Abstract
A quantitative assessment of Parkinson’s disease (PD) progression is critical for optimizing clinical trials design. Disease progression model was developed using pooled data from the Progression Marker Initiative study and the Incidence of Cognitive Impairment in Cohorts with Longitudinal Evaluation in Parkinson’s Disease study. Age, gender, concomitant medication, and study arms were predictors of baseline. A mutation in the leucine-rich repeat kinase 2 (LRRK2) encoding gene was associated with the disease progression rate. The progression rate in subjects with PD who carried LRRK2 mutation was slightly slower (~0.170 points/month) than that in PD subjects without the mutation (~0.222 points/month). For a non-enriched placebo-controlled clinical trial, approximately 70 subjects/arm would be required to detect a drug effect of 50% reduction in the progression rate with 80% probability. Whereas, 85, 93 and 100 subjects/arm would be required for an enriched clinical trial with 30%, 50% and 70% subjects with LRRK2 mutations, respectively.
Keywords: Parkinson Disease, disease progression, clinical trial simulation
Introduction
Parkinson disease (PD) is a neurodegenerative disorder that mainly affects the motor system. The cause of Parkinson’s disease is unknown but is believed to involve both genetic and environmental factors. Quantifying the variability observed in clinical trials related to factors such as patient demographics and disease conditions is important for designing efficient trials and for patient recruitment.
Genetic forms of PD are generally rare but are of major importance for a better understanding of the pathophysiology of PD. Mutations associated with leucine-rich repeat kinase 2 (LRRK2) gene are the most frequent known cause of late-onset sporadic PD [1–3]. There are more than 50 different missense and nonsense mutations reported in the LRRK2 gene to date [4]. By far, the most frequently studied mutation is G2019S, accounting for up to 40% of cases in patients of Arab descent [5], about 20% of Ashkenazi Jewish patients [3], and 1%–7% of patients of European origin. Results from a cross-sectional study conducted by Alcalay et al [5] suggested slower progression of PD among patients with LRRK2 G2019S mutation compared to patients without the mutation. These findings were confirmed by Saunders et al [6] in a longitudinal follow-up of a large cohort of PD patients. Interestingly, Oosterveld et al [7] reported faster motor progression after 4 years of disease duration for PD LRRK2 G2385R, R1628P or S1647T carriers compared to non-carriers. These divergent findings opened the opportunity to quantify longitudinal progression of PD in carriers of the LRRK2 mutations.
The Unified Parkinson’s Disease Rating Scale (UPDRS) is widely used as a clinical rating scale for PD, where the maximum total UPDRS score indicates the worst possible disability from PD (i.e., 199). A modified version of UPDRS, termed MDS-UPDRS, which detects smaller changes in early PD, has been proposed by the Movement Disorder Society [8, 9]. The MDS-UPDRS retains the core structure of the original scale with reorganization of the subscales by adding several items focused on the early stages of disability (such as differentiation of “slight” vs “mild” deficits). The MDS-UPDRS has a maximum score of 272 and is composed of 4 parts: Part I) Non-Motor Aspects of Experiences of Daily Living (13 items); Part II) Motor Aspects of Experiences of Daily Living (13 items); Part III) Motor Examination (33 items); and Part IV) Motor Complications (6 items). The conversion of UPDRS scores to MDS-UPDRS scores relies on Hoehn and Yahr stages and has been described elsewhere [8, 9].
Venuto et al [10] provided an excellent review on the available longitudinal disease progression models for PD in the literature. However, the models discussed in that review did not consider the bounded nature of UPDRS score. These types of data are typically heteroscedastic, displaying more variability around the mean and less variability around the lower and upper boundary of the scale. Therefore, ignoring the bounded nature of UPDRS score could cause bias in appropriately quantifying the disease progression. Xu et al [11, 12] pioneered the implementation of beta regression models in the context of Nonlinear Mixed Effect Model (NOMEM) to characterize longitudinal progression of Alzheimer’s Disease Assessment Scale – cognitive subscale (ADAS-cog) in Alzheimer’s disease patients. Conrado et al [13] extended the implementation of beta-regression within NONMEM by including third-level random effect (i.e., between-study, between-subject, and residual variability). Rogers et al [14] used a beta regression model in a Bayesian framework to describe the longitudinal progression of Alzheimer’s disease using Alzheimer’s Disease Assessment Scale-cognitive subscale scores. Limitation of beta regression relies on assumption that there are no data at the boundaries and they should be contained within a certain interval. The re-scaling method proposed by Smithson et al. [15] was shown to be an alternative to address these limitations.
The objective of the current analysis were to: 1) characterize disease progression in PD subjects using longitudinal MDS-UPDRS23 scores, 2) identify relevant patient characteristics, including PD-LRRK2 mutations, that significantly impact disease progression rate and 3) evaluate the implication of disease progression to inform clinical trials that would optimize the efficiency of PD drug development..
Acknowledging the advantages of the beta regression to characterize bounded data, we applied it to characterize the longitudinal progression of MDS-UPDRS23.. To our knowledge, this is the first time that beta regression was used to characterize the longitudinal progression of MDS-UPDRS in PD patients.
The current analysis was carried out in collaboration with CPP Consortium (funded by Parkinson’s United Kingdom and industry sponsors) [15]. The outcome of this effort will contribute to achieve one of CPP’s goal, which is to create new tools and techniques that can be applied during the development of new treatments for PD.
Results
Data summary
Three cohorts from the Parkinson’s Progression Markers Initiative (PPMI) study were included in this analysis: 1) PD Cohort, which was comprised of subjects diagnosed with PD within two years or less and with subjects who did not initiate any PD treatment for the first 6 months from baseline (423 subjects), 2) Genetic PD Cohort, which was comprised of PD subjects with a genetic mutation including LRRK2, glucocerebrosidase (GBA), or alpha synuclein (SNCA) (114 subjects), and 3) Genetic PD Registry Cohort, which was comprised of PD subjects with a genetic mutation including LRRK2, GBA, or SNCA mutation, and who were evaluated at less frequent intervals to augment and broaden the follow-up of PD subjects (110 subjects). In the Incidence of Cognitive Impairment in Cohorts with Longitudinal Evaluation (ICICLE)-PD study, only subjects with PD were used in this analysis (215 subjects). Summary of demographic and patient characteristics for the pooled analysis dataset are presented in Table 1.
Table 1.
Distribution of patient characteristics for the analysis dataset
| Characteristic | PPMI-PD | PPMI-Genetic Cohort PD | PPMI-Genetic Registry PD | ICICLE-PD |
|---|---|---|---|---|
| Sample size (%) | 423 (49.1) | 114 (13.2) | 110 (12.8) | 215 (24.9) |
| Gender | ||||
| Male (%) | 277 (32.10) | 46 (5.34) | 50 (5.80) | 136 (15.80) |
| Female (%) | 146 (16.90) | 68 (7.89) | 60 (6.96) | 79 (9.16) |
| Baseline age (year) | ||||
| Median (range) | 62 (33,84) | 62 (32,85) | 72 (38,88) | 66 (35, 87) |
| Missing (%) | 0 (0.0) | 0 (0.0) | 2 (0.23) | 0 (0.0) |
| Baseline body weight (kg) | ||||
| Median (range) | 80.7 (40.8, 135.0) | 73.0 (46.9,110.9) | 71.2 (40.6,103.1) | 78.0 (41, 158) |
| Missing(%) | 5 (0.58) | 1 (0.12) | 0 (0.0) | 26 (3.02) |
| Baseline height(cm) | ||||
| Median (range) | 173 (132,198) | 168 (145,192) | 165 (145,189) | 170 (148,200) |
| Missing (%) | 5 (0.58) | 1 (0.12) | 0 (0.0) | 27 (3.13) |
| Disease Duration (years) | ||||
| Median (range) | 0.33 (0.00, 3.0) | 2.40 (0.083,7.5) | 9.70 (0.250,30.0) | 0.33 (0.0,2.7) |
| Missing (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Years of education (years) | ||||
| Median (range) | NA | NA | NA | 11 (3, 24) |
| Missing (%) | 423 (49.1) | 114 (13.2) | 110 (12.8) | 0 (0.0) |
| Baseline MDS-UPDRS23 | ||||
| Median (range) | 27 (7,65) | 30 (2,100) | 39 (9,120) | 36 (9, 97) |
| Missing(%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| LRRK2 mutationa | ||||
| Yes (%) | 125 (14.50) | 98 (11.40) | 97 (11.30) | 0 (0.0) |
| No (%) | 298 (34.60) | 16 (1.86) | 13 (1.51) | 215 (24.90) |
| Concomitant medication | ||||
| Levodopa/Dopamine Agonists (%) | 386 (44.80) | 112 (13.00) | 1 (0.12) | 215 (24.90) |
| Other PD medication (%) | 37 (4.29) | 2 (0.23) | 109 (12.60) | 0 (0.0) |
Abbreviations: ICICLE = Incidence of Cognitive Impairment in Cohorts with Longitudinal Evaluation, LRRK2 = Leucine-rich repeat kinase 2, MDS-UPDRS23 = Movement Disorder Society-Unified Parkinson’s Disease Rating Scale Part II plus Part III score, NA = not applicable, PD = Parkinson’s Disease, PPMI = Parkinson’s Progression Marker Initiative. In total, the analysis dataset composed to 862 number of subjects from which, 509 males, 353 females, 320 had LRRK2 mutation, 542 without LRRK2 mutation, 742 under Levodopa/Dopamine Agonists and 148 under other type of PD medication
LRRK2 = Yes consisted of subjects with at least one of the following LRRK2 mutations: p.R114C, p.R1441G, p.Y1699C, p.G2019S, rs76904798 and G2385R.
Model development
Base Model
The standard logistic model [16], as defined in Equation 1, was found to describe the MDS-UPDRS23 data adequately
| (Equation 1) |
where, Scorei is MDS-UPDRS23 score for individual i, t is time, and max(Scorei)=118 is the maximum possible Score. The standard logistic model assumed the inflexion point occurred at the middle of the scale; i.e., max(Score)/2. Additonal disease progression models that were also tested can be found in Table S1.
Equation 1 suggests an increase of the progression rate when the scores were below the inflection point, and a decrease of the progression rate when the scores were above the inflection point. Evaluation of the inter-individual variability on slope and baseline parameters led to the estimation of a correlated inter-individual variabilities on baseline and slope. The base model was stable upon perturbation of initial parameter estimates and had a low condition number (i.e., 9.58). Goodness-of-fit plots confirmed the adequacy of the base model to describe both total population and individual study populations without bias (Supplementary Figures S1 and S2).
Covariate Analysis
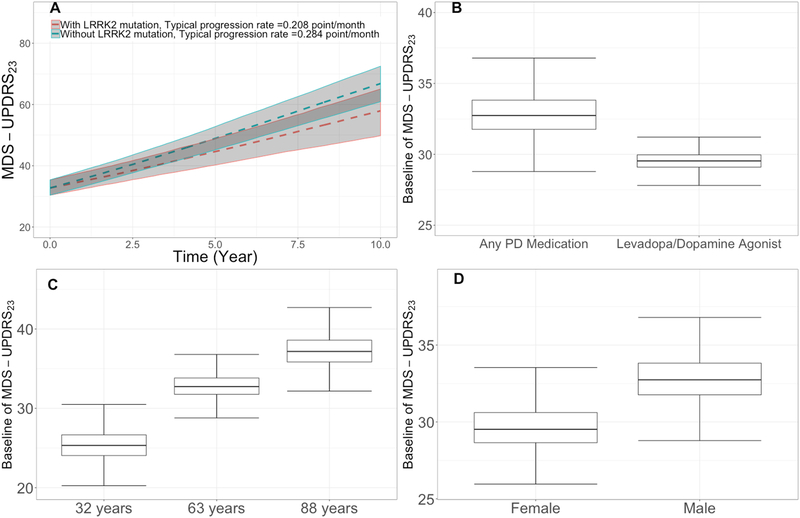
Subjects from PPMI-PD cohort had lower baseline MDS-UPDRS23 scores compared to the other cohorts, as these subjects were mostly dopamine naïve; see Figure 1. The impact of the lower baseline was assessed by considering the PPMI-PD cohort as a binary covariate (1 for PPMI-PD subjects and 0 otherwise). To quantify the impact of statistically significant covariates, simulations were performed using 1,000 virtual subjects built from the bootstrap parameters of the final model. Results of these simulations for selected covariates are presented in Figure 1. Females had approximately 10% lower baseline motor scores compared to males (Figure 1D). Age was found to have a statistically significant effect on the baseline motor score; an increase in age from 32 to 63 years is associated with approximately 30% increase in baseline motor score (Figure 1C). Subjects on levodopa/dopamine agonists had lower baseline score (~9%) compared to subjects treated with other PD medications (Figure 1B). Subjects with LRRK2 mutations were found to progress at a slower rate (~ −27%) compared to subjects without LRRK2 mutations (Figure 2A). A slower progression rate of in UPDRS Part III motor score for subjects with LRRK2 G2019S mutation compared to subjects without LRRK2 G2019S mutations was reported by Rachel Saunders-Pullman et al. [6]. Additional details on covariate analysis are presented in Table S2.
Figure 1. Covariates’ effects on the final disease progression model.
In (a) effect of subject with LRRK2 mutations on slope, red and green dashed lines are medians of simulated progression rate of subjects without and with LRRK2 mutations, respectively. Gray areas represent 90% confidence interval of prediction, (b) effect of concomitant medication on baseline, (c) effect of age on baseline, and (d) effect of gender on baseline.
Abbreviations: LRRK2 = Leucine-rich repeat kinase 2, MDS-UPDRS23 = Movement Disorder Society-Unified Parkinson’s Disease Rating Scale Part II plus Part III score, PD = Parkinson’s disease.
Note: In Figure 1A, red and green dashed lines are medians of simulated progression rate of subjects without and with LRRK2 mutations, respectively. Grey areas represents 90% CI of prediction.
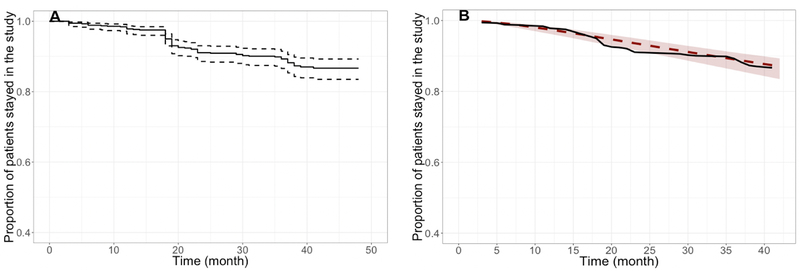
Figure 2. Kaplan Meier plots showing observed proportion of subjects remained in the study (A) and comparison between observed and simulated dropout rates with the final model (B).
Note: The pink line corresponds to Kaplan-Meier (nonparametric) estimates based on the observed data; the black line with shading corresponds to model predictions with the 95% CI.
Impact of dropout
The method used to quantify the dropout of subjects during the clinical trial is described in the Supplementary Materials. In the pooled analysis dataset, 87% of the subjects remained up to the 48-month visit (Figure 2A). Based on an exploratory analysis, dropouts in PPMI and ICICLE-PD studies were 0.06 (95% confidence interval [CI] = 0.05–0.08) and 0.31 (95% CI = 0.25–0.37]. The dropout pattern was best described by a log-normal distribution based on the Akaike information criterion (AIC) value. Univariate covariate analysis identified the presence of LRRK2 mutations, concomitant medication (i.e., levodopa/dopamine agonists) and age as statistically significant predictors of the dropout (see Table S3). The 95% CIs of the model predictions (Figure 2B) captured the observed dropout pattern, confirming the adequacy of the dropout model. The dropout model, including all the statistically significant covariates, was used in the clinical trial simulations.
Final model
Additional improvements to the model were explored, including reassessment of the covariance structure for random effects, but these analyses did not produce any further improvement in the model fit. Therefore, the final covariate model from standard logistic model was considered as the final model. The condition number (calculated as the ratio of the largest eigenvalue to the smallest eigenvalue) for the final covariate model was 29.4. Among the 1,000 runs from bootstrap, only 7 (0.7%) failed. Consistency between parameter estimates from the final model along with 95% CIs obtained from successfully converged bootstrap runs are shown in Table 2. Additional plots comparing the distribution of parameters from bootstrap estimates and the final model are presented in Supplementary Figure S4.
Table 2.
Parameter estimates of final model and stability assessment using non-parametric bootstrap analysis
| Parameters | Base Model | Final Model | Non-parametric bootstrap |
|---|---|---|---|
| Estimate (%RSE) | Estimate (%RSE) | Mean(95% CI) | |
| MDS-UPDRS23 Baseline | 30.3 (1.54%) | 29.5 (2.30%) | 29.5 (28.3, 30.8) |
| Age (Centered at 63 years old) | NE | 0.376 (22.20%) | 0.378 (0.199,0.563) |
| Female | NE | −0.0968 (27.70%) | −0.0964 (−0.148, −0.042) |
| Cohort (ICICLE-PD, PPMI Genetic Cohort PD, PPMI Genetic Registry PD) | NE | 0.134 (25.30%) | 0.133 (0.0689,0.203) |
| Any PD medications | NE | 0.112 (40.40%) | 0.111 (0.0217, 0.214) |
| Intrinsic Progression Rate (per month) | 0.00974 (8.39%) | 0.0101 (8.91%) | 0.0102 (0.0085, 0.012) |
| LRRK2 mutation | NE | −0.235 (37.40%) | −0.250 (−0.513, −0.0269) |
| Dispersion factor of Beta Distribution | 37.3 (2.53%) | 39.2 (2.57%) | 39.3 (35, 43.8) |
| Random Effect | |||
| MDS-UPDRS23 Baseline | 0.162 (5.87%) | 0.15 (6.26%) | 0.149 (0.13, 0.168) |
| Intrinsic Progression Rate | 2.67 (19.7%) | 0.000236 (9.66%) | 0.000237 (0.000185, 0.000298) |
| Correlation between Baseline and Intrinsic Progression Rate | 0.111 (40.6%) | 0.001270 (28.8%) | 0.001270 (0.000523, 0.00216) |
Abbreviations: ICICLE = Incidence of Cognitive Impairment in Cohorts with Longitudinal Evaluation, LRRK2 = Leucine-rich repeat kinase 2, MDS-UPDRS23 = Movement Disorder Society-Unified Parkinson's Disease Rating Scale part 2 plus part 3 score, NE = not evaluated, PD = Parkinson’s Disease, PPMI = Parkinson’s Progression Marker Initiative, RSE = relative standard error
Notes: 95% CI = 95% confidence interval of parameter estimate based on bootstrap results. Baseline and the typical progression rate in MDS-UPDRS23 score can be estimated as follows: MDS-UPDRS23 Baseline = 29.5*[(1 + 0.134) if Other Cohort-PD]*[(1–0.0968) if Female]]*[(1+0.112) if Any PD medication]* ((AGE/63)0.376). Intrinsic Progression Rate = 0.0101*[(1–0.235) if LRRK2 mutation]. The typical progression rate in MDS-UPDRS23 score was estimated to be dScore/dt=r*Score*(1-Score/max(Score)) = 29.5*0.0101*(1-[29.5/118]), which was approximately 0.22 point/per months.
Visual predictive check (VPC) plots stratified by subjects with/without LRRK2 mutations are presented in Figure 3. Overall, these plots illustrate the predictive ability of the model. Additional evaluations of the final model are shown in Figure S3. Together, the results of the diagnostic plots and VPCs confirm that the disease progression model was able to describe the longitudinal profile of MDS-UPDRS23.
Figure 3. Visual predictive check plots for the final model stratified by subjects with/without LRRK2 mutation.
Abbreviations: LRRK2 = Leucine-rich repeat kinase 2, MDS-UPDRS23 = Movement Disorder Society-Unified Parkinson’s Disease Rating Scale Part II plus Part III score.
Note: Black circles in the upper, middle, and lower profiles represent the 95th, 50th, and 5th percentiles of the observed data, respectively. The upper, middle, and lower lines represent the median model-based predictions for the 95th, 50th, and 5th percentiles, respectively. Shaded areas are the 95% inter-percentile ranges of the simulations.
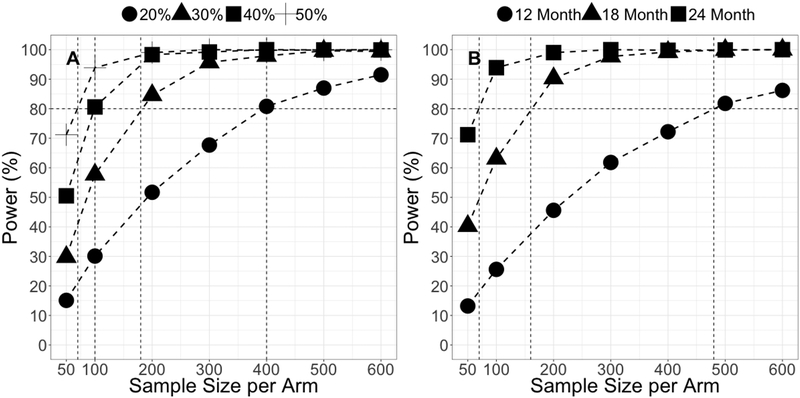
The power to detect various levels of disease modifying effect compared to placebo for a hypothetical PD therapeutic with different sample sizes is presented in Figure 4A. The sample size required per arm decreased with the increase of the drug effect size to achieve 80% power. Specifically, in order to achieve 80% power, a PD therapeutic response with a disease modifying effect of 20%, 30%, 40% or 50% will require 400, 180, 100 and 70 subjects per arm, respectively. Figure 4B shows that the sample size required to achieve 80% power for a 50% reduction in progression rate decreases with an increase in trial duration. In order to achieve 80% power, 480, 160 and 70 subjects per arm will be required for clinical trials with 12, 18 and 24 months treatment duration, respectively.
Figure 4.
Power to detect drug effect with disease modifying effect (A) and power to detect drug effect with different clinical trial duration (B)
Discussion
In recent years, there have been tremendous efforts to develop disease modifying therapies for PD that have resulted in identifying several genetically validated targets. LRRK2 kinase inhibitors were identified as a viable disease modifying treatment option and recent findings by Di Maio et al [18] suggest these inhibitors may be useful for treating patients without LRRK2 mutations in addition to subjects who carry LRRK2 mutations. Divergent findings on the rate of disease progression between subjects with or without LRRK2 mutations were reported [6, 7], which could affect the trial design for evaluating safety and efficacy of new therapeutics. One of the objectives of current analysis was to assess the difference of the progression rate for subjects with/without LRRK2 PD and to quantify the implication to clinical trial design.
Structural base model
The selection of the structural model was based on logistic models that captured different stages of PD progression, with an increase of the progression rate when the scores were below the inflection point, and a decrease of the progression rate when the scores were above the inflection point (middle of the scale). The rate of progression reported in this work (~0.22 point per month) agrees with the work conducted by Holden et al. [19] on progression of MDS-UPDRS scores over five years, in de novo Parkinson Disease from the PPMI Cohort. They reported an natural disease progression of 4.7 points per year for MDS-UPDRS total scores (i.e. non-motor and motor score) from which 0.99 points per year for MDS-UPDRS2 and 2.4 points per year for MDS-UPDRS3. The residual variability was assumed to be beta distributed to account for the bounded nature of MDS-UPDRS23 [20]. Beta regression (i.e., combination of logistic model and beta-distributed residual variability) has been previously used to characterize disease progression in Alzheimer’s disease [10–14] and rheumatoid arthritis [21]. Herein, we have applied for the first time beta regression to characterize the longitudinal progression of MDS-UPDRS in PD patients.
Covariate analysis
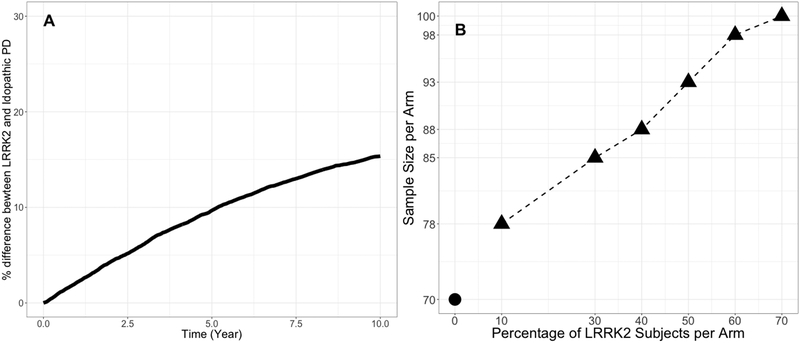
The covariate analysis was conducted to identify predictors of PD progression. A relatively stringent significance level was used for covariate testing (P < 0.01 in the forward step, P < 0.001 in the backward step) to mitigate the likelihood of false positives given that multiple hypothesis testing was applied during the covariate search [22, 23]. Using these criteria, age, gender, concomitant medication, and study arms (ICICLE-PD, PPMI Genetic Cohort PD, PPMI Genetic Registry PD) were identified as predictors of baseline MDS-UPDRS23. In addition, LRRK2 mutations were identified as predictors of the disease progression rate. The effect of other cohort (i.e., ICICLE-PD, PPMI Genetic Cohort PD and PPMI Genetic Registry PD) on the baseline was not surprising as these subjects had higher baseline disease severity compared to PPMI-PD cohort. There was correlation between age and high baseline disease severity, suggesting that PD onset in old age is associated with greater motor impairment than subjects with PD onset in middle age. This finding is in line with the common understanding of PD progression in elderly subjects [24]. Also, female subjects were found to be associated with lower baseline disease severity compared to male subjects. Several differences between men and women in cognition has been reported [25] including verbal fluency, the recognition of facial emotion, visuospatial cognition, etc. These gender differences in the clinical and cognitive characteristics of PD may partly be explained by the effect of estrogen on dopaminergic neurons and pathways in the brain [25]. PD subjects who carried LRRK2 mutations were found to have slower progression rate than non-carriers. This finding was consistent with the recent literature findings [5,6,26]. For instance, Healy et al [26] reported that motor symptoms (e.g., disease severity, rate of progression, occurrence of falls, and dyskinesia) and non-motor symptoms (e.g., cognition and olfaction) in subjects with LRRK2 mutations were more benign compared to those in non-carriers. Current understanding suggests that activation of LRRK2 by mutation promotes proinflammatory responses offering protection against infection and survival benefit earlier in life, before developing PD, a concept called antagonistic pleiotropy [27]. A simulation showing the longitudinal difference in progression rate between PD subjects with and without LRRK2 mutation is shown in Figure 5A.
Figure 5. Longitudinal percentage difference between subjects with/without LRRK2 mutation (A) and the power to detect drug effect between subjects with/without LRRK2 mutation (B).
Abbreviations: LRRK2 = Leucine-rich repeat kinase 2, PD = Parkinson’s disease
Clinical trial Simulation enrichment
Findings from final model were used to inform clinical trial enrichment strategies (i.e., 10%, 30%, 40%, 50%, 60% and 70% PD subjects with LRRK2 mutations) for future LRRK2 mutation targeted agents using the same clinical trial design as described previously with a disease modifying effect of 50% for subjects with or without LRRK2 muation. Based on these simulations, approximately 70 subjects/arm would be required in a non-enriched placebo-controlled clinical trial to detect a drug effect of 50% reduction in the progression rate with 80% probability. Whereas, for example, 85, 93 and 100 subjects/arm would be required for enriched clinical trials with 30%, 50% and 70% subjects with LRRK2 mutations, respectively, to detect a 50% drug effect with 80% power, see Figure 5–b. These modest increases in sample size suggested that enrichment with LRRK2-PD for a LRRK2 inhibitor clinical trial may not be beneficial if PD subjects with and without LRRK2 mutation have the same treatment effect.
Limitations of current analysis
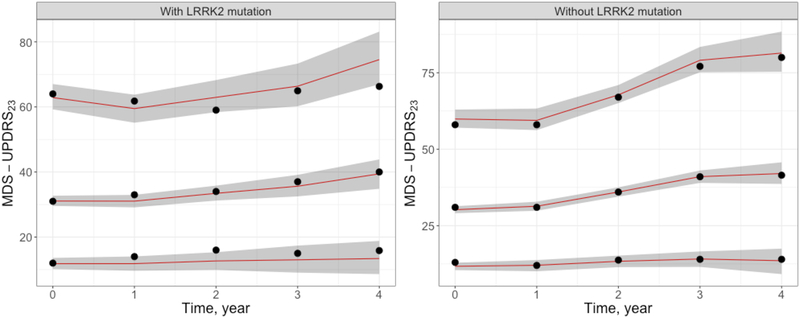
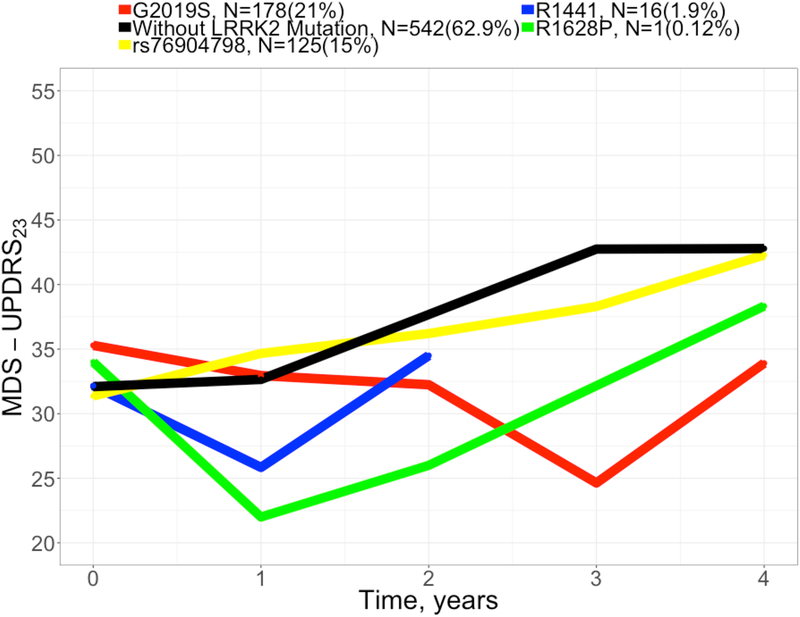
Our choice on PD subjects with LRRK2 mutation was driven by the available analysis data that reflected the challenge of recruiting PD subjects with LRRK2 mutation when designing clinical trial. The trend of the progression rate of each of these LRRK2 mutations from the analysis dataset is shown in Figure 6. Despite the fact that these trends could be cofounded with other covariates (e.g., age, gender, etc.), the disease progression of PD subjects with each of these LRRK2 mutations was slower compared to those who did not carry these LRRK2 mutations.
Figure 6. Observed disease progression for subjects with/without LRRK2 mutation.
Abbreviations: LRRK2 = Leucine-rich repeat kinase 2, MDS-UPDRS23 = Movement Disorder Society-Unified Parkinson’s Disease Rating Scale Part II plus Part III score.
At baseline, subjects who took PD medications other than levodopa/dopamine agonists were found to have higher baseline disease severity compared to subjects who took levodopa/dopamine agonists. This finding should be interpreted with caution as the criteria for background therapy allowed in PPMI and ICICLE-PD studies may not be similar to PD de novo interventional studies. Furthermore, the fact that approximately 41% dopamine-naïve subjects from the analysis dataset took levodopa/dopamine agonists after 6 months suggested that the time varying aspect of concomitant medication should be taken into account during the model development. This approach could not be considered in this analysis due limitations of the analysis data; e.g., the dose received by the patient was not recorded in the database.
Mixed cohorts (Genetic and PD cohort) considered in this analysis could have influenced the overall estimates of the progression rate in PD subjects with LRRK2 mutation as defined in this paper. To assess the impact of these cohorts on the overall progression rate of PD subjects with LRRK2 mutation, sensitivity analysis was performed by comparing progression rates with the full dataset, PD cohort and Genetics cohort. The estimate of impact of LRRK2 mutation on progression rate for the full dataset was −0.235, while it was −0.175 for the PD cohort and −1.56 for the genetic cohort, which resulted in ~20%, ~17% and ~156% reductions, respectively. All estimates from the sensitivity analysis suggested a slow progression for PD subjects with LRRK2 mutation compared to subjects without LRRK2 mutations, with a strong reduction with Genetic cohort. These results confirmed robustness of the predicted trend of the progression rate in PD subjects with LRRK2 mutation obtained from the current analysis. Correlation between disease duration and disease severity was significant for subjects enrolled with disease duration greater than two years, see supplementary Figure S4. This correlation was not statistically significant to be captured during the covariate analysis, based on the established selection criteria of SCM. Finally, the sample size estimates presented from clinical trial simulation are for reference purposes. This is due to our inability to estimate the placebo response, given that we did not have access to clinical trial data in this newly diagnosed PD population. Therefore, if a significant placebo response exists, this would impact the estimated trial sizes presented herein. In summary, the approach reported here provides a quantitative tool that characterizes progression of PD and accounts for the effect of relevant covariates in this population (age, gender, LRRK2 mutations and concomitant PD medication). Such a tool can be used in drug development to optimize clinical trial design for PD genetherapy.
Methods
Data
Patient-level longitudinal clinical data were from PPMI (www.ppmi-info.org) and ICICLE-PD (https://portal.dementiasplatform.uk/) observational studies and were downloaded from the CPP integrated database (https://codr.c-path.org/login.do). The CPP integrated Parkinson’s database is a data platform designed to curate, standardize and make data available to CPP members to expedite the development of treatments for PD. CPP used existing data standards published by the Clinical Data Interchange Standards Consortium (www.cdisc.org), a nonprofit organization that focuses on developing global standards for clinical trial data collection. These standards included the foundational Study Data Tabulation Model and the Therapeutic-Area User Guide version 1.0 for PD (https://www.cdisc.org/standards/therapeutic-areas/parkinsons-disease).
The PPMI is an international, multicenter, prospective study designed to identify clinical, imaging and biologic markers of PD progression for use in clinical trials of disease modifying therapies (National Clinical Trials identifier NCT01141023) [28]. ICICLE-PD was a twin center longitudinal observational study designed to better understand the mechanisms underlying the evolution of PD dementia from disease onset [29]. A summary of the analysis dataset is presented in Table 1. The pooled data included records from subjects assessed at baseline shortly after diagnosis, demographics, time from diagnosis, medications (PD and non-PD), motor and cognitive tests, functional tests, and genetic status.
The time metric was the time in the study in months and MDS-UPDRS23 was the endpoint used for the analysis. MDS-UPDRS23 values were generated by summing up MDS-UPDRS Part II and Part III subitems of each observation obtained from the CPP integrated database. Records were excluded if the time of MDS–UPDRS23 records was missing.
Model building process
The disease progression model was developed using nonlinear mixed-effects modeling, implemented in NONMEM [30]. The model building process was staged as follows: 1) selection of the base model structure with incorporation of two levels of random effects (inter-individual and residual variability), 2) identification of statistically significant covariates of model parameters, and 3) evaluation of model performance with simulation-based diagnostics.
The evaluation of the base model structure was initiated with a simple linear model followed by more complex nonlinear logistic functions [17]. Previously published nonlinear logistic functions [14, 28, 31] were assessed to inform the model structure. Inter-individual random variability was incorporated on the baseline and intrinsic rate of progression. Log-normally distributed inter-individual variability was estimated for the baseline in order to prevent the prediction of nonsensical scores at the individual level. Normally and log-normally distributed inter-individual variability for the intrinsic rate was evaluated.
Residual variability was assumed to be beta distributed to account for the bounded nature of MDS-UPDRS23 score [20]. The scaling method reported by Smithson et al. [15], as described in the equation (2), was used to transform the score from original scale to unit interval.
| Equation (2) |
Where n is the sample size; min(Score) is the lowest possible score and max(Score) is the highest possible score.
The NONMEM Laplacian estimation method was used for optimization to handle the second derivative of the inter-individual variability parameters. Pearl Speaks NONMEM (PsN) tool [32] and R (R-project, www.r-project.org) software packages were used for the exploratory analysis and post-processing of NONMEM output.
Concomitant medication
Subjects in both PPMI and ICICLE-PD studies received various PD medications; e.g., dopamine agonists, levodopa, monoamine oxidase B (MAO-B) inhibitors, or others. Concomitant medication covariate was defined as a binary variable. Subjects who received levodopa/dopamine agonists during the study were pooled into one category. Subjects from the PPMI-PD cohort did not take any type of PD medications (i.e., dopamine agonists, levodopa or others) for more than 60 days prior to the baseline visit, and were not currently on and did not expect to require PD medications within at least 6 months from the baseline visit. Tanya Simuni et al [33] reported no statistical difference on progression rate between subjects treated with levodopa/dopamine agonists compared to other PD medications within the PPMI-PD arm. In this analysis, we also evaluated the benefit of levodopa/dopamine agonists compared to other PD medications. Approximately 41% of dopamine-naïve subjects from the analysis dataset were required to take levodopa/dopamine agonists after 6 months.
Methods used to: 1) formally selecting covariates, 2) quantify the impact the dropout and 3) evaluate the robustness of the final model are described in the Supplementary Materials.
Simulations to inform PD clinical trial design
Once the robustness of the final model was established, Monte Carlo-based clinical trial simulations were performed in R (R-project, www.r-project.org) to showcase the utility of the disease progression model in informing trial designs, with a high likelihood of detecting a treatment effect with different effect sizes. One thousand placebo-controlled clinical trials with and without enrichment were simulated using the estimated parameter values with the final model. Sample sizes ranging from 50–600 subjects per arm and a hypothetical drug effect of 20%, 30%, 40% and 50% reduction in the disease progression rate were simulated. The identified dropout model was incorporated in the clinical trial simulations. The power of detecting the drug effect was calculated as the proportion of trials for which the slope difference between treated and untreated arms showed a beneficial drug effect with a two-tailed P value < 0.05.
Study Highlights
What is the current knowledge on the topic?
There is no therapy that halts or slows the progression of Parkinson’s disease. Leucine rich repeat (LRRK2) kinase inhibitors hold promise as potential genetic based candidates that may impact the underlying pathogenesis. There is a lack of quantitative assessment of the impact of progression rate of LRRK2-PD subjects to design clinical trial for future LRRK2 targeted agents.
What question did this study address?
This analysis 1) characterized a longitudinal disease progression model for PD, 2) Identified a subgroup of subjects including those with LRRK2 mutations that significantly impacted the rate of disease progressionand 3) quantified the implication of disease progression to to inform efficient design of clinical trials evaluating LRRK2 inhibitors.
What does this study add to our knowledge?
PD subjects carrying LRRK2 mutations were showed to have an unexpectedly slower rate of disease progression. Enrichment with LRRK2-PD for a LRRK2 inhibitor clinical trial may not be beneficial if PD subjects with and without LRRK2 mutation have the same magnitude of treatment effect.
How might this change clinical pharmacology or translational science?
Quantifying the disease progression of PD subjects with LRRK2 mutation is critical to efficiently inform clinical trial design for emerging LRRK2 targeted agents.
Supplementary Material
Acknowledgments
We acknowledge additional CPP member organizations, including the Parkinson’s Disease Foundation, The Michael J. Fox Foundation, the Davis Phinney Foundation, The Cure Parkinson’s Trust, the University of Oxford, University of Cambridge, Newcastle University, University of Glasgow, as well as the NINDS, US Food and Drug Administration, and the European Medicines Agency. We also acknowledge The Michael J. Fox Foundation for sponsoring of PPMI. Data were obtained from the PPMI database (www.ppmi-info.org/data). The PPMI is sponsored and partially funded by The Michael J. Fox Foundation for Parkinson’s Research and funding partners, including AbbVie, Avid, Biogen, Bristol-Myers Squibb, Covance, GE Healthcare, Genentech, GSK, Lilly, Lundbeck, MSD, Meso Scale Discovery, Pfizer, Piramal, Roche, Sanofi Genzyme, Servier, TEVA, UCB, and Golub Capital. For current information on the study, visit www.ppmi-info.org. With special recognition to Critical Path Institute’s data management team. Medical Writing was coordinated and supported by Certara Strategic Consulting and Medical Writing Innovations, LLC. Charles Venuto was supported in part by a grant from the National Institute of Neurological Disorders and Stroke, National Institutes of Health 1P50NS108676–01 (to CV).
Funding: Merck & Co., Inc., Kenilworth, NJ USA and the Critical Path for Parkinson’s (CPP) Consortium. The CPP Consortium is funded by Parkinson’s United Kingdom and the following industry members: AbbVie, Biogen, Eli Lilly, Lundbeck, MSD, Takeda, Sanofi and UCB, Biogen, Ixico, Denali, Roche, Merck and GSK.
Footnotes
Conflict of Interest: The authors declared no competing interests for this work.
Supplementary Materials
(Supplemental Material.pdf)
References
- 1.Brice A Genetics of Parkinson’s disease: LRRK2 on the rise. Brain 128(12), 2760–2762 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Lesage S, et al. LRRK2 G2019S as a cause of Parkinson’s disease in North African Arabs. N. Engl. J. Med 354, 422–423 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Ozelius LJ, et al. LRRK2 G2019S as a cause of Parkinson’s disease in Ashkenazi Jews. N. Engl. J. Med 354(4), 424–425 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Nuytemans K, Theuns J, Cruts M, Van Broeckhoven C Genetic etiology of Parkinson disease associated with mutations in the SNCA, PARK2, PINK1, PARK7, and LRRK2 genes: a mutation update. Hum. Mutat 31(7), 763–780 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alcalay RN, et al. Parkinson disease phenotype in Ashkenazi Jews with and without LRRK2 G2019S mutations. Mov. Disord 28(14), 1966–1971 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saunders-Pullman R, et al. JAMA. Neurol 75(3), 312–319 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oosterveld LP, et al. Greater motor progression in patients with Parkinson disease who carry LRRK2 risk variants. Neurology 85(12), 1039–1042 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Goetz CG, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov. Disord 22(1), 41–47 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Goetz CG, Stebbins GT, Tilley BC Calibration of unified Parkinson’s Disease Rating Scale scores to Movement Disorder Society/Unified Parkinson’s Disease Rating Scale scores. Mov. Disord 27, 1239–1242 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Venuto CS, Potter NB, Dorsey ER, Kieburtz K A review of disease progression models of Parkinson’s disease and applications in clinical trials. Mov. Disord 31(7), 947–956 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu XS, et al. Mixed-effects beta regression for modeling continuous bounded outcome scores using NONMEM when data are not on the boundaries. J. Pharmacokinet. Pharmacodyn 40(4), 537–544 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Xu XS, Samtani M, Yuan M, Nandy P Modeling of bounded outcome scores with data on the boundaries: application to disability assessment for dementia scores in Alzheimer’s disease. AAPS. J 16(6), 1271–1281 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conrado DJ, Denney WS, Chen D, Ito K An updated Alzheimer’s disease progression model: incorporating non-linearity, beta regression, and a third-level random effect in NONMEM. J. Pharmacokinet. Pharmacodyn 41(6), 581–598 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Rogers JA, et al. Combining patient-level and summary-level data for Alzheimer’s disease modeling and simulation: a beta regression meta-analysis. J. Pharmacokinet. Pharmacodyn 39(5), 479–498 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Smithson M, Verkuilen J A better lemon squeezer? Maximum-likelihood regression with beta-distributed dependent variables. Psychol. Methods 11(1), 54–71 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Stephenson D, et al. Precompetitive data sharing as a catalyst to address unmet needs in Parkinson’s disease. J. Parkinsons Dis 5(3), 581–594 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsoularis A, Wallace J Analysis of logistic growth models. Math. Biosci 179(1), 21–55 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Di Maio R et al. LRRK2 activation in idiopathic Parkinson’s disease. Sci. Transl. Med 10(451), pii: eaar5429. doi: 10.1126/scitranslmed.aar5429 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holden Samantha K., Finseth Taylor, Sillau Stefan H., Berman Brian D., Progression of MDS-UPDRS Scores Over Five Years in De Novo Parkinson Disease from the Parkinson’s Progression Markers Initiative Cohort, MOVEMENT DISORDERS CLINICAL PRACTICE 2018; 5(1): 47–53. doi: 10.1002/mdc3.12553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nemes G New asymptotic expansion for the gammafunction. Arch. Math 95(2), 161–169 (2010). [Google Scholar]
- 21.Conrado DJ, et al. Predicting the probability of successful efficacy of a dissociated agonist of the glucocorticoid receptor from dose–response analysis. J. Pharmacokinet. Pharmacodyn 43(3), 325–341 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Jonsson EN, Karlsson MO Automated covariate model building within NONMEM. Pharm. Res 15(9), 1463–1468 (1998). [DOI] [PubMed] [Google Scholar]
- 23.Ahamadi Malidi, et al. Operating characteristics of stepwise covariate selection in pharmacometric modeling. J. Pharmacokinet. Pharmacodyn 46:273–285 (2019) [DOI] [PubMed] [Google Scholar]
- 24.Blin J, Dubois B, Bonnet AM, Vidailhet M, Brandabur M, Agid Y Does ageing aggravate parkinsonian disability? J. Neurol. Neurosurg. Psychiatry 54(9), 780–782 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller IN, Cronin-Golomb A Gender differences in Parkinson’s disease: clinical characteristic and cognition. Mov. Disord 25(16), 2695–2703 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Healy DG, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case–control study. Lancet Neurol 7(7), 583–590 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alessi DR, Sammler E LRRK2 kinase in Parkinson’s disease. Science 360(6384), 36–37 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Marek K, et al. The Parkinson’s progression markers initiative (PPMI) –establishing a PD biomarker cohort. Ann. Clin. Transl. Neurol 5(12), 1460–1477 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yarnall AJ, et al. Characterizing mild cognitive impairment in incident Parkinson disease: the ICICLE-PD study. Neurology 82(4), 308–316 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beal SL, Sheiner LB NONMEM Users Guide: Part V (University of California - San Francisco, San Francisco, California, 1992). [Google Scholar]
- 31.Samtani MN, et al. An improved model for disease progression in patients from the Alzheimer’s disease neuroimaging initiative. J. Clin. Pharmacol 52(5), 629–644 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Lindbom L, Pihlgren P, Jonsson EN PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput. Methods Programs Biomed 79(3), 241–257 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Simuni T, et al. Longitudinal change of clinical and biological measures in early Parkinson’s disease: Parkinson’s progression markers initiative cohort. Mov. Disord 33(5), 771–782 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.