Abstract
Mortality of critically ill patients with coronavirus disease 2019 (COVID-19) was high. Aims to examine whether time from symptoms onset to intensive care unit (ICU) admission affects incidence of extra-pulmonary complications and prognosis in order to provide a new insight for reducing the mortality. A single-centered, retrospective, observational study investigated 45 critically ill patients with COVID-19 hospitalized in ICU of The Third People's Hospital of Yichang from January 17 to March 29, 2020. Patients were divided into 2 groups according to time from symptoms onset to ICU admission (>7 and ≤7 days) and into 2 groups according to prognosis (survivors and non-survivors). Epidemiological, clinical, laboratory, radiological characteristics and treatment data were studied. Compared with patients who admitted to the ICU since symptoms onset ≤7 days (55.6%), patients who admitted to the ICU since symptoms onset >7 days (44.4%) were more likely to have extra-pulmonary complications (19 [95.0%] vs 16 [64.0%], P = .034), including acute kidney injury, cardiac injury, acute heart failure, liver dysfunction, gastrointestinal hemorrhage, hyperamylasemia, and hypernatremia. The incidence rates of acute respiratory distress syndrome, pneumothorax, and hospital-acquired pneumonia had no difference between the 2 groups. Except activated partial thromboplastin and Na+ concentration, the laboratory findings were worse in group of time from symptoms onset to ICU admission >7 days. There was no difference in mortality between the 2 groups. Of the 45 cases in the ICU, 19 (42.2%) were non-survivors, and 16 (35.6%) were with hospital-acquired pneumonia. Among these non-survivors, hospital-acquired pneumonia was up to 12 (63.2%) besides higher incidence of extra-pulmonary complications. However, hospital-acquired pneumonia occurred in only 4 (15.4%) survivors. Critically ill patients with COVID-19 who admitted to ICU at once might get benefit from intensive care via lower rate of extra-pulmonary complications.
Keywords: COVID-19, extra-pulmonary complications, hospital-acquired pneumonia, SARS-CoV-2
1. Introduction
At the end of December 2019, a novel coronavirus was identified as the cause of a cluster of pneumonia cases in Wuhan, the capital of Central Hubei Province, China. The pneumonia cases were characterized primarily by fever, cough, and bilateral infiltrates on chest imaging.[1–3] The World Health Organization (WHO) named it coronavirus disease 2019 (COVID-19) which was caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Till now, SARS-CoV-2 has spread rapidly all over the world, but there are few specific tools to control the growing epidemic and treat those who are seriously sick. Previous researches have demonstrated human-to-human transmission of the COVID-19 through droplets or direct contact.[4,5] The most effective methods are quarantine, isolation, infection-control measures and on supportive care for those who become ill.[6] However, the still insufficient testing capacity for COVID-19 in other countries means that many suspected cases and asymptomatic carrier are not yet counted completely.[7] As of September 15, 2020, there were more than 29 million cases worldwide in total, which threatened global public health. Previous study indicated that the mortality of critically ill patients with COVID-19 was considerable, which was up to 61.5% in Wuhan Jinyin Tan Hospital.[8] Nevertheless, specific data about the clinical characteristics of critically ill patients of COVID-19 is remain unknown, which is paramount importance to reduce mortality. Consequently, it is vital for collection and analysis data about critically patients of COVID-19.
In this study, we investigated critically ill patients with confirmed COVID-19 who were admitted to The Third People's Hospital of Yichang, nearby Wuhan in Hubei province. The baseline COVID-19 associated morbidity and mortality data from this study will be considerable value for who are most likely to intensive care unit (ICU) treatment. By collecting data of the clinical characteristics and laboratory findings of critically ill patients infected with COVID-19, we described the relation of time from symptoms onset to ICU admission and prognosis. Our study provides a new insight into the strategy to reduce the mortality of COVID-19.
2. Materials and methods
2.1. Study design and participants
This single-centered, retrospective, observational study was conducted in The Third People's Hospital of Yichang. The hospital located in Hubei Province, nearby Wuhan, the epidemic areas of COVID-19. And the hospital is one of the major teaching hospitals and responsible for the treatments for COVID-19 assigned by the government. All consecutive patients with confirmed COVID-19 admitted to The Third People's Hospital of Yichang from January 17 to March 29, 2020, were enrolled. The reporting guidelines for STROBE were used in the design and implementation of our research.
From January 17 to March 29, 2020, there were 398 patients with confirmed SARS-CoV-2 infection hospitalized in The Third People's Hospital of Yichang in total, and of which, 49 cases were critically ill and admission to the ICU. Excluded 1 case who was <18 years old, 2 cases who died soon after ICU admission, and 1 case who refused life support in the ICU. Oral consent was obtained from patients or law of agents. 45 patients with COVID-19 enrolled in this study were diagnosed according to World Health Organization interim guidance. Laboratory confirmation of SARS-CoV-2 infection was performed via real-time reverse transcriptase polymerase chain reaction (RT-PCR) assay (Daan Gene Co., Ltd. Of Sun Yat-sen University) of nasal and pharyngeal swab specimens or lower respiratory tract secretions. Only laboratory-confirmed cases were included in this analysis. Critically ill patients were defined as these admitted to the ICU who required mechanical ventilation or had a fraction of inspired oxygen (FiO2) of at least 60% or more. Identification of critically ill patients was achieved by reviewing and analyzing admission logs and histories from all available electronic medical records and patient care resources. The Ethics Commission of The Third People's Hospital of Yichang approved this study. Written informed consent was waived due to the rapid emergence of this infectious disease and the retrospective nature of the study.
2.2. Data collection
Epidemiological, clinical, laboratory, radiological characteristics, and treatment data were obtained with data collection forms from electronic medical records. The data were reviewed by a trained team of physicians. Information recorded included age, sex, exposure history, chronic medical histories (chronic cardiac disease, chronic pulmonary disease, chronic kidney disease, cerebrovascular disease, diabetes, malnutrition, schizophrenia, malignancy, hemopathy and autoimmune disease), symptoms from onset to ICU admission (fever, cough, dyspnea, myalgia, rhinorrhea, arthralgia, chest pain, headache, coma, vomiting, bellyache and diarrhea), complications (acute respiratory distress syndrome (ARDS), acute kidney injury, cardiac injury, acute heart failure, liver dysfunction, gastrointestinal hemorrhage, pneumothorax, hyperamylasemia, hypernatremia, hospital-acquired pneumonia), laboratory values on ICU admission (arterial blood gas analysis, FiO2, partial pressure of oxygen, PaO2, white blood cell count, lymphocyte count, platelet count, prothrombin times, activated partial thromboplastin time, D-dimer, lactate concentration, creatine kinase-MB, NT-pro BNP, creatinine, total bilirubin and procalcitonin), treatments (oxygen therapy, prone position ventilation, renal replacement therapy, vasoconstrictive agents, antiviral agents, antibacterial agents, glucocorticoids and immunoglobulin), as well as living status. Any missing or uncertain records were collected and clarified through direct communication with involved health-care providers and their families.
2.3. Statistical analysis
Continuous variables were expressed as mean (standard deviation [SD]) or median (interquartile range [IQR]) and compared with the Mann–Whitney U test; categorical variables were expressed as number (%) and compared by χ2 test or Fisher's exact test between data of patients who admitted to the ICU since symptoms onset >7 days and those who admitted to the ICU since symptoms onset ≤7 days. A 2-sided α of less than 0.05 was considered statistically significant. The analyses have not been adjusted for multiple comparisons, and given the potential for type I error, the findings should be interpreted as exploratory and descriptive. Statistical analyses were done using the IBM SPSS (Statistical Package for the Social Sciences), version 23.0 software, unless otherwise indicated.
3. Results
3.1. Demographics and baseline characteristics
By 29 March 2020, clinical data were collected on 45 critically ill patients in The Third People's Hospital of Yichang with laboratory confirmed SARS-CoV-2 infection. These patients were admitted or transferred to the ICU because of the development of organ dysfunction. The median age was 64 years (IQR, 47–71), and 20 (44.4%) were older than 65 years. As of March 29, 2020, more than half of the 45 patients (30, 66.7%) were men. There was no statistical difference in age and sex ratio between with patients admitted to the ICU since symptoms onset >7 days and those admitted to the ICU since symptoms onset ≤7 days. 28 (62.2%) patients had a history of exposure to Wuhan or contact with confirmed or highly suspected COVID-19 patients. Interestingly, there was significant difference in exposure history between patients admitted to the ICU since symptoms onset >7 days and those admitted to the ICU since symptoms onset ≤7 days (7 [35.0%] vs 21 [84.0%], P = .001). Of the 45 patients, 29 (64.4%) had one or more chronic medical illness. The most common chronic medical illness was cardiac disease (46.7%), diabetes (26.7%) and pulmonary disease (15.6%). The mortality rates of patients admitted to the ICU since symptoms onset >7 days and those admitted to the ICU since symptoms onset ≤7 days were 50.0% and 36.0% respectively. However, there was no difference in prognosis between the 2 groups. The median ICU length of stay of the 2 groups were 19 days (IQR, 8–26) and 17 days (IQR, 6–28) respectively, which had no difference either (Table 1).
Table 1.
Demographics and baseline characteristics of critically ill patients with COVID-19.
| Time from symptoms onset to ICU admission | ||||
| All patients (n = 45) | >7 d (n = 20) | ≤7 d (n = 25) | P valuea | |
| Median age (yr) | 64 (47–71) | 65 (57–72) | 56 (39–70) | .098 |
| Age groups (yr) | ||||
| ≤65 | 25 (55.6%) | 10 (40.0%) | 15 (60.0%) | .502 |
| >65 | 20 (44.4%) | 10 (50.0%) | 10 (50.0%) | |
| Sex | ||||
| Female | 15 (33.3%) | 7 (35.0%) | 8 (32.0%) | .832 |
| Male | 30 (66.7%) | 13 (65.0%) | 17 (68.0%) | |
| Prognosis | ||||
| Survivors | 26 (57.8%) | 10 (50.0%) | 16 (64.0%) | .345 |
| Non-survivors | 19 (42.2%) | 10 (50.0%) | 9 (36.0%) | |
| ICU length of stay (d) | 17 (7–27) | 19 (8–26) | 17 (6–28) | .714 |
| Exposure to Wuhan or contact with patient | ||||
| Yes | 28 (62.2%) | 7 (35.0%) | 21 (84.0%) | .001 |
| No | 17 (37.8%) | 13 (65.0%) | 4 (16.0%) | |
| Chronic medical illness | 29 (64.4%) | 13 (65.0%) | 16 (64.0%) | .944 |
| Cardiac disease | 21 (46.7%) | 9 (45.0%) | 12 (48.0%) | .841 |
| Pulmonary disease | 7 (15.6%) | 3 (15.0%) | 4 (16.0%) | 1.000 |
| Cerebrovascular disease | 4 (8.9%) | 1 (5.0%) | 3 (12.0%) | .770 |
| Malignancy | 1 (2.2%) | 0 (0.0%) | 1 (4.0%) | 1.000 |
| Schizophrenia | 1 (2.2%) | 0 (0.0%) | 1 (4.0%) | 1.000 |
| Malnutrition | 1 (2.2%) | 0 (0.0%) | 1 (4.0%) | 1.000 |
| Diabetes | 12 (26.7%) | 4 (20.0%) | 8 (32.0%) | .366 |
| Chronic kidney disease | 3 (6.7%) | 1 (5.0%) | 2 (8.0%) | 1.000 |
| Hemopathy | 3 (6.7%) | 2 (10.0%) | 1 (4.0%) | .841 |
| Autoimmune disease | 1 (2.2%) | 0 (0.0%) | 1 (4.0%) | 1.000 |
| Smoking | 12 (26.7%) | 9 (45.0%) | 3 (12.0%) | .013 |
3.2. Clinical features
Similarities of clinical features between SARS-CoV-2 and previous β-coronavirus infections had been noted. In this cohort, the most common symptoms were fever (40, 88.9%), cough (39, 86.7%), and dyspnea (37, 82.2%). Less common symptoms were vomiting, myalgia, headache, rhinorrhea, diarrhea, chest pain (Table 2). Compared with patients admitted to the ICU since symptoms onset ≤7 days, the number of patients admitted to the ICU since symptoms onset >7 days were significantly fewer in fever (15 [75.0%] vs 25 [100.0%], P = .030), and were more likely to have extra-pulmonary complications (19 [95.0%] vs 16 [64.0%], P = .034), including acute kidney injury (10 [50.0%] vs 5 [20.0%], P = .034), cardiac injury (8 [40%] vs 2 [8%], P = .027), gastrointestinal hemorrhage (9 [45.0%] vs 3 [12.0%], P = .013), and hypernatremia (18 [90.0%] vs 15 [60.0%], P = .024). However, incidence rates of ARDS (14 [70.0%] vs 18 [72.0%], P = .883), pneumothorax, (2 [10.0%] vs 2 [8.0%], P = 1.000), hospital-acquired pneumonia (9 [45.0%] vs 7 [28.0%], P = .236), acute heart failure (8 [40.0%] vs 5 [20.0%], P = .141), liver dysfunction (14 [70.0%] vs 13 [52.0%], P = .221) and hyperamylasemia (0 [0.0%] vs 1 [4.0%], P = 1.000) had no difference between the 2 groups.
Table 2.
Symptoms, complications, and treatments of critically ill patients with COVID-19.
| Time from symptoms onset to ICU admission | ||||
| All patients (n = 45) | >7 d (n = 20) | ≤7 d (n = 25) | P valuea | |
| Symptoms | ||||
| Fever | 40 (88.9%) | 15 (75.0%) | 25 (100.0%) | .030 |
| Cough | 39 (86.7%) | 16 (80.0%) | 23 (92.0%) | .462 |
| Dyspnea | 37 (82.2%) | 16 (80.0%) | 21 (84.0%) | 1.000 |
| Myalgia | 10 (22.2%) | 7 (35.0%) | 3 (12.0%) | .138 |
| Rhinorrhea | 7 (15.6%) | 3 (15.0%) | 4 (16.0%) | 1.000 |
| Arthralgia | 4 (8.9%) | 3 (15.0%) | 1 (4.0%) | .446 |
| Chest pain | 4 (8.9%) | 1 (5.0%) | 3 (12.0%) | .770 |
| Headache | 7 (15.6%) | 2 (10.0%) | 5 (20.0%) | .613 |
| Coma | 2 (4.4%) | 2 (10.0%) | 0 (0.0%) | .192 |
| Vomiting | 12 (26.7%) | 5 (25.0%) | 7 (28.0%) | .821 |
| Bellyache | 1 (2.2%) | 1 (5.0%) | 0 (0.0%) | .444 |
| Diarrhea | 7 (15.6%) | 3 (15.0%) | 4 (16.0%) | 1.000 |
| Complications | ||||
| Acute respiratory distress syndrome | 32 (71.1%) | 14 (70.0%) | 18 (72.0%) | .883 |
| Pneumothorax | 4 (8.9%) | 2 (10.0%) | 2 (8.0%) | 1.000 |
| Hospital-acquired pneumonia | 16 (35.6%) | 9 (45.0%) | 7 (28.0%) | .236 |
| Extra-pulmonary complications | 35 (77.8%) | 19 (95.0%) | 16 (64.0%) | .034 |
| Acute kidney injury | 15 (33.3%) | 10 (50.0%) | 5 (20.0%) | .034 |
| Cardiac injury | 10 (22.2%) | 8 (40.0%) | 2 (8.0%) | .027 |
| Acute heart failure | 13 (28.9%) | 8 (40.0%) | 5 (20.0%) | .141 |
| Liver dysfunction | 27 (60.0%) | 14 (70.0%) | 13 (52.0%) | .221 |
| Gastrointestinal hemorrhage | 12 (26.7%) | 9 (45.0%) | 3 (12.0%) | .013 |
| Hyperamylasemia | 1 (2.2%) | 0 (0.0%) | 1 (4.0%) | 1.000 |
| Hypernatremia | 33 (73.3%) | 18 (90.0%) | 15 (60.0%) | .024 |
| Treatments | ||||
| High flow nasal cannula | 14 (31.1%) | 3 (15.0%) | 11 (44.0%) | .037 |
| Mechanical ventilation | 35 (77.8%) | 20 (100.0%) | 15 (60.0%) | .004 |
| Invasive mechanical ventilation | 14 (31.1%) | 10 (50.0%) | 4 (16.0%) | .014 |
| Non-invasive mechanical ventilation | 34 (75.6%) | 20 (100%) | 14 (56.0%) | .002 |
| Prone position ventilation | 8 (17.8%) | 6 (30.0%) | 2 (8.0%) | .127 |
| Renal replacement therapy | 6 (13.3%) | 4 (20.0%) | 2 (8.0%) | .462 |
| Vasoconstrictive agents | 16 (35.6%) | 10 (50.0%) | 6 (24.0%) | .070 |
| Antiviral agents | 44 (97.8%) | 19 (95.0%) | 25 (100.0%) | .444 |
| Antibacterial agents | 36 (80.0%) | 19 (95.0%) | 17 (68.0%) | .061 |
| Glucocorticoids | 33 (73.3%) | 16 (80.0%) | 17 (68.0%) | .366 |
| Immunoglobulin | 26 (57.8%) | 14 (70.0%) | 12 (48.0%) | .138 |
In the ICU, 14 (31.1%) patients received high flow nasal cannula and 35 (77.8%) patients received mechanical ventilation. Thereinto, 14 (31.1%) patients received invasive mechanical ventilation and 34 (75.6%) patients received non-invasive mechanical ventilation. In all of above treatments, there was significant difference between patients admitted to the ICU since symptoms onset >7 days and those admitted to the ICU since symptoms onset ≤7 days. However, there was no statistical difference in treatments of prone position ventilation (6 [30.0%] vs 2 [8.0%], P = .127), renal replacement therapy (4 [20.0%] vs 2 [8.0%], P = .462), antibacterial agents (19 [95.0%] vs 17 [68.0%], P = .061), antiviral agents (19 [95.0%] vs 25 [100%], P = .444), vasoconstrictive agents (10 [50%] vs 6 [24.0%], P = .07), glucocorticoids therapy (16 [80.0%] vs 17 [68.0%], P = .366), and immunoglobulin (14 [70.0%] vs 12 [48.0%], P = .138) between patients admitted to the ICU since symptoms onset >7 days and those admitted to the ICU since symptoms onset ≤7 days (Table 2).
3.3. Laboratory findings and vital signs
These measures were recorded on day of ICU admission for all patients. Compared with patients who admitted to the ICU since symptoms onset ≤7 days, patients who admitted to the ICU since symptoms onset >7 days had lower blood levels of ratio of PaO2 to FiO2 (105.85 mmHg [70.08–140.00] vs 150.00 mmHg [112.80–217.50], P = .009), lymphocyte count (0.58 × 109/L [0.34–0.74] vs 0.94 × 109/L [0.48–1.15], P = .032), and higher blood levels of white blood cell count (10.25 × 109/L [6.28–18.83] vs 4.30 × 109/L [3.00–10.10], D-dimer (9.34 mg/L [2.37–21.30] vs 0.76 mg/L [0.57–3.97], P = .000), lactate concentration (2.59 mmol/L [1.96–3.95] vs 1.68 mmol/L [1.12–2.65], P = .024), creatine kinase-MB (26.00 U/L [10.60–118.20] vs 12.20 U/L [9.00–21.05], P = .027), NT-pro BNP (393 pg/mL [110.5–1494] vs 68 pg/mL [0–307.5], P = .031), creatinine (114.70 μmol/L [65.48–171.58] vs 70.30 μmol/L [57.00–83.65], P = .037), procalcitonin (0.30 ng/mL [0.11–3.40] vs 0.09 ng/mL [0.05–0.38], P = .014). And prothrombin time of patients who admitted to the ICU since symptoms onset >7 days was longer than patients who admitted to the ICU since symptoms onset ≤7 days (11.9 seconds [10.93–14.40] vs 11.0 seconds [10.45–11.60], P = .043). Platelet count (98.50 × 109/L [76.50–197.50] vs 134.00 × 109/L [96.50–175.00], P = .398), activated partial thromboplastin time (31.95 seconds [27.33–37.63] vs 29.40 seconds [26.00–32.50], P = .185), total bilirubin (20.78 μmol/L [8.85–33.66] vs 10.81 μmol/L [8.13–22.14], P = .150) and Na+ concentration (145.95 μmol/L [142.10–149.80] vs 145.10 μmol/L [141.60–146.55], P = .181) had no difference between patients who admitted to the ICU since symptom onset >7 days and those who admitted to the ICU since symptoms onset ≤7 days (Table 3).
Table 3.
Laboratory Findings of Critically Ill Patients with COVID-2019 on Admission to ICU.
| Median (IQR) | |||||
| Normal range | All patients (n = 45) | >7 d (n = 20) | ≤7 d (n = 25) | P valuea | |
| Ratio of PaO2 to FiO2, mmHg | >300 | 126.00 (88.00–167.00) | 105.85 (70.08–140.00) | 150.00 (112.80–217.50) | .009 |
| White blood cell count, ×109/L | 4-10 | 6.50 (3.85–13.15) | 10.25 (6.28–18.83) | 4.30 (3.00–10.10) | .004 |
| Lymphocyte count, ×109/L | 1.26–3.35 | 0.67 (0.44–1.03) | 0.58 (0.34–0.74) | 0.94 (0.48–1.15) | .032 |
| Platelet count, ×109/L | 100-300 | 121.00 (85.00–178.50) | 98.50 (76.50–197.50) | 134.00 (96.50–175.00) | .398 |
| Prothrombin time, s | 10-13 | 11.20 (10.60–12.35) | 11.90 (10.93–14.40) | 11.00 (10.45–11.60) | .043 |
| Activated partial thromboplastin time, s | 23-37 | 30.60 (26.60–34.20) | 31.95 (27.33–37.63) | 29.40 (26.00–32.50) | .185 |
| D-dimer, mg/L | 0–1.35 | 2.29 (0.66–14.60) | 9.34 (2.37–21.30) | 0.76 (0.57–3.97) | .000 |
| Lactate concentration, mmol/L | 0.36–1.2 | 2.19 (1.32–3.27) | 2.59 (1.96–3.95) | 1.68 (1.12–2.65) | .024 |
| Creatine kinase-MB, U/L | 0-24 | 13.5 (9.15–51.00) | 26.00 (10.60–118.20) | 12.20 (9.00–21.05) | .027 |
| NT-pro BNP, pg/mL | ≤300 | 158.00 (38.00–1020.50) | 393.00 (110.50–1494.00) | 68.00 (0.00–307.50) | .031 |
| Creatinine, μmol/L | 35–97 | 72.20 (61.05–124.55) | 114.70 (65.48–171.58) | 70.30 (57.00–83.65) | .037 |
| Total bilirubin, μmol/L | 2–20.4 | 15.00 (8.45–24.38) | 20.78 (8.85–33.66) | 10.81 (8.13–22.14) | .150 |
| Procalcitonin, ng/mL | 0–0.5 | 0.17 (0.06–0.63) | 0.30 (0.11–3.40) | 0.09 (0.05–0.38) | .014 |
| Na+ concentration, mmol/L | 136–145 | 145.50 (141.65–148.00) | 145.95 (142.10–149.80) | 145.10 (141.60–146.55) | .181 |
3.4. CT findings
Chest computed tomography (CT) findings of critically ill patients with SARS-CoV-2 infection were bilateral ground glass opacity and consolidation (Fig. 1).
Figure 1.
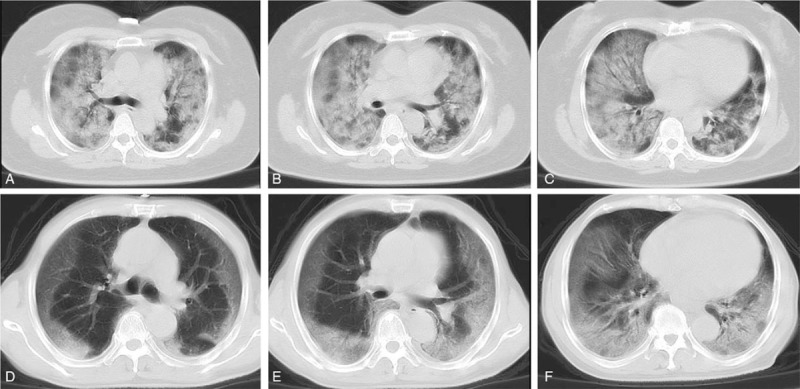
Chest computed tomographic images of 2 patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2). (A–C) CT images obtained in a 54-year-old woman on day 9 after symptom onset, show ground glass opacity and consolidation in both lungs. (D–F) CT images obtained in a 68-year-old man on day 5 after symptom onset, show ground glass opacity and consolidation in peripheral areas. Both patients were diagnosed ARDS with low ratio of PaO2 to FiO2: 57.0 and 88.0 mmHg, respectively. Written informed consent was provided by the 2 patients.
3.5. Prognosis
Of the 45 critically ill patients, the mortality rate was 42.2% in total. And the mortality rates of patients who admitted to the ICU since symptoms onset >7 days and those who admitted to the ICU since symptoms onset ≤7 days were 50.0% and 36.0% respectively. However, there was no difference in prognosis between the 2 groups (P = .345).
Furthermore, compared with survivors in this cohort, non-survivors had much higher incidence rates of hospital-acquired pneumonia (12 [63.2%] vs 4 [15.4%], P = .001) and extra-pulmonary complications (19 [100.0%] vs 16 [61.5%], P = .007) (Fig. 2). Besides, mortality rate was positive associated with incidence rates of extra-pulmonary complications (coefficient of contingency = 0.416, P = .002) and hospital-acquired pneumonia (coefficient of contingency = 0.442, P = .001). The incidence of ARDS had no difference between survivors and non-survivors (16 [61.5%] vs 16 [84.2%], P = .097) (Fig. 2).
Figure 2.

Comparison between the incidence rates of hospital-acquired pneumonia, extra-pulmonary complications, and acute respiratory distress syndrome of survivors and non-survivors. ∗, # mean P < .05.
4. Discussion
As of September 15, 2020, nearly 30 million laboratory confirmed cases of infection with the novel coronavirus (SARS-CoV-2) in total were reported all over the world. And about 900,000 patients were killed by the disease. Compared with moderate patients, the incidence of complications was higher in severe and critically ill patients.[9] As of March 29, 2020 we reported on 45 critically ill patients with COVID-19, characterized by severe hypoxemia. Of all included patients, 32 (71.1%) had ARDS and 35 (77.8%) had extra-pulmonary complications (e.g. acute kidney injury, cardiac injury, acute heart failure, liver dysfunction, gastrointestinal haemorrhage, etc). 20 (44.4%) of critically ill patients were admitted to the ICU more than 7 days since symptoms onset, and 25 (55.6%) were on or less than 7 days since symptoms onset. Interestingly, our study showed that fever symptom and history of exposure to Wuhan or close contact with some individuals with confirmed or highly suspected SARS-CoV-2 infection seemed to be dominant reason for promoting patients to see the doctors and caused their attention.
Although the novel coronavirus was quickly isolated and sequenced,[10] there are no proven, effective drugs to treat COVID-19. Until now, the mainstay of treatment has still been isolation and supportive care. All critically ill patients who suffer from severe hypoxemia, unstable hemodynamic and even multiple organ dysfunction caused by COVID-19 should be admitted to ICU. However, a considerable problem is the shortage of sufficient ICU beds to treat these critically ill patients, which leads to delay in ICU admission. It would make the critically ill patients miss the opportune for best treatment, if they are not admitted to ICU or admitted too late. Recently, this report, to our knowledge, is the first case series to investigate time from symptoms onset to ICU admission of critically ill patients with SARS-CoV-2 infection.
Since Chinese government has taken action quickly and effectively, the overall mortality of COVID-19 is about 4.0% in China, and the mortality is about 0.7% except Wuhan.[11] The number of patients with COVID-19 is increasing rapidly in other countries out of China, which might lead to higher mortality rate in the countries which are lack of medical resources, especially. Despite lower mortality rate than severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS),[12,13] COVID-19 has led to more total deaths due to the large number of cases. For reducing mortality rate, it is important to know the clinical characteristics of critically ill patients.
Previous studies showed that old age, male, and presence of chronic medical illness might be associated with increased mortality.[1,8,14] The mortality rate in critically ill patients with SARS-CoV-2 infection in our cohort was 42.2%. Our study showed that non-survivors had higher rates of extra-pulmonary complications and hospital-acquired pneumonia than survivors. The rates of ARDS had no difference between survivors and non-survivors. Pathology of critically ill patients who died from COVID-19 manifested that while the SARS-CoV-2 was mainly distributed in lung, the infection also involved in the damages of heart, vessels, liver, kidney and other organs.[15,16] On the basis of observation from COVID-19 patients, Bin Cao and his fellows hypothesized that in severe or critical COVID-19 cases, in response to the infection of SARS-CoV-2, alveolar macrophages or epithelia cells could produce various proinflammatory cytokines and chemokines, and then monocytes and neutrophils were chemotactic to the infection site to clear these exudates with virus particles and infected cells, resulting in uncontrolled inflammation which led a further worsening of lung injury.[17] Furthermore, extra-pulmonary organs could be attacked directly by disseminated SARS-CoV-2 or injured by the systemic cytokine.[17] Therefore, we supposed that ICU physicians interfered at early phase of SARS-CoV-2 infection, which might get benefit for critically ill patients from prognosis. We analyzed the clinical data of 20 critical COVID-19 cases admitted to the ICU since symptoms onset >7 days, and 25 admitted to the ICU since symptoms onset ≤7 days as well. Of the 45 cases, 30 (66.7%) were male. There was no difference in gender and age structure, and chronic medical illness between the 2 groups. This might be because all of patients who admitted to the ICU were critically ill, and this had no relationship with the time of ICU admission.
Similarities of clinical features between our cohort and previous reports had been noted. Most patients presented with fever, cough, dyspnea, and bilateral ground-glass opacities on chest CT scans. These features of COVID-19 bear some resemblance to SARS-CoV and MERS-CoV infections.[18,19] Upon the probable mechanisms mentioned above, besides respiratory failure, in our cohort, most critical COVID-19 cases [35 (77.8%)] progressed rapidly with extra-pulmonary organ injury or complications. The extra-pulmonary complications in our study were hypernatremia [33 (73.3%)], liver dysfunction [27 (60.0%)], Acute kidney injury [15 (33.3%)], acute heart failure [13 (28.9%)], gastrointestinal hemorrhage [12 (26.7%)], cardiac injury [10 (22.2%)], and hyperamylasemia [1 (2.2%)]. Critically ill patients with COVID-19 who admitted to the ICU since symptoms onset ≤7 days had significant lower incidence rates of hypernatremia, acute kidney injury, gastrointestinal hemorrhage, and cardiac injury than those who admitted to the ICU since symptoms onset >7 days. Therefore, we speculated that, for critically patients with COVID-19, time from symptoms onset to ICU was directly associated with the extra-pulmonary complications which responsible for multiple organ dysfunction, or even failure. It is generally known that, for sepsis patients, the mortality rate has positive correlation to the exhaustion of the organic function, and it is in direct proportion to the number of the failure organ. So, we supposed that once patients with SARS-CoV-2 infection had been classified critical cases, should be admitted to ICU at once, and these patients might get benefit from intensive care via lower rate of extra-pulmonary complications. However, we did not conclude that there was significant difference in prognosis between the 2 groups. Meanwhile, in our cohort, incidence rates of hospital-acquired pneumonia had no difference between the patients who admitted to the ICU since symptoms onset >7 days and those who admitted to the ICU since symptoms onset ≤7 days as well, which had significant difference between survivors and non-survivors. Incidence rate of hospital-acquired pneumonia in non-survivors was much higher than survivors. So, hospital-acquired pneumonia might be another reason for the mortality.
Critical COVID-19 cases had numerous laboratory abnormalities. Firstly, the most common laboratory abnormalities observed in our study were depressed absolute value of lymphocytes. This phenomenon suggests that SARS-CoV-2 might mainly act on lymphocytes, especially T lymphocytes, as does SARS-CoV. Pathology of critically ill patients who died from COVID-19[16] found decreased numbers of lymphocytes in spleen. Virus particles spread through the respiratory mucosa and infect other cells, induce a cytokine storm in the critically ill patients, generate a series of immune responses with altering in peripheral white blood cells and immune cells such as lymphocytes. Previous researchers had clarified that apoptosis of T and B lymphocytes was in models of sepsis, post-mortem analysis of septic patients,[20–22] and in the circulation of patients with septic shock.[23] Therefore, we speculated that necrosis or apoptosis of lymphocytes also induced depressed lymphocytes in critically ill patients with COVID-19. It suggested that the level of depressed lymphocytes reflected the severity of COVID-19, which might provide a new index for predicting severity of illness. Furthermore, the other laboratory abnormalities in critically ill patients were prolonged prothrombin time, increased D-dimer, up-regulated creatine kinase-MB and creatinine, elevated lactate dehydrogenase. These laboratory abnormalities were similar to those previously observed in patients with MERS-CoV and SARS-CoV infection,[24] which suggested that COVID-19 might be associated with cellular immune deficiency, coagulation activation, myocardia injury, hepatic injury, and kidney injury.
Denstaedt et al had found that depressed lymphocytes were associated with increased mortality and risk of hospital-acquired infection and occurs commonly in patients with persistent critical illness.[23] The hospital-acquired pneumonia incidence rate in our cohort was about 35.6% what might be attributed to depressed lymphocytes. We found prognosis of the 45 critical COVID-19 cases in our cohort was positive associated with extra-pulmonary complications (coefficient of contingency = 0.416, P = .002) and hospital-acquired pneumonia (coefficient of contingency = 0.442, P = .001). Between critically ill patients who admitted to the ICU since symptoms onset >7 days and those who admitted to the ICU since symptoms onset ≤7 days, there was significant difference in extra-pulmonary complications, while no difference in the prognosis of the 2 groups. We suspected that it might be caused by hospital-acquired pneumonia due to decreased numbers of lymphocytes.
The limitations of this study include the small number of patients from a single center. Collection of standardized data for a larger cohort would help to further define the clinical presentation, natural history, and risk factors. At the same time, finding of statistical tests and P values should be interpreted with caution, and non-significant P values do not necessarily rule out difference between critically ill patients with COVID-19 who admitted to the ICU since symptoms onset >7 days and those who admitted to the ICU since symptoms onset ≤7 days.
5. Conclusion
In this single-center case series of 45 critically ill patients with COVID-19 in Yichang, Hubei, China, the shorter duration from symptom onset to ICU admission, the less extra-pulmonary complications. So critically ill patients with SARS-Cov-2 infection who admitted to ICU at once might get benefit from intensive care via lower rate of extra-pulmonary complications. Our study provides a new insight for fighting COVID-19 globally.
Acknowledgments
We thank all the patients and their families involved in the study. And we would like to thank Editage for help with language editing of the manuscript.
Author contributions
PW, XT, QL, MQ, AC, BM, PW, XZ, CG, MS, MQ and MY collected the epidemiological, clinical, and laboratory data. PW, XT, and QL summarized all the data and drafted the manuscript. XT and MY revised the final manuscript. The author(s) read and approved the final manuscript.
Conceptualization: Min Yu, Xiang Tan.
Data curation: Peng Wang, Xiang Tan, Qian Li, Min Qian, Aiguo Cheng, Baohua Ma, Peng Wan, Xinli Zhang, Changyun Guo, Mengting Sheng, Mengqiu Yi.
Formal analysis: Peng Wang, Xiang Tan, Qian Li, Min Qian, Min Yu.
Investigation: Peng Wang, Xiang Tan, Qian Li, Min Qian, Aiguo Cheng.
Methodology: Peng Wang, Xiang Tan, Min Yu.
Project administration: Peng Wang, Xiang Tan, Min Qian, Aiguo Cheng, Min Yu.
Resources: Peng Wang, Xiang Tan, Min Yu.
Software: Peng Wang, Xiang Tan.
Validation: Peng Wang, Xiang Tan, Qian Li, Min Qian, Min Yu.
Writing – original draft: Peng Wang, Xiang Tan, Qian Li.
Writing – review & editing: Peng Wang, Xiang Tan, Min Yu.
Conceptualization: Min Yu.
Data curation: Peng Wang, Xiang Tan, Qian Li, Min Qian, Aiguo Cheng, Baohua Ma, Peng Wan, Xinli Zhang, Changyun Guo, Mengting Sheng, Mengqiu Yi.
Formal analysis: Peng Wang, Xiang Tan, Qian Li, Min Qian, Min Yu.
Investigation: Peng Wang, Xiang Tan, Qian Li, Min Qian, Aiguo Cheng.
Methodology: Peng Wang, Xiang Tan, Min Yu.
Project administration: Peng Wang, Xiang Tan, Min Qian, Aiguo Cheng, Min Yu.
Resources: Peng Wang, Xiang Tan, Min Yu.
Software: Peng Wang, Xiang Tan.
Validation: Peng Wang, Xiang Tan, Qian Li, Min Qian, Min Yu.
Writing – original draft: Peng Wang, Xiang Tan, Qian Li.
Writing – review & editing: Peng Wang, Xiang Tan, Min Yu.
Footnotes
Abbreviations: ARDS = acute respiratory distress syndrome, COVID-19 = coronavirus disease 2019, CT = computed tomography, FiO2 = fraction of inspired oxygen, ICU = intensive care unit, IQR = interquartile range, MERS = Middle East respiratory syndrome, RT-PCR = real-time reverse transcriptase polymerase chain reaction, SARS = severe acute respiratory syndrome, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, SD = standard deviation, WHO = World Health Organization.
How to cite this article: Wang P, Tan X, Li Q, Qian M, Cheng A, Ma B, Wan P, Zhang X, Guo C, Sheng M, Yi M, Yu M. Extra-pulmonary complications of 45 critically ill patients with COVID-19 in Yichang, Hubei province, China a single-centered, retrospective, observation study. Medicine. 2021;100:9(e24604).
PW, XT, and QL contributed equally to this work.
This study had no external funding source.
All the authors state that there are no conflicts of interest related to this study.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Values are numbers or percentages unless stated otherwise.
P < .05 was considered statistically significant.
P values indicate differences between duration from onset of symptom to ICU admission >7 and ≤7 days.
Values are numbers or percentages unless stated otherwise.
P < .05 was considered statistically significant.
P values indicate differences between duration from onset of symptom to ICU admission >7 and ≤7 days.
P < .05 was considered statistically significant.
IQR = interquartile range, MB = muscle and brain type.
P values indicate differences between duration from onset of symptom to ICU admission >7 and ≤7 days.
References
- [1].Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liu X, Zhang S. COVID-19: face masks and human-to-human transmission. Influenza Other Respir Viruses 2020;14:472–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mu A. A COVID-19 transmission within a family cluster by presymptomatic infectors in China. J Chem Inf Model 2019;53:1689–99. [Google Scholar]
- [6].Baden LR, Rubin EJ. Covid-19 – the search for effective therapy. N Engl J Med 2020;382:1851–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Biswas A, Bhattacharjee U, Chakrabarti AK, et al. Emergence of Novel Coronavirus and COVID-19: whether to stay or die out? Crit Rev Microbiol 2020;46:182–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Feng Y, Ling Y, Bai T, et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med 2020;201:1380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Z W., J.M M.. Characteristics of and important lessons from the coronavirus disease 2019(COVID-19) outbreak in China. JAMA 2020;10:2648. [DOI] [PubMed] [Google Scholar]
- [12].Olsen SJ, Chang HL, Cheung TYY, et al. Transmission of the severe acute respiratory syndrome on aircraft. N Engl J Med 2003;349:2416–22. [DOI] [PubMed] [Google Scholar]
- [13].Azhar EI, El-Kafrawy SA, Farraj SA, et al. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med 2014;370:2499–505. [DOI] [PubMed] [Google Scholar]
- [14].Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tian S, Hu W, Niu L, et al. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol 2020;15:700–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xiaohong Y, Tingyuan L, Zhicheng H, et al. A pathological report of three COVID-19 cases by minimally invasive autopsies. Chin J Pathol 2020;49:291–3. [Google Scholar]
- [17].Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet 2020;395:1517–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Xue X, Gao Z, Xu Y, et al. Clinical analysis of 45 patients with severe acute respiratory syndrome. Chin Med J (Engl) 2003;116:819–22. [PubMed] [Google Scholar]
- [19].Badawi A, Gwan S. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis 2016;49:129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 2011;306:2594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nicolas W, Cortes-Penfield, Barbara W, et al. Evidence for persistent immune suppression in patients who develop chronic critical illness after sepsis. Physiol Behav 2017;176:139–48.28363838 [Google Scholar]
- [22].Drewry A, Samra N, Skrupky L, et al. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock 2014;42:383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Denstaedt SJ, Singer BH, Standiford TJ. Sepsis and nosocomial infection: patient characteristics, mechanisms, and modulation. Front Immunol 2018;9:2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].de Wit E, van Doremalen N, Falzarano D, et al. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol 2016;14:523–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


