Abstract
Background:
The aim of this meta-analysis with trial sequential analysis (TSA) was to evaluate the effect of a polyurethane (PU) tracheal tube cuff on the prevention of ventilator-associated pneumonia (VAP).
Methods:
We performed a systematic search using the MEDLINE database through PubMed, Cochrane Central Register of Controlled Trial, SCOPUS, and Web of Science.
Randomized controlled trials comparing the incidence of VAP and clinically relevant outcomes between PU cuff tubes and polyvinyl chloride (PVC) cuff tubes in adult patients. Two authors independently extracted study details, patient characteristics, and clinical outcomes such as incidence of VAP, bacterial colonization of tracheal aspirate, duration of mechanical ventilation, ICU stay, and ICU mortality.
Results:
From 309 studies identified as potentially eligible, six studies with 1226 patients were included in this meta-analysis. All studies compared the incidence of VAP between PU cuffs and PVC cuffs. Use of a PU cuff was not associated with a reduction in VAP incidence (RR = 0.68; 95% CI, 0.45–1.03) with significant statistical heterogeneity (I2 = 65%). The quality of evidence was “very low.” According to the TSA, the actual sample size was only 15.8% of the target sample size, and the cumulative Z score did not cross the trial sequential monitoring boundary for benefit. No positive impact was reported for the other relevant outcomes for PU cuffs.
Conclusions:
The use of a PU cuff for mechanical ventilation did not prevent VAP. Further trials with a low risk of bias need to be performed.
Keywords: artificial, epidemiology, equipment and supplies, intratracheal, intubation, pneumonia, primary prevention, ventilation, ventilator-associated
1. Introduction
Ventilator-associated pneumonia (VAP) remains as a significant problem in critically ill patients. A multimodal approach to decrease the risk of VAP, such as the use of subglottic secretion drainage (SSD), cuff pressure monitoring, positive end-expiratory pressure (PEEP), and caring for patients in the head-up position has been conducted in these patients.[1–7] However, we must continue to explore additional means to minimize the risk of VAP. Air inflation of a high-volume low-pressure (HVLP) cuff of a tracheal tube in the trachea causes folding because the diameter of the inflated cuff is always larger than the tracheal diameter. The longitudinal folds that develop in the cuff work as channels to allow supra-pharyngeal secretions into the trachea,[8,9] which could increase the risk of VAP. Due to its ultra-thin cuff membrane, the polyurethane (PU) cuff was expected to minimize this leakage. Not surprisingly, the PU cuff showed efficacy for the prevention of fluid leakage in laboratory studies and microaspiration in clinical studies,[5,10–19] compared to the conventional polyvinyl chloride (PVC) cuff. In addition, some studies showed that a PU cuff with or without SSD decreased the incidence of VAP or shortened the intensive care unit (ICU) stay.[20–24] However, the results are still conflicting.[25,26]
In this study, we conducted a systematic review with a meta-analysis of randomized controlled trials (RCTs) to compare clinical effectiveness between the PU cuff and the PVC cuff for the prevention of VAP.
2. Methods
This systematic review and meta-analysis of RCTs with trial sequential analysis (TSA) to evaluate the efficacy of the PU cuff for the prevention of VAP was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines.[27] Meta-analysis is a study utilizing publically available data which would not link with personally identifiable information. From the standpoint of the ethical standards, meta-analysis is out of application range for review and approval by the local institutional review board. In addition, the data was already anonymized in the primary study. Therefore, informed consent could not be obtained from the patients for this study.
2.1. Search strategy
We performed a literature search using the MEDLINE database through the PubMed search engine, Cochrane Central Register of Controlled Trials, SCOPUS, and Web of Science without language restriction. The search parameters used were ventilator-associated pneumonia/cuff/RCT. We also conducted manual searches of the references from studies, reviews, and the Web. We commenced a literature search from October 2018, and the most recent access to these electronic databases was on December 10, 2019.
After duplicate publications were excluded, two authors (KM and MS) independently scanned the title and abstract of each report to eliminate irrelevant search results. Thereafter, they separately read the full text of the potential studies to assess them for inclusion in the meta-analysis. Any divergence of views was resolved by thorough discussion.
Eligible trials were prospective RCTs that compared PVC and PU tube cuffs in relation to the incidence of VAP in adult patients and that contained relevant outcomes of interest. We also excluded data from observational studies, retrospective studies, case reports, letters to the editor, reviews, and animal studies.
2.2. Primary and secondary outcomes
The primary outcome of this meta-analysis was the incidence of VAP. The secondary outcomes were bacterial colonization of tracheal aspirate, the duration of mechanical ventilation, ICU stay, and ICU mortality.
2.3. Data extraction
Two authors (KM and MS) extracted the available data from the included studies. The following items were extracted: first author, publication year, study design, country and type of ICU, number of patients, tracheal tube type, cuff material and shape, internal diameter of the tracheal tube, standard care including VAP bundle; SSD, head elevation, nutrition, oral care, ulcer prevention, cuff pressure control during care, use of PEEP, diagnostic criteria of VAP, and duration of follow-up. If further information was required, attempts were made to contact the study authors through e-mail.
2.4. Risk of bias assessment in individual studies
We used a version 2 of the Cochrane tool for assessing risk of bias in randomized trials (RoB 2) with five domains, as follows[28]:
-
1.
bias arising from the randomization process;
-
2.
bias due to deviations from intended interventions;
-
3.
bias due to missing outcome data;
-
4.
bias in measurement of the outcome; and
-
5.
bias in selection of the reported results. No funnel plot was applied because of the small number of included studies.
2.5. Quality of evidence assessment
To assess the quality of the evidence in this systematic review, we used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach. Five factors can lower the quality of evidence in this approach: limitations of detailed design and execution (risk of bias), inconsistency, indirectness, imprecision, and publication bias. We determined the quality of evidence for each outcome by developing the summary of findings table using GRADEpro GDT software (available at https://gradepro.org/).[29]
2.6. Statistical analysis
The relative risk with 95% confidence interval (CI) for categorical variables and the mean difference (MD) with 95% CI for continuous variables were used for the summary. Several studies reported continuous variables as the median and range. Therefore, we estimated median and standard deviation (SD) from these values using two simple formulae and included them in this meta-analysis.[30] We combined data using a random-effect model (DerSimonian–Laird method). Statistical inter-study heterogeneity was quantified by using I2 statistics. A value more than 50% of the I2 statistic was considered to indicate heterogeneity.
SSD is known to be beneficial in decreasing VAP.[31] Therefore, we added subgroup analysis of data without SSD to eliminate the positive bias of SSD in the sensitivity analysis. A P value <.05 was deemed statistically significant.
We also conducted TSA in this meta-analysis to prevent type I error caused by multiple testing of the effect in the meta-analysis.[32–36] First, we calculated heterogeneity-adjusted target sample size called the required information size (RIS), which is a similar concept to that of sample size calculation when conducting a RCT. We calculated the RIS based on a minimum clinically meaningful risk ratio of 0.75 for the incidence of VAP and bacterial colonization, 0.9 for ICU mortality, 0.5 days for the duration of mechanical ventilation, and 1 day for ICU stay. The risk of type I and type II errors was set at 5% and 10%, respectively. Control event rates and duration were calculated from those of the PVC cuff group. Second, the TSA monitoring boundaries were quantified using an alpha spending function, and adjusted CIs were calculated. Then, a Z statistic was calculated for each trial, and a cumulative Z curve was plotted. We assessed the risk of type I and type II error and the demand for further trials in the conducted meta-analysis using the provided graphical relationship of the cumulative Z-curve of the meta-analysis, monitoring boundaries, and RIS.[37] When the cumulative Z-curve enters the futility area or crosses the TSA monitoring boundary, a firm conclusion can be drawn that the anticipated intervention effect may reach a sufficient level of evidence and further trials will not be necessary. When the cumulative Z-curve does not cross any of the boundaries or reach the RIS, evidence is insufficient for drawing a conclusion.
The conventional meta-analysis was performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics.[37] The TSA was performed using TSA Viewer, Version 0.9.5.10 beta (www.ctu.dk/tsa).
3. Results
3.1. Study description
After discarding duplicate studies, we identified 309 studies from five electronic databases. Six studies with 1226 patients met our criteria and were included in this meta-analysis[20–22,24–26] (Fig. 1). Details of the included studies are listed in Table 1. A clinical diagnosis of VAP was made if new or progressive infiltration on chest X-ray was present with multiple criteria as follows: purulent bronchial sputum, fever or hypothermia, leukocytosis,[20] along with increase of C-reactive protein and deterioration of oxygenation,[22] or CDC definition.[26] The Clinical Pulmonary Infection Score (CPIS) was used in two studies.[21,24] Diagnosis was made only with noninvasive or invasive sampling with quantitative culture results performed in suspected patients in one study.[25] Otherwise, diagnosis was microbiologically confirmed in two studies.[20,24]
Figure 1.
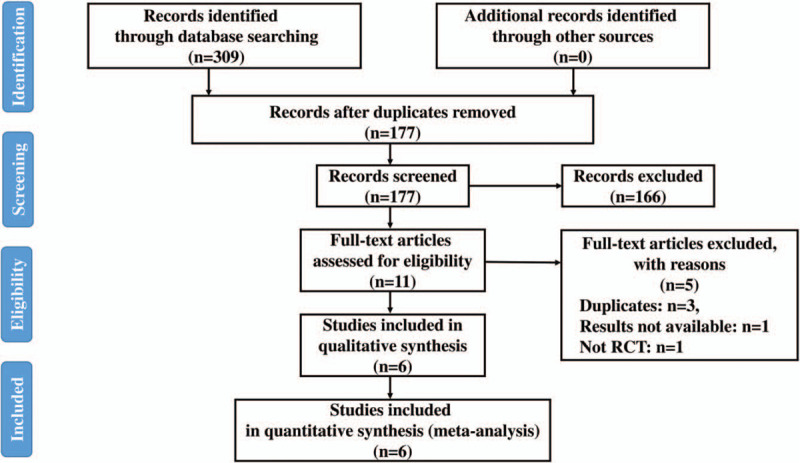
Flow diagram of the literature search. RCT = randomized controlled trials.
Table 1.
Characteristics of Included Studies.
| Author | Lorente L[20] | Poelaert J[22] | Mahmoodpoor A[21] | Phillippart F[25] | Suhas P[24] | Deem S[26] |
| Published year | 2007 | 2008 | 2013 | 2015 | 2016 | 2016 |
| Setting | Medical, Spain | Surgical (post-cardiac), Belgium | Mixed, Iran | Mixed, France and Tunisia | Surgical, India | Medical, USA |
| Number of patients | 280 | 134 | 96 | 534 | 80 | 102 |
| Tracheal tube type | PU: conical cuff with SDD PVC: cylindrical cuff | PU: conical cuff PVC: cylindrical cuff | PU: cylindrical/conical cuff with SDD PVC: cylindrical | PU: cylindrical/conical cuff PVC: cylindrical/conical cuff | PU: cylindrical cuff PVC: cylindrical cuff | PU: conical cuff with and without SDD PVC: cylindrical |
| Internal diameter | NR | 8 mm for females, 9 mm for males | 7–7.5 mm for females, 8–8.5 for males | 7.5 or 8 mm | Not described | 7 mm for females 7.5 mm for males |
| Standard care | ||||||
| SSD | Yes (every hour) | N/A | Yes (every hour) | No | N/A | Yes |
| Head elevation | Yes | NR | Yes | Yes | Yes | Yes |
| Enteric nutrition | Yes | NR | Yes | NR | Yes | Yes |
| Oral care | CHX (every 8 h) | NR | NR | 0.12% CHX (every 6 h) | CHX (every 4 h) | 0.12% CHX (every 8 h) |
| Ulcer prevention | H2-blocker | H2-blocker (every 8 h) | PPI or H2-blocker | PPI in case of hypocoagulability | NR | Conducted in case at risk |
| Cuff pressure | 25 cm H2O (checked every 4 h) | 20–26 cm H2O (checked every 4 h) | 20–30 mm Hg (checked every 3 h) | 25–30 cm H2O (checked every 6 h) | ≥25 cm H2O | 25–30 cm H2O (checked every 8 h) |
| PEEP | NR | NR | 5 mm Hg | ≥5 cm H2O | NR | NR |
| Duration of follow-up | During ICU stay | Within 7 days after surgery | 3 days | During ICU stay | During ICU stay | 7 days after tracheal intubation |
All six studies were prospective RCTs that provided the incidence of VAP and one or more data associated with morbidity and mortality in the ICU. The risk of bias is summarized in Table 2 and Supplemental Table 1.
Table 2.
Risk of bias summary.
| Author | Lorente L[20] | Poelaert J[22] | Mahmoodpoor A[21] | Phillippart F[25] | Suhas P[24] | Deem S[26] |
| Domain 1: Bias arising from the randomization process | Some concerns | Some concerns | Some concerns | Low | Some concerns | Low |
| Domain 2: Bias due to deviations from intended interventions | Some concerns | Some concerns | Some concerns | Some concerns | Some concerns | Some concerns |
| Domain 3: Bias due to missing outcome data | Low | Low | Low | Low | Low | Low |
| Domain 4: Bias in measurement of the outcome | Some concerns | Low | Some concerns | Some concerns | Some concerns | Some concerns |
| Domain 5: Bias in selection of the reported result | Low | Low | Low | Low | Low | Low |
| Overall risk of bias judgement | Some concerns | Some concerns | Some concerns | Some concerns | Some concerns | Some concerns |
3.2. Main outcomes: incidence of VAP
All six trials reported the incidence of VAP.[20–22,24–26] The combined results for the incidence of VAP showed that the tracheal tube with PU cuff did not significantly decrease the morbidity of VAP compared with the PVC cuff (RR = 0.68; 95% CI, 0.45–1.03) with significant statistical heterogeneity (I2 = 65.4%) (Fig. 2A). The RIS was calculated as 7737 using TSA. The accrued information size was 1226, which was only 15.8% of the estimated RIS. The cumulative Z score did not cross the trial sequential monitoring boundary for benefit (Fig. 2B). The TSA-adjusted 95% CI was 0.11 to 4.01.
Figure 2.
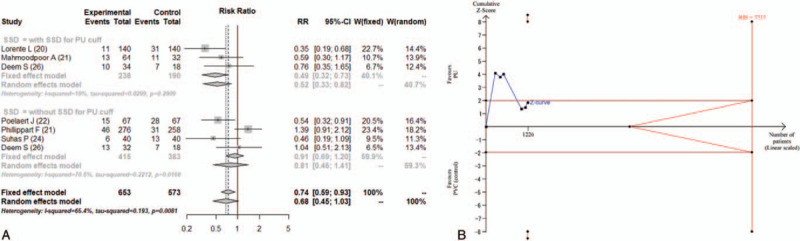
(A) Forest plot for the incidence of ventilator-associated pneumonia. (B) Trial sequential analysis for the incidence of ventilator-associated pneumonia. Risk of type 1 error was maintained at 5% with a power of 90%. The variance was calculated from the data obtained from the included trials. A clinically meaningful risk ratio was set at 0.75. The blue line is the cumulative Z curve, and each black square dot represents 1 trial. The brown horizontal lines indicate a conventional significant P value of .05. The red diagonal lines represent the futility region. The red vertical lines are the trial sequential monitoring boundaries. In total, 1226 patients were analyzed, and the Z curve did not cross the monitoring boundary. CI = confidence interval, PU = polyurethane, PVC = polyvinyl chloride, RIS = required information size, RR = relative risk, SSD = subglottic secretion drainage.
3.3. Secondary outcomes
3.3.1. Incidence of bacterial colonization of tracheal aspirate
Four studies reported the incidence of bacterial colonization of tracheal aspirate.[20,22,25,26] The definition of bacterial colonization varied among the studies. It was defined as the quantitative culture of respiratory secretions by tracheal aspirate of more than 106 cfu/mL in the studies by Lorente et al and Deem et al,[20,26] and 105 cfu/mL by Poelaert et al.[22] Philippart et al reported incidences of bacterial colonization at levels from 103 to106 cfu/mL.[25] We adopted 105 cfu/mL as the indicator of bacterial colonization. The combined results are shown in Figure 3A. There was no significant difference between the PU cuff and PVC cuff (RR = 0.69; 95% CI, 0.45–1.04) in the incidence of bacterial colonization with significant heterogeneity (I2 = 60.6%). The RIS was 7477, and the accrued information size reached only 14.0% of the estimated RIS. The cumulative Z score did not cross the trial sequential monitoring boundary for benefit (Fig. 3B). The TSA-adjusted 95% CI was 0.11 to 4.22.
Figure 3.
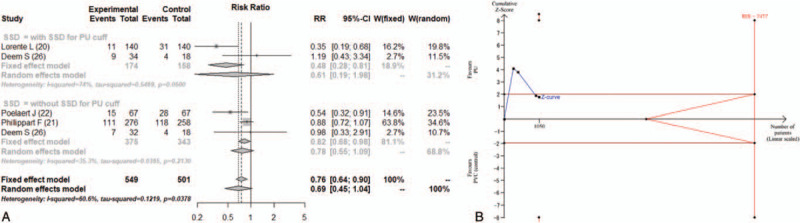
(A) Forest plot for the incidence of bacterial colonization of tracheal aspirate. (B) Trial sequential analysis for the incidence of bacterial colonization of tracheal aspirate. Risk of type 1 error was maintained at 5% with a power of 90%. The variance was calculated from the data obtained from the included trials. A clinically meaningful risk ratio was set at 0.75. The blue line is the cumulative Z curve, and each black square dot represents 1 trial. The brown horizontal lines indicate a conventional significant P value of .05. The red diagonal lines represent the futility region. The red vertical lines are the trial sequential monitoring boundaries. In total, 1050 patients were analyzed, and the Z curve did not cross the monitoring boundary. CI = confidence interval, PU = polyurethane, PVC = polyvinyl chloride, RIS = required information size, RR = relative risk, SSD = subglottic secretion drainage.
3.3.2. Duration of mechanical ventilation
Five studies reported duration of mechanical ventilation.[20,22,24–26] Two of these five studies reported the duration of ventilation as median and range.[24,25] The combined results failed to show a significant difference between the PU cuff and PVC cuff (MD = −0.36; 95% CI, −1.15 to 0.44, I2 = 47.5%) in duration of ventilation (Fig. 4A) The estimated RIS was 8284, and the accrued information size (n = 1200) reached only 14.5% of the estimated RIS. The cumulative Z score did not cross the trial sequential monitoring boundary for benefit (Fig. 4B). The TSA-adjusted 95% CI was −0.36 to 2.88.
Figure 4.
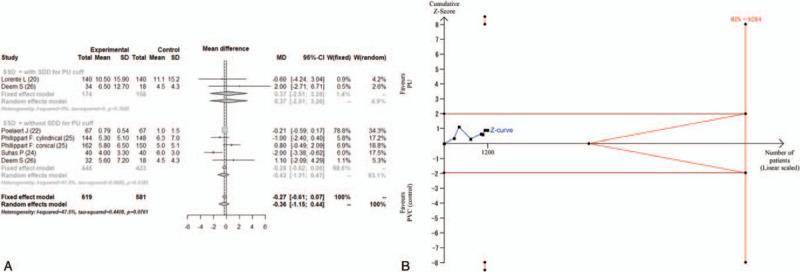
(A) Forest plot for the duration of mechanical ventilation. (B) Trial sequential analysis for the duration of mechanical ventilation. Risk of type 1 error was maintained at 5% with a power of 90%. The variance was calculated from the data obtained from the included trials. A clinically significant anticipated mean difference of duration of ventilation was set at 0.5 days. The blue line is the cumulative Z curve, and each black square dot represents 1 trial. The brown horizontal lines indicate a conventional significant P value of .05. The red diagonal lines represent the futility region. The red vertical lines are the trial sequential monitoring boundaries. In total, 1200 patients were analyzed, and the Z curve did not cross the monitoring boundary. CI = confidence interval, MD = mean difference, PU = polyurethane, PVC = polyvinyl chloride, RIS = required information size, SSD = subglottic secretion drainage.
3.3.3. ICU stay
Five studies reported ICU stay.[20,22,24–26] Two of these studies reported the ICU stay as median and range.[24,25] The combined results did not show a significant difference in ICU stay between the PU cuff and PVC cuff (MD = −0.22; 95% CI, −1.81 to 1.38, I2 = 65.7%) (Fig. 5A). The estimated RIS was 7641, and the accrued information size (n = 1098) reached only 14.4% of the estimated RIS. The cumulative Z score did not cross the trial sequential monitoring boundary for benefit (Fig. 5B). The TSA-adjusted 95% CI was −6.73 to 6.29.
Figure 5.
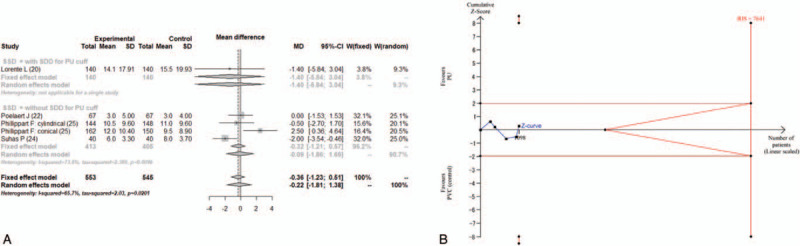
(A) Forest plot for ICU stay. (B) Trial sequential analysis for ICU stay. Risk of type 1 error was maintained at 5% with a power of 90%. The variance was calculated from the data obtained from the included trials. A clinically significant anticipated mean difference of duration of ventilation was set at 1 day. The blue line is the cumulative Z curve, and each black square dot represents 1 trial. The brown horizontal lines indicate a conventional significant P value of .05. The red diagonal lines represent the futility region. The red vertical lines are the trial sequential monitoring boundaries. In total, 1098 patients were analyzed, and the Z curve did not cross the monitoring boundary. CI = confidence interval, MD = mean difference, PU = polyurethane, PVC = polyvinyl chloride, RIS = required information size, SSD = subglottic secretion drainage.
3.3.4. ICU mortality
The ICU mortality was reported in four studies.[20,21,24,26] The combined results showed no significant difference between the PU cuff and PVC cuff (RR = 0.81, 95% CI, 0.57–1.14, I2 = 0%) in ICU mortality (Fig. 6). The accrued information size (n = 558) was far from the RIS (16,373), and the TSA-adjusted 95% CI could not be calculated.
Figure 6.
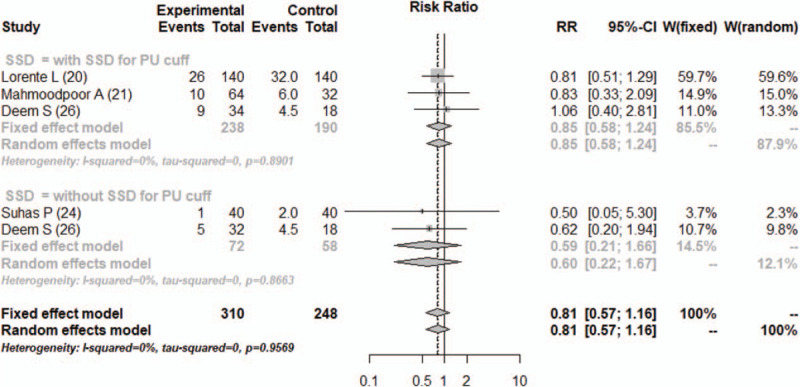
Forest plot for ICU mortality. CI = confidence interval, PU = polyurethane, RR = relative risk, SSD = subglottic secretion drainage.
3.4. Sensitivity analysis
We conducted sensitivity analysis in the subgroup of tracheal tube with or without SSD. Although the incidence of VAP was significantly reduced in the patients with PU cuff with SDD, compared with the PVC cuff (RR = 0.52; 95% CI, 0.33–0.82, I2 = 19%), there was no difference in VAP incidence between the PU cuff without SDD and the PVC cuff (RR = 0.81; 95% CI, 0.46–1.41, I2 = 70.6%) (Fig. 2A). We also compared the other combined results both a fixed-effect model and a random-effect model. As a result, the sensitivity did not change the direction of these results.
3.5. Quality of evidence assessment
The quality of the evidence of the primary outcomes was graded as “very low” (Table 3). This was downgraded due to the risk of bias being of some concern, significant heterogeneity, small sample size, and the possibility of publication bias.
Table 3.
Summary of findings.
| [PU cuff] compared to [PVC cuff] for [VAP incidence] | ||||||
| Patient or population: [Patients who were intubated and mechanically ventilated] | ||||||
| Setting: Adult patients in ICU | ||||||
| Intervention: [PU cuff] | ||||||
| Comparison: [PVC cuff] | ||||||
| Outcomes | Anticipated absolute effects∗ (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with [PVC cuff] | Risk with [PU cuff] | |||||
| VAP incidence | 223 per 1000 | 152 per 1000 (101–230) | RR 0.68 (0.45–1.03) | 988 (6 RCTs) |
 VERY LOW†,‡,§,¶ VERY LOW†,‡,§,¶
|
|
4. Discussion
Although some of the individual trials showed the effectiveness of the PU cuff and PVC cuff to decrease VAP,[20–24] our meta-analysis failed to show a significant difference in the incidence of VAP between the two cuff types. The quality of this evidence as assessed by GRADE was “very low.” Additionally, the result of the TSA indicated that the present evidence would have a risk of type II error due to the lack of adequate information size. These findings indicated that this meta-analysis should be considered as hypothesis generating. Likewise, this meta-analysis did not identify a difference in the incidence of bacterial colonization of tracheal aspirate, the duration of mechanical ventilation, ICU stay, or ICU mortality between the PU cuff and the PVC cuff. High-quality trials should be explored to reach firm conclusions.
Another systematic review without meta-analysis published earlier assessed the clinical evidence for the use of the PU cuff to counter VAP to be fragile because the cuff is approved for use only in high-risk surgical patients.[38] In contrast, the major strength of our systematic review is that to our best knowledge, it is the first quantitative review conducting a meta-analysis of accumulated RCTs that offer a comprehensive overview of the current knowledge. We used the GRADE approach to assess the level of evidence and TSA to interpret the combined results more carefully.
When fully inflated, the diameter of the HVLP cuff expands to 1.5 to 2 times that of the adult trachea.[39] Therefore, expansion of the cuff leads to folding of the excess material of the cuff over itself, which can work as a channel for microaspiration of subglottic secretions. Preventing fluid leakage via this channel is an inherent challenge to overcome with HVLP cuffs to decrease the risk of VAP, and PU is one of the promising materials used to decrease fluid leakage around cuffs. The thickness of the PU cuff material is 7 to 10 μm, which is much thinner than that of the PVC cuff (50–70 μm).[10] Therefore, the channels formed by the PU cuff also appear to be narrower than those formed by the PVC cuff. A previous systematic review revealed that numerous studies showed a decrease in fluid leakage with PU cuffs in laboratory investigations,[39] which might have a beneficial effect in preventing VAP. However, no positive effect on preventing VAP with the PU cuff was shown in the present meta-analysis. This discrepancy between the laboratory findings and the combined results of this meta-analysis may be explained as follows. There was difference in outcomes between the laboratory and clinical studies. The main outcome of the laboratory studies with the PU cuff was the decreased amount of static fluid leak, whereas that of the clinical studies was the incidence of VAP. A decrease in fluid leakage might not be linked directly to the decrease of VAP. In addition to static leakage of subglottic secretions, dynamic microaspiration can occur repetitively during standard ICU care such as rapid and excessive dilatation of the tracheal diameter with bucking or accidental downward fluctuation of the cuff pressure when the manometer is disconnected from the cuff pilot balloon after checking cuff pressure. Therefore, we assume that the superior static sealing of the PU cuff would not achieve the expected outcome.
Considerable heterogeneity exists among our selected studies in terms of the population and clinical setting of the ICU. There is a significant difference between medical and surgical patients in the attributes of their clinical conditions. One of the studies reporting a preventative effect of VAP was conducted with post-cardiac patients whose mean duration of ventilation was 25 h.[22] Compared with surgical patients, a longer duration of ventilation would frequently be expected in medical patients, and this is an important risk factor for VAP development due to the longer exposure of these patients to microaspiration of oropharyngeal secretions. However, there was a lack of information on the incidence per number of ventilation days in each study. Additionally, the shape of cuff also differed among the included studies. Introduction of tapered cuff seemed another step forward in cuff technology with improved air, fluid, and dye sealing characteristics.[11,17,40–45] Despite demonstrably positive in vitro study results, the previous systematic review with meta-analysis showed that the tapered cuff did not reduce VAP incidence compared with conventional cuff.[46] Therefore, we assume that the difference in cuff shape did not have a significant implication for the results of this study.
In the standard care for VAP, the use of SSD would be an especially significant factor that could decrease the incidence of VAP. In previous reports and a meta-analysis, SSD was reported to decrease VAP or delay its onset, which may result in positive bias for VAP prevention.[31] In the subgroup analysis, the PU cuff with SDD did decrease the incidence of VAP compared with PVC cuff. However, this effect was not found for the PU cuff without SDD, which indicating that the PU would show no advantage as a cuff material compared with PVC.
We also combined the results of the incidence of bacterial colonization of tracheal aspirate, the duration of ventilation, ICU stay, and ICU mortality. The results showed that the PU cuff had no impact on these relevant outcomes of interest. However, the interpretation of these results was still difficult because the TSA for these outcomes also revealed the lack of an appropriate sample size to detect the difference.
There are several limitations in our meta-analysis. First, there was a lack of an adequate number of patients to increase the certainty of the findings. As the results of TSA indicated, the evidence was insufficient for drawing a conclusion related to the outcomes of this meta-analysis. Second, we included only 6 trials, and thus, a funnel plot to evaluate publication bias could not be drawn. Therefore, the possibility of publication bias could remain in this meta-analysis.
5. Conclusions
The present meta-analysis suggests that the PU cuff was not effective in decreasing the incidence of VAP. However, significant concerns remain in the quality of evidence and sample size in this meta-analysis. Therefore, further accumulation of RCTs exploring the effect of the PU cuff is essential to reach a firm conclusion.
Author contributions
Conceptualization: Koichi Maruyama.
Data curation: Minami Saito, Koichi Maruyama.
Formal analysis: Koichi Maruyama, Takahiro Mihara.
Funding acquisition: Go Hirabayashi, Tomio Andoh.
Investigation: Minami Saito, Koichi Maruyama.
Methodology: Koichi Maruyama, Hiroshi Hoshijima.
Project administration: Koichi Maruyama.
Resources: Koichi Maruyama, Go Hirabayashi.
Software: Takahiro Mihara.
Supervision: Koichi Maruyama, Hiroshi Hoshijima.
Validation: Koichi Maruyama.
Visualization: Koichi Maruyama.
Writing – original draft: Koichi Maruyama.
Writing – review & editing: Koichi Maruyama, Tomio Andoh.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, GRADE = Grading of Recommendations Assessment, Development, and Evaluation, HVLP = high-volume low-pressure, ICU = intensive care unit, MD = mean difference, PEEP = positive end-expiratory pressure, PRISMA = Preferred Reporting Items for Systematic Review and Meta-Analysis, PU = polyurethane, PVC = polyvinyl chloride, RCTs = randomized controlled trials, RIS = required information size, RR = relative risk, SSD = subglottic secretion drainage, TSA = trial sequential analysis, VAP = ventilator-associated pneumonia.
How to cite this article: Saito M, Maruyama K, Mihara T, Hoshijima H, Hirabayashi G, Andoh T. Comparison of polyurethane tracheal tube cuffs and conventional polyvinyl chloride tube cuff for prevention of ventilator-associated pneumonia: a systematic review with meta-analysis. Medicine. 2021;100:9(e24906).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the present study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
CHX = chlorhexidine, N/A = not applicable, NR = not reported, PPI = proton pump inhibitor, PU = polyurethane, PVC = polyvinyl chloride, SDD = subglottic secretion drainage.
GRADE Working Group grades of evidence.
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect.
Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect
CI = confidence interval, ICU = intensive care unit , PVC = polyvinylchloride, PU = polyurethane, RCTs = random controlled trials, RR = risk ratio, VAP = ventilator-associated pneumonia.
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
The overall risk of bias was at some concerns in all studies.
I2 was significant.
Small sample size.
Possibility of publication bias could not be denied.
References
- [1].Muscedere J, Rewa O, McKechnie K, et al. Subglottic secretion drainage for the prevention of ventilator-assisted pneumonia: a systematic review and meta-analysis. Crit Care Med 2011;39:1985–91. [DOI] [PubMed] [Google Scholar]
- [2].Lacherade JC, De Jonghe B, Guezennec P, et al. Intermittent subglottic secretion drainage and ventilator- assisted pneumonia: a multicenter trial. Am J Resp Crit Care Med 2010;182:910–7. [DOI] [PubMed] [Google Scholar]
- [3].Nseir S, Zerimech F, Fournier C, et al. Continuous control of tracheal cuff pressure and microaspiration of gastric contents in critically ill patients. Am J Respir Crit Care Med 2011;184:1041–7. [DOI] [PubMed] [Google Scholar]
- [4].Lorente L, Blot S, Rello J. New issues and controversies in the prevention of ventilator-assisted pneumonia. Am J Respir Crit Care Med 2010;182:870–6. [DOI] [PubMed] [Google Scholar]
- [5].Lucangelo U, Zin WA, Antonaglia V, et al. Effect of positive expiratory pressure and type of tracheal cuff on the incidence of aspiration in mechanically ventilated patients in an intensive care unit. Crit Care Med 2008;36:409–13. [DOI] [PubMed] [Google Scholar]
- [6].Manzano F, Fernández-Mondéjar E, Colmenero M, et al. Positive-end expiratory pressure reduces incidence of ventilator-associated pneumonia in nonhypoxemic patients. Crit Care Med 2008;36:2225–31. [DOI] [PubMed] [Google Scholar]
- [7].Alexiou VG, Ierodiakonou V, Dimopoulos G, et al. Impact of patient position on the incidence of ventilator-associated pneumonia: a meta-analysis of randomized controlled trials. J Crit Care 2009;24:515–22. [DOI] [PubMed] [Google Scholar]
- [8].Young PJ, Ridley SA, Downward G. Evaluation of a new design of tracheal tube cuff to prevent leakage of fluid to the lungs. Br J Anaesth 1998;80:796–9. [DOI] [PubMed] [Google Scholar]
- [9].Young PJ, Rollinson M, Downward G, et al. Leakage of fluid past the tracheal tube cuff in a bench-top model. Br J Anaesth 1997;78:557–62. [DOI] [PubMed] [Google Scholar]
- [10].Dullenkopf A, Gerber A, Weiss M. Fluid leakage past tracheal tube cuffs: evaluation of the new Microcuff endotracheal tube. Intensive Care Med 2003;29:1849–53. [DOI] [PubMed] [Google Scholar]
- [11].Dave MH, Frotzler A, Spielmann N, et al. Effect of tracheal tube cuff shape on fluid leakage across the cuff: an in vitro study. Br J Anaesth 2010;105:538–43. [DOI] [PubMed] [Google Scholar]
- [12].Dave MH, Frotzler A, Weiss M. Closed tracheal suction and fluid aspiration past the tracheal tube. Impact of tube cuff and airway pressure. Minerva Anestesiol 2011;77:166–71. [PubMed] [Google Scholar]
- [13].Kolobow T, Cressoni M, Epp M, et al. Comparison of a novel lycra endotracheal tube cuff to standard polyvinyl chloride cuff and polyurethane cuff for fluid leak prevention. Respir Care 2011;56:1095–9. [DOI] [PubMed] [Google Scholar]
- [14].Ouanes I, Lyazidi A, Danin PE, et al. Mechanical influences on fluid leakage past the tracheal tube cuff in a benchtop model. Intensive Care Med 2011;37:695–700. [DOI] [PubMed] [Google Scholar]
- [15].Zanella A, Scaravilli V, Isgrò S, et al. Fluid leakage across tracheal tube cuff, effect of different cuff material, shape, and positive expiratory pressure: a bench-top study. Intensive Care Med 2011;37:343–7. [DOI] [PubMed] [Google Scholar]
- [16].Li Bassi G, Ranzani OT, Martí JD, et al. An in vitro study to assess determinant features associated with fluid sealing in the design of endotracheal tube cuffs and exerted tracheal pressures. Crit Care Med 2013;41:518–26. [DOI] [PubMed] [Google Scholar]
- [17].Lau AC, Lam SM, Yan WW. Benchtop study of leakages across the Portex, TaperGuard, and Microcuff endotracheal tubes under simulated clinical conditions. Hong Kong Med J 2014;20:7–15. [DOI] [PubMed] [Google Scholar]
- [18].Li Bassi G, Luque N, Martí JD, et al. Endotracheal tubes for critically ill patients: an in vivo analysis of associated tracheal injury, mucociliary clearance, and sealing efficacy. Chest 2015;147:1327–35. [DOI] [PubMed] [Google Scholar]
- [19].Nseir S, Zerimech F, De Jonckheere J, et al. Impact of polyurethane on variations in tracheal cuff pressure in critically ill patients: a prospective observational study. Intensive Care Med 2010;36:1156–63. [DOI] [PubMed] [Google Scholar]
- [20].Lorente L, Lecuona M, Jiménez A, et al. Influence of an endotracheal tube with polyurethane cuff and subglottic secretion drainage on pneumonia. Am J Respir Crit Care Med 2007;176:1079–83. [DOI] [PubMed] [Google Scholar]
- [21].Mahmoodpoor A, Peyrovi-far A, Hamishehkar H, et al. Comparison of prophylactic effects of polyurethane cylindrical or tapered cuff and polyvinyl chloride cuff endotracheal tubes on ventilator-associated pneumonia. Acta Med Iran 2013;51:461–6. [PubMed] [Google Scholar]
- [22].Poelaert J, Depuydt P, De Wolf A, et al. Polyurethane cuffed endotracheal tubes to prevent early postoperative pneumonia after cardiac surgery: a pilot study. J Thorac Cardiovasc Surg 2008;135:771–6. [DOI] [PubMed] [Google Scholar]
- [23].Miller MA, Arndt JL, Konkle MA, et al. A polyurethane cuffed endotracheal tube is associated with decreased rates of ventilator-associated pneumonia. J Crit Care 2011;26:280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Suhas P, Kundra P, Cherian A. Polyurethane cuffed versus conventional endotracheal tubes: effect on ventilator-associated pneumonia rates and length of Intensive Care Unit stay. Indian J Anaesth 2016;60:163–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Philippart F, Gaudry S, Quinquis L, et al. Randomized intubation with polyurethane or conical cuffs to prevent pneumonia in ventilated patients. Am J Respir Crit Care Med 2015;191:637–45. [DOI] [PubMed] [Google Scholar]
- [26].Deem S, Yanez D, Sissons-Ross L, et al. Randomized pilot trial of two modified endotracheal tubes to prevent ventilator-associated pneumonia. Ann Am Thorac Soc 2016;13:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. [DOI] [PubMed] [Google Scholar]
- [28].Sterne JA, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:I4898. [DOI] [PubMed] [Google Scholar]
- [29].Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mao Z, Gao L, Wang G, et al. Subglottic secretion suction for preventing ventilator-associated pneumonia: an updated meta-analysis and trial sequential analysis. Crit Care 2016;20:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Brok J, Thorlund K, Wetterslev J, et al. Apparently conclusive meta-analyses may be inconclusive--Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol 2009;38:287–98. [DOI] [PubMed] [Google Scholar]
- [33].Brok J, Thorlund K, Gluud C, et al. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol 2008;61:763–9. [DOI] [PubMed] [Google Scholar]
- [34].Wetterslev J, Thorlund K, Brok J, et al. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 2008;61:64–75. [DOI] [PubMed] [Google Scholar]
- [35].Thorlund K, Devereaux PJ, Wetterslev J, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int J Epidemiol 2009;38:276–86. [DOI] [PubMed] [Google Scholar]
- [36].Afshari A, Wetterslev J. When may systematic reviews and meta-analyses be considered reliable? Eur J Anaesthesiol 2015;32:85–7. [DOI] [PubMed] [Google Scholar]
- [37].Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Blot SI, Rello J, Koulenti D. The value of polyurethane-cuffed endotracheal tubes to reduce microaspiration and intubation-related pneumonia: a systematic review of laboratory and clinical studies. Crit Care 2016;20:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Seegobin RD, van Hasselt GL. Aspiration beyond endotracheal cuffs. Can Anaesth Soc J 1986;33:273–9. [DOI] [PubMed] [Google Scholar]
- [40].Madjdpour C, Mauch J, Dave MH, et al. Comparison of air-sealing characteristics of tapered- vs. cylindrical-shaped high-volume, low-pressure tube cuffs. Acta Anaesthesiol Scand 2012;56:230–5. [DOI] [PubMed] [Google Scholar]
- [41].Kimijima T, Edanaga M, Yamakage M. Comparison of fluid leakage across endotracheal tube cuffs using a three-dimensional printed model of the human trachea. J Anesth 2016;30:510–3. [DOI] [PubMed] [Google Scholar]
- [42].Maguire S, Haury F, Jew K. An in vitro comparison of tracheostomy tube cuffs. Med Devices (Auckl) 2015;21:185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Komasawa N, Fujiwara S, Miyazaki S, et al. Comparison of fluid leakage from four different cuffed pediatric endotracheal tubes using a pediatric airway simulation model. Pediatr Int 2014;56:634–6. [DOI] [PubMed] [Google Scholar]
- [44].Shiotsuka J, Lefor AT, Sanui M, et al. A quantitative evaluation of fluid leakage around a polyvinyl chloride tapered endotracheal tube cuff using an in-vitro model. HSR Proc Intensive Care Cardiovasc Anesth 2012;4:169–75. [PMC free article] [PubMed] [Google Scholar]
- [45].Lichtenthal PR, Maul D, Borg U. Do tracheal tubes prevent microaspiration? Br J Anaesth 2011;107:821–2. [DOI] [PubMed] [Google Scholar]
- [46].Maertens B, Blot K, Blot S. Prevention of ventilator-associated and early postoperative pneumonia through tapered endotracheal tube cuffs: a systematic review and meta-analysis of randomized controlled trials. Crit Care Med 2018;46:316–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


