not completely eliminate pericytes or their interactions with vessels, suggesting that other molecular regulators must also be operative. In addition, disruptions in the blood-brain barrier were observed using similar genetic deletions, suggesting an important role for PDGF-BB signaling and pericytes in blood barrier function28, 29. Other growth factors that have been implicated in EC-mural cell interactions affecting either larger vessels or capillaries include TGFβ and EGF isoforms and receptors, as well as PDGF-DD26, 41–48. Previous work from our laboratory, using a defined serum-free culture system, demonstrated a role for both PDGF-BB and HB-EGF in pericyte recruitment to EC tubes and, also pericyte proliferation26. Furthermore, we showed that the presence of ECs was required for pericytes to migrate and proliferate in 3D collagen matrices and that combined blockade of PDGF-BB and HB-EGF, strongly reduced, but did not eliminate pericyte responsiveness to the presence of ECs26. One issue that was not addressed in the previous work was the direct functional role of these growth factors on pericyte abilities to invade 3D matrices, proliferate, and recruit to EC-lined tube networks.
In this work, we have re-examined the question of what EC-derived factors promote pericyte recruitment and pericyte-induced capillary tube maturation using highly defined in vitro model systems. We have developed novel pericyte only invasion assays and analyses which allow us to evaluate the direct role of factors that promote pericyte invasion, proliferation and recruitment to tubes. In addition, we integrate this information using a detailed analysis of pericyte recruitment to EC-lined tubes in an EC-pericyte co-culture system that recapitulates the assembly of capillary tube networks in vivo. Our data suggests that the primary direct drivers of pericyte invasion are PDGF-BB, PDGF-DD, and ET-1, individually and in combination. In contrast, other important factors that show weak or no ability to promote invasion include TGFβ isoforms and HB-EGF. In contrast, TGFβ has an ability to act as an upstream primer of pericytes so that they invade more vigorously in response to PDGFs and ET-1, while HB-EGF promotes pericyte proliferation along with PDGFs and ET-1. In co-culture assays, we demonstrate that each of these EC-derived factors plays a role in EC-pericyte tube co-assembly, although PDGF-BB and ET-1 appear to be the most dominant. Single and combined blockade of these ligands or their receptors selectively and markedly impairs pericyte recruitment and proliferation without interfering with EC tubulogenesis. When each of these factors or their receptors are blocked in combination, minimal to no pericyte recruitment or responsiveness to EC tubes is observed, suggesting that these EC-derived factors (i.e. PDGF-BB, PDGF-DD, ET-1, TGFβs, and HB-EGF) are the critical ones that promote pericyte recruitment to tubes. These pericyte signaling factors also critically affect pericyte-induced capillary maturation events (i.e. narrow and elongated tube networks with basement membrane deposition). Under these blocked conditions, EC tubes remodel to become much wider and less branched just like they appear when ECs form tubes in the absence of pericytes. Overall, this novel work defines the key factors from ECs that control pericyte recruitment, proliferation, and pericyte-induced basement membrane deposition during capillary network assembly.
MATERIALS AND METHODS
The authors declare that all supporting data are available within the article [and its online supplementary files].
Cell culture
Human umbilical vein ECs (HUVECs) were used from passage 3–6. Human brain vascular pericytes (HBVPs) were used from passage 4–12. HUVECs and HBVPs were passaged on gelatin-coated flasks. Both were grown in our own ‘Supermedia,’ with M199 as a base, 20% fetal bovine serum, bovine hypothalamus extract, heparin sodium salt, gentamicin and amphotericin B as described49. Cells were grown in incubators set at 37°C and 5% CO2.
Pericyte only assays
Invasion: Type I collagen matrices (2.5 mg/mL) were polymerized and equilibrated at 37°C in 5% CO2 for 45 minutes. After polymerization, pericytes (at 1.4 × 106 cells/mL) were seeded on top of gel with reduced-serum II supplement (RSII) in M199 (RSII media). Pericytes then invaded over a period time (0–72 hr). Starting In 3D: Same as invasion except pericytes were mixed directly into collagen gel (at 0.4 × 106 cells/mL) before polymerization. After gel polymerized, RSII media was added to cultures. Molecules for different conditions were added directly into the pre-polymerized gel. After 72 hr, pericytes were fixed in 3% glutaraldehyde or 3% paraformaldehyde and stained with 0.1% toluidine blue in 30% methanol for non-fluorescent imaging or immunostained for fluorescent imaging. Pericyte invasion was quantified and imaged at predetermined depths. Four images per depth at 2–3 depths (8–12 fields per experiment); ≥3 validating experimental replicates were conducted in total.
Vasculogenic assays
HUVECs (at 2 × 106 cells/mL) and pericytes (0.4 × 106 cells/mL) were co-cultured in 2.5 mg/mL type I collagen matrices. After polymerization, a five-growth factor system was added in the media. RSII media (containing insulin) served as a base to which fibroblast growth factor-2 (FGF-2) at 50 ng/mL, stem cell factor (SCF) at 40 ng/mL, interleukin-3 (IL-3) at 40 ng/ml, and stromal cell-derived factor 1α (SDF-1α) at 40 ng/mL were all added6, 50. These cultures assembled for a period of 0–72 hr or 0–120 hr and then fixed in 3% paraformaldehyde. To quantify pericyte recruitment, ≥5 pictures were taken from predetermined locations per experiment, and ≥3 validating experimental replicates were conducted in total for all quantitative data. Pericyte recruitment was assessed by two criteria: 1. To be recruited the majority of the pericyte body must be on an EC tube and 2. The pericyte must be elongated on that tube (rounded up pericytes did not qualify as recruited).
Immunostaining of 3D cultures
After fixation in 3% paraformaldehyde, collagen gels were plucked and rinsed in a Tris-glycine buffer solution for 1 hr. To stain any intercellular components, a 1% Triton-X100 solution was added for 1 hr (basement membrane components were not permeabilized). These were then put into a blocking solution containing 5% serum specific to secondary antibody (either goat or rabbit in this paper) for 1 hr. After which the primary antibody was added directly into blocking solution and allowed to incubate overnight. After incubation, the solution containing the primary antibody was removed and the gels were washed several times with Tris buffered saline (TBS). Blocking solution containing 5% serum was added with secondary fluorescent antibody. After 2 hr this solution was removed and washed again several times with TBS. Samples could then be imaged by immunofluorescence microscopy.
Microscopy and imaging
Images for quantification of pericyte recruitment and pericyte invasion were obtained using DIC images of HUVECs, which were then overlaid with fluorescent GFP pericytes on an Olympus CKX41 microscope (Olympus) and imaging software (Olympus). Time-lapse movies on living cells, as well as immunofluorescence microscopy were both obtained using a DMI6000B microscope with environmental chamber (Leica Microsystems) and controlled using MetaMorph 7.8 software (Molecular Devices). 10X objective was used for all movies, pericyte only DIC movies used an additional 1.5X magnification for a total of 15X. To create time-lapse movies, images were taken every 10 minutes with a monochromatic Hamamatsu ORCA-ER C4742–80 camera (Hamamatsu) in different stage locations over a period of 0–72 hr and 72–120 hr. These images could then be compiled into a movie using MetaMorph software. MetaMorph was also used to evaluate EC tube widths, EC tube lengths, and average aggregate area. This data was exported into an excel (Microsoft) sheet and analyzed. Movies were stabilized and edited using Adobe Creative Cloud: Adobe After Effects (Adobe Systems). Confocal images were obtained using a Leica SP8 LIGHTNING White light laser confocal scanning microscope. All images were obtained using a 10X HC PL APO, 0.4NA, WD 2.2 mm lens. Confocal reconstructions, imaging and rotational movies were created using either LAS X (Leica Microsystems) or Fiji (ImageJ).
Tracking pericyte movement
Vasculogenic assays were set up in the same way described above with EC-pericyte co-cultures or with pericytes alone in the collagen gel. Nuclear GFP-labeled pericytes were used for these purposes. In pericyte only assays, various factors and inhibitors were added directly to the media, to demonstrate the stimulation effect of these molecules on pericytes, as well as our ability to inhibit those factors with either pharmacologically or with neutralizing antibodies. Similarly, co-cultures of ECs and pericytes were set up and blocked by placing pharmaceutical blockers or neutralizing antibodies into the media to investigate the reduction in movement. Time-lapse movies were created showing movement of the pericyte nuclei using immunofluorescence microscopy. The entire time-lapse movie was then compressed into an individual image, to show the “footprints” of pericyte movement using MetaMorph (Molecular Devices). These were quantitated as described above. Only a single field of view was used for each experiment, and ≥6 validating experimental replicates were conducted in total.
Pericyte aggregate invasion assays
Pericytes were aggregated in 35 mm polystyrene dishes, coated with 1% fatty acid free bovine serum albumin (BSA) in PBS for 30 minutes. After 30 minutes, 1% BSA in PBS was removed, and pericytes in RSII media were added to the dish at a density of ~2.5 × 106 cells/ ml and permitted to aggregate for a period of 3 hr, with occasional swirling of dish. After 3 hr, pericytes were suspended in 3D collagen I gel as described above. After pericytes formed for a period of 24 hr under various conditions, assay was fixed with 3% glutaraldehyde and stained with toluidine blue. Only a single field of view was used for each experiment with a 4X lens, and ≥6 validating experimental replicates were conducted in total.
Pericyte Proliferation Assays
Pericytes were suspended in a 3D collagen I gel as described above with RSII feeding media under control conditions vs. the addition of EC-derived molecules. After 72 hr, MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) was directly added to each well to create a final concentration of 0.5 mg/mL and incubated for 4 hr. After 4 hr, the MTT media was removed, washed with DMSO, immediately removed, then 100 μL of DMSO was added to each well of a 96 well A/2 plate, and allowed to sit for 2 hr. At which point the plate was read using a SYNERGY HI microplate reader (BioTek Instruments) and analyzed using the software Gen5 3.04 (BioTek Instruments) using an absorbance of 570 nm to measure cell number.
Statistics
Student t tests were performed using Microsoft Excel (Microsoft) to assess statistical significance between means of various conditions. Data were analyzed for normality and equal variances were obtained. When it was necessary to cross compare the means of multiple conditions within a given experiment, Prism 8 (Graphpad) was used for analysis of variance (ANOVA) with follow up post-hoc Tukey tests. P < .05 was set as the minimum statistical significance. All experiments were performed with ≥3 validating experimental replicates in total. All experiments using ANOVA were performed with ≥3 validating experimental replicates in total.
RESULTS
EC-pericyte tube co-assembly mimicking capillary network formation in 3D collagen matrices
For many years, our laboratory has developed and utilized 3D, serum-free defined systems to elucidate the underlying cell, molecular and functional biology of vascular cells such as human ECs and pericytes2, 5, 6. Our major focus has been to investigate them in the context of vascular morphogenesis (i.e. lumen formation and sprouting behavior) and capillary network assembly6, 51. In this work, we utilize pericyte-only invasion assays or co-cultures of ECs and pericytes to identify and characterize the functional role of the key EC-derived molecules that induce pericyte recruitment to EC tube networks. An important consequence of pericyte recruitment is EC tube remodeling and maturation events that leads to narrow, branched and elongated tube networks as well as basement membrane matrix deposition25. To illustrate the capillary-like tube networks that form in this co-culture model, we compiled confocal z-stack reconstructions of EC-pericyte co-cultures (500 μm thick) at 120 hr as rotational movies (Movies I and II). Recruitment of pericytes to EC tube networks, can be observed in ‘x’ and ‘y’, and ‘z’ axes and the tube networks are highly interconnected throughout these axes and thickness of the gel which mimic the appearance of capillary networks in vivo (Suppl. Figure I). Dynamic assembly of these EC tube-pericyte networks is illustrated using real-time video analysis over a 72 hr period. Overlay movies of ECs and GFP-pericytes imaged under light and fluorescence microscopy, respectively are shown (Movies III and IV). Activation of GFP-pericytes is observed (i.e. elongated appearance with accompanying process extension) and then recruitment of these pericytes to the developing and assembling EC tube networks. The presence of pericytes is necessary for EC tubes to remain narrow and elongated in these highly branched networks that resemble capillaries, since tubes consisting of ECs by themselves remodel over time into larger, wider and less branched networks which leaves large areas of avascular space (Suppl. Figure IIA). Thus, capillary network assembly requires the presence of pericytes and, it is essential to; i) elucidate the molecular basis for how they recruit to EC tubes, ii) identify the factors that stimulate this recruitment and control proliferation, and iii) determine how they work with ECs to control the deposition of the capillary basement membrane, an essential step in capillary maturation.
A broad screen of EC-derived factors reveals key roles for PDGF-BB, PDGF-DD, and Endothelin-1 as direct stimulators of pericyte invasion
Previously, we reported that pericyte motility in 3D collagen gels was completely dependent on the presence of ECs, as pericytes did not migrate without ECs under our serum-free defined conditions26. Here, our goal was to identify the key EC-derived proteins, peptides or other small molecules that stimulate pericyte invasion into 3D collagen matrices. To accomplish this goal, we added different factors (23 in total and all known to be made or activated by ECs) into 3D matrices, and seeded pericytes onto collagen gel surfaces to assess their invasive ability (i.e. since pericytes invade such matrices during EC-pericyte tube co-assembly). Only three major factors strongly stimulated pericyte invasion including PDGF-BB, PDGF-DD, and the peptide, endothelin-1 (ET-1) (Figure 1A, Suppl. Figure IIIA–C). Dose response curves are shown demonstrating the potent influence of PDGF-BB and PDGF-DD in stimulating pericyte invasion (Suppl. Figure IIIB,C). Weaker effects were observed with TGFβ2 and the peptide apelin. TGFβ1 also induced weak effects in this assay but was not significant compared to control. Interestingly, HB-EGF, a growth factor that we had previously identified as playing a role during EC-pericyte tube co-assembly, did not directly stimulate pericyte invasion. In further support of this conclusion is that addition of HB-EGF did not influence the invasion dose-response curves of either PDGF-BB or PDGF-DD, suggesting that it also does not appear to contribute to this process by effecting PDGF-dependent invasion (Suppl. Figure IIIB,C). We also assessed the mRNA expression of these growth factors/ peptide ligands in ECs vs. pericytes using RT-PCR analysis. ECs predominantly expressed PDGF-B, PDGF-D, ET-1, HB-EGF, and TGFβ1 compared to pericytes, while pericytes expressed much higher levels of TGFβ2. With regard to receptors for these ligands, pericytes predominantly expressed PDGFRβ, ET-1 receptor A (EDNRA), as well as ErbB1 and ErbB2 (EGF ligand receptors), while Alk-5 (TGFβ receptor) was observed at comparable levels on both cell types. ET-1 receptor B (EDNRB) was found predominantly on ECs (Suppl. Figure IIB).
Figure 1. Broad screen of endothelial cell-derived factors that stimulate pericyte invasion.

(A) Various EC-derived proteins, peptides and other small molecules were screened by placing each factor, individually, into a collagen gel, under serum-free conditions. Pericytes were seeded on-top of the gel, allowed to invade downward for 72 hr, and the invasion response was quantitated (n≥3). Asterisks indicate significance relative to control, p<.01. PDGF (platelet-derived growth factor)-BB, PDGF-DD, HB-EGF (heparin-binding EGF-like growth factor), Angiopoietin 2, SDF (stromal cell-derived factor 1-alpha), MCP-1/CCL2 (monocyte chemoattractant protein-1), FGF-2 (fibroblast growth factor), IL-8 (interleukin-8), SCF (stem cell factor), IGF2 (insulin-like growth factor 2), BMP-2 (bone morphogenetic protein 2), BMP-4, and BMP-6 were added at a final concentration of 40 ng/mL; ET-1 (endothelin 1), apelin ([Pyr1]-Apelin-13), PGI2 (treprostinil [prostacyclin analog]), ADM2 (adrenomedullin 2), ADM (adrenomedullin) and angiotensin II were added at a final concentration of 10−7 M; TGFβ2 (transforming growth factor beta 2) and TGFβ1 were added at a final concentration of 10 ng/mL; SNAP ([S]-Nitroso-N-acetylpenicillamine – NO donor) was added at a final concentration of 10−4 M; and S1P (sphingosine-1-phosphate) at 10−6 M. (B) Molecules from Figure 1A that induced the greatest invasion response (along with HB-EGF) were utilized in a separate experiment and after 72 hr, fixed cultures were imaged using confocal z-stack, 3D reconstructions. White arrowheads denote top of gel (i.e. the monolayer from which the pericytes invade into the gel). These were viewed from the top as a “Downward” view, as well as rotated 90° and observed from side as a “Cross Section” view. GFP-pericytes were used for these confocal images. Bar equals 200 μm.
The invasion screening experiment was separately evaluated in a different way by performing confocal z-stack reconstructions of GFP-pericytes invading into collagen gels under the different conditions (Figure 1B). This allowed us to rotate the image and obtain the top-view of the assay “Downward” or rotate it 90° for a side-view “Cross Section” (Figure 1B). PDGF-BB and -DD were the strongest stimulators of pericyte invasion and the deepest invasion was observed here also. ET-1 also induced strong invasion, which was more superficial, but showed more fluorescence intensity, which might be reflective of a positive influence of ET-1 on pericyte proliferation (Figure 1B). There were some modest increases in invasion observed with TGFβ2 and TGFβ1 compared to control, while HB-EGF was very similar to control. Remarkably, when PDGF-BB, PDGF-DD, and ET-1 were combined, dramatic and deep invasion was observed (Figure 1B). These responses are invasion responses, rather than just effects on motility since the matrix metalloproteinase (MMP) inhibitor, GM6001, completely interferes with the pericyte invasion promoting activity of PDGFs, ET-1 and their combination (Suppl. Figure IV). Previous work in our laboratory demonstrated that while GM6001 blocks invasive responses of cells in 3D collagen matrices such as ECs and malignant tumor cells, it has no ability to block cell motility on 2D matrix surfaces52–54. Overall, these experiments strongly support the conclusion that PDGF-BB, PDGF-DD and ET-1, singly and in combination, represent the major EC-derived factors that directly stimulate pericyte invasion and recruitment to EC tubes.
Another key issue to consider in our serum-free defined assays, are whether EC-derived factors could influence proliferative functions for pericytes, since pericyte numbers do increase over time during EC-pericyte tube co-assembly in our previous studies26. Thus, we also performed the same broad screen to determine if EC-derived factors could influence pericyte proliferation in pericyte-only assays when they were seeded as single cells and at the same densities in 3D collagen matrices that are utilized when they are co-cultured with ECs. Remarkably, the same factors identified in the invasion assays are observed here (i.e. PDGF-BB, PDGF-DD and ET-1- and their combination) except for HB-EGF which also showed increased pericyte numbers (Suppl. Figure V). In addition, neither TGFβ1 nor TGFβ2 supported increased proliferation, while a few factors including BMP-6, IL-8 and the nitric oxide donor, SNAP, showed very weak inhibition of pericyte number following addition. This data further supports the likelihood that these key EC-derived factors (Figure 1 and Suppl. Figure V) are important during EC-pericyte tube (i.e. capillary) assembly by controlling both pericyte invasion/ recruitment and proliferation.
Addition of PDGF-BB, PDGF-DD, and ET-1 to pericytes mimics the influence of ECs during EC-pericyte tube co-assembly
To address whether we could induce pericyte motility (i.e. invasion) in 3D matrices (in the absence of ECs) by suspending them at the same density that is used during EC-pericyte tube co-assembly assays, we added PDGF-BB, PDGF-DD and ET-1 together vs. control conditions and assessed pericyte motility over time. Real-time videos illustrate the strong ability of these EC factors to dramatically regulate pericyte motility and process extension compared to control conditions (Movies V and VI). Blockade of these factors using neutralizing antibodies to PDGF-BB and PDGF-DD as well as an ET-1 receptor A antagonist markedly interferes with the pericyte response to these EC-derived factors (Movie VII). A major goal of this experiment was to determine whether we can mimic the pericyte motility that we reported previously, and which occurred only when ECs were co-cultured with the pericytes. For these analyses, we utilized nuclear-GFP labeled pericytes whose motility can be tracked over time using time-lapse fluorescence microscopy. Each time lapse movie was compressed into a single image, leaving “footprints” and “tracks” of pericyte movement. Here, we demonstrate again that pericyte motility is strongly stimulated in the presence of ECs but is largely absent without ECs (Figure 2A). Next, we assessed whether the addition of the combination of PDGF-BB, PDGF-DD and ET-1 to pericytes (and in the absence of ECs) could mimic the effect of adding ECs. Strong motility of pericytes was observed following addition of these three factors compared to controls when visualized from 0–72 hr or 72–120 hr time periods (Figure 2B–E) which does resemble the addition of ECs. Furthermore, we have identified a combination of pharmacologic agents which block ET-1 receptor A (CI 1020-C), PDGF receptors (Imatinib-I), epidermal growth factor receptor (gefitinib-G), and TGF-beta receptor (SB431542-S) (abbreviated CIGS) that together markedly inhibit this pericyte motility response in 3D matrices (Figure 2) (also see later on for co-culture assays). Additional support for our findings is shown utilizing a novel pericyte aggregate invasion assay that we have developed (Figure 2F). The addition of PDGF-BB, PDGF-DD and ET-1 markedly stimulate pericyte invasion from these pericyte aggregates compared to controls which shows modest invasion (Figure 2F,G). Addition of CIGS markedly blocks this invasion response from the aggregated pericytes (Figure 2F,G). Overall, this data provides strong support for the concept that PDGF-BB, PDGF-DD, and ET-1, in combination, can substitute for ECs and induce pericyte invasive and motile behavior in a 3D matrix environment.
Figure 2. Marked ability of the EC-derived factors, PDGF-BB, PDGF-DD and ET-1, to stimulate pericyte motility in 3D matrices and identification of pharmacologic inhibitors that block this response.

(A, B, and C) Nuclear-GFP labeled pericyte motility was analyzed over the indicated time frames and experimental conditions. These time lapse image stacks were compressed into a single image, so that the “footprints” or “tracks” of pericyte movements can be observed. All cells begin suspended in the 3D collagen gel. (A) Nuclear tracking of pericyte motility over the course of 72 hours. Motility of pericytes when seeded on their own (top row), pericyte motility when co-cultured with ECs (bottom row). (B-G) No additional growth factors = Control; PDGF-BB (20 ng/ml), PDGF-DD (20 ng/ml) and ET-1 (2×10−8 M) = +PDGFs and ET-1; 20 μM CI 1020 (ET-1 receptor A antagonist), 1 μM imatinib (PDGFR inhibitor), 1 μM gefitinib (EGFR inhibitor), 10 μM SB 431542 (ALK5 inhibitor) = +CIGS. (B) Pericytes 0–72 hr compressed time lapse movie, (D) 0–72 hr pericyte motility quantitation, (C) Pericyte 72–120 hr compressed time lapse movie (E) 72–120 hr pericyte motility quantitation. (A-E) Bar equals 200 μm; n≥8. (F) Aggregated pericytes suspended in 3D collagen gel for 24 hr and allowed to invade radially outward. (G) Quantitation of aggregate invasion area. (E-F) Bar equals 100 μm; n≥6. Asterisks indicate significance relative to control at p≤.01.
Combined pharmacologic blockade of PDGF-BB/-DD, endothelin-1, HB-EGF, and TGFβ receptors completely interferes with pericyte recruitment and responsiveness to EC tubes
While our broad screen identified three major EC-derived factors that control pericyte invasion, we needed to test whether these factors and potentially others were relevant for the ability of pericytes to recruit to EC tubes and impact capillary network assembly in EC-pericyte co-culture assays. Pericyte recruitment was assessed based on three criteria: total pericyte number, pericytes that were elongated on the EC tube networks (indicative of motile and functional pericytes) and the percentage of pericytes elongated on EC tubes. Finally, we investigated a key consequence of pericyte recruitment which is capillary basement membrane deposition. We initially screened both ECs and pericytes for basement membrane genes by RT-PCR and found that both cell types express the major basement membrane matrix components (Suppl. Figure IIB). To address these issues in detail, we utilized the CIGS drug combination, with components added individually or in combination, and with up to all four drugs added together to assess if pericyte recruitment was impacted during EC-pericyte tube co-assembly (Figure 3A–C). In each case, individual addition of each of the CIGS components leads to significant blockade of total pericyte number, number of pericytes elongated on tubes and the percentage of pericytes elongated on tubes as a measure of pericyte recruitment. In this latter case, the addition of all four components of CIGS significantly blocks compared to all other individual and combinations of CIGS drugs (Figure 3C). In the other two analyses, when all four components of CIGS were added, this was significant against all combinations but a combination of three which was CIG (Figure 3A,B). Thus, in only a couple of cases, the addition of SB 431542 to CIG did not significantly enhance blockade of pericyte number or functional response to the presence of ECs compared to CIG treatment alone. A major conclusion here is that multiple factors and pericyte receptors are necessary to promote pericyte proliferation and recruitment to EC-lined tubes during capillary network assembly. The addition of CIGS blocks pericyte recruitment whether it is analyzed from the beginning of culture from 0–72 hr or later from 72–120 hr (Figure 4B–D) Another key point is that EC tube formation occurs normally in the presence of CIGS, except that EC tubes are wider and less branched compared to controls (Figure 3D, Figure 4A), in a manner very similar to that observed when ECs are seeded and allowed to form tubes in the absence of pericytes (Suppl. Figure IIA). We also performed real-time video analysis to further evaluate the influence of CIGS addition vs. control using GFP-labeled ECs and mCherry labeled pericytes (analyzed from 0–72 hr or 72–120 hr) (Movies VIII–XI) or using unlabeled ECs and GFP-labeled pericytes (analyzed from 0–72 hr) (Movies XII, XIII). In each instance, the addition of CIGS resulted in marked inhibition of pericyte responsiveness and recruitment to developing EC tube networks. Finally, the addition of CIGS, compared to control, led to strongly reduced basement membrane deposition of fibronectin, collagen type IV, perlecan, nidogen 1 and nidogen 2 staining around EC tubes, while laminin staining was less affected (Figure 4E). Overall, this data provides considerable evidence that a combination of EC-derived factors including PDGFs, ET-1, HB-EGF, and TGFβs and their pericyte receptors, play a critical functional role in promoting pericyte invasion, recruitment to tubes, proliferation and capillary basement membrane assembly.
Figure 3. Combinations of pharmacologic inhibitors (CIGS) directed to pericyte receptors for EC-derived molecules involved in pericyte recruitment, markedly interferes with pericyte accumulation along EC-lined tubes during capillary formation.

EC-pericyte co-cultures were established in the absence or presence of the indicated pharmacologic inhibitors [20 μM CI-1020 (ET-1 receptor A antagonist), 1 μM imatinib (PDGFR inhibitor), 1 μM gefitinib (EGFR inhibitor), 10 μM SB 431542 (ALK5 inhibitor)], that were added singly or in the indicated combinations including CIGS, which is a mixture of all 4 of the drugs. Cultures were fixed at 72 hr and were quantitated for total pericytes (A), the number of pericytes elongated on EC tubes (B) and the percentage of pericytes elongated on EC tubes (C). n≥6; Asterisks indicate significance at p<.05 relative to control, † indicates p<.05 significance at p<.05 from all others unless specified “ns” = not significant. Analyzed using ANOVA with post hoc Tukey tests. (D) Co-cultures were established in the absence or presence of CIGS, were fixed at 120 hr, and immunostained with CD31 antibodies to label ECs. Bar equals 200 μm.
Figure 4. Pharmacologic inhibition using CIGS markedly blocks pericyte motility and recruitment as well as pericyte-induced capillary basement membrane deposition.

(A) EC-pericyte co-cultures were established under control conditions or with the CIGS drug combination added [20 μM CI-1020 (ET-1 receptor A antagonist), 1 μM imatinib (PDGFR inhibitor), 1 μM gefitinib (EGFR inhibitor), 10 μM SB 431542 (ALK5 inhibitor)]. Cultures were fixed at 72 hr and were immunostained with anti-CD31 antibodies to visualize ECs (red), while pericytes were GFP-labeled (green). Overlay images are shown in the upper and lower panels, while the middle panels show pericytes alone. Bar equals 200 μm. (B-D) EC-pericyte co-cultures were established with nuclear GFP-labeled pericytes and pericyte motility using time-lapse movie analysis was performed (B). Motility was examined in control cultures or cultures treated with CIGS from 0–72 hr (C) or separately, from 72–120 hr (D). Quantitation of pericyte motility under these conditions was performed. n≥8; Asterisks indicate significance relative to control at p<.05. (E) Control or CIGS treated co-cultures (120 hr) were immunostained for the indicated basement membrane components and imaged by confocal microscopy. Bar equals 200 μm.
Ligand blocking reagents reveals that a combination of EC-derived factors is necessary to promote pericyte recruitment and responsiveness to EC tube networks
To examine these results and conclusions using a different approach, we employed the use of neutralizing antibodies directed to PDGF-BB, PDGF-DD, and HB-EGF, and a receptor trap, Alk-5-Fc, to inhibit TGFβ isoforms. We utilized these blocking reagents along with the ET-1 receptor A inhibitor, CI-1020, as individual inhibitors or as a combination of five inhibitors. Before starting these experiments, we confirmed that each reagent selectively inhibited only its specific ligand or receptor and that was indeed the case (not shown). To further confirm their blocking activities, we compared a real-time movie analysis of pericytes treated with PDGF-BB, PDGF-DD, and ET-1 (Movie VI) (pericyte-only assay), vs. a culture treated with the same three factors, but also with blocking antibodies directed to PDGF-BB and PDGF-DD, in combination with the ET-1 receptor A inhibitor. The addition of these blocking agents led to marked inhibition of the pericyte response to the three factors (Movie VII).
Next, we utilized these and other neutralizing and blocking agents (i.e. directed to PDGF-BB, PDGF-DD, ET-1, TGFβs, HB-EGF) in EC-pericyte co-culture assays (Figure 5). Each of the individual blocking reagents significantly inhibited pericyte recruitment to tubes and the combination of all five inhibitors (i.e. antibodies, trap and drug) completely disrupted pericyte proliferation, responsiveness, and recruitment that was significant compared to all other conditions (Figure 5A–C). As individual factors, the most dominant factors were PDGF-BB and ET-1, as its neutralizing antibodies or the ET-1 receptor A chemical inhibitor, respectively, had the greatest blocking influence, while there was less influence of a comparable factor, PDGF-DD (Figure 5A–C). In the presence of all five neutralizing agents, the EC tubes again resemble EC only cultures without pericytes where the tubes are widened and less elongated (Figures 5D and 6A,B). Blockade of PDGF-BB or ET-1 signaling alone did not show this dramatic change in EC tube morphology (Figure 5D), suggesting again that multiple factors are acting together to control this pericyte recruitment process. The EC tubes with the ligand neutralizing agents also resemble what we observe following addition of the pharmacologic receptor inhibitors, CIGS (Figure 6A,B). In both cases, the addition of these inhibitor combinations results in significant reductions in EC tube length, and large increases in EC tube widths (Figure 6C,D). Overall, our novel findings using different classes of blocking reagents, demonstrates that multiple EC-derived growth factors (i.e. PDGF-BB, PDGF-DD, HB-EGF, TGFβs) and the peptide, ET-1, play key functional roles in controlling pericyte recruitment, proliferation and pericyte-induced tube maturation events that are necessary to create capillary tube networks.
Figure 5. Marked blockade of pericyte recruitment using combinations of neutralizing agents including blocking antibodies directed to PDGF-BB, PDGF-DD and HB-EGF, a receptor trap directed to TGFβs and an ET-1 receptor A antagonist.

EC-pericyte co-cultures were established under control conditions or in the presence of indicated neutralizing agents individually or in combination. Pericytes co-assembled with ECs for a period of 72 hours and were fixed, and quantitated for total pericytes (A), the number of pericytes elongated on tubes (B) or the percentage of pericytes elongated on tubes (C). Neutralizing agents included: neutralizing antibodies (all added at 10 μg/ml) (αPDGF-BB, αPDGF-DD, αHB-EGF, αIL-6- polyclonal antibody-pAb, αIL-6 monoclonal antibody-mAb); 10 μg/ml of ALK5-Fc trap, and CI 1020 (ET-1 receptor A antagonist). The All condition was a combination of αPDGF-BB, αPDGF-DD, αHB-EGF, ALK5-Fc, and CI 1020. n≥6; Asterisks indicate significance at p<.05 compared to control with t-test. † p<.05 compared to any other condition. Analyzed using ANOVA with post hoc Tukey tests and student t-tests. (D) EC-pericyte co-cultures were established under the indicated conditions using GFP-F HUVECs (further immunostained with CD31 antibodies –green) and mCherry-labeled (red) pericytes which were fixed after 120 hr. Confocal microscopy was performed, and 3D reconstructions of these images are shown. ECs and pericytes shown together (top row), EC only shown (middle row), and pericyte only shown (bottom row) of these co-cultures. Bar equals 200 μm.
Figure 6. Blockade of pericyte recruitment with the pharmacologic drug combination, CIGS, and comparison to a combined set of neutralizing agents.

(A-D) EC-pericyte co-cultures were established under control conditions vs. the addition of CIGS or a combined set of neutralizing agents and the cultures were fixed after 120 hr. CIGS doses: 20 μM CI 1020 (ET-1 receptor A antagonist), 1 μM imatinib (PDGFR inhibitor), 1 μM gefitinib (EGFR inhibitor), 10 μM SB 431542 (ALK5 inhibitor). Neutralizing Agents (added in combination): 10 μg/ml of αPDGF-BB, αPDGF-DD, and αHB-EGF; 10 μg/ml of ALK5-Fc trap, and 20 μM CI-1020. (A) Control (left column), +CIGS (middle column), and +Neutralizing agents (right column); co-culture with CD31 (red) and GFP-pericytes (green) (top row), pericyte only shown (middle row), and EC only shown (bottom row). (B) anti-CD31 (red) antibodies used to stain all tubes. Control EC-pericyte co-culture (top left); EC only culture (bottom-left); EC-pericyte co-culture +neutralizing agents (top-right); EC-pericyte co-culture +CIGS (bottom-right). Bar equals 200 μm. Control vs. treated cultures were quantitated for total tube length (C) or average tube width (D). n≥3; Asterisks indicate significance at p<.01.
TGFβ1 can act as an upstream pericyte primer to stimulate pericyte responsiveness to EC-derived factors that promote pericyte recruitment
Our findings presented above suggest a role for TGFβ and its key receptor, Alk-5, in pericyte recruitment in conjunction with other EC-derived factors. TGFβ1 and TGFβ2 had only modest ability to stimulate pericyte invasion (Figure 1). We hypothesized that TGFβs might have a separate function during this EC-pericyte co-assembly process. To address such a possibility, we pre-treated pericytes with TGFβ1, the major isoform made by ECs (Suppl. Figure IIB) and, determined if their responsiveness to other EC-derived factors was affected using pericyte-only invasion assays. Remarkably, TGFβ1-pretreatment (or priming) of pericytes leads to substantially greater pericyte invasion in response to PDGF-BB, PDGF-DD, and ET-1 compared to control (Figure 7A,B). Furthermore, this TGFβ1 priming effect was completely inhibited by the Alk-5 inhibitor, SB 431542 (Figure 7C,D), one of the key components of CIGS that blocks pericyte recruitment (Figures 3 and 4). Also, the addition of SB 431542 did not block the direct ability of PDGF-BB, PDGF-DD or ET-1 to stimulate pericyte invasion (Figure 7E). Thus, it appears that TGFβ, acting through Alk-5, can strongly accelerate pericyte responses to PDGFs and ET-1, and function as an upstream primer to facilitate capillary assembly.
Figure 7. TGFβ acts as an upstream primer to stimulate downstream pericyte invasion responses to PDGF-BB, PDGF-DD, and ET-1.

(A) Pericytes were either left untreated or were treated with TGFβ1 at 10 ng/mL for 24 hr (i.e. priming step) prior to the assay setup. The cells were then tested in invasion assays in response to the indicated factors [PDGF-BB, PDGF-DD, HB-EGF (40 ng/mL final concentration); ET-1 (2×10−8 M final concentration); TGFβ1 and TGFβ2 (10 ng/mL final concentration)] vs. control. Cultures were fixed after 72 hr and the invasion response was quantitated. n≥3; Asterisks indicate significance at p<.05. and their response to invasion during 72-hour invasion assays. (B) Toluidine blue stained representative images of the indicated conditions. Bar equals 100 μm. (C,D) TGFβ1 primed pericytes in the absence or presence of the Alk5 inhibitor (10 μM SB431542) were tested in invasion assays with the indicated factors at the same doses as in (A). (E) Pericytes were induced to invade in response to the indicated factors and in the absence or presence of 10 μM SB431542. Cultures were fixed after 72 hr and were quantitated for invasion. n≥3; Asterisks indicate significance at p<.05.
DISCUSSION
In this work, we address a fundamental question in vascular biology which is to identify and determine the functional role of the critical EC-derived factors that stimulate pericyte recruitment to EC tubes, a process that is required for the assembly of capillary networks. These findings may also be important and relevant to the process of EC-mural cell assembly in larger vessels such as arteries and veins. Our laboratory has developed defined serum-free models of human capillary network formation in 3D matrices using ECs and pericytes that allow us to identify and investigate the specific role of key growth factors, peptides and other small molecules during this process6, 50. Here, we specifically have focused on using this model to address how pericytes respond to ECs and, identify the key factors that stimulate pericyte recruitment and pericyte proliferation, and then determine how these factors affect the ability of ECs and pericytes to work together to assemble capillary basement membranes2, 25. Furthermore, we report the development of highly defined pericyte invasion assays (in the absence of ECs) that have allowed us to broadly screen and rapidly identify the key regulators of pericyte recruitment. Finally, we have identified pharmacologic drug combinations and combinations of neutralizing antibodies and ligand binding traps that have allowed us to selectively block pericyte recruitment, proliferation and basement membrane deposition, without interfering with the ability of ECs to form tube networks.
The marked blockade of pericyte recruitment that we have achieved results in EC tubes that are markedly widened, and which progressively become shorter and less branched over time as if pericytes were never added. In contrast, control cultures with extensive pericyte recruitment leads to EC tubes with narrow lumens that remain highly elongated and branched, which are key morphologic features of capillary networks in vivo. Our work suggests that pericyte recruitment is necessary during the development of the vasculature to form and stabilize capillary tube networks and to prevent EC tubes from remodeling into larger, wider and less branched structures. This latter case occurs when ECs undergo tube formation without pericytes or when pericyte recruitment is disrupted (as shown here) but might also happen in disease states where EC-pericyte interactions are abnormal including diabetes, malignant cancers and vascular malformations. The identification of the key EC-derived drivers of pericyte recruitment which control capillary assembly is clearly essential to understand human disease states where EC-pericyte interactions are abnormal. Our novel findings demonstrating the co-contribution of PDGF-BB, PDGF-DD, ET-1, TGFβ, and HB-EGF (Pericyte Factors) (Figure 8B), and delineating their specific roles during these processes is necessary to understand both normal developmental processes but also to begin to address why abnormalities arise in specific human diseases such as diabetes. Pericyte loss has been described as a central feature of diabetes, and yet there is currently limited information addressing the underlying basis for this phenomenon. We think it likely that the pericyte factors or pericyte factor receptors that we have identified which control normal capillary assembly might be dysregulated in diabetes. In support of this conclusion is previous work showing that genetic disruption of PDGF-BB or PDGFRβ in mice can result in abnormalities such as excessive vascularization and microaneurysms, which are observed in diabetes35, 38. Similarly, the “Pericyte Factors” that we have identified may be dysregulated in other key diseases with abnormal EC-pericyte interactions (e.g. malignant cancers or vascular malformations). One important approach to further addressing such questions would be to develop in vitro systems modeled on the work reported here under diabetic conditions or under circumstances mimicking vascular malformations. The central issue here is to elucidate the underlying basis of the abnormalities that are present in the disease. There is considerable complexity here, and these issues will not be readily solvable without serious efforts and coordination using both in vitro and in vivo model approaches.
Figure 8. Schematic diagram illustrating molecular regulators of EC-pericyte tube co-assembly during capillary network formation.
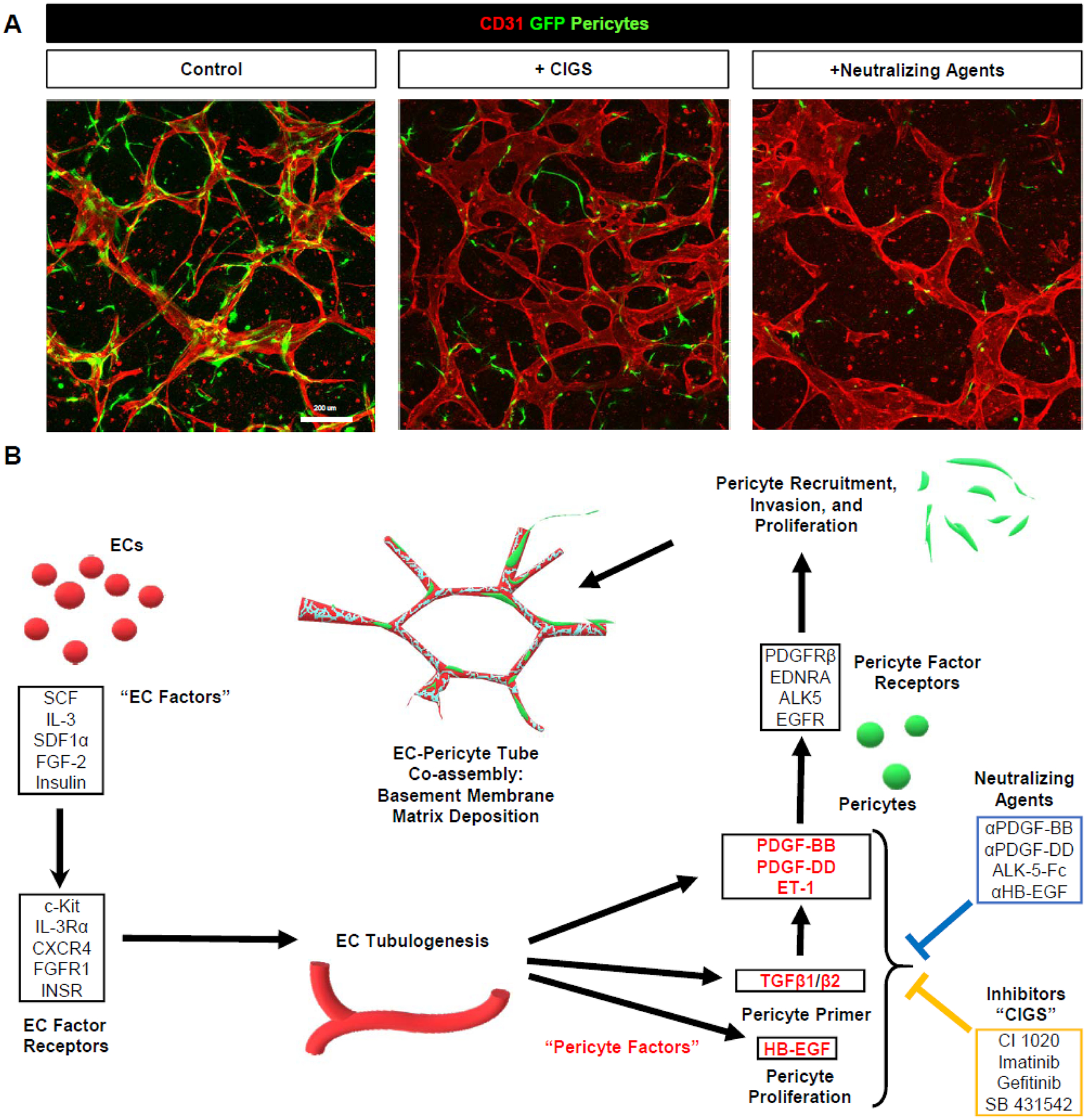
(A) EC-pericyte co-cultures were established in the absence or presence of pharmacologic inhibitors (CIGS) or neutralizing agents that interfere with pericyte recruitment, and after 120 hr, cultures were fixed and stained with anti-CD31 antibodies. Representative confocal images of these cultures are shown. Bar equals 200 μm. (B) Schematic illustration of the key growth factors and peptides that stimulate EC tubulogenesis (EC Factors- IL-3, SCF, SDF-1α, FGF-2, Insulin) and pericyte recruitment to EC tube networks (Pericyte Factors- PDGF-BB, PDGF-DD, ET-1, TGFβs, HB-EGF) that are each necessary to create capillary networks. Combined blockade of the Pericyte Factors or their receptors markedly interferes with pericyte invasion, recruitment, basement membrane deposition, and establishment of narrow and elongated networks of capillary tubes.
It is very intriguing that pericyte recruitment to EC tubes to create capillary networks requires multiple growth factors (i.e. PDGF-BB, PDGF-DD, TGFβs, and HB-EGF, a peptide (ET-1) (i.e. Pericyte Factors and their pericyte receptors) (Figure 8). Over recent years, our laboratory has discovered that a combination of 5 growth factors including SCF, IL-3, SDF-1α, FGF-2 and insulin (i.e. EC Factors and their receptors) are necessary for human ECs to form tubes and sprout in either collagen or fibrin matrices6, 50, 55 (Figure 8B). These EC Factors are required for the EC-pericyte co-cultures to properly form and assemble and for the ECs to produce, activate and present the multiple Pericyte Factors that are required for pericyte recruitment, proliferation, and basement membrane deposition (Figure 8B). When considered together, these studies indicate that 10 or more Factors are necessary for human capillary networks to assemble. This fundamental information is necessary for us to understand how capillaries form, stabilize and interact with adjacent parenchymal cells to promote tissue health. We recently proposed the concept that healthy capillaries are disease suppressors5, and when capillary networks become disorganized, disassemble or regress, which occurs in many major human disease states, such capillary abnormalities appear to be directly contributing to the underlying pathogenesis of key diseases. Our novel studies and approaches presented here, which define the EC-derived factors that are necessary for pericytes to co-assemble onto EC tubes to create capillary networks, adds to our growing knowledge of this normal process which should strongly aid in new approaches to repair the vasculature, restore tissue health and prevent disease.
Supplementary Material
HIGHLIGHTS.
A key combination of EC-derived factors, including PDGF-BB, PDGF-DD, ET-1, TGFβs and HB-EGF control pericyte interactions with EC tubes to create capillary networks.
PDGF-BB, PDGF-DD, and ET-1 directly control pericyte invasion, TGFβ1 primes pericyte invasion responses, and HB-EGF controls pericyte proliferation along with PDGFs and ET-1.
Combined neutralization of the above EC-derived factors or pharmacologic blockade of their pericyte receptors markedly interferes with pericyte recruitment and responsiveness to EC-lined tubes, which behave as if pericytes were not present.
ACKNOWLEDGEMENTS
Sources of funding: This work was supported by NIH grants HL136139, HL149748 and HL128584 (GED).
ABBREVIATIONS
- PDGF-BB
Platelet-derived growth factor-BB
- PDGF-DD
Platelet-derived growth factor-DD
- ET-1
Endothelin-1
- TGFβ
Transforming growth factor beta
- HB-EGF
Heparin binding epidermal growth factor
- EC
Endothelial cell
- 3D
Three-dimensional
- PDGFRβ
Platelet-derived growth factor receptor beta
- Alk-5
Transforming growth factor beta receptor 1
- GFP
Green fluorescent protein
- CIGS
CI 1020+ Imatinib+ Getinib+ SB 431542
Footnotes
Disclosures: The authors declare no competing financial interests related to these studies.
REFERENCES
- 1.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478 [DOI] [PubMed] [Google Scholar]
- 2.Stratman AN, Davis GE. Endothelial cell-pericyte interactions stimulate basement membrane matrix assembly: Influence on vascular tube remodeling, maturation, and stabilization. Microsc Microanal. 2012;18:68–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armulik A, Genove G, Betsholtz C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215 [DOI] [PubMed] [Google Scholar]
- 4.Ramasamy SK, Kusumbe AP, Adams RH. Regulation of tissue morphogenesis by endothelial cell-derived signals. Trends Cell Biol. 2015;25:148–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis GE, Norden PR, Bowers SL. Molecular control of capillary morphogenesis and maturation by recognition and remodeling of the extracellular matrix: Functional roles of endothelial cells and pericytes in health and disease. Connect Tissue Res. 2015;56:392–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowers SL, Norden PR, Davis GE. Molecular signaling pathways controlling vascular tube morphogenesis and pericyte-induced tube maturation in 3d extracellular matrices. Adv Pharmacol. 2016;77:241–280 [DOI] [PubMed] [Google Scholar]
- 7.Majesky MW. Vascular development. Arterioscler Thromb Vasc Biol. 2018;38:e17–e24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaengel K, Genove G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:630–638 [DOI] [PubMed] [Google Scholar]
- 9.Koller GM, Schafer C, Kemp SS, Aguera KN, Lin PK, Forgy JC, Griffin CT, Davis GE. Proinflammatory mediators, il (interleukin)-1beta, tnf (tumor necrosis factor) alpha, and thrombin directly induce capillary tube regression. Arterioscler Thromb Vasc Biol. 2020;40:365–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Ischemia/reperfusion. Compr Physiol. 2016;7:113–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammes HP. Pericytes and the pathogenesis of diabetic retinopathy. Horm Metab Res. 2005;37 Suppl 1:39–43 [DOI] [PubMed] [Google Scholar]
- 12.Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: A potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens. 2004;13:1–7 [DOI] [PubMed] [Google Scholar]
- 13.Battegay EJ, de Miguel LS, Petrimpol M, Humar R. Effects of anti-hypertensive drugs on vessel rarefaction. Curr Opin Pharmacol. 2007;7:151–157 [DOI] [PubMed] [Google Scholar]
- 14.Durham JT, Herman IM. Microvascular modifications in diabetic retinopathy. Curr Diab Rep. 2011;11:253–264 [DOI] [PubMed] [Google Scholar]
- 15.Goldenberg NM, Steinberg BE, Slutsky AS, Lee WL. Broken barriers: A new take on sepsis pathogenesis. Sci Transl Med. 2011;3:88ps25 [DOI] [PubMed] [Google Scholar]
- 16.Granger DN, Kvietys PR. Reperfusion therapy-what’s with the obstructed, leaky and broken capillaries? Pathophysiology. 2017;24:213–228 [DOI] [PubMed] [Google Scholar]
- 17.Hinkel R, Howe A, Renner S, Ng J, Lee S, Klett K, Kaczmarek V, Moretti A, Laugwitz KL, Skroblin P, Mayr M, Milting H, Dendorfer A, Reichart B, Wolf E, Kupatt C. Diabetes mellitus-induced microvascular destabilization in the myocardium. J Am Coll Cardiol. 2017;69:131–143 [DOI] [PubMed] [Google Scholar]
- 18.Noon JP, Walker BR, Webb DJ, Shore AC, Holton DW, Edwards HV, Watt GC. Impaired microvascular dilatation and capillary rarefaction in young adults with a predisposition to high blood pressure. J Clin Invest. 1997;99:1873–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostergaard L, Granfeldt A, Secher N, Tietze A, Iversen NK, Jensen MS, Andersen KK, Nagenthiraja K, Gutierrez-Lizardi P, Mouridsen K, Jespersen SN, Tonnesen EK. Microcirculatory dysfunction and tissue oxygenation in critical illness. Acta Anaesthesiol Scand. 2015;59:1246–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uemura MT, Maki T, Ihara M, Lee VMY, Trojanowski JQ. Brain microvascular pericytes in vascular cognitive impairment and dementia. Front Aging Neurosci. 2020;12:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng H, Chen JX. Microvascular rarefaction and heart failure with preserved ejection fraction. Front Cardiovasc Med. 2019;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 2005;15:102–111 [DOI] [PubMed] [Google Scholar]
- 23.Park KM, Gerecht S. Harnessing developmental processes for vascular engineering and regeneration. Development. 2014;141:2760–2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song HG, Rumma RT, Ozaki CK, Edelman ER, Chen CS. Vascular tissue engineering: Progress, challenges, and clinical promise. Cell Stem Cell. 2018;22:340–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114:5091–5101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stratman AN, Schwindt AE, Malotte KM, Davis GE. Endothelial-derived pdgf-bb and hb-egf coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood. 2010;116:4720–4730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stratman AN, Pezoa SA, Farrelly OM, Castranova D, Dye LE 3rd, Butler MG, Sidik H, Talbot WS, Weinstein BM. Interactions between mural cells and endothelial cells stabilize the developing zebrafish dorsal aorta. Development. 2017;144:115–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561 [DOI] [PubMed] [Google Scholar]
- 29.Armulik A, Mae M, Betsholtz C. Pericytes and the blood-brain barrier: Recent advances and implications for the delivery of cns therapy. Ther Deliv. 2011;2:419–422 [DOI] [PubMed] [Google Scholar]
- 30.Saunders WB, Bohnsack BL, Faske JB, Anthis NJ, Bayless KJ, Hirschi KK, Davis GE. Coregulation of vascular tube stabilization by endothelial cell timp-2 and pericyte timp-3. J Cell Biol. 2006;175:179–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He L, Vanlandewijck M, Raschperger E, Andaloussi Mae M, Jung B, Lebouvier T, Ando K, Hofmann J, Keller A, Betsholtz C. Analysis of the brain mural cell transcriptome. Sci Rep. 2016;6:35108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volz KS, Jacobs AH, Chen HI, Poduri A, McKay AS, Riordan DP, Kofler N, Kitajewski J, Weissman I, Red-Horse K. Pericytes are progenitors for coronary artery smooth muscle. Elife. 2015;4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stallcup WB. The ng2 proteoglycan in pericyte biology. Adv Exp Med Biol. 2018;1109:5–19 [DOI] [PubMed] [Google Scholar]
- 34.Howson KM, Aplin AC, Gelati M, Alessandri G, Parati EA, Nicosia RF. The postnatal rat aorta contains pericyte progenitor cells that form spheroidal colonies in suspension culture. Am J Physiol Cell Physiol. 2005;289:C1396–1407 [DOI] [PubMed] [Google Scholar]
- 35.Enge M, Bjarnegard M, Gerhardt H, Gustafsson E, Kalen M, Asker N, Hammes HP, Shani M, Fassler R, Betsholtz C. Endothelium-specific platelet-derived growth factor-b ablation mimics diabetic retinopathy. EMBO J. 2002;21:4307–4316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammes HP, Lin J, Renner O, Shani M, Lundqvist A, Betsholtz C, Brownlee M, Deutsch U. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002;51:3107–3112 [DOI] [PubMed] [Google Scholar]
- 37.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of pdgf-b and pdgfr-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055 [DOI] [PubMed] [Google Scholar]
- 38.Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in pdgf-b-deficient mice. Science. 1997;277:242–245 [DOI] [PubMed] [Google Scholar]
- 40.Bjarnegard M, Enge M, Norlin J, Gustafsdottir S, Fredriksson S, Abramsson A, Takemoto M, Gustafsson E, Fassler R, Betsholtz C. Endothelium-specific ablation of pdgfb leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development. 2004;131:1847–1857 [DOI] [PubMed] [Google Scholar]
- 41.Hirschi KK, Rohovsky SA, D’Amore PA. Pdgf, tgf-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10t1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141:805–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirschi KK, Rohovsky SA, Beck LH, Smith SR, D’Amore PA. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-bb and heterotypic cell contact. Circ Res. 1999;84:298–305 [DOI] [PubMed] [Google Scholar]
- 43.Oshima M, Oshima H, Taketo MM. Tgf-beta receptor type ii deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev Biol. 1996;179:297–302 [DOI] [PubMed] [Google Scholar]
- 44.Orlova VV, Liu Z, Goumans MJ, ten Dijke P. Controlling angiogenesis by two unique tgf-beta type i receptor signaling pathways. Histol Histopathol. 2011;26:1219–1230 [DOI] [PubMed] [Google Scholar]
- 45.Dave JM, Mirabella T, Weatherbee SD, Greif DM. Pericyte alk5/timp3 axis contributes to endothelial morphogenesis in the developing brain. Dev Cell. 2018;44:665–678 e666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furuhashi M, Sjoblom T, Abramsson A, Ellingsen J, Micke P, Li H, Bergsten-Folestad E, Eriksson U, Heuchel R, Betsholtz C, Heldin CH, Ostman A. Platelet-derived growth factor production by b16 melanoma cells leads to increased pericyte abundance in tumors and an associated increase in tumor growth rate. Cancer Res. 2004;64:2725–2733 [DOI] [PubMed] [Google Scholar]
- 47.Gladh H, Folestad EB, Muhl L, Ehnman M, Tannenberg P, Lawrence AL, Betsholtz C, Eriksson U. Mice lacking platelet-derived growth factor d display a mild vascular phenotype. PLoS One. 2016;11:e0152276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iivanainen E, Nelimarkka L, Elenius V, Heikkinen SM, Junttila TT, Sihombing L, Sundvall M, Maatta JA, Laine VJ, Yla-Herttuala S, Higashiyama S, Alitalo K, Elenius K. Angiopoietin-regulated recruitment of vascular smooth muscle cells by endothelial-derived heparin binding egf-like growth factor. FASEB J. 2003;17:1609–1621 [DOI] [PubMed] [Google Scholar]
- 49.Koh W, Stratman AN, Sacharidou A, Davis GE. In vitro three dimensional collagen matrix models of endothelial lumen formation during vasculogenesis and angiogenesis. Methods Enzymol. 2008;443:83–101 [DOI] [PubMed] [Google Scholar]
- 50.Stratman AN, Davis MJ, Davis GE. Vegf and fgf prime vascular tube morphogenesis and sprouting directed by hematopoietic stem cell cytokines. Blood. 2011;117:3709–3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davis GE, Stratman AN, Sacharidou A, Koh W. Molecular basis for endothelial lumen formation and tubulogenesis during vasculogenesis and angiogenic sprouting. Int Rev Cell Mol Biol. 2011;288:101–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stratman AN, Saunders WB, Sacharidou A, Koh W, Fisher KE, Zawieja DC, Davis MJ, Davis GE. Endothelial cell lumen and vascular guidance tunnel formation requires mt1-mmp-dependent proteolysis in 3-dimensional collagen matrices. Blood. 2009;114:237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fisher KE, Pop A, Koh W, Anthis NJ, Saunders WB, Davis GE. Tumor cell invasion of collagen matrices requires coordinate lipid agonist-induced g-protein and membrane-type matrix metalloproteinase-1-dependent signaling. Mol Cancer. 2006;5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fisher KE, Sacharidou A, Stratman AN, Mayo AM, Fisher SB, Mahan RD, Davis MJ, Davis GE. Mt1-mmp- and cdc42-dependent signaling co-regulate cell invasion and tunnel formation in 3d collagen matrices. J Cell Sci. 2009;122:4558–4569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith AO, Bowers SL, Stratman AN, Davis GE. Hematopoietic stem cell cytokines and fibroblast growth factor-2 stimulate human endothelial cell-pericyte tube co-assembly in 3d fibrin matrices under serum-free defined conditions. PLoS One. 2013;8:e85147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


