Abstract
Rationale:
With the easy access, rodenticide poisoning has been a public health problem in many countries. Characteristics of central nervous system (CNS) lesions induced by rodenticides are scarcely reported.
Patient concerns:
We presented a case of a 40-year-old man with seizure and consciousness disorder, coagulation dysfunction, and symmetric lesions in white matter and corpus callosum.
Diagnosis:
He was diagnosed with rodenticide poisoning due to bromadiolone and fluoroacetamide.
Interventions:
He was treated with vitamin K, hemoperfusion, acetamide, and calcium gluconate.
Outcomes:
His leukoencephalopathy was reversed rapidly with the improvement of clinical symptoms.
Lessons:
This report presented the impact of rodenticide poisoning on CNS and the dynamic changes of brain lesions, and highlighted the importance of timely targeted treatments.
Keywords: central nervous system, coagulopathy, corpus callosum, leukoencephalopathy, rodenticide
1. Introduction
Anticoagulants are the main component of the rodenticide. The first generation of anticoagulant rodenticide was developed in 1948 and was gradually replaced in the 1970s by the second generation, namely superwarfarins, due to drug resistance.[1,2] As a type of superwarfarins with high potency, bromadiolone is a common rodenticide used all over the world. Bromadiolone inhibits the carboxylation of vitamin K–dependent coagulation factors (II, VII, IX, and X) and exerts a prolonged anticoagulant effect.[3,4] Fluoroacetamide is another common rodenticide which induces an accumulation of citrate and cellular metabolic disorder by blocking the tricarboxylic acid cycle.[5]
With the easy access, rodenticide poisoning has been a public health problem in many countries.[6–8] The clinical effect of bromadiolone is associated with the exposure dosage. Most patients with bromadiolone poisoning have only minor or no effects due to the small exposure.[7,9] Interfering with blood coagulation, bromadiolone induces varying degrees of hemorrhage, such as ecchymoses, gingival bleeding, epistaxis, gastrointestinal bleeding, hematuria, vaginal bleeding, and rarely SAH.[6,10–14] Besides coagulopathy, patients with bromadiolone poisoning sometimes present with headache, seizure, hallucinations, dizziness, consciousness impairment and a few other symptoms of central nervous system (CNS).[7] Fluoroacetamide poisoning often causes damages in heart (QT prolongation, arrhythmia, myocardial damage), digestive system (vomiting, nausea, burning sensation in the epigastrium), and CNS (seizure, aphasia, myasthenia, and coma).[15–18] However, characteristics of CNS lesions induced by rodenticides are scarcely reported. Here, we presented a case with reversible leukoencephalopathy caused by bromadiolone and fluoroacetamide poisoning.
2. Case presentation
2.1. Clinical history
A 40-year-old man was referred to our neurological intensive care unit (NICU) due to unconsciousness for 1 day. He had headache 4 days ago, then he started to feel lack of energy and had generalized tonic-clonic seizure for 5 minutes. Blood tests of coagulation function in the emergency unit showed a prothrombin time of 92.6 seconds (normal: 11.0–14.5 seconds), an activated partial thromboplastin time of 52.8 seconds (normal: 28.0–45.0) and an international normalized ratio of 8.88 (normal 0.80–1.20). He received fresh frozen plasma transfusion and intravenous treatments of diazepam and valproate and in the emergency unit. This patient was unconscious on admission, so he could not provide any history of poison ingestion. His family reported no history of familial diseases and no awareness of poisoning.
2.2. Clinical examination and diagnosis
He had eyes opening to sound, no spontaneous motor movements, no motor movements to commands, and no speech or vocalization. His 4 limbs had normal flexion to pain stimuli. His pupil diameters are 2.0 mm. Direct and indirect light reflexes of both pupils were slow. All the brainstem reflexes were present. His muscle tone was normal, and no involuntary movements were observed. Tendon reflexes of upper limbs were normal, patellar reflexes of both legs were exaggerated. Babinski signs of both sides were positive, and meningeal irritation signs were negative. No ecchymoses or any other hemorrhage was found in his skin, conjunctiva, nose, and mouth. He had no hemoptysis, haematemesis, hematochezia, or hematuria.
His blood tests showed a mild elevation in total bilirubin (35.1 μmol/L), unconjugated bilirubin (27.2 μmol/L), and ammonia (44.0 μmol/L). Liver enzymes, creatinine, electrolytes in the blood were normal. Gastric occult blood test was positive (3+), and fecal occult blood test was negative. Brain Diffusion Weighted Imaging (DWI) showed hyperintense lesions throughout the corpus callosum and in both sides of brachium pontis, posterior limb of internal capsule, periventricular white matter, centrum semiovale, and corona radiata (Fig. 1A). Toxin testing identified bromadiolone (58 ng/ml, liquid chromatography-mass spectrometry) and fluoroacetamide (chromatography-mass spectrometry) in his blood, and fluorine (25.21 mg/g creatinine, WS/T 30-1996) in his urine. He was diagnosed with rodenticide poisoning.
Figure 1.
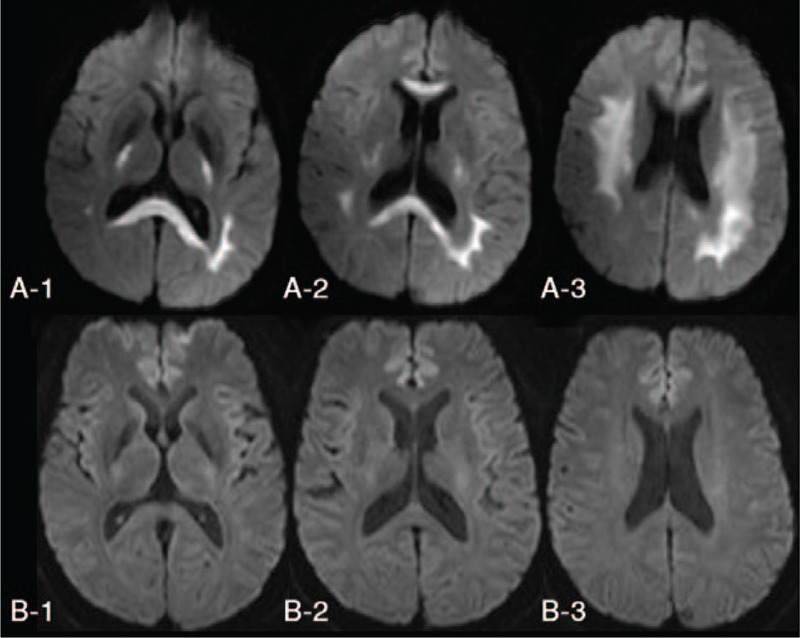
Brain Diffusion Weighted Imaging. A. On admission; B. 7 days after consciousness recovery.
2.3. Treatment
He received fresh frozen plasma transfusion and hemoperfusion upon his admission to NICU, together with vitamin K1 10 mg iv, pantoprazole 40 mg iv, and sodium valproate 600 mg po. As soon as the result of toxin detection came out (4 hours after NICU admission), vitamin K1 30 mg/d iv, acetamide 5 g tid im., and calcium gluconate 2 g/d iv were administered for 12 days.
2.4. Outcome
On the second day in NICU, he opened eyes spontaneously with accurate but slightly slurred speech and normal muscle strength in all his limbs. On the third day in NICU, his speech became clear. His coagulation function became completely normal on the fourth day in NICU, then he was transferred out of NICU. He took brain MRI examination again 7 days after consciousness recovery: previous high DWI signals in corpus callosum and in both sides of brachium pontis, posterior limb of internal capsule, PWN, centrum semiovale, and corona radiata all disappeared (Fig. 1B). He was discharged from hospital 2 weeks from the onset, and he did not take vitamin K1, acetamide, or calcium gluconate after hospital discharge. He reported no symptoms at 5-month follow-up.
3. Discussion
Ecchymoses and bleeding were the most common initial symptoms of rodenticide poisoning.[6,19] There was even a case reporting the misdiagnosis of rodenticide poisoning as ectopic pregnancy in an 18-year-old woman.[14] In this case, sudden impairment of consciousness, coagulation disorder, and symmetric lesions in periventricular white matter and corpus callosum indicated a possibility of toxic encephalopathy. Accordingly, plasma transfusion and vitamin K were given to improve coagulation function, and hemoperfusion was performed to remove toxins. As soon as the identification of toxins (bromadiolone and fluoroacetamide), vitamin K1 30 mg/d iv, acetamide 5 g tid im, and calcium gluconate 2 g/d iv were administered. His consciousness level and coagulation function were improved rapidly, and cerebral lesions were reversed.
Previous cohort studies investigated the clinical characteristics and outcomes of rodenticide poisoning. Bromadiolone and bromethalin were the most common toxicants found in rodenticide intoxication, and rodenticide exposures were mainly pediatric (under 12 years old).[7,20] Accidental ingestion was the most common cause of poisoning in children, and no effects or only minor effects were usually seen due to low exposure.[7,11] Intentional ingestion and unknown intake were the most frequent causes of rodenticide intoxication in adults.[7] Unknown ingestion needs longer time to make a diagnosis and give targeted treatments than intentional ingestion and usually contains greater dosage than accidental intake. Therefore, rodenticide poisoning in adults due to unknown ingestion usually leads to more severe symptoms. Once the type of rodenticides was identified, targeted treatment should be administered as soon as possible.
The supplement of Vitamin K can directly ameliorate the K-dependent coagulation factor deficiency caused by long-acting anticoagulant rodenticides. So far there were no consensuses on the loading and maintenance dosage of Vitamin K. The loading dose reported by previous studies was 10 to 100 mg/d intravenously,[21–23] and the maximal loading dosage was 800 mg/d orally.[24] The maintenance dosage of Vitamin K reported by previous studies was quite different as well: 5 to 600 mg/d orally.[21–24] A study of 56 patients with anticoagulant rodenticides poisoning showed that there was not a significant dose–effect relationship between the concentration of rodenticides and the requirement of vitamin K1 during the maintenance period.[10] For severe cases, transfusion of fresh frozen plasma, prothrombin complex, and/or recombinant coagulation factor VIIa should be given. Muscle injection of acetamide is the targeted treatment for fluoroacetamide,[16] and calcium therapy can ameliorate cardiac arrhythmias induced by fluoroacetamide.[25,26]
Several case repots presented the effects of rodenticide on CNS (Table 1). Due to the anticoagulant effect, bromadiolone can cause intracerebral hematoma.[27] The brain MRI of a bromadiolone poisoning case found symmetrical patchy lesions in bilateral posterior limb of the internal capsule, splenium of corporis callosum, and bilateral centrum semiovale which were similar to the affected locations in our case.[28] Tetramine and fluoroacetamide were reported to cause hypoxic–ischemic changes at hippocampal regions and cerebral cortex.[15] β-fluoroethyl acetate can cause cerebellar atrophy,[29] and bromethalin may lead to leukoencephalopathy.[7] In this case, we found that leukoencephalopathy was reversed with the improvement of clinical symptoms.
Table 1.
Reported neuroimaging findings associated with rodenticides.
| Case | Rodenticides | Neuroimaging findings | Treatment | Outcome |
| Zuo et al, 2019[27] | bromadiolone | CT: intracerebral haematoma | In-hospital: vitamin K (30 mg q8h) + fresh frozen plasma (800 ml in total)After hospital discharge: Vitamin K (30 mg, q8 h) for 6 months. | No obvious hemorrhage in brain |
| Wang et al, 2017[28] | bromadiolone | MRI: symmetrical patchy lesions in bilateral posterior limb of the internal capsule, splenium of corporis callosum, and bilateral centrum semiovale. | Vitamin K and blood plasma (unknown dosage) | Relief from confusion and dysphoria |
| Wang et al, 2016[15] | tetramine +fluoroacetamide | CT: hypoxic–ischemic changes lightly at hippocampal regions and cerebral cortex | Case 1: no treatment.Case 2: unknown. | Case 1: Death.Case 2: Recovered. |
| Jin et al, 2017[29] | β-fluoroethyl acetate | MRI: cerebellar atrophy | Unknown | Unknown |
| Feldman et al, 2019[7] | bromethalin | MRI: leukoencephalopathy (non-specified) | Unknown | Pediatric: 96.38% had no effects, 3.32% had minor effects, and 0.45% had moderate effects.Patients >12 yrs: 65.73% had no effect, 25.58% had minor effects, 5.88% had moderate effects, 2.30% had major effects, and 0.51% died. |
4. Conclusions
We reported a case of rodenticide poisoning presented with seizure, consciousness disorder, and coagulation dysfunction. His brain DWI showed symmetric lesions in white matter and corpus callosum. After receiving vitamin K, hemoperfusion, acetamide, and calcium gluconate, he restored consciousness and his leukoencephalopathy was rapidly reversed. This report presented the impact of rodenticide poisoning on CNS and the dynamic changes of brain lesions, and highlighted the importance of timely targeted treatments.
Author contributions
Conceptualization: Aili Lu, Fang Yuan.
Data curation: Yufei Yao, Wanxin Wen, Hongji Lu, Shibiao Wu.
Formal analysis: Aili Lu, Fang Yuan.
Investigation: Yufei Yao, Wanxin Wen, Hongji Lu, Shibiao Wu.
Supervision: Lixin Wang.
Writing – original draft: Aili Lu, Fang Yuan, Yufei Yao.
Writing – review & editing: Lixin Wang.
Footnotes
Abbreviations: CNS = central nervous system, DWI = diffusion weighted imaging, NICU = neurological intensive care unit.
How to cite this article: Lu A, Yuan F, Yao Y, Wen W, Lu H, Wu S, Wang L. Reversible leukoencephalopathy caused by 2 rodenticides bromadiolone and fluroacetamide: a case report and literature review. Medicine. 2021;100:9(e25053).
AL and FY contributed equally.
The raw data supporting the conclusions of this manuscript will be made available by the authors to any qualified researcher.
Ethics approval or consent to participate was not applicable. Consent for publication was obtained from the patient.
The authors have no conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Routh CR, Triplett DA, Murphy MJ, et al. Superwarfarin ingestion and detection. Am J Hematol 1991;36:50–4. [DOI] [PubMed] [Google Scholar]
- [2].Sharma P, Bentley P. Of rats and men: superwarfarin toxicity. Lancet 2005;365:552–4. [DOI] [PubMed] [Google Scholar]
- [3].Chua JD, Friedenberg WR. Superwarfarin poisoning. Arch Intern Med 1998;158:1929–32. [DOI] [PubMed] [Google Scholar]
- [4].Vindenes V, Karinen R, Hasvold I, et al. Bromadiolone poisoning: LC-MS method and pharmacokinetic data. J Forensic Sci 2008;53:993–6. [DOI] [PubMed] [Google Scholar]
- [5].Alex T Proudfoot, Sally M Bradberry, J Allister Vale. Sodium fluoroacetate poisoning. Toxicol Rev 2006;25:213–9. [DOI] [PubMed] [Google Scholar]
- [6].Liao Xiang, Zhang Min, Zhao Alan, et al. Retrospective study of twenty-four patients with prolonged coagulopathy due to long-acting anti-vitamin K rodenticide poisoning. Am J Med Sci 2014;347:299–304. [DOI] [PubMed] [Google Scholar]
- [7].Feldman R, Stanton M, Borys D, et al. Medical outcomes of bromethalin rodenticide exposures reported to US poison centers after federal restriction of anticoagulants. Clin Toxicol 2019;57:1109–14. [DOI] [PubMed] [Google Scholar]
- [8].Karen M, Shabnam H, Muhammed R, et al. Survival benefits of N- Acetylcysteine in rodenticide poisoning retrospective evidence from an Indian tertiary care setting. Curr Clin Pharmacol 2020. [DOI] [PubMed] [Google Scholar]
- [9].Ingels M, Lai C, Tai W, et al. A prospective study of acute, unintentional, pediatric superwarfarin ingestions managed without decontamination. Ann Emerg Med 2002;40:73–8. [DOI] [PubMed] [Google Scholar]
- [10].Long J, Peng X, Luo Y, et al. Treatment of a long-acting anticoagulant rodenticide poisoning cohort with vitamin K1 during the maintenance period. Medicine (Baltimore) 2016;95:e5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Parsons BJ, Day LM, Ozanne-Smith J, et al. Rodenticide poisoning among children. Aust N Z J Public Health 1996;20:488–92. [DOI] [PubMed] [Google Scholar]
- [12].Rohit B Sangal, Lauren W Conlon. Rodenticide causing lower gastrointestinal bleeding resident simulation. MedEdPORTAL 2018;14:10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hsin-Ying Yu, Ja-Liang Lin, Jen-Fen Fu, et al. Outcomes of patients with rodenticide poisoning at a far east poison center. Springerplus 2013;2:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Singhal SR, Paul A, Dahiya P. Misdiagnosis of rodenticide poisoning as ectopic pregnancy: a case report. Eur J Obstet Gynecol Reprod Biol 2012;163:119–20. [DOI] [PubMed] [Google Scholar]
- [15].Wang R, Zhuo L, Wang Y, et al. Lessons learned from poisoning cases caused by 2 illegal rodenticides: tetramine and fluoroacetamide. Medicine (Baltimore) 2016;95:e5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wen W, Gao H, Kang N, et al. Treatment of severe fluoroacetamide poisoning in patient with combined multiple organ dysfunction syndrome by evidence-based integrated Chinese and Western medicines: A case report. Medicine (Baltimore) 2017;96:e7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jieming Lin, Chaoqiang Jiang, Jianping Ou, et al. <Acute fluoroacetamide poisoning with main damage to the heart. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2002;20:344–6. [PubMed] [Google Scholar]
- [18].Taitelman U, Roy A, Hoffer E. Fluoroacetamide poisoning in man the role of ionized calcium. Arch Toxicol Suppl 1983;6:228–31. [DOI] [PubMed] [Google Scholar]
- [19].Ng WY, Ching CK, Chong YK, et al. Retrospective study of the characteristics of anticoagulant-type rodenticide poisoning in Hong Kong. J Med Toxicol 2018;14:218–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yan H, Zhu L, Zhuo X, et al. Anticoagulant rodenticide intoxication in east China: a three-year analysis. Forensic Sci Res 2016;1:22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hong J, Yhim HY, Bang SM, et al. Korean patients with superwarfarin intoxication and their outcome. J Korean Med Sci 2010;25:1754–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].King N, Tran MH. Long-acting anticoagulant rodenticide (superwarfarin) poisoning: a review of its historical development, epidemiology, and clinical management. Transfus Med Rev 2015;29:250–8. [DOI] [PubMed] [Google Scholar]
- [23].Gunja N, Coggins A, Bidny S. Management of intentional superwarfarin poisoning with long-term vitamin K and brodifacoum levels. Clin Toxicol (Phila) 2011;49:385–90. [DOI] [PubMed] [Google Scholar]
- [24].Tsutaoka BT, Miller M, Fung SM, et al. Superwarfarin and glass ingestion with prolonged coagulopathy requiring high-dose vitamin K1 therapy. Pharmacotherapy 2003;23:1186–9. [DOI] [PubMed] [Google Scholar]
- [25].Taitelman U, Roy A, Hoffer E. Fluoroacetamide poisoning in man: the role of ionized calcium. Arch Toxicol Suppl 1983;6:228–31. [DOI] [PubMed] [Google Scholar]
- [26].Jieming Lin, Chaoqiang Jiang, Jianping Ou, et al. Acute fluoroacetamide poisoning with main damage to the heart. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2002;20:344–6. [PubMed] [Google Scholar]
- [27].Zuo W, Zhang X, Chang JB, et al. Bromadiolone poisoning leading to subarachnoid haemorrhage: a case report and review of the literature. J Clin Pharm Ther 2019;44:958–62. [DOI] [PubMed] [Google Scholar]
- [28].Wang M, Yang Y, Hou Y, et al. Effects of bromadiolone poisoning on the central nervous system. Neuropsychiatr Dis Treat 2017;13:2297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jin JH, Lee ES, Choi JY, et al. Isolated cerebellar atrophy due to rodenticide (beta-fluoroethyl acetate) intoxication. J Neurol Sci 2017;373:208–9. [DOI] [PubMed] [Google Scholar]


