Abstract
Activation of the Wnt/β-catenin pathway is one of the hallmarks of colorectal cancer (CRC). Sirtuin 2 (SIRT2) protein has been shown to inhibit CRC proliferation. Previously, we reported that SIRT2 plays an important role in the maintenance of normal intestinal cell homeostasis. Here, we show that SIRT2 is a direct target gene of Wnt/β-catenin signaling in CRC cells. Inhibition or knockdown of Wnt/β-catenin increased SIRT2 promoter activity and mRNA and protein expression, whereas activation of Wnt/β-catenin decreased SIRT2 promoter activity and expression. β-catenin was recruited to the promoter of SIRT2 and transcriptionally regulated SIRT2 expression. Wnt/β-catenin inhibition increased mitochondrial oxidative phosphorylation (OXPHOS) and CRC cell differentiation. Moreover, inhibition of OXPHOS attenuated the differentiation of CRC cells induced by Wnt/β-catenin inhibition. In contrast, inhibition or knockdown of SIRT2 decreased, while overexpression of SIRT2 increased, OXPHOS activity and differentiation in CRC cells. Consistently, inhibition or knockdown or SIRT2 attenuated the differentiation induced by Wnt/β-catenin inhibition. These results demonstrate that SIRT2 is a novel target gene of the Wnt/β-catenin signaling and contributes to the differentiation of CRC cells.
Keywords: Cell signaling, Protein expression, Cell differentiation
1. INTRODUCTION
Wnt/β-catenin signaling is critical for normal intestinal epithelial cell proliferation and differentiation; conversely, aberrant regulation of the Wnt signaling pathway contributes to tumorigenesis [1, 2]. Wnt signaling stabilizes the transcription factor β-catenin, which enters the nucleus to regulate the transcription of Wnt pathway target genes through its interaction with the T-cell factor/lymphoid enhancer-binding factor (TCF/LEF) family of members [1, 3]. The Wnt/β-catenin pathway controls the self-renewal of intestinal stem cells and is crucial for preserving intestinal homeostasis [4]. The majority of colorectal cancers (CRCs) contain a mutation in Adenomatous polyposis coli (APC), which suppresses Wnt signaling, or β-catenin; these mutations result in the stabilization of β-catenin and thus activate Wnt signaling [5]. Sustained activation of Wnt/β-catenin signaling contributes to hyper-proliferation and oncogenic transformation of intestinal epithelial cells leading to CRC [4, 6]. Targeting the Wnt pathway signaling cascade is a promising approach for CRC chemoprevention and therapeutics.
The sirtuins (SIRT1–7) are a family of proteins possessing an NAD-dependent deacetylase activity [7]. Sirtuin proteins participate in a wide range of biological processes [8]. Different from other sirtuin family members, sirtuin 2 (SIRT2) is mainly found in the cytoplasm, mitochondria, and the nucleus [9, 10]. With a growing number of substrates, SIRT2 plays an important role in cell proliferation, differentiation and metabolism [11]. Recently, SIRT2 demonstrated an inhibitory role in the growth of tumors including CRC [12–14]. SIRT2 deficiency induces chromosome alterations and subsequent tumor development in the liver [12, 13]. Knockdown of SIRT2 in human fibroblasts decreased mitochondrial oxidative phosphorylation (OXPHOS) and increased glycolytic activity while SIRT2 overexpression in human pluripotent stem cells affected energy metabolism and stem cell function [15]. SIRT2 protects against the development of inflammatory processes in the intestine [16]. Recently, we have shown a novel role for SIRT2 in the maintenance of intestinal cell homeostasis [17]. However, the signaling pathways controlling the expression of SIRT2 are largely undefined.
Effective cancer treatments require activation of tumor suppressors or inactivation of tumor promoters that results in tumor cell death or the re-programming of tumor cells to differentiate [18]. Hyper-activation of the Wnt pathway, through APC inactivation in intestinal stem cells (ISCs), promotes the rapid accumulation of ISCs [19]. Restoration of APC function promotes tumor cell differentiation and causes complete tumor regression [18, 20]. Along the intestinal crypt-villus axis, high glycolytic metabolism is noted in the proliferative crypt cells and an OXPHOS phenotype in the differentiated cells of the villus. Recent findings have shown that mitochondrial OXPHOS activates reactive oxygen species to induce intestinal crypt cell differentiation [21]. Moreover, inhibition of histone deacetylase induces cancer cell differentiation and thus inhibits CRC cell proliferation [22].
In our current study, we show that SIRT2 is a target gene of the Wnt/β-catenin signaling pathway in CRCs. Wnt/β-catenin/SIRT2 regulates CRC cell differentiation through the regulation of mitochondrial OXPHOS. Previously, we have shown the inhibitory role of SIRT2 on Wnt/β-catenin in intestinal cells. Our findings provide novel insights regarding the interaction between Wnt/β-catenin and SIRT2 in the regulation of CRC cell proliferation and differentiation.
2. MATERIALS AND METHODS
Materials.
FH535, sodium butyrate (NaBT), nicotinamide, oligomycin, rotenone and antibodies to β-actin, MUC2 and KRT20 were purchased from Sigma-Aldrich (St. Louis, MO). ICG001 and AGK2 were purchased from Selleckchem (Radnor, PA). Antibodies to SIRT2, p21Waf1, α-tubulin, ac-α-tubulin, β-catenin and Na,K-ATPase were purchased from Cell Signaling (Beverly, MA). Anti-villin and anti-p27Kip1 antibodies were from BD (San Jose, CA). Human β-catenin and non-targeting control siRNA SMARTpool were purchased from Dharmacon, Inc. (Lafayette, CO). MISSION control shRNA and two shRNA sequences (SIRT2 shRNA: TRCN0000012120; SIRT2 shRNA 2: TRCN0000012119) targeting human SIRT2 constructed in pLKO.1-puro vector were purchased from Sigma-Aldrich.
Cell culture and transfection.
The human colon cancer cell lines HT29, HCT116 and Caco-2 were purchased from ATCC (Manassas, VA, USA). HT29 and HCT116 were maintained in McCoy’s 5A supplemented with 10% fetal calf serum (FCS). Caco-2 cells were cultured in MEM with 15% FBS, 1% sodium pyruvate, and 1% nonessential amino acids. Authentications were confirmed by a 100% match in comparison to the reference STR profiles from ATCC. Moreover, cells were tested for mycoplasma contamination and found to be negative. The human CRC cell line LS174T, purchased in February 2016 from ATCC, were maintained in EMEM supplemented with 10% FCS and DMEM supplemented with 10% FCS, respectively. To knock down SIRT2 or β-catenin expression, cells were transfected with siRNA duplexes (100 nmol/L) by electroporation. Cells were also infected with lentiviral vectors containing control shRNA or shRNA targeting human SIRT2 followed by selection with puromycin (5 μg/ml). SIRT2 gene was sub-cloned into the pCW57-GFP-2A-MCS lentiviral vector (Addgene, Watertown, MA) and SIRT2 expression was induced using doxycycline. Cells were infected with lentiviral particles containing protein expression vector encoding gene for SIRT2, selected with puromycin (5 μg/ml), and induced by doxycycline (400 ng/ml).
SIRT2 promoter cloning and dual-luciferase assay.
Genomic DNA from HCT116 cells was extracted. SIRT2 gene promoter fragments 2900 bp (from −2800 bp to +100 bp from the transcription start site), 1100 bp (−1000 bp to +100 bp) and 400 bp (−300 to +100 bp) were produced by PCR. The primers were synthesized based on the genomic sequence lying upstream of the human SIRT2 cDNA (NCBI, NC_000019.10). The PCR primers with NheI and XhoI sites are shown in (Table 1). PCR products were cloned into the reporter vector (pGL3-basic), sequenced and confirmed to contain contiguous genomic sequences. The pRL-Tk-luc plasmid was co-transfected together with SIRT2 promoter constructs to normalize for variation in transfection efficiency. Luciferase assays were performed as described [23].
Table 1.
PCR primers were synthesized based on the genomic sequence lying upstream of the human SIRT2 cDNA.
| Primer names | Primer sequences | Position | Product sizes |
|---|---|---|---|
| SIRT2-PF1 | 5’- (NheI) cataacaaaaagaggaatatc-3’ | −2800 | 2900 bp |
| SIRT2-PF2 | 5’- (NheI) cttagagtcagggaccaagt-3’ | −1000 | 1100 bp |
| SIRT2-PF3 | 5’- (NheI) aatgataatatgctgaatcg-3’ | −300 | 400 bp |
| SIRT2-PR | 5’- (XhoI) catgggcgcggtgctgaagc-3’ | +100 | |
Chromatin immunoprecipitation analysis.
Chromatin immunoprecipitation (ChIP) assay was performed using the Active Motif ChIP-IT Express Enzymatic Kit following the manufacturer’s protocol as described previously [24]. The human SIRT2 promoter containing the TCF/β-catenin binding site was amplified by PCR was from total (input) or immunoprecipitated chromatin. The following pair of oligonucleotide primers were used: 5′-CAGATGAATCCTTGGTCTC-3′ and 5′-TCTTTGATTCTTAGTTCCCGA-3′.
Quantitative real-time RT-PCR and western blot analysis.
Total RNA was extracted followed by treatment with DNase (Promega, Madison, WI). Equal amounts of total RNA (1 μg) were transcribed to synthesize cDNA using the TaqMan Reverse Transcription Reagents Kit (Applied Biosystems, Foster City, CA). TaqMan probe and primers were obtained from Applied Biosystems. Quantitative real-time RT-PCR was performed as described previously [25].
Total protein was separated on a 10% polyacrylamide gel and resolved. Proteins were electrotransferred to PVDF membranes. Membranes were incubated with primary antibodies followed by incubation with secondary antibodies as described previously [25].
Intestinal alkaline phosphatase (IAP) activity assay.
Cells were lysed and proteins were extracted and concentrations determined. IAP activity was accessed using Alkaline Phosphatase Yellow (pNPP) Liquid Substrate System (Sigma) as described [25].
Live cell metabolic analysis.
Cells were plated into 96-well XF cell culture microplates followed by incubation at 37°C in a CO2 incubator for 24 h. Oxygen consumption rate (OCR) was measured using the XFe96 analyzer (Agilent Technologies) according to the manufacturer’s instructions as described [26]. Each plotted value was normalized to total protein.
Statistical analysis.
Descriptive statistics, including means and standard deviations (SD) are presented for each experimental group and displayed in bar graphs. Multiple comparisons of RT-PCR and IAP were performed using analysis of variance with pairwise comparisons using contrast statements. Metabolic analysis utilized repeated measures linear mixed models of OCR levels over time. Using the Holm’s step-down procedure, adjustment in p-values due to multiple pairwise testing between groups was performed. Data were log transformed as necessary for the parametric tests. Each experiment was repeated at least 3 times and representative data are presented. Statistical analyses were performed using SAS software version 9.4 (SAS Inc., Cary, NC, USA).
3. RESULTS
SIRT2 contributes to CRC cell differentiation.
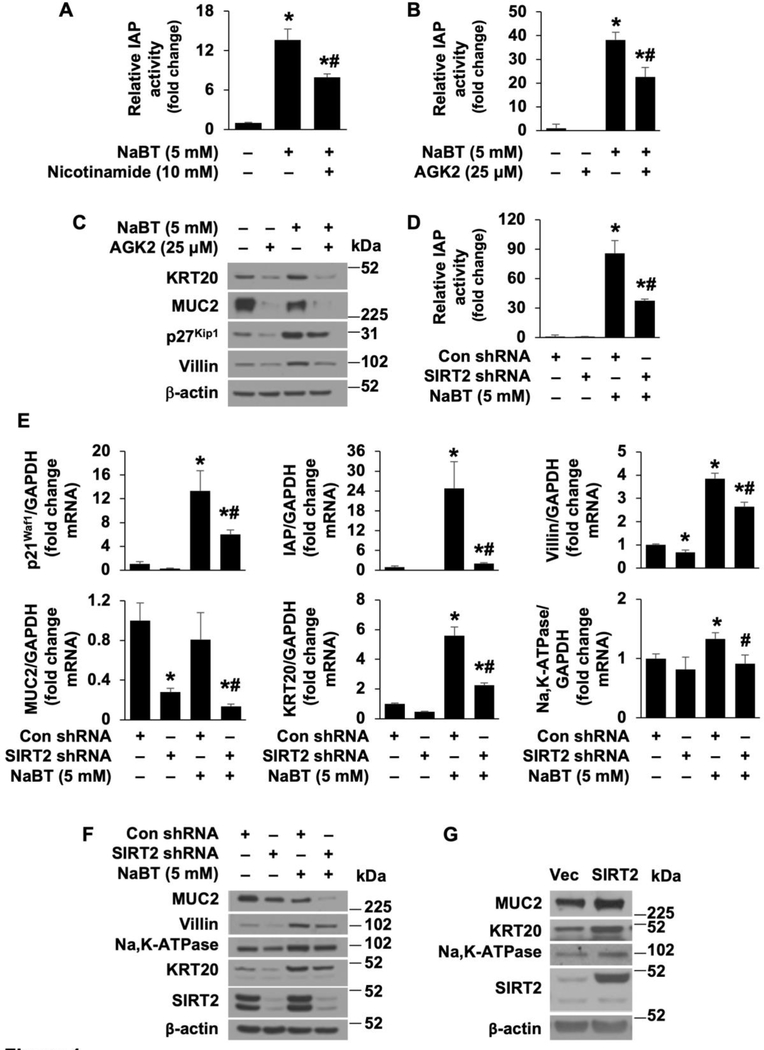
Epigenetic alternations such as histone modifications play an important role in tumorigenesis [27]. Inhibition of histone deacetylase induces cancer cell differentiation and thus inhibits CRC cell proliferation [22]. SIRT2, acting as a tumor suppressor, decreases CRC cell proliferation [14]. We first determined the effects of SIRT2 on CRC cell differentiation. As shown in Fig. 1A–C and Supplemental Figure 1A and B, treatment with sodium butyrate (NaBT), a histone deacetylase inhibitor which induces CRC cell differentiation and inhibits CRC cell growth [28, 29], induced differentiation as noted by increased IAP activity in HT29 cells (Fig. 1A and B) and Caco-2 cells (Supplemental Fig. 1A and B) and increased protein expression of KRT20, p27Kip1 and villin as determined by western blotting in HT29 cells (Fig. 1C) and Na,K-ATPase and p27Kipl in Caco-2 cells (Supplemental Fig. 1C); these increases were significantly attenuated by treatment with either nicotinamide, a pan inhibitor of sirtuins, or AGK2, a selective inhibitor of SIRT2 [30]. These results suggest that SIRT2 contributes to the process of CRC cell differentiation.
Figure 1. SIRT2 is required for NaBT-induced CRC cell differentiation.
A. HT29 cells were treated with NaBT with or without the sirtuin inhibitor nicotinamide for 48 h. IAP activity was assayed. (n=3, data represent mean ± SD; *p<0.01 vs control; #p<0.01 vs NaBT alone). B and C. HT29 cell cells were treated with NaBT with or without the SIRT2 inhibitor AGK2 for 48 h. IAP activity assay (B). (n=3, data represent mean ± SD; *p<0.01 vs control; #p<0.01 vs NaBT alone). Western blot analysis using antibodies as indicated (C). D-F. HT29 cells, stably transfected with control or SIRT2 shRNA, were treated with NaBT for 48 h. Cell lysates were used for IAP activity assay (D). (n=3, data represent mean ± SD; *p<0.01 vs con shRNA; #p<0.01 vs NaBT plus Con shRNA). Total RNA was extracted and p21Waf1, IAP, villin, MUC2, KRT20, and Na,K-ATPase mRNA levels were determined by real-time RT-PCR (E). (n=3, data represent mean ± SD; *p<0.01 vs con shRNA; #p<0.01 vs NaBT plus Con shRNA). Western blot analysis using antibodies as indicated (F). G. Doxycycline-induced overexpression of SIRT2 in HT29 cells. Western blot analysis was performed.
To further confirm the role of SIRT2 in CRC cell differentiation, HT29 and Caco-2 cells were transfected with shRNA targeting SIRT2 to determine whether knockdown of SIRT2 can attenuate the NaBT-induced differentiation. As shown in Fig. 1D–F, HT29 cells were treated with NaBT and differentiation was confirmed by increased IAP activity (Fig. 1D), increased mRNA expression of p21Waf1, IAP, villin, KRT20 and Na,K-ATPase (Fig. 1E), and increased protein expression of villin, Na,K-ATPase and KRT20 (Fig. 1F). Similar results were also found using Caco-2 cells (Supplemental Fig. 1D–G). These increased expression of the differentiation markers were significantly attenuated by knockdown of SIRT2. The role of SIRT2 in CRC differentiation has been further confirmed using another shRNA sequence targeting human SIRT2 (Supplemental Fig. 2A–B). Consistent with the results from others [31], treatment with NaBT decreased MUC2 expression in HT29 as expected (Fig. 1C, E and F). Next, we used a doxycycline-inducible vector to overexpress SIRT2 in HT29 and Caco-2 cells; we noted increased expression of MUC2, KRT20, and Na,K-ATPase in HT29 cells (Fig. 1G) and of p21Waf1 and Na,K-ATPase in Caco-2 cells (Supplemental Fig. 1H), respectively. Collectively, these results indicate that SIRT2 protein contributes to CRC cell differentiation.
SIRT2 promotes oxidative catabolism contributing to CRC cell differentiation.
Activation of mitochondrial OXPHOS enhances crypt formation and intestinal cell differentiation [21]. We next determined if SIRT2 regulates CRC cell differentiation via regulation of OXPHOS. First, we determined whether altered SIRT2 expression affects OXPHOS in CRC cells utilizing an Agilent Seahorse XFe96 extracellular flux analyzer (Fig. 2A and B). Knockdown of SIRT2 in HT29 cells resulted in the decreased OXPHOS as noted by the decreased OCR compared to control cells (Fig. 2A), whereas overexpression of SIRT2 increased OXPHOS capacity (Fig. 2B), suggesting that SIRT2 promotes OXPHOS in CRC cells.
Figure 2. SIRT2 promotes oxidative catabolism to increases CRC cell differentiation.
A and B. HT29 cells stably transfected with control shRNA or SIRT2 shRNA (A) or with overexpression of SIRT2 (B) was assessed for oxygen consumption using the Seahorse Bioscience XFe96 extracellular flux analyzer. The normalized OCR was shown. (n=10, data represent mean± S.D.; *P <0.05 compared with control shRNA or control vector). C and D. HT29 cell cells were treated with OXPHOS inhibitors oligomycin (1 uM) or rotenone (100 nM) in the presence or absence of NaBT for 48 h. IAP activity assay (C). (n=3, data represent mean ± SD; *p<0.01 vs control; #p<0.01 vs NaBT alone). Western blotting analysis using antibodies as indicated (D).
We next determined if inhibition of OXPHOS inhibits differentiation in HT29 cells. Treatment with oligomycin or rotenone, which inhibit OXPHOS [21], inhibited differentiation as shown by the attenuation of NaBT-induced IAP activity and decreased protein expression of MUC2 and villin and villin mRNA expression in HT29 cells (Fig. 2C and 2D; Supplemental Fig. 3). Together, these results demonstrate that SIRT2 promotes OXPHOS and thus contributes to CRC cell differentiation.
Negative regulation of SIRT2 expression by Wnt/β-catenin signaling pathway in CRC cells.
Inhibition of Wnt/β-catenin signaling by restoration of APC function promotes tumor cell differentiation and causes complete tumor regression [18, 20]. To determine the interaction between SIRT2 and the Wnt/β-catenin signaling pathway, HT29 and LS174T cells were treated with ICG001, a molecule inhibitor of Wnt signaling [32]. As shown in Fig. 3A, treatment with ICG001 resulted in a dose- and time-dependent increase in SIRT2 mRNA (Fig. 3A and Fig. 3B) and protein (Fig. 3C) expression in HT29 and LS174T cells. Similarly, treatment with FH535, which inhibits Wnt signaling[33], also increased SIRT2 mRNA and protein expression (Supplemental figure 4).
Figure 3. Inhibition of Wnt/β-catenin signaling increases SIRT2 protein expression.
A and B. HT29 and LS174T cells were treated with Wnt inhibitor ICG001 at various dosages for 48 h (A) or at 40 μM at various times (B). Total RNA was extracted and SIRT2 mRNA levels were determined by real-time RT-PCR. (n=3, data represent mean ± SD; *p<0.01 vs control). C. HT29 and LS174T cells were treated with ICG001 at various dosages for 48 h. Western blot analysis was performed using antibodies against SIRT2 and β-actin.
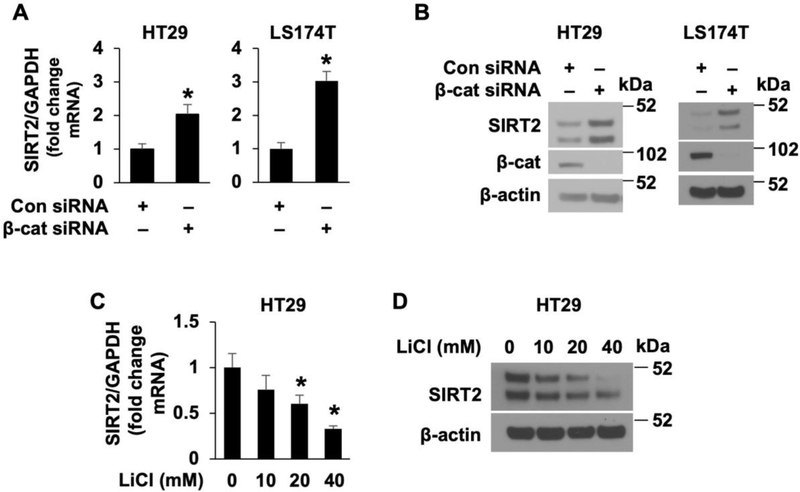
To further confirm the increase of SIRT2 expression by Wnt/β-catenin pathway inhibition, HT29 and LS174T cells were transfected with control siRNA or β-catenin siRNA. As shown in Fig. 4, knockdown of β-catenin significantly increased SIRT2 mRNA (Fig. 4A) and protein (Fig. 4B) expression. These results demonstrate that inhibition of Wnt/β-catenin increased SIRT2 expression in CRC cells.
Figure 4. Regulation of SIRT2 expression by Wnt/β-catenin signaling.
A and B. Knockdown of β-catenin results in the induction of SIRT2 expression. HT29 and LS174T cells were transfected with β-catenin siRNA or nontargeting control siRNA. After 48 h incubation, SIRT2 expression was determined by real-time RT-PCR (A) or western blot analysis (B), respectively. (n=3, data represent mean ± SD; * p < 0.05 vs. control). C and D. Activation of Wnt/β-catenin pathway suppressed SIRT2 expression. HT29 and LS174T cells were treated with LiCl at with various dosages or 40 mM of NaCl as control for 24 h. SIRT2 mRNA levels were assessed by real-time RT-PCR (C). (n=3, data represent mean ± SD; * p < 0.05 vs. control). Western blot analysis using the antibodies as indicated (D).
Finally, to determine whether activation of Wnt/β-catenin inhibited SIRT2 expression, we treated HT29 cells with LiCl, which activates Wnt/β-catenin signaling [34, 35]. In contrast to Wnt/β-catenin inhibition, treatment with LiCl resulted in a dose-dependent repression of SIRT2 mRNA (Fig. 4C) and protein (Fig. 4D) expression in HT29 cells. Thus, our results demonstrate that the expression of SIRT2 is negatively regulated by the Wnt/β-catenin signaling pathway in CRC cells.
β-catenin directly binds the SIRT2 promoter and regulates SIRT2 expression in CRC cells.
To determine whether β-catenin directly regulates the transcription of the SIRT2 gene, SIRT2 gene promoters were cloned into a luciferase reporter vector (pGL3-basic) (Fig. 5A). Transfection of the 2800 bp promoter fragment resulted in an approximate 7.6-fold increase in SIRT2 promoter activity compared with pGL3-basic in Caco-2 cells (Fig. 5B). Deletion from −2800 to −300 increased SIRT2 promoter activity, suggesting that the important regulatory elements for basal SIRT2 promoter activity are contained between −2800 to −300. To determine whether Wnt/β-catenin regulates SIRT2 promoter activity, a 2800 bp construct was transfected into Caco-2 cells (Fig. 5C). Inhibition of Wnt by treatment with Wnt inhibitor ICG001 increased SIRT2 promoter activity. To further demonstrate the role of Wnt/β-catenin, 2800 bp construct was transfected with or without HA-tagged β-catenin plasmid into Caco-2 cells. As shown in Fig. 5D, overexpression of β-catenin inhibited SIRT2 promoter activity. Importantly, these results demonstrate transcriptional regulation of SIRT2 by Wnt/β-catenin signaling.
Figure 5. Transcriptional regulation of SIRT2 expression by Wnt/β-catenin signaling.
A. SIRT2 promoter cloning. B. Basal SIRT2 promoter activity in Caco-2 cells. Caco-2 cells were transfected with SIRT2 promoter deletion constructs and relative luciferase activity was measured. C. Inhibition of Wnt/β-catenin by treatment with ICG001 increases SIRT2 promoter activity. SIRT2 −2800 promoter construct were transfected into Caco-2 cells and then treated with or without ICG001 (20 uM). Luciferase activity was measured 24 h after treatment. D. Overexpression of β-catenin decreased SIRT2 promoter activity. Caco-2 cells were transfected with construct containing −2800 bp SIRT2 promoter together with either the control vector, or Flag-tagged β-catenin. After incubation for 48 h, cells were lysed and luciferase activity determined. Results were normalized for transfection efficiency using the pRL-Tk-luc plasmid (Promega). (n=3, data shown as mean ± SD; *, P<0.05 vs control). E. Promoter sequences of the SIRT2 gene with a putative TCF/β-catenin binding site. F, ChIP assays were used to elucidate the binding of β-catenin to the SIRT2 promoter. Chromatin DNA fragments were precipitated using normal rabbit IgG and anti–β-catenin antibody as indicated.
TCF/LEF transcription factors bind TCF motifs and recruit the β-catenin transcriptional coactivator to activate target gene expression [36]. An essential binding sequence for TCF/β-catenin is 5′ CTTTGAA-3′ [37]. We have identified this binding sequence in the SIRT2 promoter (Fig. 5E). To determine whether β-catenin binds the SIRT2 promoter, cross-linked chromatin was extracted from Caco-2 cells, and ChIP assay was performed. The sequence (−1145/−926 bp) containing the putative TCF/β-catenin binding site was amplified by PCR (Fig. 5F) and showed β-catenin binding the SIRT2 promoter. Together, these results demonstrate β-catenin binding to the SIRT2 promoter, suggesting that Wnt/β-catenin represses SIRT2 expression via the recruitment of β-catenin transcriptional coactivator to the promoter.
SIRT2 is involved in Wnt/β-catenin regulation of CRC differentiation.
During differentiation the intestinal proliferative cells near the base of crypts progress up the crypt-villus axis with cessation of proliferation and subsequent differentiation [38, 39]. This process is characterized by decreased Wnt signaling activity associated with a change from glycolysis to OXPHOS along the crypt-villus axis [38]. Activation of Wnt signaling results in a metabolic switch from mitochondrial OXPHOS to glycolysis in cancer cells [40]. Consistently, expression of APC, which suppresses Wnt signaling, increases mitochondrial oxidative metabolism in HT29 cells [38].
To determine the interaction between Wnt/β-catenin and SIRT2 in the regulation of CRC cell differentiation, we first determined the effects of Wnt/β-catenin on the differentiation in CRC cells. As shown in Fig. 6, knockdown of β-catenin increased differentiation as noted by the increased MUC2, villin, and KRT20 protein (Fig. 6A) and mRNA (Fig. 6B) expression in HT29 and LS174T cells, demonstrating the inhibition of CRC cell differentiation by Wnt/β-catenin signaling.
Figure 6. Knockdown of β-catenin increases OXPHOS activity and induces CRC cell differentiation.
A and B. HT29 and LS174T cells were transfected with control siRNA or β-catenin siRNA. (A) Western blot analysis was performed using antibodies as indicated. (B) MUC2, villin and KRT20 mRNA expression was determined by real-time RT-PCR (n=3, data represent mean ± SD; *p<0.01 vs control siRNA). C and D. HT29 and LS174T cells transfected with control siRNA or β-catenin siRNA was assessed for oxygen consumption using the Seahorse Bioscience XFe96 extracellular flux analyzer. The normalized OCR was shown. (n=10, data represent mean± S.D.; *P <0.05 compared with control siRNA). E. HT29 cells, transfected with control or β-catenin siRNA, were treated with oligomycin (1 μM) for 48 h. MUC2, villin and KRT20 mRNA levels were determined by real-time RT-PCR (E). (n=3, data represent mean ± SD; *p<0.05 vs con siRNA; #p<0.05 vs β-catenin siRNA).
Given that an increase in OXPHOS activity contributes to CRC cell differentiation (Fig. 2), we next determined the effect of Wnt/β-catenin signaling inhibition on mitochondrial oxidative activity in CRC cells with β-catenin knockdown. As shown in Fig. 6C&D, knockdown of β-catenin increased mitochondrial oxidative activity as noted by increased OCR in HT29 and LS174T cells. Importantly, knockdown of β-catenin increased differentiation as shown by increased MUC2, villin, and KRT20 in HT29 cells; these increases were attenuated by treatment with oligomycin, which inhibits OXPHOS (Fig. 6E). Together, these results demonstrate that Wnt/β-catenin inhibits CRC cell differentiation through the inhibition of mitochondrial oxidative activity.
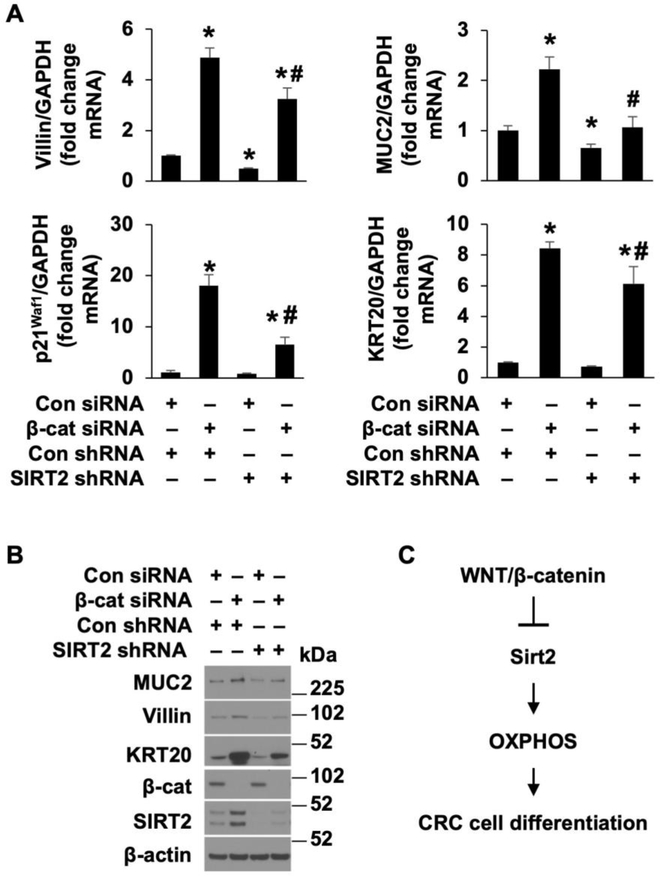
To determine if SIRT2 participates in the regulation of CRC cell differentiation mediated by Wnt/β-catenin signaling, HT29 cells stably transfected with SIRT2 or control shRNA were subsequently transfected with siRNA directed to β-catenin or control and the levels of CRC differentiation markers were determined (Fig. 7 A&B). Knockdown of β-catenin increased differentiation as shown by increased MUC2, villin, KRT20 and p21Waf1 mRNA (Fig. 7A) and MUC2, villin, and KRT20 protein expression (Fig. 7B); these increases were repressed by knockdown of SIRT2, demonstrating a role of SIRT2 in Wnt/β-catenin regulation of CRC cell differentiation. The role of SIRT2 in CRC differentiation, mediated by Wnt/β-catenin signaling, has been further confirmed using another shRNA sequence targeting human SIRT2 (Supplemental Fig. 5 A&B). To further demonstrate the role of SIRT2, HT29 cells transfected with control or β-catenin siRNA were treated with the SIRT2 inhibitor AGK2 and CRC cell differentiation was determined. In agreement with our SIRT2 knockdown results, treatment with AGK2 attenuated the increased differentiation mediated by β-catenin knockdown (Supplemental Fig. 6 A&B). Taken together, our results demonstrate a novel role of SIRT2, acting as a downstream target of Wnt/β-catenin, in the regulation of mitochondrial oxidative activity and differentiation of CRC cells (Fig. 7C).
Figure 7. β-catenin regulation of CRC cell differentiation through, at least in part, the regulation of SIRT2 expression.
HT29 cells, stably transfected with control or SIRT2 shRNA, were transfected with control or β-catenin siRNA. A and B. Forty-eight h after transfection, cells were lysed and total RNA was extracted and villin, MUC2, p21Waf1 and KRT20 mRNA levels were determined by real-time RT-PCR (A). (n=3, data represent mean ± SD; *p<0.05 vs con siRNA; #p<0.05 vs β-catenin siRNA). Western blot analysis using antibodies as indicated (B). C. Schematic diagram summarizing the overall findings.
4. DISCUSSION
Wnt/β-catenin signaling drives CRC growth. We and others have shown that inhibition of Wnt/β-catenin results in the inhibition of CRC growth [1, 41, 42]. In this study, we demonstrate that SIRT2 is a novel target gene of the Wnt/β-catenin pathway in CRC cells. SIRT2 induces differentiation in CRC cells through increasing OXPHOS activity. Moreover, we found that inhibition of Wnt/β-catenin increases OXPHOS activity and CRC cell differentiation; this increase was mediated, at least in part, through the induction of SIRT2 in CRC cells.
In our current study, we found that SIRT2 contributes to the differentiation of CRCs. In agreement with these findings, we have shown that SIRT2 contributes to normal intestinal cell differentiation [17]. In addition, upregulation of SIRT2 contributes to human pluripotent stem cell differentiation [15]. Induction of cancer cell differentiation is an effective strategy for the treatment of cancers including CRCs [43–45]. APC restoration results in the inhibition of Wnt signaling and induction of CRC cell differentiation [5]. Results from our laboratory have showed that Wnt signaling pathway plays an important role in the regulation of intestinal cell proliferation and differentiation [24, 46, 47]. SIRT2 deficiency can result in chromosome alterations and subsequent tumor development in the liver [12, 13]. Moreover, SIRT2 plays an important role in growth suppression in tumors including CRC [12–14]. Together, these results suggest that SIRT2-mediated CRC cell differentiation contributes to its role in CRC growth suppression.
We showed that SIRT2 regulates OXPHOS; inhibition of OXPHOS inhibits CRC cell differentiation. Our results suggest that SIRT2 regulates CRC cell differentiation through regulation of OXPHOS in intestinal cells. Mitochondrial OXPHOS drives normal intestinal cell differentiation and crypt formation [21]. Recent findings have shown that mitochondrial OXPHOS activates reactive oxygen species and the p38 MAPK signaling pathway to induce intestinal crypt cellular differentiation [21]. In agreement with the contributory role of SIRT2 in CRC cell differentiation, we showed that OXPHOS was decreased with SIRT2 knockdown and increased with SIRT2 overexpression. In support of our findings, knockdown of SIRT2 resulted in significantly decreased OXPHOS and increased glycolysis in human fibroblasts [15]. In contrast, inhibition of SIRT2 increased OXPHOS in non-small cell lung cancer [48]. Sirtuin proteins have been shown to differentially regulate OXPHOS activity [49]. For example, SIRT3 interacts with PDHA1 and activates OXPHOS activity by alterations in protein acetylation [50]. In contrast, SIRT4 acts as a cellular lipoamidase that inhibits OXPHOS activity [49, 51]. The distinct functional roles of sirtuin proteins in the regulation of OXPHOS may reflect variation in the expression levels or activity of metabolic enzymes in various type of cells.
To our knowledge, we are the first to show that β-catenin directly binds to the SIRT2 promoter and transcriptionally inhibits SIRT2 expression. SIRT2 has shown distinct effects on Wnt/β-catenin signaling. For example, SIRT2 activates Wnt/β-catenin in hepatocellular carcinomas [52, 53] but inhibits Wnt/β-catenin signaling in other cell types [54–56]. Moreover, we and others showed that SIRT2 negatively regulates Wnt/β-catenin signaling in intestinal cells [56]. Deletion of SIRT2 in mouse fibroblasts increased β-catenin acetylation and Wnt activation [56]. Together, these results suggest that SIRT2 can be an upstream regulator or a downstream target of Wnt/β-catenin, thus demonstrating a cross-talk between SIRT2 and Wnt. Although we have previously shown that SIRT2 inhibits Wnt signaling through deacetylation of β-catenin, which results in the destabilization of β-catenin protein in normal intestinal epithelial cells, we did not find the inhibitory effects of SIRT2 on Wnt/β-catenin signaling in the CRC cells used in this study (Li et al. unpublished data). These results suggest a differential interaction between SIRT2 and Wnt/β-catenin in normal and malignant cells.
We showed that SIRT2 is a novel target gene of Wnt/β-catenin and contributes to the differentiation of CRCs. SIRT2 plays a critical role in the regulation of mitosis and genome integrity; SIRT2-deficient mice develop mammary tumors or hepatocellular carcinoma [13]. Consistent with the enhanced intestinal growth noted with SIRT2 deficiency, SIRT2 plays an important role in growth suppression of tumors including CRC [12–14]. Moreover, downregulated SIRT2 is noted in CRC biopsy samples compared with the adjacent non-cancerous tissues [57]. Although our results showed an inhibitory role for Wnt/β-catenin in the regulation of SIRT2 expression in CRC cell lines, whether Wnt/β-catenin also inhibits SIRT2 expression in CRC tumors in vivo remains to be further verified. Nevertheless, our current study demonstrates a novel role for SIRT2, acting as a downstream target Wnt/β-catenin signaling, in the regulation of CRC cell differentiation. These findings shed new light on how SIRT2 functions as a tumor suppressor and highlight its potential value in CRC treatment.
Supplementary Material
HIGHLIGHTS.
SIRT2, acting as a downstream target of Wnt/β-catenin signaling, contributes to the differentiation of colorectal cancer cells.
ACKNOWLEDGEMENTS
The authors thank Donna Gilbreath for manuscript preparation; the Redox Metabolism, and Biostatistics and Bioinformatics Shared Resource Facilities of the University of Kentucky Markey Cancer Center (supported by National Cancer Institute grant P30 CA177558).
FUNDING: This work was supported by the National Institutes of Health [grant numbers R01 DK048498 and P30 CA177558 to BME].
ABBREVIATIONS
- SIRT
sirtuin
- MUC2
mucin2
- IAP
intestinal alkaline phosphatase
- KRT20
keratin 20
- OXPHOS
oxidative phosphorylation
- OCR
oxygen consumption rate
- NaBT
sodium butyrate
Footnotes
DISCLOSURES: The authors have no potential conflicts of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. REFERENCES
- [1].Bahrami A, Amerizadeh F, ShahidSales S, Khazaei M, Ghayour-Mobarhan M, Sadeghnia HR, Maftouh M, Hassanian SM, Avan A, Therapeutic Potential of Targeting Wnt/beta-Catenin Pathway in Treatment of Colorectal Cancer: Rational and Progress, Journal of cellular biochemistry, (2017). [DOI] [PubMed] [Google Scholar]
- [2].Schatoff EM, Leach BI, Dow LE, Wnt Signaling and Colorectal Cancer, Current colorectal cancer reports, 13 (2017) 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gordon MD, Nusse R, Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors, J Biol Chem, 281 (2006) 22429–22433. [DOI] [PubMed] [Google Scholar]
- [4].Nusse R, Clevers H, Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities, Cell, 169 (2017) 985–999. [DOI] [PubMed] [Google Scholar]
- [5].Dow LE, O’Rourke KP, Simon J, Tschaharganeh DF, van Es JH, Clevers H, Lowe SW, Apc Restoration Promotes Cellular Differentiation and Reestablishes Crypt Homeostasis in Colorectal Cancer, Cell, 161 (2015) 1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Prossomariti A, Piazzi G, Alquati C, Ricciardiello L, Are Wnt/beta-catenin and PI3K/AKT/mTORC1 distinct pathways in colorectal cancer?, Cell Mol Gastroenterol Hepatol, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Feldman JL, Dittenhafer-Reed KE, Denu JM, Sirtuin catalysis and regulation, J Biol Chem, 287 (2012) 42419–42427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fang Y, Tang S, Li X, Sirtuins in Metabolic and Epigenetic Regulation of Stem Cells, Trends in endocrinology and metabolism: TEM, 30 (2019) 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kitada M, Ogura Y, Monno I, Koya D, Sirtuins and Type 2 Diabetes: Role in Inflammation, Oxidative Stress, and Mitochondrial Function, Front Endocrinol (Lausanne), 10 (2019) 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Eldridge MJG, Pereira JM, Impens F, Hamon MA, Active nuclear import of the deacetylase Sirtuin-2 is controlled by its C-terminus and importins, Sci Rep, 10 (2020) 2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang Y, Yang J, Hong T, Chen X, Cui L, SIRT2: Controversy and multiple roles in disease and physiology, Ageing Res Rev, 55 (2019) 100961. [DOI] [PubMed] [Google Scholar]
- [12].Park SH, Zhu Y, Ozden O, Kim HS, Jiang H, Deng CX, Gius D, Vassilopoulos A, SIRT2 is a tumor suppressor that connects aging, acetylome, cell cycle signaling, and carcinogenesis, Transl Cancer Res, 1 (2012) 15–21. [PMC free article] [PubMed] [Google Scholar]
- [13].Kim HS, Vassilopoulos A, Wang RH, Lahusen T, Xiao Z, Xu X, Li C, Veenstra TD, Li B, Yu H, Ji J, Wang XW, Park SH, Cha YI, Gius D, Deng CX, SIRT2 maintains genome integrity and suppresses tumorigenesis through regulating APC/C activity, Cancer Cell, 20 (2011) 487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang B, Ye Y, Yang X, Liu B, Wang Z, Chen S, Jiang K, Zhang W, Jiang H, Mustonen H, Puolakkainen P, Wang S, Luo J, Shen Z, SIRT2-dependent IDH1 deacetylation inhibits colorectal cancer and liver metastases, EMBO Rep, 21 (2020) e48183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cha Y, Han MJ, Cha HJ, Zoldan J, Burkart A, Jung JH, Jang Y, Kim CH, Jeong HC, Kim BG, Langer R, Kahn CR, Guarente L, Kim KS, Metabolic control of primed human pluripotent stem cell fate and function by the miR-200c-SIRT2 axis, Nat Cell Biol, 19 (2017) 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lo Sasso G, Menzies KJ, Mottis A, Piersigilli A, Perino A, Yamamoto H, Schoonjans K, Auwerx J, SIRT2 deficiency modulates macrophage polarization and susceptibility to experimental colitis, PLoS One, 9 (2014) e103573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li C, Zhou Y, Rychahou P, Weiss HL, Lee EY, Perry CL, Barrett TA, Wang Q, Evers BM, SIRT2 Contributes to the Regulation of Intestinal Cell Proliferation and Differentiation, Cellular and molecular gastroenterology and hepatology, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Krimpenfort P, Berns A, Wnt Down, Tumors Wind Up?, Cell, 161 (2015) 1494–1496. [DOI] [PubMed] [Google Scholar]
- [19].Bruschi M, Garnier L, Cleroux E, Giordano A, Dumas M, Bardet AF, Kergrohen T, Quesada S, Cesses P, Weber M, Gerbe F, Jay P, Loss of APC Rapidly Impairs DNA Methylation Programs and Cell Fate Decisions in LGR5+ Intestinal Stem Cells, Cancer Res, (2020). [DOI] [PubMed] [Google Scholar]
- [20].Dow LE, O’Rourke KP, Simon J, Tschaharganeh DF, van Es JH, Clevers H, Lowe SW, Apc Restoration Promotes Cellular Differentiation and Reestablishes Crypt Homeostasis in Colorectal Cancer, Cell, 161 (2015) 1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rodriguez-Colman MJ, Schewe M, Meerlo M, Stigter E, Gerrits J, Pras-Raves M, Sacchetti A, Hornsveld M, Oost KC, Snippert HJ, Verhoeven-Duif N, Fodde R, Burgering BM, Interplay between metabolic identities in the intestinal crypt supports stem cell function, Nature, 543 (2017) 424–427. [DOI] [PubMed] [Google Scholar]
- [22].Sanaei M, Kavoosi F, Histone Deacetylases and Histone Deacetylase Inhibitors: Molecular Mechanisms of Action in Various Cancers, Adv Biomed Res, 8 (2019) 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang Q, Ji Y, Wang X, Evers BM, Isolation and molecular characterization of the 5’-upstream region of the human TRAIL gene, Biochem Biophys Res Commun, 276 (2000) 466–471. [DOI] [PubMed] [Google Scholar]
- [24].Wang Q, Zhou Y, Rychahou P, Harris JW, Zaytseva YY, Liu J, Wang C, Weiss HL, Liu C, Lee EY, Evers BM, Deptor Is a Novel Target of Wnt/beta-Catenin/c-Myc and Contributes to Colorectal Cancer Cell Growth, Cancer Res, 78 (2018) 3163–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang Q, Zhou Y, Rychahou P, Fan TW, Lane AN, Weiss HL, Evers BM, Ketogenesis contributes to intestinal cell differentiation, Cell Death Differ, 24 (2017) 458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wen YA, Xiong X, Zaytseva YY, Napier DL, Vallee E, Li AT, Wang C, Weiss HL, Evers BM, Gao T, Downregulation of SREBP inhibits tumor growth and initiation by altering cellular metabolism in colon cancer, Cell death & disease, 9 (2018) 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cheng Y, He C, Wang M, Ma X, Mo F, Yang S, Han J, Wei X, Targeting epigenetic regulators for cancer therapy: mechanisms and advances in clinical trials, Signal Transduct Target Ther, 4 (2019) 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang Q, Wang X, Hernandez A, Kim S, Evers BM, Inhibition of the phosphatidylinositol 3-kinase pathway contributes to HT29 and Caco-2 intestinal cell differentiation, Gastroenterology, 120 (2001) 1381–1392. [DOI] [PubMed] [Google Scholar]
- [29].Wang Q, Li N, Wang X, Kim MM, Evers BM, Augmentation of sodium butyrate-induced apoptosis by phosphatidylinositol 3’-kinase inhibition in the KM20 human colon cancer cell line, Clin Cancer Res, 8 (2002) 1940–1947. [PubMed] [Google Scholar]
- [30].Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet JC, McLean PJ, Young AB, Abagyan R, Feany MB, Hyman BT, Kazantsev AG, Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease, Science (New York, N.Y.), 317 (2007) 516–519. [DOI] [PubMed] [Google Scholar]
- [31].Augenlicht L, Shi L, Mariadason J, Laboisse C, Velcich A, Repression of MUC2 gene expression by butyrate, a physiological regulator of intestinal cell maturation, Oncogene, 22 (2003) 4983–4992. [DOI] [PubMed] [Google Scholar]
- [32].Takahashi-Yanaga F, Kahn M, Targeting Wnt signaling: can we safely eradicate cancer stem cells?, Clin Cancer Res, 16 (2010) 3153–3162. [DOI] [PubMed] [Google Scholar]
- [33].Handeli S, Simon JA, A small-molecule inhibitor of Tcf/beta-catenin signaling down-regulates PPARgamma and PPARdelta activities, Mol Cancer Ther, 7 (2008) 521–529. [DOI] [PubMed] [Google Scholar]
- [34].Caspi M, Zilberberg A, Eldar-Finkelman H, Rosin-Arbesfeld R, Nuclear GSK-3beta inhibits the canonical Wnt signalling pathway in a beta-catenin phosphorylation-independent manner, Oncogene, 27 (2008) 3546–3555. [DOI] [PubMed] [Google Scholar]
- [35].Vidal F, de Araujo WM, Cruz AL, Tanaka MN, Viola JP, Morgado-Diaz JA, Lithium reduces tumorigenic potential in response to EGF signaling in human colorectal cancer cells, Int J Oncol, 38 (2011) 1365–1373. [DOI] [PubMed] [Google Scholar]
- [36].Clevers H, Wnt/beta-catenin signaling in development and disease, Cell, 127 (2006) 469–480. [DOI] [PubMed] [Google Scholar]
- [37].Yochum GS, McWeeney S, Rajaraman V, Cleland R, Peters S, Goodman RH, Serial analysis of chromatin occupancy identifies beta-catenin target genes in colorectal carcinoma cells, Proc Natl Acad Sci U S A, 104 (2007) 3324–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cristofaro M, Contursi A, D’Amore S, Martelli N, Spaziante AF, Moschetta A, Villani G, Adenomatous polyposis coli (APC)-induced apoptosis of HT29 colorectal cancer cells depends on mitochondrial oxidative metabolism, Biochimica et biophysica acta, 1852 (2015) 1719–1728. [DOI] [PubMed] [Google Scholar]
- [39].de Lau W, Barker N, Clevers H, WNT signaling in the normal intestine and colorectal cancer, Front Biosci, 12 (2007) 471–491. [DOI] [PubMed] [Google Scholar]
- [40].Lecarpentier Y, Schussler O, Hebert JL, Vallee A, Multiple Targets of the Canonical WNT/beta-Catenin Signaling in Cancers, Front Oncol, 9 (2019) 1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhang W, Chen X, Kato Y, Evans PM, Yuan S, Yang J, Rychahou PG, Yang VW, He X, Evers BM, Liu C, Novel cross talk of Kruppel-like factor 4 and beta-catenin regulates normal intestinal homeostasis and tumor repression, Molecular and cellular biology, 26 (2006) 2055–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang W, Sviripa V, Kril LM, Chen X, Yu T, Shi J, Rychahou P, Evers BM, Watt DS, Liu C, Fluorinated N,N-dialkylaminostilbenes for Wnt pathway inhibition and colon cancer repression, Journal of medicinal chemistry, 54 (2011) 1288–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].de The H, Differentiation therapy revisited, Nat Rev Cancer, 18 (2018) 117–127. [DOI] [PubMed] [Google Scholar]
- [44].Yan M, Liu Q, Differentiation therapy: a promising strategy for cancer treatment, Chin J Cancer, 35 (2016) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cruz FD, Matushansky I, Solid tumor differentiation therapy - is it possible?, Oncotarget, 3 (2012) 559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kim JT, Li C, Weiss HL, Zhou Y, Liu C, Wang Q, Evers BM, Regulation of Ketogenic Enzyme HMGCS2 by Wnt/beta-catenin/PPARgamma Pathway in Intestinal Cells, Cells, 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wang Q, Zhou Y, Rychahou P, Liu C, Weiss HL, Evers BM, NFAT5 represses canonical Wnt signaling via inhibition of beta-catenin acetylation and participates in regulating intestinal cell differentiation, Cell death & disease, 4 (2013) e671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ma W, Zhao X, Wang K, Liu J, Huang G, Dichloroacetic acid (DCA) synergizes with the SIRT2 inhibitor Sirtinol and AGK2 to enhance anti-tumor efficacy in non-small cell lung cancer, Cancer Biol Ther, 19 (2018) 835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zhu S, Dong Z, Ke X, Hou J, Zhao E, Zhang K, Wang F, Yang L, Xiang Z, Cui H, The roles of sirtuins family in cell metabolism during tumor development, Semin Cancer Biol, 57 (2019) 59–71. [DOI] [PubMed] [Google Scholar]
- [50].Ozden O, Park SH, Wagner BA, Song HY, Zhu Y, Vassilopoulos A, Jung B, Buettner GR, Gius D, SIRT3 deacetylates and increases pyruvate dehydrogenase activity in cancer cells, Free Radic Biol Med, 76 (2014) 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mathias RA, Greco TM, Oberstein A, Budayeva HG, Chakrabarti R, Rowland EA, Kang Y, Shenk T, Cristea IM, Sirtuin 4 is a lipoamidase regulating pyruvate dehydrogenase complex activity, Cell, 159 (2014) 1615–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Chen J, Chan AW, To KF, Chen W, Zhang Z, Ren J, Song C, Cheung YS, Lai PB, Cheng SH, Ng MH, Huang A, Ko BC, SIRT2 overexpression in hepatocellular carcinoma mediates epithelial to mesenchymal transition by protein kinase B/glycogen synthase kinase-3beta/beta-catenin signaling, Hepatology (Baltimore, Md.), 57 (2013) 2287–2298. [DOI] [PubMed] [Google Scholar]
- [53].Piracha ZZ, Kwon H, Saeed U, Kim J, Jung J, Chwae YJ, Park S, Shin HJ, Kim K, Sirtuin 2 Isoform 1 Enhances Hepatitis B Virus RNA Transcription and DNA Synthesis through the AKT/GSK-3beta/beta-Catenin Signaling Pathway, Journal of virology, 92 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [54].Zhao Y, Yu T, Zhang N, Chen J, Zhang P, Li S, Luo L, Cui Z, Qin Y, Liu F, Nuclear E-Cadherin Acetylation Promotes Colorectal Tumorigenesis via Enhancing beta-Catenin Activity, Molecular cancer research : MCR, 17 (2019) 655–665. [DOI] [PubMed] [Google Scholar]
- [55].Sarikhani M, Mishra S, Maity S, Kotyada C, Wolfgeher D, Gupta MP, Singh M, Sundaresan NR, SIRT2 deacetylase regulates the activity of GSK3 isoforms independent of inhibitory phosphorylation, eLife, 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Nguyen P, Lee S, Lorang-Leins D, Trepel J, Smart DK, SIRT2 interacts with beta-catenin to inhibit Wnt signaling output in response to radiation-induced stress, Molecular cancer research : MCR, 12 (2014) 1244–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zhang LL, Zhan L, Jin YD, Min ZL, Wei C, Wang Q, Chen YJ, Wu QM, Hu XM, Yuan Q, SIRT2 mediated antitumor effects of shikonin on metastatic colorectal cancer, European journal of pharmacology, 797 (2017) 1–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.