Abstract
Objective:
Primary plasma cell leukemia (pPCL) is an aggressive form of multiple myeloma (MM) with dismal overall survival (OS) outcomes. We evaluated the clinical outcomes of pPCL patients defined by ≥5% circulating clonal plasma cells (cPCs) on peripheral blood (PB) smear and treated with novel agent induction therapies.
Patients and Methods:
To evaluates a cohort of 68 pPCL patients diagnosed at the Mayo Clinic in Rochester, MN from January 1, 2000 – December 31, 2019 and treated with novel agent induction therapies.
Results:
The median follow up was 46 months (95% CI: 41 – 90). The median bone marrow plasma cell content was 84% (Range: 10 – 100) and the median cPC% on the PB smear was 23% (range: 5 – 93). There was a preponderance of t(11;14) primary cytogenetic abnormality in this cohort. The median time to next therapy (TTNT) and OS for all pPCL patients in this cohort was 13 months (95% CI: 9 – 17) and 24 months (95% CI: 19 – 40) respectively. However, when stratified by cytogenetic risk, the median TTNT and OS was 16 months and 51 months for standard risk vs. 10 months and 19 months for high risk (P = 0.01 for OS).
Conclusion:
pPCL remains an aggressive disease with poor prognosis despite novel agent-based therapies. Some patients have better than expected survival and this phenomenon may be influenced by the absence of high-risk cytogenetics. Newer treatment regimens are needed to improve the prognosis of this devastating disease.
INTRODUCTION
Primary plasma cell leukemia (pPCL) is an aggressive form of multiple myeloma (MM) that appears in about 2–4% of all newly diagnosed MM patients.1–6 pPCL arises de novo in patients with no history of MM in contrast to secondary PCL (sPCL) where there is a leukemic progression of previously treated MM. The improvement in overall survival (OS) of pPCL has been relatively modest compared to that experienced by MM.7 pPCL is defined by the presence of ≥20% circulating plasma cells (cPCs) on a peripheral blood (PB) smear by morphological assessment and/or an absolute plasma cell count greater than 2 × 109/L.5, 6 The International Myeloma Working Group (IMWG), has recommended that the criteria to define pPCL be less restrictive by including any newly diagnosed MM patient with ≥5% cPCs detected morphologically on a PB smear.8 However, this recommendation has yet to be formally endorsed but is informally recognized by hematologists specializing in plasma cell malignancies. Given the limited data due to the rarity of this disease, we evaluated the disease course, clinical outcomes and cytogenetic features of patients diagnosed with pPCL at our institution with the new proposed definition of pPCL of ≥5% cPCs on PB smear that were treated with novel agent induction therapies.
METHODS
We evaluated all patients with pPCL diagnosed from 2000 – 2019 at the Mayo Clinic in Rochester, MN. The cohort identified for this study was defined as patients with MM who have ≥5% cPCs present on morphological evaluation of their PB smear. Data regarding these pPCL patients were extracted from their electronic medical records with their consent. The study was conducted with approval of the institutional review board and in accordance with the principles of the Helsinki Declaration.
Fluorescent in situ hybridization (FISH) analysis performed on bone marrow aspirates at diagnosis were categorized as having high risk cytogenetics with any of the following abnormalities: t(4;14), t(14;16), t(14;20) and del17p.9 The variables at diagnosis that were recorded and examined for prognostic significance included: age, presence of high-risk cytogenetics by FISH, plasma cell labeling index (PCLI), serum albumin, bone marrow plasma cell percentage (BMPC%), β2-microglobulin, serum and urine M spike, hemoglobin, creatinine and LDH. Hematological responses to therapy were assessed based on the International Myeloma Working Group (IMWG) consensus criteria.10 Time to next therapy (TTNT) and OS were determined in this study. TTNT was calculated from the day of diagnosis to the day of initiating the next systemic therapy due to a documented relapse or progression of disease. Those patients who were either alive or dead but relapse free were censored at the day of last follow up. Furthermore, those patients who started a different therapy due to toxicity and not due to disease progression were censored. OS was calculated from the day of diagnosis to the day of death from any cause with censoring performed at the date of last contact if alive.
Statistical analysis was performed using the SAS biostatistical software JMP 14.0.1 (SAS Institute Inc., Cary, NC). Differences between sub-groups in this study cohort were compared by using either the Chi-square or Fisher exact statistical test. A Kaplan-Meier analysis was used to analyze and create the OS and TTNT curves, and log rank test was used to compare these curves. Finally, univariate and multivariate analysis was performed using the Cox proportional hazards model to assess the influence of various prognostic factors and their ability to predict TTNT and OS.
RESULTS
Patient characteristics
This cohort consisted of 68 patients with pPCL diagnosed between January 1, 2000 to December 31, 2019. The median age was 62 years (range: 34–91) and 33 (49%) were male. The median follow up was 46 months (95% CI: 29 – 90). At diagnosis, the median bone marrow plasma cell percentage (BMPC%) involvement was 85% (Range: 10 – 100) and the median cPC% detected by morphological evaluation of their PB smear was 26% (range: 5 – 93). There were 46 (68%) patients with ≥20% cPCs on their PB smear evaluations. Only 23 (32%) patients had concurrent information on the number of cPCs in their PB assessed by multiparametric flow cytometry at diagnosis and the median number of cPCs detected was 32,807 per 150,000 events analyzed (Range: 354 – 132,256). A total of 17/55 (31%) patients for whom baseline serum calcium data was available presented with hypercalcemia and a total of 15/53 (28%) patients for whom baseline serum creatinine data was available presented with a serum creatinine of 2 mg/dL or greater. A total of 23/43 (54%) patients who had their baseline platelet count known had levels less than 100 ×10(9)/L at diagnosis. There were six patients who had evidence of extramedullary lesions at diagnosis of which five were identified on cross sectional imaging and one presented with cutaneous involvement of the lip.
22/41 (54%) patients for whom baseline data was available presented with an elevated serum LDH level at diagnosis and a total of 10/22 (28%) patients presented with a PCLI% of 2% or greater. Data for ISS classification was available in 50 patients and is as follows: Stage I- 3 (6%) patients, Stage II- 7 (14%) patients and Stage III- 40 (80%) patients. Data on the presence of primary cytogenetic abnormalities were available in 58 (85%) patients of which 29 (50%) had high risk cytogenetics. The distribution of primary cytogenetic abnormalities among this cohort was as follows: t(11;14) – 27 (47%), t(4;14) – 5 (9%), t(14;16) – 7 (12%), t(14;20)- 3 (5%) and del 17p- 16 (28%).
First-line treatment regimens and hematologic response
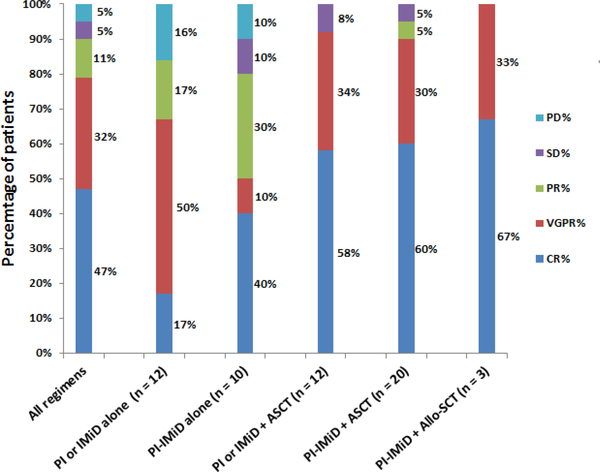
Data regarding first line treatment utilized was available for all 68 patients, with 24 (35%) receiving proteasome inhibitor (PI) alone, 4 (6%) receiving immunomodulator (IMiD) alone and 38 (56%) receiving a combination of PI and IMiD. The individual therapies used inf the first line setting are listed in Supplementary Table 1. All 11 patients who had a relatively more intense induction regimen upfront with a VDT-PACE/VDR-PACE like regimen had 20% or more cPCs detected on their PB smear. The best overall hematological response to first line of therapy was available for only 57 (84%) patients and was 91%. Due to the lack of an accepted response criteria for pPCL, the IMWG-based hematologic response criteria was utilized and their distribution among patients during their first line therapy is detailed in Figure 1 and Supplementary Table 2. The utilization of any form of stem cell transplantation (SCT) [ Autologous SCT(ASCT), tandem ASCT or Allogeneic-SCT (allo-SCT)] as upfront consolidation was associated with higher complete response (CR) to first line therapy compared to not using any form of early SCT (62% vs. 38%; P = 0.03). The presence of high-risk cytogenetics at diagnosis did not affect the probability of achieving a CR or better compared to the presence of standard risk cytogenetics (48% vs. 56%; p = 0.78). Of the 6 patients who experienced either progressive disease or stable disease with their initial induction therapy, 5 patients were switched within 3 months of starting first line therapy to a second line treatment regimen containing an anthracycline in combination with PI (VD-PACE or Velcade-Doxil etc.) and one patient initiated a daratumumab-based salvage regimen.
Figure 1:
Distribution of hematological response categories based on first line therapy.
Stem cell transplant utilization and post-transplant therapies
Of the 61 patients for whom information on sequential therapies were available, a total of 36 (53%) patients received at least one ASCT (3 of which received tandem ASCTs) and 4 (6%) patients underwent an allogeneic stem cell transplant. Of the 36 patients who underwent an ASCT, 34 (96%) were performed in the upfront setting. A total of 29 patients who underwent an ASCT at any time received post-ASCT maintenance therapy as follows: PI alone – 14, IMiD alone – 9 and PI/IMiD combination – 3, other – 3. The 7 patients who did not receive any maintenance therapy had a fulminant hematologic relapse at the time of post-ASCT assessments requiring salvage chemotherapy.
First line treatment duration and overall survival outcomes
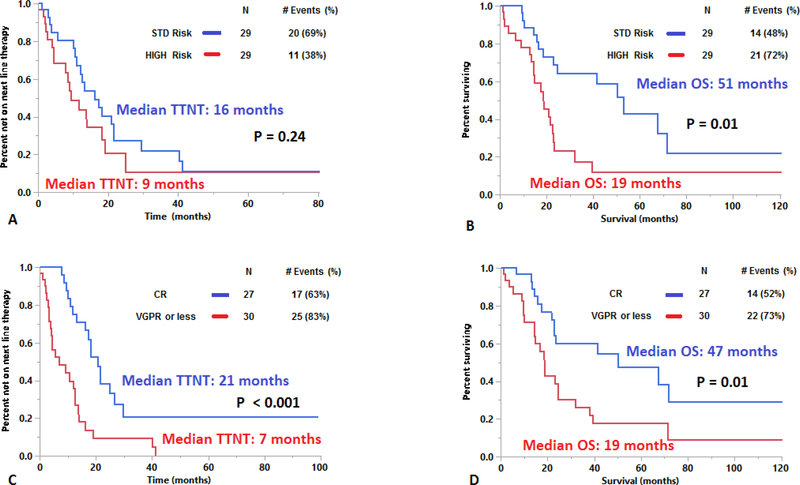
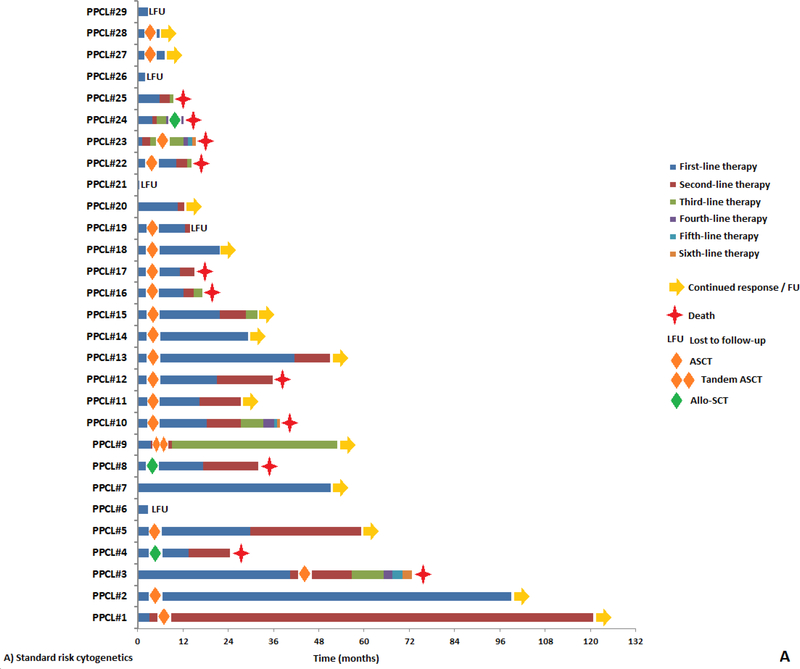
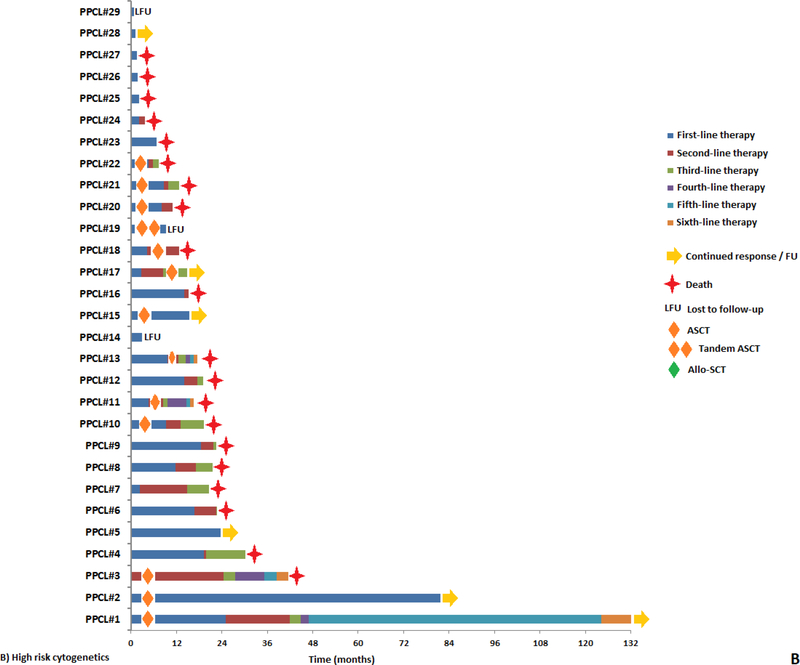
The median TTNT after first line therapy was 13 months (95% CI: 9 – 17). The median overall survival (OS) for this cohort was 23 months (95% CI: 19 – 38). The TTNT and OS was similar in patients with 5–19% cPCs vs. ≥20% cPCs (TTNT: 16 vs. 12 months, P = 0.93 and OS: 25 vs. 21 months, P = 0.72; Supplementary Figure 1A and 1B). When stratifying by cytogenetic risk, the median TTNT after first line therapy was 16 months for those with standard risk cytogenetics compared to 9 months for those with high risk cytogenetics (P = 0.24; Figure 2A). However, the median OS was 51 months for standard risk cytogenetics vs. 19 months for high risk cytogenetics (P = 0.01; Figure 2B). When stratifying by the presence of a gain of chromosome 1q by FISH, the median TTNT after first line therapy was 13 months for those without a gain of chromosome 1q compared to 14 months for those with the presence of a gain of chromosome 1q (P = 0.82). Whereas the median OS was 32 months for those without a gain of chromosome 1q vs. 19 months for the presence of a gain of chromosome 1q (P = 0.27). When stratifying by the presence of a t(11;14) by FISH, the median TTNT after first line therapy for patients with a) standard risk cytogenetics without t(11;14), b) high risk cytogenetics and without t(11;14), c) standard risk cytogenetics with t(11;14), d) high risk cytogenetics with t(11;14) were 13, 10, 17 and 19 months respectively (P = 0.28). Whereas the median OS for patients with a) standard risk cytogenetics without t(11;14), b) high risk cytogenetics without t(11;14), c) standard risk cytogenetics with t(11;14) and d) high risk cytogenetics with t(11;14) were 60, 19, 47 and 12 months respectively (P = 0.01). The TTNT after the first line therapy for patients who experienced a CR to first line therapy was 21 months compared to 7 months in patients whose response was a very good partial response (VGPR) or less (P < 0.001; Figure 2C). Similarly, the OS for patients who experienced a CR to first line therapy was 47 months compared to 19 months in patients whose response was a VGPR or less (P = 0.01; Figure 2D). Among the 40 (59%) patients who were not alive at the time of analysis but who had sequential treatment information, the median number of lines of therapy received during their disease course was 3 (range: 1 – 9). The duration of response to each subsequent line of therapy based on cytogenetic risk at diagnosis is depicted in Figure 3 (A and B). The rate of early mortality defined by survival under 1 month from diagnosis was 4.6% (3 of 65 patients).
Figure 2:
Kaplan-Meier plot evaluating a) TTNT and b) OS of pPCL patients based on baseline cytogenetic risk and c) TTNT and d) OS of pPCL patients based on achievement of a CR to first line therapy.
Figure 3:
Swimmer plot demonstrating duration of sequential lines of therapy in pPCL patients with a) standard-risk cytogenetics and b) high-risk cytogenetics.
Since t(11;14) cytogenetic abnormality was present in a little less than 50% of all pPCL patients in this cohort, its prognostic significance was evaluated. The TTNT and OS was numerically better for patients with a t(11;14) cytogenetic abnormality which was 16 months and 40 months in comparison to 12 months and 21 months in those without this abnormality; however, neither results were statistically significant (TTNT: P= 0.47; OS: P = 0.52). Among 22 patients who had information on their PCLI% at diagnosis, the TTNT and OS of patients with a PCLI > 2% was numerically worse at 10 months and 17 months in comparison to 19 months and 51 months in those with a PCLI ≤ 2%; however, these results were not statistically significant (TTNT: P= 0.62; OS: P = 0.08). Among the following variables: age of 75 years and older, achieving a CR as the best hematological response with first line therapy, the presence of high risk cytogenetics by FISH, presence of an elevated serum LDH level at diagnosis, a platelets count of less than 100,000 at diagnosis and a PCLI% greater than 2%, only the presence of high-risk cytogenetics by FISH at diagnosis and achieving a CR to first line therapy were found to be prognostic in terms of OS in a univariate analysis and both retained significance in a multivariate analysis (Table 1). Whereas only achieving a CR as the best hematological response with first line therapy was found to be prognostic in terms of TTNT.
Table 1:
Univariate and multivariate analysis of clinical and laboratory characteristics predictive of OS and TTNT.
| Characteristic | Overall Survival (OS) | Time to next therapy (TTNT) | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||
| HR (95% CI) | P – value | HR (95% CI) | P – value | HR (95% CI) | P – value | HR (95% CI) | P – value | |
| Age ≥ 75 | 1.68 (0.77 – 3.64) | 0.19 | – | – | 0.57 (0.20 – 1.60) | 0.29 | – | – |
| CR w/1st line therapy | 0.43 (0.22 – 0.83) | 0.01 | 0.47 (0.23 – 0.96) | 0.04 | 0.29 (0.16 – 0.55) | < 0.001 | 0.29 (0.16 – 0.55) | < 0.001 |
| High risk cytogenetics | 2.66 (1.35 – 5.24) | 0.01 | 2.95 (1.37 – 6.26) | 0.01 | 1.54 (0.81 – 2.91) | 0.19 | – | – |
| Elevated serum LDH | 0.67 (0.30 – 1.49) | 0.33 | – | – | 0.76 (0.35 – 1.62) | 0.47 | – | – |
| PCLI > 2% | 2.62 (0.92 – 7.55) | 0.07 | – | – | 1.31 (0.45 – 3.85) | 0.62 | – | – |
| Platelets < 100k | 1.40 (0.67 – 2.93) | 0.38 | – | – | 1.45 (0.71 – 2.97) | 0.31 | – | – |
| ≥20% cPCs by PB smear | 1.12 (0.60 – 2.08) | 0.72 | – | – | 0.97 (0.54 – 1.76) | 0.93 | – | – |
Characteristics of patients with longer than expected survival
There were 6 (9%) patients who have not relapsed after first line therapy and who have had at least 12 months of follow up at the time of this analysis (median follow up: 40 months (95% CI: 15 – 90)). There were 14 (21%) patients in this cohort who were alive for 48 months or longer since diagnosis (i.e. about twice the median OS of this cohort). The induction regimens used were variable and included bortezomib-lenalidomide-dexamethasone (N = 4), cyclophosphamide-bortezomib-dexamethasone (N = 4), bortezomib, thalidomide or lenalidomide, dexamethasone, cisplatin, adriamycin, cyclophosphamide and etoposide (N = 2), thalidomide and dexamethasone (N = 1), carfilzomib, lenalidomide and dexamethasone (N = 1), elotuzumab, bortezomib, lenalidomide and dexamethasone (N = 1) and cyclophosphamide, lenalidomide and dexamethasone (N = 1). Only the absence of high risk cytogenetics (P = 0.049), achievement of at least CR as best hematological response to the first line of therapy (P = 0.046) and receiving any form of SCT (ASCT, tandem ASCT or allo-SCT) during the disease course (P = 0.02) were predictors for an OS of 48 months or longer.
DISCUSSION
The survival outcomes of pPCL patients in the US have been poor with only modest improvement over the last decade despite the incorporation of novel agents such as PIs, IMiDs, and ASCT into clinical practice.7 This in-depth, patient-level review of all cases of pPCL diagnosed in the last 20 years and treated with novel agent-based induction therapy supports the aforementioned observation since the median OS of this cohort was still under 2 years. However, this study also demonstrates that the survival outcomes of pPCL patients can be heterogeneous as a little over one-fourth of this cohort had survived for 4 years or longer which is twice the median OS for the entire cohort. This is supported by a recent multi-institutional study demonstrating the different outcomes in patients with pPCL based on baseline age, platelet counts and levels of cPCs in the PB.11 Furthermore, even though the improvement in OS of patients with pPCL lags behind that of MM, our cohort had an early mortality (within 1 month of diagnosis) of under 5% in comparison to population-based registry data suggesting levels as high as 15% from 2006 – 2009.7 Referral and selection bias associated with tertiary referral centers likely has a some role to play in this aforementioned observation of low early mortality in addition to improved therapies.
The strongest predictor of better than expected OS outcomes in this study appears to be cytogenetic risk at diagnosis. Achieving a CR as the best hematologic response to first line therapy was also a strong predictor of better than expected OS outcomes and it was independent of the presence of standard risk cytogenetics at diagnosis in a multivariate analysis. While it is not possible to determine if achieving a CR is just reflective of less aggressive disease biology, the likelihood of achieving a CR as the best hematologic response to first line therapy was at least not associated with the cytogenetic risk at diagnosis. Nevertheless, given that achievement of a CR has been known to be especially beneficial to those patients with high risk disease biology12, it is plausible that such deep hematological responses are critical to achieve in pPCL patients also.
As a result, a more intensive treatment approach with novel agent induction therapy followed by consolidation and maintenance is an accepted standard of management for patients with pPCL.8, 13, 14 The phase II IFM study that utilized bortezomib-based induction regimens followed by consolidation with either a tandem ASCT followed by novel agent maintenance therapy or an ASCT followed by a reduced-intensity conditioning allograft (RIC-allo) has the longest reported median OS reported at 36.3 months with one-third of the study cohort having a best hematological response of a CR or better to this first line of therapy.15 The ongoing, non-randomized, phase II multicenter (EMN12) study is also evaluating such intensive treatment regimens comprising of induction therapy with carfilzomib, lenalidomide and dexamethasone followed by either consolidation with a tandem ASCT or an ASCT-alloSCT and then carfilzomib maintenance or carfilzomib and lenalidomide doublet maintenance in patients not eligible for SCT.16 Preliminary evaluation of 15 patients shows deep hematologic responses with a ≥VGPR in 80% and ≥CR in 33%.16 This study demonstrated a CR or better rate of 61% in the 23 patients treated with a combination of a PI/IMiD as induction therapy and consolidated with either an ASCT or an allo-SCT which appears to be better than the aforementioned prospective studies. However, one of the major challenges in pPCL including conventional high-risk MM that remains is to sustain the deep hematological responses for a considerable duration. Recent experiences have suggested that continuous doublet or triplet therapy carried over from induction devoid or with minimal use of corticosteroids as an extended or indefinite maintenance may be a critical approach to maintaining hematologic responses.17 Incorporation of cellular adapted therapies may also have a role in sustaining disease control in the future. At our institution, in the absence of a clinical trial, all pPCL patients would be considered to receive a triplet induction therapy regimen combining a PI and IMiD but many may receive more intensive induction regimens such as VDT/R-PACE especially in the setting of high disease burden and extramedullary disease.18 In those patients that are eligible, consolidation with an ASCT is performed and preferably a tandem ASCT. In select younger patients, an allo-SCT may be performed after a thoughtful discussion on the risks and benefits. All patients not transplant eligible or post-ASCT are kept on prolonged maintenance therapy that includes both PI and IMiDs if feasible.18
The current diagnostic criteria for pPCL requiring either ≥20% cPCs and/or an absolute count greater than 2 × 109/L cPCs in the PB limits the experiences and outcomes reported on this entity. With two separate studies demonstrating the similar OS outcomes of patients with 5–19% cPCs in the PB to that of the traditional pPCL patients with ≥20% cPCs, the IMWG has recommended that the diagnostic criteria include newly diagnosed MM patients with ≥5% cPCs.19, 20 Thus, this is the first study to evaluate in detail the clinical characteristics, disease course and outcomes of pPCL patients based on the new ≥5% cPCs cutoff on a PB smear. However, it is important to note that many patients in this cohort who had between 5% to 19% cPCs on their PB smear and were diagnosed prior to the aforementioned publications demonstrating the rationale for considering ≥5% cPCs as the new cutoff were classified as MM rather than pPCL in their clinical records which nevertheless was accurate at that time. As a result, such patients may not have been treated any differently than conventional MM patients rather than with a more chemo-intensive approach. Finally, this study re-confirmed the predisposition the t(11;14) primary cytogenetic abnormality in patients with pPCL.4 This preponderance is ever so important given that t(11;14) serves as a useful biomarker predicting sensitivity to the small molecule bcl-2 inhibitor venetoclax.21, 22 This cytogenetic subset is associated with high bcl-2, and low bcl-XL and MCL-1 mRNA expression, resulting in greater sensitivity to bcl-2 inhibition.23 Several recent case reports have highlighted venetoclax as a promising therapeutic agent in patients with refractory pPCL.24, 25
There are several limitations to our study, the first being its retrospective nature derived from a single institution experience. Second, information on the achievement of minimal residual disease negativity (MRD-) by next generation technology (10−5 or 10−6) is of critical prognostic importance especially in patients with high risk disease.26 The pPCL patients included in this study lacked information on this response end-point. Third, with the advent of immunotherapy as the future of the therapeutic landscape in MM, agents such as chimeric antigen receptor T-cells (CAR-T), bispecific T-cell engagers and antibody-drug conjugates are showing unexpected superior responses in the late line setting.27–29 Thus, the natural history of pPCL both newly diagnosed and in the relapsed setting is set to change in the future. None of the patients in this cohort received any form of aforementioned immunotherapy agents in the relapsed setting. Nevertheless, this study demonstrates the heterogeneous outcomes of patients with pPCL that appears to be driven primarily by baseline cytogenetic risk and possibly by initial depth of hematological response to therapy. These findings have implications on evaluating the role of risk-adapted treatment approaches on the management of patients in pPCL.
Supplementary Material
ACKNOWLEDGEMENTS
Research reported in this publication was supported by grants from the National Cancer Institute of the National Institutes of Health under Award Number K23CA218742. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Finally, this research is also supported in part by the Marion Schwartz Foundation for Multiple Myeloma.
Footnotes
Conflict-of-interest disclosure:
These authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Dimopoulos MA, Palumbo A, Delasalle KB, Alexanian R. Primary plasma cell leukaemia. British Journal of Haematology 1994; 88(4): 754–759. [DOI] [PubMed] [Google Scholar]
- 2.García-Sanz R, Orfão A, González M, Tabernero MD, Bladé J, Moro MJ et al. Primary Plasma Cell Leukemia: Clinical, Immunophenotypic, DNA Ploidy, and Cytogenetic Characteristics. Blood 1999; 93(3): 1032–1037. [PubMed] [Google Scholar]
- 3.Ramsingh G, Mehan P, Luo J, Vij R, Morgensztern D. Primary plasma cell leukemia. Cancer 2009; 115(24): 5734–5739. [DOI] [PubMed] [Google Scholar]
- 4.Tiedemann RE, Gonzalez-Paz N, Kyle RA, Santana-Davila R, Price-Troska T, Van Wier SA et al. Genetic aberrations and survival in plasma cell leukemia. Leukemia 2008; 22(5): 1044–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyle RA, Maldonado JE, Bayrd ED. Plasma cell leukemia. Report on 17 cases. Arch Intern Med 1974; 133(5): 813–8. [DOI] [PubMed] [Google Scholar]
- 6.Noel P, Kyle RA. Plasma cell leukemia: an evaluation of response to therapy. Am J Med 1987; 83(6): 1062–8. [DOI] [PubMed] [Google Scholar]
- 7.Gonsalves WI, Rajkumar SV, Go RS, Dispenzieri A, Gupta V, Singh PP et al. Trends in survival of patients with primary plasma cell leukemia: a population-based analysis. Blood 2014; 124(6): 907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez de Larrea C, Kyle RA, Durie BG, Ludwig H, Usmani S, Vesole DH et al. Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia 2013; 27(4): 780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mikhael JR, Dingli D, Roy V, Reeder CB, Buadi FK, Hayman SR et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines 2013. Mayo Clin Proc 2013; 88(4): 360–76. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 2016; 17(8): e328–e346. [DOI] [PubMed] [Google Scholar]
- 11.Jurczyszyn A, Radocha J, Davila J, Fiala MA, Gozzetti A, Grzasko N et al. Prognostic indicators in primary plasma cell leukaemia: a multicentre retrospective study of 117 patients. Br J Haematol 2018; 180(6): 831–839. [DOI] [PubMed] [Google Scholar]
- 12.Haessler J, Shaughnessy JD Jr., Zhan F, Crowley J, Epstein J, van Rhee F et al. Benefit of complete response in multiple myeloma limited to high-risk subgroup identified by gene expression profiling. Clin Cancer Res 2007; 13(23): 7073–9. [DOI] [PubMed] [Google Scholar]
- 13.van de Donk NW, Lokhorst HM, Anderson KC, Richardson PG. How I treat plasma cell leukemia. Blood 2012; 120(12): 2376–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavriatopoulou M, Musto P, Caers J, Merlini G, Kastritis E, van de Donk N et al. European myeloma network recommendations on diagnosis and management of patients with rare plasma cell dyscrasias. Leukemia 2018; 32(9): 1883–1898. [DOI] [PubMed] [Google Scholar]
- 15.Royer B, Minvielle S, Diouf M, Roussel M, Karlin L, Hulin C et al. Bortezomib, Doxorubicin, Cyclophosphamide, Dexamethasone Induction Followed by Stem Cell Transplantation for Primary Plasma Cell Leukemia: A Prospective Phase II Study of the Intergroupe Francophone du Myelome. J Clin Oncol 2016; 34(18): 2125–32. [DOI] [PubMed] [Google Scholar]
- 16.Van De Donk NWCJ, van der Holt B, Schjesvold FH, Wu KL, Spada S, Broyl A et al. Treatment of Primary Plasma Cell Leukemia with Carfilzomib and Lenalidomide-Based Therapy: Results of the First Interim Analysis of the Phase 2 EMN12/HOVON129 Study. Blood 2019; 134(Supplement_1): 693–693. [Google Scholar]
- 17.Nooka AK, Kaufman JL, Muppidi S, Langston A, Heffner LT, Gleason C et al. Consolidation and maintenance therapy with lenalidomide, bortezomib and dexamethasone (RVD) in high-risk myeloma patients. Leukemia 2014; 28(3): 690–3. [DOI] [PubMed] [Google Scholar]
- 18.Gonsalves WI. Primary Plasma Cell Leukemia: A Practical Approach to Diagnosis and Clinical Management. American Journal of Hematology and Oncology 2017; 13(3): 21–25. [Google Scholar]
- 19.Granell M, Calvo X, Garcia-Guinon A, Escoda L, Abella E, Martinez CM et al. Prognostic impact of circulating plasma cells in patients with multiple myeloma: implications for plasma cell leukemia definition. Haematologica 2017; 102(6): 1099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravi P, Kumar SK, Roeker L, Gonsalves W, Buadi F, Lacy MQ et al. Revised diagnostic criteria for plasma cell leukemia: results of a Mayo Clinic study with comparison of outcomes to multiple myeloma. Blood Cancer J 2018; 8(12): 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar S, Kaufman JL, Gasparetto C, Mikhael J, Vij R, Pegourie B et al. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood 2017; 130(22): 2401–2409. [DOI] [PubMed] [Google Scholar]
- 22.Moreau P, Chanan-Khan A, Roberts AW, Agarwal AB, Facon T, Kumar S et al. Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood 2017; 130(22): 2392–2400. [DOI] [PubMed] [Google Scholar]
- 23.Touzeau C, Maciag P, Amiot M, Moreau P. Targeting Bcl-2 for the treatment of multiple myeloma. Leukemia 2018; 32(9): 1899–1907. [DOI] [PubMed] [Google Scholar]
- 24.Jelinek T, Mihalyova J, Kascak M, Duras J, Popkova T, Benkova K et al. Single-agent venetoclax induces MRD-negative response in relapsed primary plasma cell leukemia with t(11;14). Am J Hematol 2019; 94(1): E35–E37. [DOI] [PubMed] [Google Scholar]
- 25.Gonsalves WI, Buadi FK, Kumar SK. Combination therapy incorporating Bcl-2 inhibition with Venetoclax for the treatment of refractory primary plasma cell leukemia with t (11;14). Eur J Haematol 2018; 100(2): 215–217. [DOI] [PubMed] [Google Scholar]
- 26.Lahuerta JJ, Paiva B, Vidriales MB, Cordon L, Cedena MT, Puig N et al. Depth of Response in Multiple Myeloma: A Pooled Analysis of Three PETHEMA/GEM Clinical Trials. J Clin Oncol 2017; 35(25): 2900–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sidana S, Shah N. CAR T-cell therapy: is it prime time in myeloma? Blood Adv 2019; 3(21): 3473–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.BiTE Therapy Active in Multiple Myeloma. Cancer Discov 2019; 9(2): 157–158. [DOI] [PubMed] [Google Scholar]
- 29.Cohen AD. Myeloma: next generation immunotherapy. Hematology Am Soc Hematol Educ Program 2019; 2019(1): 266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.