Abstract
Atherosclerosis, the major underlying cause of cardiovascular diseases (CVD), is the number one killer globally. The disease pathogenesis involves a complex interplay between metabolic and immune components. Although lipid-lowering drugs such as statins curb the risks associated with CVD, significant residual inflammatory risk remains. Substantial evidence from experimental models and clinical studies has established the role of inflammation and immune effector mechanisms in the pathogenesis of atherosclerosis. Several stages of the disease are affected by host-mediated antigen-specific adaptive immune responses that play either protective or proatherogenic roles. Therefore, strategies to boost an anti-atherogenic humoral and T regulatory cell response are emerging as preventative or therapeutic strategies to lowering inflammatory residual risks. Vaccination holds promise as an efficient, durable and relatively inexpensive approach to induce protective adaptive immunity in atherosclerotic patients. In this review, we discuss the status and opportunities for a human atherosclerosis vaccine. We describe 1) some of the immunomodulatory therapeutic interventions tested in atherosclerosis 2) the immune targets identified in pre-clinical and clinical investigations 3) immunization strategies evaluated in animal models 4) past and ongoing clinical trials to examine the safety and efficacy of human atherosclerosis vaccines and 5) strategies to improve and optimize vaccination in humans (antigen selection, formulation, dose and delivery).
Keywords: Atherosclerosis, Immunomodulation, Antigen-specific, Tregs, Peptide-based vaccine
1. Introduction
Cardiovascular diseases (CVD) constitute a heterogeneous group of heart and blood vessel disorders that together are the leading cause of death in the United States [1] and worldwide. Atherosclerosis, a disease of large- and medium-sized arteries characterised by lipid-rich atherosclerotic plaques, is the most common pathology of CVD. When plaques rupture or erode, Major Adverse Cardiovascular Events (MACE) ensue, including stroke and myocardial infarction (MI). The etiology of atherosclerosis is complex, with contributions from genetic, dietary, lifestyle, metabolic and immune components. However, the primary events involve accumulation of modified lipoproteins, especially low density lipoprotein (LDL) in the vessel walls, which then triggers a cascade of pro-inflammatory events [2]. During atherogenesis, LDL accumulates in the artery wall, where it becomes oxidized, resulting in reactive aldehyde groups such as malonaldehyde (MDA). Scavenger receptor mediated uptake of oxLDL by macrophages results in foam cell formation. It is controversial whether foam cells are pro-inflammatory. Current therapies available for atherosclerosis, such as statins and Proprotein Convertase Subtilisin/Kexin type 9 (PCSK9) inhibitors, decrease LDL cholesterol levels in the blood. Although these interventions are among the most successful prevention strategies known in medicine, significant residual risk persists due to ongoing inflammation [3].
Recently, immunomodulatory therapeutic approaches to combat atherosclerosis have been explored. Anti-inflammatory drugs and monoclonal antibodies (mAbs) that target the ongoing immune reaction have been tested in both pre-clinical and clinical studies. For example, in a large cohort of patients with coronary heart disease, the Canakinumab Anti-inflammatory thrombosis outcomes study (CANTOS) trial tested the effects of the anti-IL-1β mAb canakinumab that successfully reduced MACE [4]. However, systemic suppression of IL-1β negatively impacted host defence, leaving subjects more susceptible to lethal and nonlethal infections.
2. New interventions based on immunomodulation
Along with the innate immune system, the adaptive immune system also contributes to atherogenesis. The relevance of both T and B cells to disease progression was demonstrated using animal models of atherosclerosis in which deficiency of both cell types reduced the aortic root lesion area by 80% [5]. A growing body of evidence supports an atherogenic role of T-helper type 1 (Th1) cells [6, 7] and a protective role of T regulatory (Treg) cells [8–10], whereas the role of T-helper type 17 (Th17) and follicular helper T (Tfh) cells in atherosclerosis remains controversial [11–13]. Among the B cell subsets, the B-1a cells confer atheroprotection, possibly through production of natural IgM autoantibodies against oxLDL [14, 15]. B-2 cells secrete isotype-switched antibodies, mainly IgG, whose role in atherogenesis remains unclear [16]. Two recent studies have illustrated an important role of Germinal-center (GC) derived B cells in atherosclerosis. One study highlights the role of the FcγRIIb, an IgG-binding inhibitory receptor expressed on B cells, in differentially modulating proatherogenic adaptive GC B cell and atheroprotective B-1 responses and IgM production in atherosclerotic male and female mice [17]. The other study illustrates an important role of antibodies, particularly the IgG isotype, in promoting the growth and stability of plaques in mice aorta [18].
2.1. Antibody-based immunotherapy
In the past decade, several strategies aimed at activating atheroprotective adaptive immunity (Figure 1) have been developed. Different vaccination approaches tested in atherosclerosis aim to directly modulate either inflammation (MDA-p45) or lipid biology (PCSK9 Abs). Passive immunization of Apoe−/− mice with recombinant IgG antibody against an MDA-modified ApoB-100 peptide, MDA-p45 (Table 1), reduced atherosclerotic lesions by 50% [19]. Another successful strategy includes inhibition of PCSK9 [20], a serine protease secreted from hepatocytes. PCSK9 promotes LDL receptor (LDL-R) degradation in acidic lysosomes and prevents LDLR recycling to the plasma membrane [21]. Two fully human mAbs against PCSK9, Evolocumab and Alirocumab, are FDA-approved and used to treat patients with homozygous familial hypercholesterolemia and patients at high risk for atherosclerotic cardiovascular disease. These antibodies have significant therapeutic potential, but they require frequent administration and are costly [22].
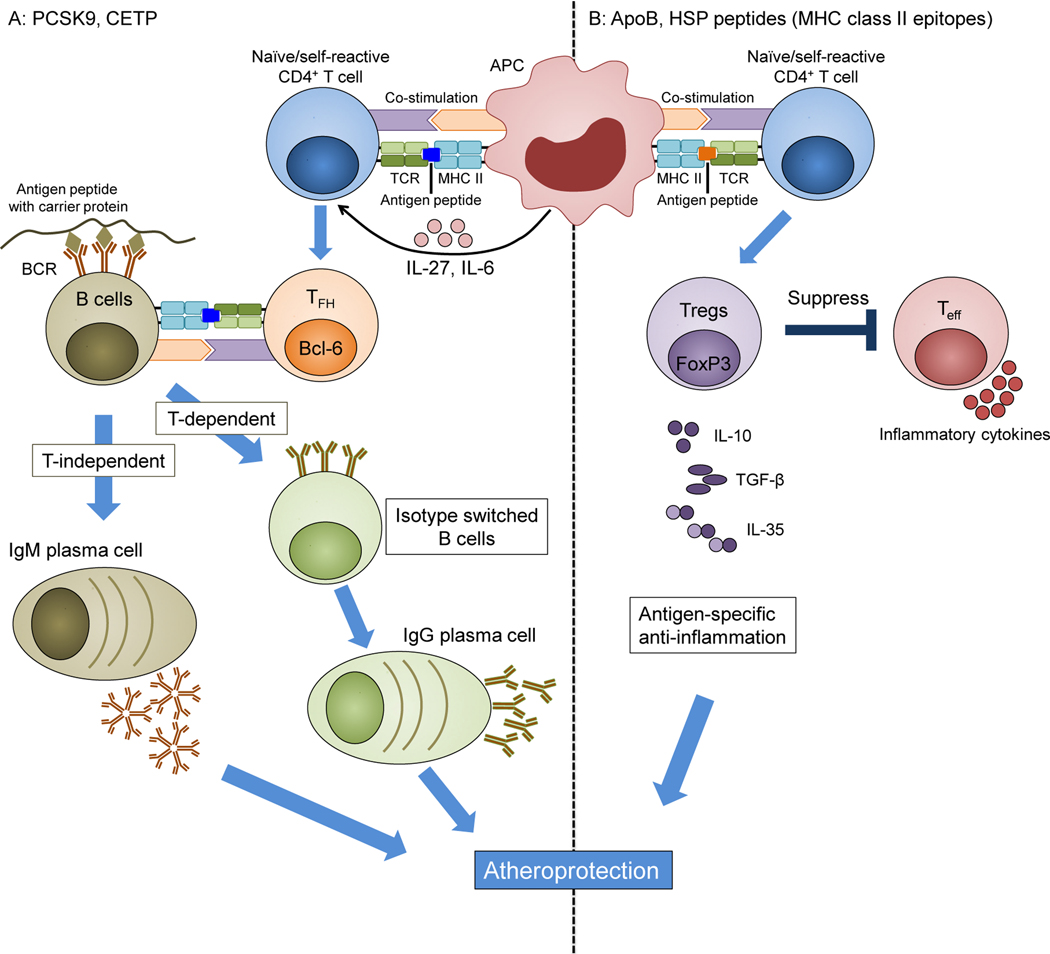
Figure 1. Approaches to atherosclerosis vaccines.
A: Antibody-based vaccines may target PCSK9, CETP or other proteins. The antigenic protein is engineered, possibly using a carrier protein (tan). MHC-II-restricted T cell epitopes induce a CD4 T cell response that, in the presence of IL-6 and IL-27, can result in follicular helper TFH cells, characterized by the transcription factor Bcl6 (orange). In germinal centres, TFH cells provide help to B cells, induce antibody isotype switch from IgM to IgG and support the maturation of B cells to long-lived IgG-secreting plasma cells (green). Without T cell help, B cells can mature into IgM-producing plasma cells (tan). B: Self-reactive Tregs can be targeted when self- peptides are administered through an appropriate route and formulated in a suitable adjuvant. After vaccination, antigen-specific peptides are presented to naïve self-reactive T cells through MHC class II on APCs. This results in activation of antigen-specific Tregs. Activated Tregs suppress effector T cells by cell contact-dependent mechanisms and by secreting anti-inflammatory cytokines, such as IL-10, IL-35, and TGFβ. ApoB: apolipoprotein B, APC: antigen-presenting cell, TFH: T follicular cell, Treg: regulatory T cell, Teff: T effector cell, MHC: major histocompatibility complex, TCR: T cell receptor.
Table 1:
Summary of atheroprotective peptides used for vaccination
| Target protein | Peptide name | Peptide sequence | Reference |
|---|---|---|---|
| Human APOB | Native P2 | ATRFKHLRKYTYNYEAESSS | [40] |
| MDA-P74 | VISIPRLQAEARSEILAHWS | [39] | |
| MDA-P45 | IEIGLEGKGFEPTLEALFGK | [39] | |
| P45 | CIEIGLEGKGFEPTLEALFGK | [41] | |
| P210 | KTTKQSFDLSVKAQYKKNKH | [41] | |
| P18 | SLFFSAQPFEITAST | [47] | |
| Mouse ApoB | P33501–3516 | SQEYSGSVANEANVY | [45] |
| P6978–993 | TGAYSNASSTESASY | ||
| P183030–3044 | SLFFSAQPFEITAST | [47] | |
| P101705–720 | FGKQGFFPDSVNKALY | [46] | |
| P102441–456 | TLYALSHAVNSYFDVD | ||
| P1033953–3968 | LYYKEDKTSLSASAAS | ||
| Human PCSK9 | P153–163 | SIPWNLERITP | [44] |
| P68–76 | AKDPWRLPG | ||
| P207–223 | NVPEEDGTRFHRQASKC | ||
| Variants of the PCSK9 fragment having amino acid sequence SIPWNLERITPPR | [43] and Patent application: PCT/EP2013/067797 | ||
| CGGGSIPWNLERITPVRKKAQYIKANSKFIGITEL | [42] | ||
| HSP65 | P34166–180 | KVGNEGVITVEESNT | [52] |
| P67331–345 | VEGAGDTDAIAGRVA | ||
| P84416–430 | TLLQAAPTLDELKLE | ||
| P85421–435 | APTLDELKLEGDEAT | ||
| HSP60 | P253–268 | EGEALSTLVVNKIRGT | [53] |
| Human HSP60 | CAELKKQSKPVT | [55] | |
2.2. Cell-based immunotherapy
Treg numbers and the Treg:Teff ratio decrease progressively in atherosclerotic mice [23]. Adoptive transfer of CD4+CD25+ Treg cells into Apoe−/− mice is atheroprotective and significantly reduces the extent of atherosclerosis when compared to mice injected with CD4+CD25− T cells or PBS [10]. Further research aimed at boosting Treg expansion in vivo via the administration of either non-mitogenic anti-CD3 specific antibody [24] or IL2/anti-IL2 complex [25]. Increased functional Tregs in the circulation and in lymphoid organs correlated with reduced initial and established atherosclerosis.
The protective role of autoantigen-specific T cells has been harnessed in a recent study where native human LDL specific T cells expressing the TCRβ segment TRBV31 were shown to attenuate atherosclerosis when adoptively transferred into human ApoB100-transgenic Ldlr−/− (HuBL) mice [13]. Transfer of ex vivo expanded polyclonal Tregs to treat autoimmune disorders has already been successful in preclinical and phase I clinical trials of type1 diabetes mellitus (T1D) [26, 27]. However, Tregs may lose their functionality and adopt a CD4+ T effector phenotype under inflammatory conditions [12, 28], which raises safety concerns. Furthermore, the best dose and the frequency of cell infusions is unknown.
3. Vaccination as a promising strategy to combat atherosclerosis
In contrast to the aforementioned passive immunization strategies such as cell-based therapies or antibody administration, vaccination involves an active immunization process that can induce a durable and highly specific anti-atherogenic adaptive immune response in the recipient. However, unlike vaccines for infectious diseases and cancer that aim to boost pro-inflammatory and lytic T cell response, atherosclerosis vaccine formulations need to induce immunological tolerance and/or functional neutralization to alleviate the inflammatory response. Fundamentally, there are two strategies: inducing B cell-dependent production of neutralizing antibodies that can either block protein function (eg: anti-PCSK9) to improve lipid profiles or may facilitate increased oxLDL uptake by phagocytosis and subsequent clearance from circulation (eg: anti-oxLDL); inducing a durable Treg or Tr1 response. Ideally, vaccination promises specific and long-term protection, is inexpensive and thus accessible to millions of people around the globe.
3.1. Vaccines aimed at producing neutralizing antibodies
3.1.1. oxLDL and ApoB
Structural modifications of LDL such as oxLDL or MDA-LDL create new epitopes (neoepitopes) that elicit T cell independent B1 cell responses producing low-affinity IgM autoantibodies [14]. Detection of such autoantibodies in animal and human atherosclerotic lesions [29, 30] led to early studies aimed at restraining atherosclerosis by immunizing animals with MDA-LDL, oxLDL or native LDL [31, 32], (Table 1). In all these studies, a significant reduction in atherosclerotic lesion formation was demonstrated. Immunization with MDA-LDL but not native LDL generated antibodies against oxidation-specific epitopes, which correlated with atheroprotection, but causality was not rigorously tested [31]. Specific MDA-adducts have been shown to be antigenic and could induce hapten-specific Th2-biased humoral and cellular response upon immunization [33]. This study proved important for the assessment of atheroprotective properties of anti-MDA specific antibodies.
Several studies showed that high levels of IgG reactive to MDA-p45 and native-p210 epitopes from ApoB-100 were associated with atheroprotection [34–36]. A protective role of IgM antibodies against MDA-modified APOB-100 peptides has also been suggested by a clinical study where higher levels of IgM antibodies were associated with lower risks of coronary disease [37]. In an attempt to find the antigenic determinant within ApoB-100, Fredrikson et al., screened native and MDA-modified ApoB-100 for epitopes reactive to autoantibodies in sera from patients with coronary heart disease, and identified a number of highly reactive peptides such as p210, p45 and p32 [38]. Later reports showed that subcutaneous immunization of Apoe−/− mice with some of these peptides along with alum as adjuvant had an anti-atherogenic effect, and was associated with antibodies recognizing these peptides [39–41].
3.1.2. PCSK9
Momtazi-Borojeni et al. developed a nanoliposomal peptide-based immunization approach to inhibit PCSK9 activity [42]. Alum formulated nanoliposomal PCSK9-specific peptide was administered subcutaneously, four times in bi-weekly intervals, to BALB/c mice. The immunization induced a humoral response against PCSK9 and caused a significant reduction in plasma PCSK9 that was persistent up to one year. In another study, APOE*3Leiden.CETP mice were used as a model for atherosclerosis. In these mice, administration of a PCSK9-peptide vaccine reduced aortic lesion size and significantly decreased plasma lipid by inducing a long-term humoral response against PCSK9 [43]. Similarly, vaccination with PCSK9 peptides conjugated to virus-like particle from bacteriophage Qβ induced a strong humoral response in BALB/c mice and macaques [44].
3.2. Vaccines aimed at inducing an atheroprotective Treg response
3.2.1. ApoB
Given that oxLDL and its major protein component ApoB are highly associated with autoimmune-mediated atherosclerotic response, our group sought to evaluate MHC ClassII-restricted murine ApoB peptides (Table 1). Using a competitive peptide binding assay, we identified 65 ApoB peptides that bind I-Ab with <1μM affinity [45, 46]. One of these peptides, namely P18, is sequence identical in mouse and human APOB and binds both mouse MHC-II (I-Ab) and human (DRB1*0101, DRB1*0701 and several other) MHC-II alleles [47]. Selected peptides were emulsified in Complete Freund’s adjuvant (CFA) and administered by the subcutaneous route to 8-week old Apoe−/− mice. This was followed by four booster injections emulsified in Incomplete Freund’s adjuvant (IFA), administered by the intraperitoneal route every 4 weeks. This regimen reduced the lesion size in whole aorta by 40% when compared with adjuvant alone. Moreover, immunization with two of these peptides caused a reduction in aortic-root lesion area [45, 46]. The protection induced by these vaccinations was associated with an increase in IL-10 producing T-cells [45–47] as well as an induction of Foxp3+ Tregs [46, 47] in the peritoneal cavity. Activation of peptide-specific CD4+ T cells was demonstrated by antigen-specific proliferation assays in vitro [45] or through in vivo evaluation of activation by immunizing Nur77GFP mice [46, 47], in which T cells transiently upregulate GFP after antigen encounter but not by inflammatory stimuli [48]. The Nur77 GFP mouse expresses the green fluorescent reporter protein under the promoter of Nr4a1 (Nur77), an immediate early gene whose expression is rapidly and transiently upregulated in activated lymphocytes. This mouse is an important transgenic model to distinguish between true antigen-mediated T cell stimulation from bystander activation by inflammatory stimuli. pMHC-II tetramer staining was used to examine the number and phenotype of ApoB-100-specific CD4+ T cells (p18:I-Ab+) [47]. pMHC-II are tetramers of recombinant MHC-II molecules loaded with the relevant peptide. 40% of the p18:I-Ab+ CD4+ T cells upregulated Foxp3 upon immunization with P18 peptide, compared to only 9% in the p18:I-Ab− CD4+ T population.
3.2.2. Heat shock Proteins
Human and microbial HSPs share high sequence homology. This is thought to cause cross-reactivity, possibly triggering pathological autoimmune responses. Human HSP60/65 is expressed by endothelial cells in pathologic conditions such as atherosclerosis [49]. Antibodies against HSP60/65 have been shown to be elevated in serum of patients suffering from cardiovascular diseases [50]. This prompted the evaluation of the therapeutic potential of HSP60/65 vaccination in atherosclerosis (Table 1). Indeed, immunization with whole HSP60/65 protein or HSP60 peptides was shown to reduce early atherosclerotic lesions [51–55]. The protection observed in these studies was accompanied by an increase in IL-10 producing cells and expansion of CD4+CD25+Foxp3+ Treg cells.
4. Correlative evidence for the role of the adaptive immune system in CVD patients
The progress made in unravelling the key role of inflammation and the immune system at various stages of CVD in animal models motivated further research to find evidence for similar contributions of the immune components in the pathogenesis of human atherosclerosis. Using computational approaches such as genome-wide association studies (GWAS), large-scale population studies have identified Single Nucleotide Polymorphisms in inflammatory genes and non-coding regulatory loci that correlate with CAD and MI [56–58].
4.1. Network analysis of gene expression to find immune-associated signatures in the blood of participants in the Framingham Heart Study
Huan et.al., undertook a systems biology approach that integrated whole blood transcriptomes with network approaches, genome-wide association studies (GWAS), and genetics [59]. Blood samples from 188 pairs of CHD cases and controls from the Framingham Heart Study, a longitudinal cardiovascular cohort study, were used for analysis. Their analysis identified co-expression modules that differ between cases and controls. Next, they screened for an enrichment of CHD-associated Expression Quantitative Trait Loci (eQTLs), which are genomic regions whose variations account for differences in expression of the differentially regulated genes. Finally, using Bayesian networks (BNs) and protein-protein interaction (PPI) circuits, they identified putative upstream modulators of the differentially expressed genes. The genes that were most strongly enriched in the controls, but not in the CHD patients, were those associated with B cell activation, indicating a protective role of the humoral immune response in atherosclerosis.
4.2.1. Correlations between antibodies and carotid plaque characteristics
A large number of clinical observational studies have demonstrated an association between CVD and antibodies against self-antigens. In a population-based cohort of 1,022 subjects, levels of plasma IgM against oxLDL inversely correlated with carotid artery intima media thickness (IMT) [60]. Clinical studies measuring autoantibodies have shown that high levels of plasma IgG correlated with increased lipid content of the plaques, while IgM levels were associated with less lipids and macrophages [61]. A study by Khamis et.al. showed that in a hypertensive population, total serum IgG levels, and to a lesser extent IgM levels, correlate inversely with CHD risks [62]. Importantly, they show that IgG and IgM antibodies against MDA-LDL are no longer associated with CV events if adjusted for total serum IgG and IgM levels, respectively. Human atherosclerosis is also associated with abundant expression of HSPs and elevated levels of anti-HSP65 in the serum [63].
4.2.2. T cells in human atherosclerosis
Immunohistochemical methods have detected CD3+ T cells in both human coronary arteries and ruptured coronary plaques [64]. Evidence for a local antigen-driven immune response emerged from T cell repertoire analysis in coronary plaque specimens from acute coronary syndrome (ACS) patients [65]. Unstable plaques not only had greater T cell content (as demonstrated by quantitative RT-PCR) but the array of their Complementarity Determining Region (CDR3) motifs, as assessed by spectratyping, was also skewed, indicating selective expansion of specific T-cell clones [65]. Spectratyping involves PCR-based amplification of CDR3 variable region whose sequence variations account for most of the variability associated with T cell Receptors (TCR). The length and sequence pattern is unique to individual T cell clones.
HLA-restricted proliferative capacity and cytokine production have been demonstrated against oxLDL and human HSP60 epitopes in T cells from atherosclerotic lesions [66]. Based on FACS-based Intracellular-cytokine staining after PMA ionomycin stimulation, Interferon gamma (IFN-γ) producing CD4+ effector memory T cells (TEM) seemed to be the dominant T cell subtype in the lesions [66].
Immunohistochemistry and qRT-PCR to detect IL-2 and IFN-γ in human carotid plaques [7] revealed expansion of Th1 cells in patients with unstable CAD, accompanied by a concomitant decrease in CD4+CD25+Foxp3+ T lymphocytes [67]. FACS analysis of T cell subset-specific gene expression and ELISA of plasma cytokines demonstrated increased Th1 cells and decreased Tregs in the peripheral blood of patients with ACS [68]. This was accompanied by elevated levels of IFN-γ in the plasma of CAD patients, while plasma TGF-β1 was decreased in ACS patients as compared to those with normal coronary arteries. A perturbed Th17/Treg balance was also reported in ACS patients with increased ratio of RORγt+/Foxp3+ T cells in circulation, in conjunction with higher levels of Th17 related cytokines (IL-17, IL-6 and IL-23) and diminished Treg related cytokines (IL-10 and TGF-β1) [69].
Immunohistochemical staining of stable and unstable carotid plaques revealed reduced numbers of Tregs in vulnerable plaques, which are characterised by thin caps, increased immune cell infiltration and hence enhanced susceptibility to rupture [70]. CD4+CD25+ Tregs present in the circulation of ACS patients were not only low in number but also functionally compromised [71]. The in vitro suppressive ability of Tregs is often mediated by IL-10. Low plasma levels of IL-10 have been associated with deterioration of coronary disease, highlighting a protective role of IL-10 in CAD [67].
Using DRB1*0701 tetramers loaded with the APOB peptide p18 showed that APOB-specific CD4+ T cells in blood from donors without cardiovascular disease were mostly Foxp3+ Tregs. In donors with subclinical CVD as detected by carotid artery ultrasound, the APOB specific CD4+ T cells increased in number and the bulk of them expressed both FoxP3 and the Th17 transcription factor RORγt [47].
4.2.3. B cells in human atherosclerosis
B cells have been identified in the adventitia and intima of coronary artery samples [64]. Antigen-driven clonal expansion of B cells has been detected in coronary plaques [72]. Hamze et al characterized the immunoglobulin repertoire in human carotid endarterectomy samples and provided evidence for ongoing local B cell differentiation and maturation in the arterial walls [73]. Many B cells reside in artery tertiary lymphoid organs (ATLOs), which develop in aortas of old mice and are found in human coronary arteries with atherosclerosis [74]. It is important to also mention that the vast majority of experimental studies showing the impact of B cells in atherosclerosis have been conducted in young animals that typically do not present ATLOs, suggesting that B cells may influence atherosclerosis via different anatomical sites and at different stages of the disease. B-1 cells present in the ATLOs have been reported to secrete IgM antibodies locally [75]. Natural IgMs are known to protect children from Strepcoccus pneumoniae infections and cross-react with oxidation-specific epitopes (OSEs) [76]. An inverse correlation between OSE-specific IgM and carotid artery IMT has been observed in several clinical studies [60, 61]. Another study showed differential association of two different B cell subsets with risk for acute CV events [77]. While protective CD19+CD40+ B cells correlated negatively with risks of a stroke event, CD19+CD86+ B cell subset was associated with a proinflammatory phenotype and increased risk for development of stroke.
4.2.4. Cytokines
All major T-cell related cytokines such as IFN-γ (Th1), IL-17 (Th17), IL-4,5,13 (Th2) and IL-10 (Tregs) are critical modulators of atherosclerosis. Blocking IL17A signaling through administration of anti-IL17A antibody in atherosclerotic mice led to reduced formation and increased stability of plaques [78]. IL17 neutralizing antibodies such as secukinumab have been successfully tested in clinical trials for autoimmune diseases such as psoriasis. However, inhibitors of the IL17A pathway should be used with caution as more unexpected MACE were reported in the treated group compared to placebo-treated subjects [79, 80]. Administration of IL-2/anti-IL2 mAb complexes promotes Treg expansion and has proved successful in reducing lesion development and disease progression in mouse models [25]. Currently, treatment with recombinant IL2 (aldesleukin) is approved for renal cell carcinoma and metastatic melanoma. Zhao et al. proposed to use low dose IL-2 to specifically expand Treg cells, thereby limiting immune responses in patients with ACS and stable ischemic heart disease [81]. The primary endpoint of this trial (Trial registration number NCT03113773) is to show that aldesleukin administration is safe and will determine the dose needed to elevate the number of Treg cells in circulation by at least 75%.
5. Path to an atherosclerosis vaccine for humans: hurdles and opportunities
Despite the complex pathology of cardiovascular diseases in humans, it is becoming increasingly clear that disease-related immune responses in patients bear striking resemblance to those identified in animal models. The success of mAbs against PCSK9 in humans [82] provides strong support for antigen-specific immunotherapy, particularly vaccination. Here, we provide an update of the vaccines that have been evaluated in clinical trials for human atherosclerosis (Table 2).
Table 2 –
Summary of clinical trials for vaccines to modulate atherosclerosis
| Identifier | Status | Target | Study type | Subjects | Biologicals | Adjuvant, route and immunization regime | Primary outcome measures | Secondary outcome measures | Observations |
|---|---|---|---|---|---|---|---|---|---|
| NCT01284582 | Complete (Study completion date July, 2012) | Cholesterol Ester Transfer Protein (CETP) | Interventional, Non-randomized, open-label Phase 1 study | 36 healthy male subjects (aged 18–65 years) with HDLc levels in the blood equal to or below 80 mg/dl. | ATH03 | Participants were divided into three equal groups. Each group received four vaccinations of three different doses of the vaccine – either 10 or 30 or 100μg ATH03 in 0.2% alum | Occurrence of adverse events [Time Frame: 264 days] |
Immunogenicity and response to the various applied doses of ATH03 [Time Frame: 264 days] |
Different doses of ATH03 vaccine were found to be safe, without any significant clinical adverse effects |
| NCT02508896 | Complete (Study completion date August, 2017) | Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) | Interventional, single blind, single-centre, randomized, placebo-controlled, parallel group, phase I clinical trial | 72 healthy subjects of either sex (aged 18–65 years) with body weight > 50 kg and a body mass index (BMI) between 19 and 35. | PCSK9 peptides - AT04A or AT06A | Participants were divided into three equal groups – two treatment and one placebo group. Three sub-cutaneous injections of priming immunizations at a dose of 15μg were given at days 0, 28 and 56. One boost immunization at a dose of 75μg was applied one year after the 3rd immunization (day 420). Adjuvant used was Aluminium oxy-hydroxide. | Occurrence of serious and Grade3 or higher AE (Adverse Events) Evaluation of solicited local and systemic AEs and unsolicited non-serious AEs [Time Frame: 21 months] |
Evaluation of immunological activity of AFFITOPE® AT04A and AT06A - Titre of vaccination-induced antibodies against peptide components of the vaccine and the carrier Measurement of Mean Levels and change from baseline of LDLc, HDLc, VLDL, Total cholesterol, triglycerides, PCSK9 Correlating the strength of antibody responses to Lipid lowering effects [Time Frame: 21 months] |
Results are yet to be published |
| ACTRN12615000536561 | Ongoing Active, not recruiting | Pneumococcal poly-saccharide, cross-reaction to ox-LDL | Interventional, multi-centre, randomized, double-blind, placebo-controlled, parallel group clinical trial | 4725 participants of either sex, aged between 55–60 years with no history of CVD events, but with two or more risk factors for CVD. Risk factors include high cholesterol, hypertension, elevated BMI (≥27 kg/m2), and elevated waist circumference (>88 cm for women and >102 cm for men). | Pneumovax 23 (Merck Sharp & Dohme Australia Pty Ltd). A 23-valent Pneumo-coccal poly-saccharide vaccine (PPV) | Treatment group receives intramuscular injection of a single dose of (0.25μg) PPV. The control group receives equivalent volume of normal saline. | Assessment of CVD events such as fatal and non-fatal acute coronary syndrome and ischaemic stroke, through linkage of participant medical records to health databases. Diagnosis of unstable angina, acute myocardial infarction and cerebral infarction will be based on ICD-codes (International Classification of Diseases, 10th Revision). [Time point : 6 years after randomisation, with interim analysis at 3 years after randomisation] | Titres of antibodies against oxLDL and pneumococcal epitopes, measured at baseline, and at the specified time-point Change in pulse wave velocity carotid intima-media thickness at baseline and at the specified time-point [Time point : 6 years after randomisation, with interim analysis at 3 years after randomisation] | Data will be published after study completion |
| None | Completed | Adipose tissue antigens | Small-scale Phase II open label clinical trial | 13 overweight Asian participants (aged between 22–79 years) with abnormal baseline HDL and triglyceride levels, BMI and waist circumference | Athero sclerosis vaccine V-6, a tableted preparation of antigens from pig adipose tissue | Participants were given two V-6 pills, twice-daily, for 3 months (except patient #13 who received V6 for 1 month due to late entry) | Assessment of mid-arm, abdominal and thigh diameters, complete blood counts and standard biochemistry tests including liver, kidney and lipid profile tests were performed at baseline and at specified time-points. [Time point : At 2, 4, 8, and 12 weeks intervals] | Not applicable. | V6 treatment was well tolerated. Although cholesterol and LDL levels remained unchanged, triglyceride levels declined by 26.1% and HDL levels increased by 25.9%. There was statistically significant reduction in waist, mid-arm and thigh circumference, with no change in weight and BMI being observed [94,95]. |
| NCT03042741 | Ongoing Estimated completion date December, 2018 | Adipose tissue antigens | Interventional, randomized, double-blind, placebo-controlled, parallel group Phase III clinical trial | 300 obese individuals (baseline waist diameter > 90 for men and >80 cm for women), ages 18 years or above. | Athero sclerosis vaccine V-6, a tableted preparation of antigens from pig adipose tissue | The treatment group is given a daily dose of one V6 pill, administered for one month. The remaining half of the participants receive placebo pill. | Monitoring changes in lipid profile as measured by difference in LDL, HDL, triglycerides and total cholesterol before and after treatment. [Time Frame: 1 month] |
Evaluation of effects on anthropomorphic indices of obesity (changes in diameter of waist, mid-arm and hip after one month of treatment versus baseline measurements) Evaluation of effects on hypertension (systolic and diastolic blood pressures measured one month apart). Evaluation of differences in fasting blood glucose levels measured at baseline and at post-treatment time-point. [Time Frame: 1 month] |
Data will be published after study completion |
5.1. An update on clinical trials
a). to improve the lipid profile
CETP vaccine trial –
The first human atherosclerosis vaccine was directed against the Cholesterol Ester Transfer Protein (CETP), a key enzyme in HDL metabolism [83] whose inhibition changes the ratio of LDL to HDL cholesterol. Single injection of the CETi-1 vaccine induced anti-CETP antibodies in only one out of 36 patients [83]. However, a second injection resulted in specific humoral response in 53% of treated individuals, as compared to 0% in the placebo group. No significant changes in HDL levels were observed. Later, an Austrian company, AFFiRiS evaluated the safety and immunogenicity of another CETP vaccine, ATH03 (ClinicalTrials.gov NCT01284582). This non-randomized, open-label Phase 1 study included three groups, each with 12 healthy males having blood HDLc levels at or below 80 mg/dl. The study reported ATH03 to be safe and well tolerated in groups that received four vaccinations of 10 or 30 or 100 μg of ATH03 in 0.2% alum. No evaluation of HDL levels was reported.
PCSK9-based vaccine trials –
Human mAbs against PCSK9 (alirocumab and evolocumab) have already been approved by the European Commission (EC) and US Food and Drug Administration (FDA) as adjunct therapy for hypercholesterolemia. AFFiRiS, in collaboration with the Medical University of Vienna are currently testing two PCSK9-based peptide vaccines (AFFITOPE AT04A AND AT06A) ( ClinicalTrials.gov NCT02508896). In this single-blind, randomized study, 72 healthy subjects were divided into three groups – one placebo and 2 test groups. The treatment groups received three subcutaneous priming injections of either one of the two peptides adsorbed to 1 mg aluminium oxyhydroxide and a boost, one year later. The primary outcome measures included occurrence of Serious Adverse Events (SAE). These are defined by norms of FDA and include adverse outcomes such as death, life-threatening experience, inpatient or prolonged hospitalization, disability or permanent damage. Secondary outcomes involved measure of titres of antibodies directed against the test peptides and changes in mean cholesterol levels (total, VLDL, HDL), triglycerides and PCSK9 levels. This study evaluated the safety of the PCSK9 vaccines and tested their efficacies in lowering lipid levels through an induction of humoral response. Protective or potentially deleterious T cell responses were not analyzed. This Phase I trial has been completed on August 31, 2017. The results are yet to be published.
b). to directly modulate the inflammatory response
AUSPICE trial –
Vaccinating mice with a Streptococcus pneumoniae vaccine has been shown to reduce atherosclerosis burden, associated with inducing antibodies that cross-react with oxLDL [76]. A systematic meta-analysis of acute-coronary syndrome (ACS) events in 8 human observational studies that tested pneumococcal polysaccharide vaccine (PPV) as an intervention revealed that PPV is associated with a 17% reduction in the risk for ACS events in patients older than 65 years [84]. This same group has now undertaken the first registered randomized, double-blind, placebo-controlled clinical trial, the AUSPICE, to evaluate the cardioprotective potential of the 23-valent PPV [85]. (Australia and New Zealand trial registry ACTRN12615000536561). The ongoing trial includes participants in the age range of 55–60 years, with no history of CVD events, but at an increased risk of developing heart disease. While the treatment group received intramuscular injection of a single dose of PPV, the control group received saline. The primary outcome is the incidence of fatal and non-fatal CVD events such as unstable angina, acute myocardial infarction and cerebral infarction, over a period of 6 years with interim analysis at 3 years after randomization. Serum antibody titres against oxLDL and pneumococcus and surrogate measures such as carotid IMT will be compared between vaccinated and placebo groups.
V-6 vaccine trials –
Both atherosclerosis and obesity are the results of abnormal lipid metabolism, which are major risk factors for CHD. It has been demonstrated that obesity, like atherosclerosis, is also associated with chronic inflammation mediated by the host immune system against self-antigens [86]. One group hypothesised that oral administration of pooled adipose tissue antigens in overweight patients can skew the autoimmune response towards tolerance and induce a favourable lipid profile. They conducted two small-scale Phase II open label clinical trials [87, 88] to study the safety and efficacy of the atherosclerosis vaccine V-6, a tableted preparation of antigens from pig adipose tissue. Although these preliminary studies had small sample size (12–13 volunteers) with short-term follow-up of only 2–3 months, significant increases in HDL levels and reduced triglyceride levels were observed. This led to a large-scale, randomized, double-blind, placebo-controlled, phase III trial of the V-6 vaccine ( ClinicalTrials.gov NCT03042741). The study includes 300 obese individuals. While one half of the group received V-6 tablets, the remaining subjects received placebo pills once per day for one month. Primary outcome measures include a change in lipid profile (total cholesterol, LDL, HDL, and triglycerides). The secondary outcome measures monitor effects on obesity indices such as diameter of waist, mid-arm and hip, changes in systolic and diastolic blood pressures, and blood glucose levels. The estimated date for completion of this study was December 18, 2018. This clinical trial, which aims to treat atherosclerosis in overweight people, does not monitor cardiovascular risk factors or immunological parameters.
In Europe, a team of experts from academic research institutions, small and medium-sized enterprises (SMEs), and pharmaceutical industry have come together under the Vaccination in Atherosclerosis (VIA) Consortium to develop a vaccine for atherosclerosis that can substantially lower the risk of coronary disease (http://www.viavaccine.com). The major focus of the program is to optimize a therapeutic vaccine that can reverse atherosclerosis-associated inflammatory responses and restore immune homeostasis within the arterial walls. They plan to conduct phase I clinical trials to determine its safety.
6. Strategies to optimize T cell peptide vaccines for atherosclerosis
T cell vaccine candidates for atherosclerosis have been well studied in animal models, with a focus on ApoB-100 and HSPs. Human data suggests that these proteins likely drive antigen-dependent immune responses in patients as well. For the generation of a successful T cell based vaccine in atherosclerosis it will be important to consider key factors such as appropriate antigen selection, vaccine formulation, dosage and timing.
6.1. Antigen selection
The first step involves the identification and validation of human epitopes and their appropriate presentation by class-II MHC molecules. Although in silico methods predicting binding affinities provide good starting points, a more authentic yet challenging discovery approach is mass spectrometric sequencing of peptides eluted from MHC molecules on cells from human samples [89]. The next step often includes in vitro functional assays such as cytokine production in response to in vitro peptide re-stimulation. Validation steps involve tetramer staining of antigen-specific T cell populations. The enormous heterogeneity of the human HLA locus makes the manufacture of individual peptide-MHC tetramer combination a daunting task. Linking the peptide to Ii protein bypasses HLA-DR restriction since the Ii-key segment binds to all HLA molecules with high affinity [90]. This strategy also ensures preferential loading of the coupled peptide onto MHC molecules as the Ii segment replaces endogenous bound antigens [90]. This strategy has been tested for various peptide-based vaccines for cancer [91].
6.2. Immunization platform
Mucosal vaccination that elicits a tolerogenic response has been shown to trigger both humoral and T cell memory at mucosal sites as well as systemically [92]. Frequent administration of a low dose antigen or a single delivery of high antigen dose can both induce mucosal tolerance. Encapsulation of peptide epitopes within liposomes, microspheres, virus-like-particles and nanoparticle-based delivery systems can improve bioavailability of the peptides across the mucosal barrier and will also protect them from degradative enzymes encountered at mucosal sites. Another new, convenient and cost-effective delivery platform includes plant and bacteria-based vaccines [93, 94]. The ApoB-100 p210 peptide:CETP chimeric protein, fused to non-toxic B subunits of cholera toxin (CTB), expressed in tobacco plants efficiently induced an antigen-specific antibody response in mice [95].
Several reports have confirmed that the pathogenesis of CVD is associated with reduced Treg frequency and a defect in their suppressive phenotype. Therefore, inclusion of an appropriate Treg inducing adjuvant in the vaccination regime may help to dampen disease-associated inflammation. Intranasal administration of p210:CTB fusion to mice resulted in 35% reduction in atherosclerosis via induction of IL-10 producing Tr1 cells that inhibit antigen-specific effector cells [96]. TLR2 signaling has been shown to mediate induction of antigen-specific Treg responses in mouse models of Type I diabetes upon treatment with Diapep277 vaccine [97], highlighting the benefits of using TLR2 ligands as adjuvants. Diapep277 is an HSP60-derived peptide that that was proven to be safe in clinical trials for T1D [98].
6.3. Response duration and stability
Duration of response, safety and protective efficacy of the vaccine need to be thoroughly examined. Conclusions based solely on numbers of peripheral antigen-reactive Tregs may be misleading. It will be more important to consider whether circulating Tregs home efficiently to sites of inflammation and are able to function as effective suppressors. Work from our group and others have shown that Tregs in the inflamed areas undergo phenotypic changes and lose their suppressive capacity [12, 28, 99]. Hence, vaccine design may need to include strategies to maintain Treg functionality under pathological settings. Co-administration of immunosuppressive drugs such as dexamethasone, retinoic acid, vitamin D3 analogs and rapamycin analogs can enhance Treg induction via generation of tolerogenic DCs. Alternately, mAbs that target co-stimulatory pathways can be used to inhibit T effector cell proliferation. For example, Abatacept or CTLA-4-Ig (a fusion protein that blocks B7/CD28 co-stimulation) is approved for autoimmune disorders such as rheumatoid arthritis (RA). Such systemic and broad-spectrum strategies of immunomodulation may weaken host defence.
7. Conclusions
A pivotal role of the adaptive immune system has now been firmly established in animal models of atherosclerosis, with strong correlative evidence emerging from clinical studies as well. These studies have provided ample motivation for the development of novel therapeutic interventions that target inflammation. Global immunosuppressive or anti-cytokine strategies can increase the risk of infections. Monoclonal antibodies to PCSK9 are approved, successful, but inconvenient for the patient and expensive for the health care system. siRNA to PCSK9 promises longer-lived responses of up to 9 months [100]. Pre-clinical data suggests that immunization-induced humoral and cell-based adaptive immune responses can curb the progression of atherosclerosis. However, the antigen selection, vaccine design and the immunization regime need to be optimized before a human atherosclerosis vaccine may be developed (Figure 2). To achieve these goals, it is critical to have a better understanding of the human-specific molecular mechanisms underlying disease progression. Likely, the design and development of a preventative vaccine will differ substantially from that of a therapeutic vaccine.
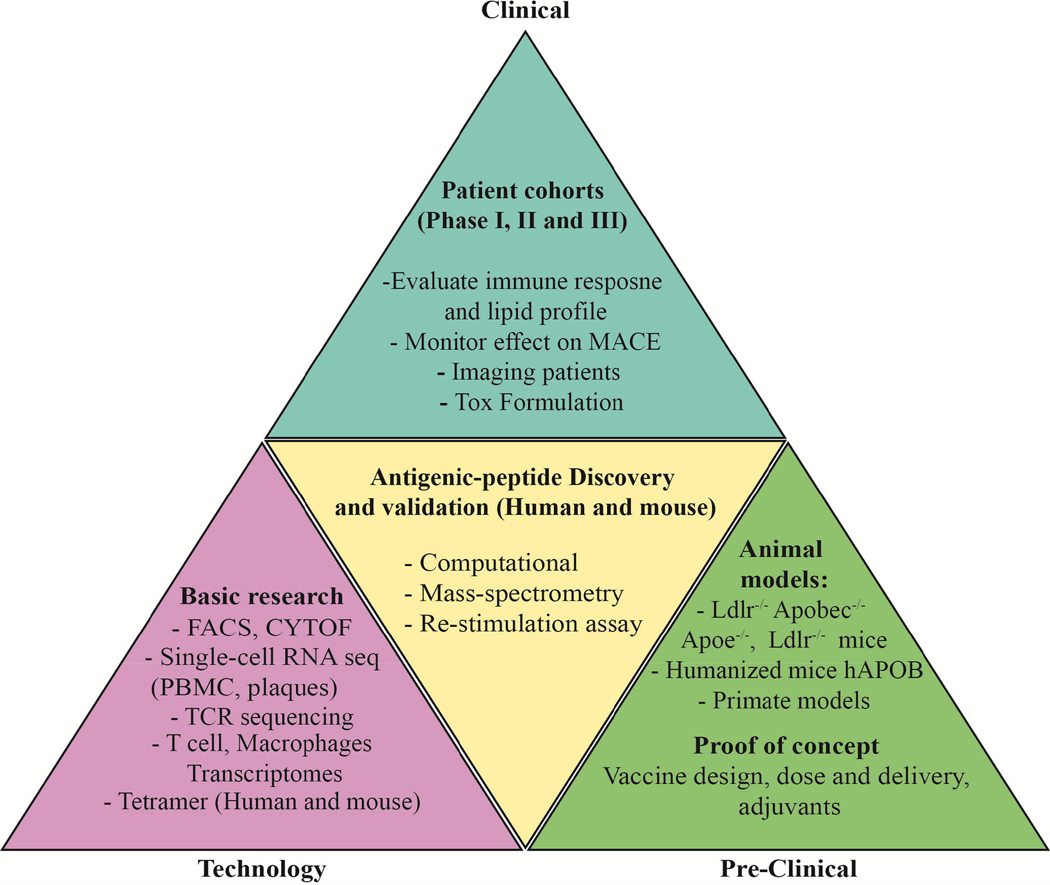
Figure 2. Overview of vaccine design development.
The triangle illustrates the stages required for the development of effective atheroprotective vaccines – the technologies (left, mauve), pre-clinical studies (right, green), antigen discovery (center, yellow) and vaccine formulation, toxicology and clinical studies (top, blue).
Acknowledgments
Conflicts of interest statement
KL received a research contract from United Bioscience (UBI). He is also a co-founder of Atherovax, Inc.
Abbreviations
- ACS
Acute Coronary Syndrome
- ApoB
Apolipoprotein B
- ApoB-100
Apolipoprotein B-100
- ApoE
Apolipoprotein E
- ATLO
Artery Tertiary Lymphoid Organ
- β2GPI
β2-glycoprotein I
- BN
Bayesian networks
- CAD
Coronary artery disease
- CANTOS
Canakinumab Anti-inflammatory thrombosis outcomes study
- CDR3
Complementarity determining region 3
- CETP
Cholesterol ester transfer protein
- CHD
coronary heart disease
- CVD
Cardiovascular diseases
- eQTL
Expression quantitative trait loci
- FDA
Food and drug administration
- FHS
Framingham heart study
- GWAS
Genome-wide association studies
- HDL
High-density lipoprotein
- HSP
Heat shock protein
- HuBL
Human ApoB100-transgenic Ldlr−/−
- IFN- γ
Interferon-γ
- IL
Interleukin
- IMT
Intima media thickness
- KLH
Keyhole Limpet Hemocyanin
- LDL
low-density lipoprotein
- LDL-C
low-density lipoprotein-cholesterol
- LDLR
low-density lipoprotein receptor
- mAb
monoclonal antibody
- MACE
Major adverse cardiovascular events
- MAPP
Mass-spectrometry-based proteomic mapping of MHC-associated eluted peptide
- MDA
Malondialdehyde-modified
- MHC
Major histocompatibility complex
- MI
Myocardial infarction
- OSE
Oxidation-specific epitope
- oxLDL
Oxidized low-density lipoprotein
- PCSK9
Proprotein convertase subtilisin/kexin 9
- PPI
Protein-protein interaction
- PPV
Pneumococcal polysaccharide vaccine
- RA
Rhematoid arthritis
- SAE
Serious Adverse Events
- SNP
Single nucleotide polymorphism
- T1D
Type 1 diabetes
- TCR
T cell receptor
- TEM
Effector memory T cell
- Tfh
Follicular helper T
- TGF-β1
Transforming Growth Factor β-1
- TLR2
Toll Like Receptor 2
- TNF
Tumor Necrosis Factor
- Treg
T regulatory cell
- VDLR
Very low-density lipoprotein receptor
- VIA
Vaccination in Atherosclerosis
- WTCCC
Wellcome Trust Case Control Consortium Study
Footnotes
Declaration of Interest Statement
This manuscript has not been submitted elsewhere.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
10. References
- [1].Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- [2].Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol. 2009;27:165–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sampson UK, Fazio S, Linton MF. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: the evidence, etiology, and therapeutic challenges. Curr Atheroscler Rep. 2012;14:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119–31. [DOI] [PubMed] [Google Scholar]
- [5].Reardon CA, Blachowicz L, White T, Cabana V, Wang Y, Lukens J, et al. Effect of immune deficiency on lipoproteins and atherosclerosis in male apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:1011–6. [DOI] [PubMed] [Google Scholar]
- [6].Buono C, Binder CJ, Stavrakis G, Witztum JL, Glimcher LH, Lichtman AH. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc Natl Acad Sci U S A. 2005;102:1596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Frostegard J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, et al. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145:33–43. [DOI] [PubMed] [Google Scholar]
- [8].Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–80. [DOI] [PubMed] [Google Scholar]
- [9].Gotsman I, Grabie N, Gupta R, Dacosta R, MacConmara M, Lederer J, et al. Impaired regulatory T-cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation. 2006;114:2047–55. [DOI] [PubMed] [Google Scholar]
- [10].Mor A, Planer D, Luboshits G, Afek A, Metzger S, Chajek-Shaul T, et al. Role of naturally occurring CD4+ CD25+ regulatory T cells in experimental atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:893–900. [DOI] [PubMed] [Google Scholar]
- [11].Akhavanpoor M, Akhavanpoor H, Gleissner CA, Wangler S, Doesch AO, Katus HA, et al. The Two Faces of Interleukin-17A in Atherosclerosis. Curr Drug Targets. 2017;18:863–73. [DOI] [PubMed] [Google Scholar]
- [12].Gaddis DE, Padgett LE, Wu R, McSkimming C, Romines V, Taylor AM, et al. Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat Commun. 2018;9:1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gistera A, Klement ML, Polyzos KA, Mailer RK, Duhlin A, Karlsson MCI, et al. LDL-Reactive T Cells Regulate Plasma Cholesterol Levels and Development of Atherosclerosis in Humanized Hypercholesterolemic Mice. Circulation. 2018;138(22):2513–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rosenfeld SM, Perry HM, Gonen A, Prohaska TA, Srikakulapu P, Grewal S, et al. B-1b Cells Secrete Atheroprotective IgM and Attenuate Atherosclerosis. Circ Res. 2015;117:e28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kyaw T, Tay C, Krishnamurthi S, Kanellakis P, Agrotis A, Tipping P, et al. B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ Res. 2011;109:830–40. [DOI] [PubMed] [Google Scholar]
- [16].Meier LA, Binstadt BA. The Contribution of Autoantibodies to Inflammatory Cardiovascular Pathology. Front Immunol. 2018;9:911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bagchi-Chakraborty J, Francis A, Bray T, Masters L, Tsiantoulas D, Nus M, et al. B Cell Fcgamma Receptor IIb Modulates Atherosclerosis in Male and Female Mice by Controlling Adaptive Germinal Center and Innate B-1-Cell Responses. Arterioscler Thromb Vasc Biol. 2019;39:1379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Centa M, Jin H, Hofste L, Hellberg S, Busch A, Baumgartner R, et al. Germinal Center-Derived Antibodies Promote Atherosclerosis Plaque Size and Stability. Circulation. 2019;139:2466–82. [DOI] [PubMed] [Google Scholar]
- [19].Schiopu A, Bengtsson J, Soderberg I, Janciauskiene S, Lindgren S, Ares MP, et al. Recombinant human antibodies against aldehyde-modified apolipoprotein B-100 peptide sequences inhibit atherosclerosis. Circulation. 2004;110:2047–52. [DOI] [PubMed] [Google Scholar]
- [20].Warden BA, Fazio S, Shapiro MD. The PCSK9 revolution: Current status, controversies, and future directions. Trends Cardiovasc Med. 2019. [DOI] [PubMed] [Google Scholar]
- [21].Zhang DW, Lagace TA, Garuti R, Zhao Z, McDonald M, Horton JD, et al. Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation. J Biol Chem. 2007;282:18602–12. [DOI] [PubMed] [Google Scholar]
- [22].Hlatky MA, Kazi DS. PCSK9 Inhibitors: Economics and Policy. J Am Coll Cardiol. 2017;70:2677–87. [DOI] [PubMed] [Google Scholar]
- [23].Maganto-Garcia E, Tarrio ML, Grabie N, Bu DX, Lichtman AH. Dynamic changes in regulatory T cells are linked to levels of diet-induced hypercholesterolemia. Circulation. 2011;124:185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kita T, Yamashita T, Sasaki N, Kasahara K, Sasaki Y, Yodoi K, et al. Regression of atherosclerosis with anti-CD3 antibody via augmenting a regulatory T-cell response in mice. Cardiovasc Res. 2014;102:107–17. [DOI] [PubMed] [Google Scholar]
- [25].Dinh TN, Kyaw TS, Kanellakis P, To K, Tipping P, Toh BH, et al. Cytokine therapy with interleukin-2/anti-interleukin-2 monoclonal antibody complexes expands CD4+CD25+Foxp3+ regulatory T cells and attenuates development and progression of atherosclerosis. Circulation. 2012;126:1256–66. [DOI] [PubMed] [Google Scholar]
- [26].Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Derkowska I, Juscinska J, et al. Therapy of type 1 diabetes with CD4(+)CD25(high)CD127-regulatory T cells prolongs survival of pancreatic islets - results of one year follow-up. Clin Immunol. 2014;153:23–30. [DOI] [PubMed] [Google Scholar]
- [27].Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7:315ra189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Butcher MJ, Filipowicz AR, Waseem TC, McGary CM, Crow KJ, Magilnick N, et al. Atherosclerosis-Driven Treg Plasticity Results in Formation of a Dysfunctional Subset of Plastic IFNgamma+ Th1/Tregs. Circ Res. 2016;119:1190–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yla-Herttuala S, Palinski W, Butler SW, Picard S, Steinberg D, Witztum JL. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb. 1994;14:32–40. [DOI] [PubMed] [Google Scholar]
- [30].Tsimikas S, Palinski W, Witztum JL. Circulating autoantibodies to oxidized LDL correlate with arterial accumulation and depletion of oxidized LDL in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:95–100. [DOI] [PubMed] [Google Scholar]
- [31].George J, Afek A, Gilburd B, Levkovitz H, Shaish A, Goldberg I, et al. Hyperimmunization of apo-E-deficient mice with homologous malondialdehyde low-density lipoprotein suppresses early atherogenesis. Atherosclerosis. 1998;138:147–52. [DOI] [PubMed] [Google Scholar]
- [32].Asgary S, Saberi SA, Azampanah S. Effect of immunization against ox-LDL with two different antigens on formation and development of atherosclerosis. Lipids Health Dis. 2007;6:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gonen A, Hansen LF, Turner WW, Montano EN, Que X, Rafia A, et al. Atheroprotective immunization with malondialdehyde-modified LDL is hapten specific and dependent on advanced MDA adducts: implications for development of an atheroprotective vaccine. J Lipid Res. 2014;55:2137–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Svenungsson E, Engelbertsen D, Wigren M, Gustafsson JT, Gunnarsson I, Elvin K, et al. Decreased levels of autoantibodies against apolipoprotein B-100 antigens are associated with cardiovascular disease in systemic lupus erythematosus. Clin Exp Immunol. 2015;181:417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McLeod O, Silveira A, Fredrikson GN, Gertow K, Baldassarre D, Veglia F, et al. Plasma autoantibodies against apolipoprotein B-100 peptide 210 in subclinical atherosclerosis. Atherosclerosis. 2014;232:242–8. [DOI] [PubMed] [Google Scholar]
- [36].Fredrikson GN, Schiopu A, Berglund G, Alm R, Shah PK, Nilsson J. Autoantibody against the amino acid sequence 661–680 in apo B-100 is associated with decreased carotid stenosis and cardiovascular events. Atherosclerosis. 2007;194:e188–92. [DOI] [PubMed] [Google Scholar]
- [37].Bjorkbacka H, Alm R, Persson M, Hedblad B, Nilsson J, Fredrikson GN. Low Levels of Apolipoprotein B-100 Autoantibodies Are Associated With Increased Risk of Coronary Events. Arterioscler Thromb Vasc Biol. 2016;36:765–71. [DOI] [PubMed] [Google Scholar]
- [38].Fredrikson GN, Hedblad B, Berglund G, Alm R, Ares M, Cercek B, et al. Identification of immune responses against aldehyde-modified peptide sequences in apoB associated with cardiovascular disease. Arterioscler Thromb Vasc Biol. 2003;23:872–8. [DOI] [PubMed] [Google Scholar]
- [39].Fredrikson GN, Andersson L, Soderberg I, Dimayuga P, Chyu KY, Shah PK, et al. Atheroprotective immunization with MDA-modified apo B-100 peptide sequences is associated with activation of Th2 specific antibody expression. Autoimmunity. 2005;38:171–9. [DOI] [PubMed] [Google Scholar]
- [40].Chyu KY, Zhao X, Reyes OS, Babbidge SM, Dimayuga PC, Yano J, et al. Immunization using an Apo B-100 related epitope reduces atherosclerosis and plaque inflammation in hypercholesterolemic apo E (−/−) mice. Biochem Biophys Res Commun. 2005;338:1982–9. [DOI] [PubMed] [Google Scholar]
- [41].Fredrikson GN, Bjorkbacka H, Soderberg I, Ljungcrantz I, Nilsson J. Treatment with apo B peptide vaccines inhibits atherosclerosis in human apo B-100 transgenic mice without inducing an increase in peptide-specific antibodies. J Intern Med. 2008;264:563–70. [DOI] [PubMed] [Google Scholar]
- [42].Momtazi-Borojeni AA, Jaafari MR, Badiee A, Sahebkar A. Long-term generation of antiPCSK9 antibody using a nanoliposome-based vaccine delivery system. Atherosclerosis. 2019;283:69–78. [DOI] [PubMed] [Google Scholar]
- [43].Landlinger C, Pouwer MG, Juno C, van der Hoorn JWA, Pieterman EJ, Jukema JW, et al. The AT04A vaccine against proprotein convertase subtilisin/kexin type 9 reduces total cholesterol, vascular inflammation, and atherosclerosis in APOE*3Leiden.CETP mice. Eur Heart J. 2017;38:2499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Crossey E, Amar MJA, Sampson M, Peabody J, Schiller JT, Chackerian B, et al. A cholesterol-lowering VLP vaccine that targets PCSK9. Vaccine. 2015;33:5747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tse K, Gonen A, Sidney J, Ouyang H, Witztum JL, Sette A, et al. Atheroprotective Vaccination with MHC-II Restricted Peptides from ApoB-100. Front Immunol. 2013;4:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kimura T, Tse K, McArdle S, Gerhardt T, Miller J, Mikulski Z, et al. Atheroprotective vaccination with MHC-II-restricted ApoB peptides induces peritoneal IL-10-producing CD4 T cells. Am J Physiol Heart Circ Physiol. 2017;312:H781–H90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kimura T, Kobiyama K, Winkels H, Tse K, Miller J, Vassallo M, et al. Regulatory CD4(+) T Cells Recognize MHC-II-Restricted Peptide Epitopes of Apolipoprotein B. Circulation. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wu T, Tanguay RM. Antibodies against heat shock proteins in environmental stresses and diseases: friend or foe? Cell Stress Chaperones. 2006;11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Grundtman C, Kreutmayer SB, Almanzar G, Wick MC, Wick G. Heat shock protein 60 and immune inflammatory responses in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:960–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jing H, Yong L, Haiyan L, Yanjun M, Yun X, Yu Z, et al. Oral administration of Lactococcus lactis delivered heat shock protein 65 attenuates atherosclerosis in low-density lipoprotein receptor-deficient mice. Vaccine. 2011;29:4102–9. [DOI] [PubMed] [Google Scholar]
- [52].Grundtman C, Jakic B, Buszko M, Onestingel E, Almanzar G, Demetz E, et al. Mycobacterial heat shock protein 65 (mbHSP65)-induced atherosclerosis: Preventive oral tolerization and definition of atheroprotective and atherogenic mbHSP65 peptides. Atherosclerosis. 2015;242:303–10. [DOI] [PubMed] [Google Scholar]
- [53].van Puijvelde GH, van Es T, van Wanrooij EJ, Habets KL, de Vos P, van der Zee R, et al. Induction of oral tolerance to HSP60 or an HSP60-peptide activates T cell regulation and reduces atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2677–83. [DOI] [PubMed] [Google Scholar]
- [54].Klingenberg R, Ketelhuth DF, Strodthoff D, Gregori S, Hansson GK. Subcutaneous immunization with heat shock protein-65 reduces atherosclerosis in Apoe(−)/(−) mice. Immunobiology. 2012;217:540–7. [DOI] [PubMed] [Google Scholar]
- [55].Mundkur LA, Varma M, Shivanandan H, Krishna D, Kumar K, Lu X, et al. Activation of inflammatory cells and cytokines by peptide epitopes in vitro: a simple in-vitro screening assay for prioritizing them for in-vivo studies. Inflamm Res. 2013;62:471–81. [DOI] [PubMed] [Google Scholar]
- [56].Fava C, Montagnana M. Atherosclerosis Is an Inflammatory Disease which Lacks a Common Anti-inflammatory Therapy: How Human Genetics Can Help to This Issue. A Narrative Review. Front Pharmacol. 2018;9:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Khera AV, Kathiresan S. Genetics of coronary artery disease: discovery, biology and clinical translation. Nat Rev Genet. 2017;18:331–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Erdmann J, Kessler T, Munoz Venegas L, Schunkert H. A decade of genome-wide association studies for coronary artery disease: the challenges ahead. Cardiovasc Res. 2018;114:1241–57. [DOI] [PubMed] [Google Scholar]
- [59].Huan T, Zhang B, Wang Z, Joehanes R, Zhu J, Johnson AD, et al. A systems biology framework identifies molecular underpinnings of coronary heart disease. Arterioscler Thromb Vasc Biol. 2013;33:1427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Karvonen J, Paivansalo M, Kesaniemi YA, Horkko S. Immunoglobulin M type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation. 2003;108:2107–12. [DOI] [PubMed] [Google Scholar]
- [61].Goncalves I, Gronholdt ML, Soderberg I, Ares MP, Nordestgaard BG, Bentzon JF, et al. Humoral immune response against defined oxidized low-density lipoprotein antigens reflects structure and disease activity of carotid plaques. Arterioscler Thromb Vasc Biol. 2005;25:1250–5. [DOI] [PubMed] [Google Scholar]
- [62].Khamis RY, Hughes AD, Caga-Anan M, Chang CL, Boyle JJ, Kojima C, et al. High Serum Immunoglobulin G and M Levels Predict Freedom From Adverse Cardiovascular Events in Hypertension: A Nested Case-Control Substudy of the Anglo-Scandinavian Cardiac Outcomes Trial. EBioMedicine. 2016;9:372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Xu Q, Willeit J, Marosi M, Kleindienst R, Oberhollenzer F, Kiechl S, et al. Association of serum antibodies to heat-shock protein 65 with carotid atherosclerosis. Lancet. 1993;341:255–9. [DOI] [PubMed] [Google Scholar]
- [64].Kortelainen ML, Porvari K. Adventitial macrophage and lymphocyte accumulation accompanying early stages of human coronary atherogenesis. Cardiovasc Pathol. 2014;23:193–7. [DOI] [PubMed] [Google Scholar]
- [65].De Palma R, Del Galdo F, Abbate G, Chiariello M, Calabro R, Forte L, et al. Patients with acute coronary syndrome show oligoclonal T-cell recruitment within unstable plaque: evidence for a local, intracoronary immunologic mechanism. Circulation. 2006;113:640–6. [DOI] [PubMed] [Google Scholar]
- [66].Almanzar G, Ollinger R, Leuenberger J, Onestingel E, Rantner B, Zehm S, et al. Autoreactive HSP60 epitope-specific T-cells in early human atherosclerotic lesions. J Autoimmun. 2012;39:441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Han SF, Liu P, Zhang W, Bu L, Shen M, Li H, et al. The opposite-direction modulation of CD4+CD25+ Tregs and T helper 1 cells in acute coronary syndromes. Clin Immunol. 2007;124:90–7. [DOI] [PubMed] [Google Scholar]
- [68].Zhao Z, Wu Y, Cheng M, Ji Y, Yang X, Liu P, et al. Activation of Th17/Th1 and Th1, but not Th17, is associated with the acute cardiac event in patients with acute coronary syndrome. Atherosclerosis. 2011;217:518–24. [DOI] [PubMed] [Google Scholar]
- [69].Cheng X, Yu X, Ding YJ, Fu QQ, Xie JJ, Tang TT, et al. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin Immunol. 2008;127:89–97. [DOI] [PubMed] [Google Scholar]
- [70].Rohm I, Atiskova Y, Drobnik S, Fritzenwanger M, Kretzschmar D, Pistulli R, et al. Decreased regulatory T cells in vulnerable atherosclerotic lesions: imbalance between pro- and anti-inflammatory cells in atherosclerosis. Mediators Inflamm. 2015;2015:364710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Mor A, Luboshits G, Planer D, Keren G, George J. Altered status of CD4(+)CD25(+) regulatory T cells in patients with acute coronary syndromes. Eur Heart J. 2006;27:2530–7. [DOI] [PubMed] [Google Scholar]
- [72].Burioni R, Canducci F, Saita D, Perotti M, Mancini N, De Marco D, et al. Antigen-driven evolution of B lymphocytes in coronary atherosclerotic plaques. J Immunol. 2009;183:2537–44. [DOI] [PubMed] [Google Scholar]
- [73].Hamze M, Desmetz C, Berthe ML, Roger P, Boulle N, Brancherau P, et al. Characterization of resident B cells of vascular walls in human atherosclerotic patients. J Immunol. 2013;191:3006–16. [DOI] [PubMed] [Google Scholar]
- [74].Yin C, Mohanta SK, Srikakulapu P, Weber C, Habenicht AJ. Artery Tertiary Lymphoid Organs: Powerhouses of Atherosclerosis Immunity. Front Immunol. 2016;7:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Srikakulapu P, Hu D, Yin C, Mohanta SK, Bontha SV, Peng L, et al. Artery Tertiary Lymphoid Organs Control Multilayered Territorialized Atherosclerosis B-Cell Responses in Aged ApoE−/− Mice. Arterioscler Thromb Vasc Biol. 2016;36:1174–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Binder CJ, Horkko S, Dewan A, Chang MK, Kieu EP, Goodyear CS, et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9:736–43. [DOI] [PubMed] [Google Scholar]
- [77].Mantani PT, Ljungcrantz I, Andersson L, Alm R, Hedblad B, Bjorkbacka H, et al. Circulating CD40+ and CD86+ B cell subsets demonstrate opposing associations with risk of stroke. Arterioscler Thromb Vasc Biol. 2014;34:211–8. [DOI] [PubMed] [Google Scholar]
- [78].Erbel C, Akhavanpoor M, Okuyucu D, Wangler S, Dietz A, Zhao L, et al. IL-17A influences essential functions of the monocyte/macrophage lineage and is involved in advanced murine and human atherosclerosis. J Immunol. 2014;193:4344–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, et al. Secukinumab Inhibition of Interleukin-17A in Patients with Psoriatic Arthritis. N Engl J Med. 2015;373:1329–39. [DOI] [PubMed] [Google Scholar]
- [80].Ryan C, Leonardi CL, Krueger JG, Kimball AB, Strober BE, Gordon KB, et al. Association between biologic therapies for chronic plaque psoriasis and cardiovascular events: a meta-analysis of randomized controlled trials. JAMA. 2011;306:864–71. [DOI] [PubMed] [Google Scholar]
- [81].Zhao TX, Kostapanos M, Griffiths C, Arbon EL, Hubsch A, Kaloyirou F, et al. Low-dose interleukin-2 in patients with stable ischaemic heart disease and acute coronary syndromes (LILACS): protocol and study rationale for a randomised, double-blind, placebo-controlled, phase I/II clinical trial. BMJ Open. 2018;8:e022452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Gencer B, Lambert G, Mach F. PCSK9 inhibitors. Swiss Med Wkly. 2015;145:w14094. [DOI] [PubMed] [Google Scholar]
- [83].Davidson MH, Maki K, Umporowicz D, Wheeler A, Rittershaus C, Ryan U. The safety and immunogenicity of a CETP vaccine in healthy adults. Atherosclerosis. 2003;169:113–20. [DOI] [PubMed] [Google Scholar]
- [84].Ren S, Newby D, Li SC, Walkom E, Miller P, Hure A, et al. Effect of the adult pneumococcal polysaccharide vaccine on cardiovascular disease: a systematic review and meta-analysis. Open Heart. 2015;2:e000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ren S, Hure A, Peel R, D’Este C, Abhayaratna W, Tonkin A, et al. Rationale and design of a randomized controlled trial of pneumococcal polysaccharide vaccine for prevention of cardiovascular events: The Australian Study for the Prevention through Immunization of Cardiovascular Events (AUSPICE). Am Heart J. 2016;177:58–65. [DOI] [PubMed] [Google Scholar]
- [86].Nishimura S, Manabe I, Nagai R. Adipose tissue inflammation in obesity and metabolic syndrome. Discov Med. 2009;8:55–60. [PubMed] [Google Scholar]
- [87].Bourinbaiar AS, Jirathitikal V. Safety and efficacy trial of adipose-tissue derived oral preparation V-6 Immunitor (V-6): results of open-label, two-month, follow-up study. Lipids Health Dis. 2010;9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Bourinbaiar AS, Jirathitikal V. Effect of oral immunization with pooled antigens derived from adipose tissue on atherosclerosis and obesity indices. Vaccine. 2010;28:2763–8. [DOI] [PubMed] [Google Scholar]
- [89].Purcell AW, Ramarathinam SH, Ternette N. Mass spectrometry-based identification of MHC-bound peptides for immunopeptidomics. Nat Protoc. 2019;14:1687–707. [DOI] [PubMed] [Google Scholar]
- [90].Kallinteris NL, Lu X, Blackwell CE, von Hofe E, Humphreys RE, Xu M. Ii-Key/MHC class II epitope hybrids: a strategy that enhances MHC class II epitope loading to create more potent peptide vaccines. Expert Opin Biol Ther. 2006;6:1311–21. [DOI] [PubMed] [Google Scholar]
- [91].Xu M, Kallinteris NL, von Hofe E. CD4+ T-cell activation for immunotherapy of malignancies using Ii-Key/MHC class II epitope hybrid vaccines. Vaccine. 2012;30:2805–10. [DOI] [PubMed] [Google Scholar]
- [92].Kim SH, Jang YS. The development of mucosal vaccines for both mucosal and systemic immune induction and the roles played by adjuvants. Clin Exp Vaccine Res. 2017;6:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Bermudez-Humaran LG, Aubry C, Motta JP, Deraison C, Steidler L, Vergnolle N, et al. Engineering lactococci and lactobacilli for human health. Curr Opin Microbiol. 2013;16:278–83. [DOI] [PubMed] [Google Scholar]
- [94].Hernandez M, Rosas G, Cervantes J, Fragoso G, Rosales-Mendoza S, Sciutto E. Transgenic plants: a 5-year update on oral antipathogen vaccine development. Expert Rev Vaccines. 2014;13:1523–36. [DOI] [PubMed] [Google Scholar]
- [95].Salazar-Gonzalez JA, Rosales-Mendoza S, Romero-Maldonado A, Monreal-Escalante E, Uresti-Rivera EE, Banuelos-Hernandez B. Production of a plant-derived immunogenic protein targeting ApoB100 and CETP: toward a plant-based atherosclerosis vaccine. Mol Biotechnol. 2014;56:1133–42. [DOI] [PubMed] [Google Scholar]
- [96].Klingenberg R, Lebens M, Hermansson A, Fredrikson GN, Strodthoff D, Rudling M, et al. Intranasal immunization with an apolipoprotein B-100 fusion protein induces antigen-specific regulatory T cells and reduces atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:946–52. [DOI] [PubMed] [Google Scholar]
- [97].Aldridge S. Toll-like receptor blocker slows beta cell death in type 1 diabetes. Nat Biotechnol. 2012;30:124. [DOI] [PubMed] [Google Scholar]
- [98].Raz I, Avron A, Tamir M, Metzger M, Symer L, Eldor R, et al. Treatment of new-onset type 1 diabetes with peptide DiaPep277 is safe and associated with preserved beta-cell function: extension of a randomized, double-blind, phase II trial. Diabetes Metab Res Rev. 2007;23:292–8. [DOI] [PubMed] [Google Scholar]
- [99].Li J, McArdle S, Gholami A, Kimura T, Wolf D, Gerhardt T, et al. CCR5+T-bet+FoxP3+ Effector CD4 T Cells Drive Atherosclerosis. Circ Res. 2016;118:1540–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Fitzgerald K, White S, Borodovsky A, Bettencourt BR, Strahs A, Clausen V, et al. A Highly Durable RNAi Therapeutic Inhibitor of PCSK9. N Engl J Med. 2017;376:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]