Abstract
Background:
Even though a variety of rehabilitative technique have been implemented to ameliorate neglect symptoms of patients with stoke, the effects of limb activation using a robotic device are still unknown. The purpose of this study was to investigate the effects of the robot-assisted hand training on hemispatial neglect of older patients with chronic stroke.
Methods:
The participants were randomly allocated to the experimental group (EG) receiving robot-assisted left-hand training (n = 12) or the control group (CG) receiving conventional treatments for neglect symptoms (n = 12). All participants received 20 sessions for 4-week. To examine the effects on hemispatial neglect, the line bisection test (LBT), the Albert test, and the Catherine Bergego Scale (CBS) were utilized. The outcome measures were analyzed before and after the 20 training sessions.
Results:
After the intervention, improvements in the LBT, the Albert test, and the CBS were found in the EG whereas there were significant improvements in the LBT and the CBS but not the Albert test in the CG. In addition, the EG showed a significantly greater gain in all outcome measures compared to the CG (p < .05).
Conclusions:
These results indicate that robot-assisted hand training was beneficial to improving the hemispatial neglect of elderly person with chronic stroke compared to the conventional treatments. Robot-assisted limb activation might be useful to ameliorate hemispatial neglect of the elderly with chronic stroke.
Keywords: attention, hemispatial neglect, limbs, stroke
1. Introduction
Hemispatial neglect is a frequent consequence of right hemispheric stroke experienced by 13 to 82% of the patients.[1] Since right hemisphere is found to play a role in spatial attention, hemispatial neglect is observed at higher frequency in stroke patients with right hemisphere damage compared to left hemisphere damage.[2] Whatever the side of brain lesions, hemispatial neglect can be characterized by a reduced or complete lack of awareness of and reaction to stimuli on the side of the body or space contralateral to a brain lesion.[3] Hemipatial neglect symptoms are naturally relieved in more than 80% of stroke patients 3 months after the onset of stroke, but still, hemispatial neglect persist even after 6 months in about 20%, which has a negative impact on the rehabilitation prognosis of patients with stroke.[2,4]
Several rehabilitation techniques including training in visual scanning, prism adaptation, neuromuscular electric simulation, limb activation have been identified to be clinically beneficial in reducing hemispatial neglect.[5,6,7,8] In some of these studies, a limb activation treatment was used to reduce the visuospatial deficits in hemispatial neglect both in the acute and chronic stage.[3,9,10,11] Furthermore, a reduction in hemispatial neglect has been observed during both passive and active left-hand movements.[12] Given the high incidence of upper limb paresis associated with the neglect syndrome, the positive effect of passive or active limb movement on hemispatial neglect could gain a lot of attention in rehabilitative field.[11,13]
The premotor theory of spatial attention, that somatosensory activation in the contralateral side by activating the limb facilitates the neural circuits underlying space representation, could explain the clinical effectiveness of limb activation for hemispatial neglect because the motor circuits and attention are closely related, which enhance the conscious perception of stimuli in the contralateral hemi-space.[5,13,14] Therefore, contralateral limb activation inducing activation of the affected hemisphere might reduce the inhibitory competition from the unaffected hemisphere.[5,11,15]
With the development of robotic devices, there is a growing interest in using them in rehabilitation since they could provide consistent and precise delivery compared to therapists.[16] Indeed, a number of robotic devices, which have been proven to improve the paretic upper limb motor function of stroke patients by using passive, assistive, and active training modes, have been developed over the past decade.[17] Contrary to upper limb motor function, few studies reported the effects of robot-assisted therapy on hemispatial neglect.[3] Even though, previous case series study found significant effects of robot-assisted limb activation on hemispatial neglect, confirmation of the effects of robot-assisted therapy on hemispatial neglect was limited by the lack of a control condition. Additionally, in a previous randomized controlled trial, since patients with acute stroke participated in robot-assisted limb activation,[18,19] it is hard to rule out spontaneous recovery effects on hemispatial neglect.
Therefore, this study with a randomized controlled trial design was conducted to identify the effects of robot-assisted hand training on hemispatial neglect in stroke patients with chronic stroke after 20 sessions’ intervention. This study was conducted to measure the effect of robot-assisted hand training in hemispatial neglect compared to a control group.
2. Methods
2.1. Design
This study was a pilot and a randomized controlled trial design. All participants were randomly assigned to the experimental group (EG) or the control group (CG) by using random numbers generated by an occupational therapist blinded to the allocation using a computer software (Excel, Microsoft), which is called simple randomization. To avoid unequal allocation, a randomization list made an occupational therapist who was not aware of participants and not participated in this study was used. The EG received robot-assisted hand training and the CG participated in conventional neglect treatments including both remedial approaches, such as visual scanning training and vibration on the left neck extensors and compensatory approaches. Outcome measures were examined at pre- and post-intervention by an assessor, an occupational therapist with more than five years of clinical experience, who was blinded to the group allocation. The intervention consisted of 20 sessions conducted five times a week for four weeks. This study was approved by the local Institutional Review Board and registered at the Thai Clinical Trials (https://www.clinicaltrials.in.th, ID: TCTR20200222005). All participants provided written informed consent before the study according to the code of ethics of the World Medical Association (Declaration of Helsinki, version 2004).
2.2. Participants
Since there is no sufficient prior information to calculate the sample size, a sample size of 12 per group would be appropriate as a pilot study.[20] Therefore, a total of 24 chronic stroke patients with hemispatial neglect were recruited by the author from a rehabilitation hospital in South Korea. The inclusion criteria derived from a previous study[5] were:
-
(1)
over 65 years of age,
-
(2)
right hemisphere stroke confirmed by a computed tomography scan or magnetic resonance imaging,
-
(3)
first-ever ischemic or hemorrhage stroke,
-
(4)
intact global cognitive function confirmed by the Korean version of Mini-Mental State Examination score ≥ 24,
-
(5)
time since stroke onset ≥ 6 months, and
-
(6)
the presence of hemispatial neglect diagnosed by performance on the Line Bisection Test and the Korean version of the Motor-free Visual Perception Test-Third Edition (MVPT-3).
The MVPT-3 is a tool to assess visual perception ability consisting of spatial relationship, visual discrimination, figure-ground discrimination, visual closure, and visual memory. In this study, the number of neglected responses in the MVPT-3 was counted. Hemispatial neglect was diagnosed by these two tests just before subjects participated in this study.
The exclusion criteria were:
-
(1)
any additional treatment for hemispatial neglect,
-
(2)
left upper limb sensory deficit or impairment,
-
(3)
visual impairment,
-
(4)
the modified Ashworth scale score for left-hand muscle tone ≥ 2,
-
(5)
below second-grade left hand muscle strength in a manual muscle test,
-
(6)
orthopedic conditions involving the left upper limb, and
-
(7)
apraxia.[5]
2.3. Intervention
The EG performed 20 sessions (five days a week for four weeks) of robot-assisted hand training using the Amadeo Robotic device (Trymotion GmbH, Graz, Austria) (Figure 1). The end-effector-based Amadeo Robot has five degrees of freedom and provides the motion of one or all five fingers through a passive rotational joint placed between the fingertip and an entity moves laterally (the thumb has two passive rotational joints). All five translational degrees of freedom are independent and almost entirely cover the fingers’ workspace. The interface between the human hand and the machine is achieved via elastic bands or plasters and the wrist is restrained from movement by a Velcro strap.
Figure 1.
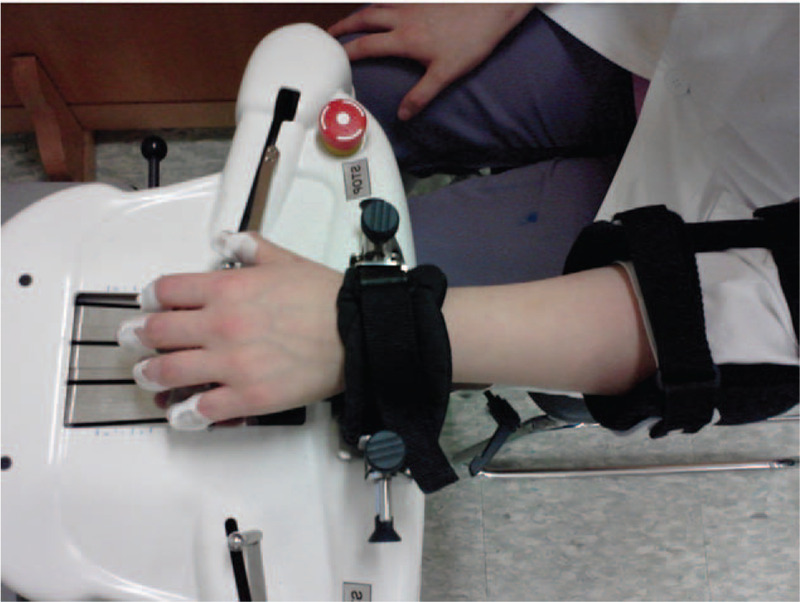
Amadeo robotic device.
Each session lasted 30 minutes. The exercises were carried out according to a previous study as follow: (1) grasp and release training (digital joint flexion/extension exercise from the thumb to the fifth finger) for 15 minutes; and (2) count training (count a number sequence from one to five) for 15 minutes.[5] The participant's hand motion was assisted by the robot and adjusted to the individual's level of function through the assistive therapy mode of the Amadeo robot. During the training, the participants in the EG received visual feedback of their hand movements via video animation presented on a monitor.
The control group received the 20 sessions of the conventional treatments that lasted 30 minutes each session for hemispatial neglect symptoms. These treatments included visual scanning training using a prism and vibration stimulation applied on the left neck extensors and a middle part of the left forearm. In addition, the participants in the CG learned the compensatory approach for ameliorating hemispatial neglect symptoms involving turning a head or trunk. Two dependent occupational therapists who had more than five years of experience conducted all sessions.
2.4. Outcome measures
Hemispatial neglect was diagnosed by the Line Bisection Test (LBT), the Star Cancellation test (SCT), and the MVPT-3. Among these tests, the LBT was also used to investigate training effectiveness. In the SCT, the number of neglected stars on a left side of a test sheet was counted.
The training effects were investigated by using the line bisection test (LBT), the Albert's Test, and the Catherine Bergego Scale (CBS). In the LBT, the distance of deviation from the objective midline of 18 lines was measured. The participant was instructed to point to the estimated center position using either a pencil or a stick. An average deviation distance less than 6.3 mm was normal, more than 6.33 mm indicated mild hemispatial neglect, and more than 12.5 mm indicated severe hemispatial neglect.[21] In the Albert's Test, the participant was asked to mark 40 lines randomly located on a test sheet. Four lines located in the middle were marked by the assessor's demonstration. The number of missing lines of 36 lines was taken as the result.[22]
The Catherine Bergego Scale (CBS) is a checklist designed to examine neglect symptoms by observing and interviewing the participant's daily function in 10 real life situations with a total score of 30. Each item is rated on a 4-point scale. A participant with a total score of 0 is considered to have no effect of neglect syndrome in performing the daily living. Higher scores mean more severe neglect symptom impact on daily life.[23] Unlike the LBT and the Albert's test, the CBS is a tool that assesses not only hemispatial neglect symptoms but also the degree of disease recognition by comparing scores.[24]
2.5. Statistical analyses
All data were analyzed using SPSS 22.0 version. All measures were described as the mean ± standard deviation. The Shapiro-Wilk test was used to determine normal distribution of outcome measures. To compare the general characteristics of the participants between both groups, the Chi-square test and independent t-test were conducted. After the training sessions, Wilcoxon-signed rank test was used to investigate changes within each group and the differences in changes between the groups were analyzed by using the Mann-Whitney U test. The effect size of each intervention group was calculated using the partial η2 value. A partial η2 ≥ 0.14 was considered a large effect; between ≥ 0.06 and <0.14, a moderate effect; and an between ≥ 0.01 and < 0.06, a small effect.[25] The statistical significance level was set at P < . 05.
3. Results
3.1. General characteristics of the participants
A total of 24 participants were selected from 32 stroke patients. All participants completed the 20 training sessions. No dropouts were recorded during the intervention and all participants fulfilled the intervention protocol (Fig. 2). There were no significant differences in the general characteristics between both groups (Table 1).
Figure 2.
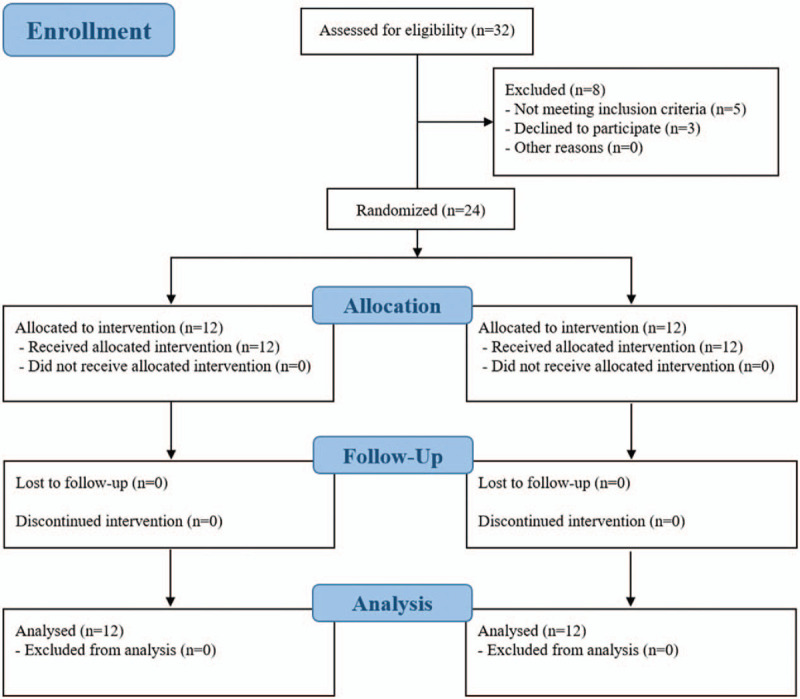
Flow diagram of subjects in the study.
Table 1.
Demographic and clinical characteristics of both groups.
| Characteristics | Experimental group (n = 12) | Control group (n = 12) | χ2/t |
| Demographic characteristics | |||
| Sex | |||
| Male | 7 (53.8%) | 6 (50.0%) | .168 |
| Female | 5 (41.7%) | 6 (50.0%) | |
| Age (yr) | 69.08 ± 4.71 | 71.58 ± 3.17 | 1.523 |
| Stroke type | |||
| Infarction | 6 (50.0%) | 7 (58.3%) | .168 |
| Hemorrhage | 6 (50.0%) | 5 (41.7%) | |
| Onset period (months) | 9.50 ± 2.61 | 9.08 ± 2.10 | .430 |
| Clinical characteristics | |||
| LBT (mm) | 9.38 ± 1.29 | 8.66 ± 1.13 | 1.447 |
| MVPT (numbers) | 11.41 ± 1.31 | 10.50 ± 1.44 | .118 |
| K-MBI (scores) | 51.08 ± 4.85 | 52.16 ± 4.52 | .578 |
3.2. Performance on hemispatial neglect tasks
After intervention, the EG showed significant improvements in the LBT (P < .001) and the Albert test (P < .01) whereas there were significant improvements in the LBT (P < .01) but not in the Albert test (P > .05) in the CG. On the other hand, there were statistically significant differences in changes in the LBT (P < .001; η2 = 0.507) and the Albert test (P < .05; η2 = 0.205), suggesting that robot-assisted hand training showed a greater improvement in ameliorating hemispatial neglect symptoms compared to the conventional treatment (Table 2).
Table 2.
Comparison of outcome measures in both groups.
| Variables | Experimental group (n = 12) | Control group (n = 12) | Between-group differences | U | η2 |
| LBT (mm) | |||||
| Pre-intervention | 9.38 ± 0.37 | 8.66 ± 0.32 | |||
| Post-intervention | 6.27 ± 0.36 | 7.89 ± 0.34 | 2.337 (1.31; 3.35) | 5.500∗∗∗ | .507 |
| Within-group changes | 3.10 ± 0.43(2.14; 4.06)††† | 0.77 ± 0.22(0.26; 1.27)†† | |||
| Albert's test (score) | |||||
| Pre-intervention | 7.67 ± 0.64 | 6.75 ± 0.46 | |||
| Post-intervention | 5.00 ± 0.38 | 5.92 ± 0.45 | 1.833 (0.23; 3.43) | 33.500∗ | .205 |
| Within-group changes | 2.66 ± 0.56(1.41; 3.91)†† | 0.83 ± 0.52(−0.31; 1.97) | |||
| CBS (score) | |||||
| Pre-intervention | 20.75 ± 0.79 | 19.75 ± 0.53 | |||
| Post-intervention | 15.83 ± 1.07 | 18.50 ± 0.37 | 3.666 (2.25; 5.07) | 11.500∗∗∗ | .569 |
| Within-group changes | 4.30 ± 0.54(3.03; 5.56)††† | 1.25 ± 0.41(0.34; 2.15)† | |||
3.3. Hemispatial neglect symptoms in activities of daily living
After intervention, both group showed a significant improvement in the CBS (EG: P < .001; CG: P < .05). On the other hand, there was a statistically significant difference in changes in the CBS (P < .001; η2 = 0.569). This finding indicated that robot-assisted hand training was more clinically beneficial in reducing hemispatial neglect symptoms in the participants’ activities of daily living (Table 2).
4. Discussion
The main purpose of this study was to investigate the effects of limb activation using a robotic device on hemispatial neglect in older adults with chronic stroke. The findings of this study suggested that robot-assisted left-hand training might improve visuospatial exploration as measured by the LBT and the Albert test, as well as functional performance in daily living measured by the CBS, which means that robot-assisted hand training is useful in ameliorating the neglect symptoms of older adults with chronic stroke.
These results are consistent with previous findings on the effectiveness of contralateral limb activation for reducing the level of impairment in stroke patients with hemispatial neglect due to stroke.[5,11,12,13] Previous studies reported that both passive and active left finger movements might reduce neglect compared to visual cueing in the neglect sides,[7,13] supporting the results of the current study. Similarly, in a previous study, it was confirmed that proprioceptive cueing related to the left finger position could enhance the impaired spatial representation of the left side.[13]
Meanwhile, previous studies have tried to conduct limb activation using robotic devices for stroke patients with neglect symptoms. The effectiveness of robot-assisted hand training on hemispatial neglect of the left hand in three patients with stroke was reported.[5,11] These findings were consistent with the results of the present study, but this study slightly differed in terms of study design. The previous studies involved patients with stroke at the acute stage, whereas this study confirmed the effects of robot-assisted limb activation of hemispatial neglect of older patients with chronic stroke so that this study could exclude spontaneous recovery effects. Moreover, since this study was a randomized controlled trial design, higher level of evidence was collected than in a case-series design or a single-subject design.[5,11]
The positive effects of robot-assisted hand training could be attributed to the fact that activation of the right hemisphere motor circuits by using left-hand movements leads to recruitment of the associated attentional mechanism, improving attention for the left side of the space.[7] Indeed, Takahashi and colleagues (2008) found robot-assisted hand training increased the primary sensorimotor cortex representational map size by using functional magnetic resonance imaging.[26] Moreover, continuous visual feedback might activate the extrapersonal space of the participants in the EG when they saw an animation of their hand movements on the monitor. Accordingly, the findings of this study suggest that robot-assisted left-hand training in the left space could enhance the neural networks sub-serving space representation in the damaged right hemisphere. Meanwhile, given that limb activation of one side could facilitate contralateral hemisphere,[5,13,14] there is a possibility that right-sided limb activation might be also effective in stroke patients with right hemispatial neglect due to left hemisphere lesions. Unfortunately, however, a previous study reported no significant effects of right-sided limb activation on ameliorating hemispatial neglect.[7] This could be attributed by the evidence that attention network is more closely correlated with right hemisphere than left hemisphere. Therefore, it is interpreted that limb activation without considering the affected side has no considerable therapeutic effect for hemispatial neglect.
On the other hand, from a rehabilitative point of view, robot-assisted limb activation also could be used to improve motor function in the upper limb. Indeed, in a previous study, gross motor function, as well as neglect symptoms of the left upper limb, was improved by robot-assisted limb activation.[5] Taken together, robot-assisted limb activation is thought to be of clinical benefit to stroke patients with neglect symptoms.
Compared to a previous study, some aspects of this study were more useful. The previous study did not investigate the transfer effects of robot-assisted limb activation to a participant's daily function.[5] However, the findings of this study further provide evidence of a partial generalization of the training effects to daily living, as shown by improvements confirmed in the CBS. In addition, according to a previous study, behavioral assessments are potentially more sensitive to the presence of neglect than paper and pencil tests such as the LBT.[24] Given that one of the main goals of rehabilitation is to maintain independent activities of daily living, these findings suggested the clinical implications. Nevertheless, given a recent previous study reporting that robot-assisted therapy could be simultaneously provided with repetitive transcranial magnetic stimulation and it was found to be clinically effective, a combination treatment for visual sensory stimulation needs to be investigated rather than an individual treatment to maximize its effects in clinical settings.[27]
While this study was able to provide evidence to support the applied therapeutic concept, there are still limitations in this study. First, the relatively small sample of this study prevents generalization of the effects of robot-assisted left-hand training to all patients with neglect due to stroke. Second, long-term effects of robot-assisted limb activation were not confirmed. Finally, this study did not examine right hemisphere activation by using neuroimaging devices so it is not clear how much right hemisphere activation is effective for hemispatial neglect. Therefore, future studies need to provide evidence for robot-assisted limb activation with larger study samples by additionally using neuroimaging devices before this approach is widely adopted in clinical settings.
5. Conclusions
The results of this study showed that after 20 robot-assisted hand training sessions, the hemispatial neglect symptoms in patients with chronic stroke were ameliorated. In a future study, randomized controlled trials with various treatment protocols (types of robotic devices and training programs such as passive mode, assistive mode, and active mode) should consider examining such changes using functional magnetic resonance imaging (fMRI) to confirm the neural mechanism underlying effects of robot-assisted left-hand training on hemispatial neglect in patients with stroke.
Acknowledgment
I would like to thank you all who gave me their unconditional supports to conduct this study.
This work was supported by the Soonchunhyang University Research Fund.
Author contributions
Conceptualization: Jin-Hyuck Park.
Data curation: Jin-Hyuck Park.
Formal analysis: Jin-Hyuck Park.
Funding acquisition: Jin-Hyuck Park.
Investigation: Jinhyuck Park.
Methodology: Jin-Hyuck Park.
Project administration: Jin-Hyuck Park.
Resources: Jinhyuck Park.
Supervision: Jinhyuck Park.
Validation: Jinhyuck Park.
Visualization: Jinhyuck Park.
Writing – original draft: Jin-Hyuck Park.
Writing – review & editing: Jin-Hyuck Park.
Footnotes
Abbreviations: CBS = catherine bergego scale, CG = control group, EG = experimental group, LBT = line bisection test.
How to cite this article: Park JH. The effects of robot-assisted left-hand training on hemispatial neglect in older patients with chronic stroke: a pilot and randomized controlled trial. Medicine. 2021;100:9(e24781).
This work was supported by the Soonchunhyang University Research Fund.
This work was supported by the Korea Institute for Advancement of Technology(KIAT) grant funded by the Korea Government(MOTIE) (P0012724, The Competency Development Program for Industry Specialist).
Role of the funding source: This work was supported by the Soonchunhyang University Research Fund and the Korea Institute for Advancement of Technology(KIAT) grant funded by the Korea Government(MOTIE) (P0012724, The Competency Development Program for Industry Specialist). The proofreading of this manuscript were conducted by these funding sources. In addition, these funding sources were used to rent places and meals when having several meetings.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Data are shown as mean ± standard deviations. K-MBI = Korean version of Modified Barthel Index, LBT = Line Bisection Test, MVPT = Motor-free Visual Perception Test.
Data are shown as mean ± standard errors and mean (95% confidence interval) for within and between-group changes. CBS = Catherine Bergego Scale, LBT = Line Bisection Test. †comparison within both groups, †P < .05, ††P < .01, †††P < .001. ∗Comparison between both groups, ∗P < .05, ∗∗∗P < .001.
References
- [1].Chechlacz M, Rotshtein P, Humphreys GW. Neuroanatomical dissections of unilateral visual neglect symptoms: ALE meta-analysis of lesion-symptom mapping. Front Hum Neurosci 2012;6:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pierce SR, Buxbaum LJ. Treatments of unilateral neglect: a review. Arch Phys Med Rehabil 2002;83:256–68. [DOI] [PubMed] [Google Scholar]
- [3].Robertson I, Halligan PW, Bergego C, et al. Right neglect following right hemisphere damage? Cortex 1994;3:199–213. [DOI] [PubMed] [Google Scholar]
- [4].Ringman JM, Saver JL, Woolson RF, et al. Frequency, risk factors, anatomy, and course of unilateral neglect in an acute stroke cohort. Neurology 2004;63:468–74. [DOI] [PubMed] [Google Scholar]
- [5].Varalta V, Picelli A, Fonte C, et al. Effects of contralesional robot-assisted hand training in patients with unilateral spatial neglect following stroke: a case series study. J Neuroeng Rehabil 2014;11:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pizzamiglio L, Fasotti L, Jehkonen M, et al. The use of optokinetic stimulation in rehabilitation of the hemineglect disorder. Cortex 2004;40:441–50. [DOI] [PubMed] [Google Scholar]
- [7].Reinhart S, Schmidt L, Kuhn C, et al. Limb activation ameliorates body-related deficits in spatial neglect. Front Hum Neurosci 2012;6:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liu KP, Hanly J, Fahey P, et al. A systematic review and meta-analysis of rehabilitative interventions for unilateral spatial neglect and hemianopia poststroke from 2006 through 2016. Arch Phys Med Rehabil 2019;100:956–79. [DOI] [PubMed] [Google Scholar]
- [9].Robertson IH, North N. Spatio-motor cueing in unilateral left neglect: the role of hemispace, hand and motor activation. Neuropsychologia 1992;30:553–63. [DOI] [PubMed] [Google Scholar]
- [10].Vallar G, Rusconi M, Barozzi S, et al. Improvement of left visuo-spatial hemineglect by left-sided transcutaneous electrical stimulation. Neuropsychologia 1995;33:73–82. [DOI] [PubMed] [Google Scholar]
- [11].Park JH. Effect of robot-assisted left hand training on unilateral neglect in patients with stroke. J Kor Soc Occup Ther 2015;23:117–27. [Google Scholar]
- [12].Frassinetti F, Rossi M, Ladavas E. Passive limb movements improve visual neglect. Neuropsychologia 2001;39:725–33. [DOI] [PubMed] [Google Scholar]
- [13].Eskes GA, Butler B. Using limb movements to improve spatial neglect: the role of functional electrical stimulation. Restor Neurol Neurosci 2006;24:385–98. [PubMed] [Google Scholar]
- [14].Rizzolatti G, Berti A. Neglect as a neural representation deficit. Rev Neurol 1990;146:626–34. [PubMed] [Google Scholar]
- [15].Robertson IH, Hogg K, McMillan TM. Rehabilitation of unilateral neglect: improving function by contralesional limb activation. Neuropsychol Rehabil 1998;8:19–29. [Google Scholar]
- [16].Veerbeek JM, Langbroek-Amersfoort AC, Van Wegen EE, et al. Effects of robot-assisted therapy for the upper limb after stroke: a systematic review and meta-analysis. Neurorehabil Neural Repair 2017;31:107–21. [DOI] [PubMed] [Google Scholar]
- [17].Sale P, Franceschini M, Mazzoleni S, et al. Effects of upper limb robot-assisted therapy on motor recovery in subacute stroke patients. J Neuroeng Rehabil 2014;11:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Maddicks R, Marzillier SL, Parker G. Rehabilitation of unilateral neglect in the acute recovery stage: The efficacy of limb activation therapy. Neuropsychol Rehabil 2003;13:391–408. [DOI] [PubMed] [Google Scholar]
- [19].Choi YS, Lee KW, Lee JH, et al. The effect of an upper limb rehabilitation robot on hemispatial neglect in stroke patients. Ann Rehabil Med 2016;40:611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat: J Appl Stat Pharm Ind 2005;4:287–91. [Google Scholar]
- [21].Schenkenberg T, Bradford D, Ajax E. Line bisection and unilateral visual neglect in patients with neurologic impairment. Neurology 1980;30:509–1509. [DOI] [PubMed] [Google Scholar]
- [22].Albert ML. A simple test of visual neglect. Neurology 1973;23:658–64. [DOI] [PubMed] [Google Scholar]
- [23].Chen P, Hreha K, Fortis P, et al. Functional assessment of spatial neglect: a review of the Catherine Bergego Scale and an introduction of the Kessler Foundation Neglect Assessment Process. Top Stroke Rehabil 2012;19:423–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Azouvi P, Bartolomeo P, Beis J-M, et al. A battery of tests for the quantitative assessment of unilateral neglect. Restor Neurol Neurosci 2006;24:273–85. [PubMed] [Google Scholar]
- [25].Cohen J. Statistical power analysis. Curr Dir Psychol Sci 1992;1:98–101. [Google Scholar]
- [26].Takahashi CD, Der-Yeghiaian L, Le V, et al. Robot-based hand motor therapy after stroke. Brain 2008;131:425–37. [DOI] [PubMed] [Google Scholar]
- [27].Kim SB, Lee KW, Lee JH, et al. Effect of combined therapy of robot and low-frequency repetitive transcranial magnetic stimulation on hemispatial neglect in stroke patients. Ann Rehabil Med 2018;42:788–97. [DOI] [PMC free article] [PubMed] [Google Scholar]


