Abstract
Although the mutual relationship between ambulation and physical activity (PA) in people with multiple sclerosis (pwMS) has been described in several studies, there is still a lack of detailed information about the way in which specific aspects of the gait cycle are associated with amount and intensity of PA. This study aimed to verify the existence of possible relationships among PA parameters and the spatio-temporal parameters of gait when both are instrumentally assessed.
Thirty-one pwMS (17F, 14 M, mean age 52.5, mean Expanded Disability Status Scale (EDSS) score 3.1) were requested to wear a tri-axial accelerometer 24 hours/day for 7 consecutive days and underwent an instrumental gait analysis, performed using an inertial sensor located on the low back, immediately before the PA assessment period. Main spatio-temporal parameters of gait (i.e., gait speed, stride length, cadence and duration of stance, swing, and double support phase) were extracted by processing trunk accelerations. PA was quantified using average number of daily steps and percentage of time spent at different PA intensity, the latter calculated using cut-point sets previously validated for MS. The existence of possible relationships between PA and gait parameters was assessed using Spearman rank correlation coefficient rho.
Gait speed and stride length were the parameters with the highest number of significant correlations with PA features. In particular, they were found moderately to largely correlated with number of daily steps (rho 0.62, P< .001), percentage of sedentary activity (rho = –0.44, P < .001) and percentage of moderate-to-vigorous activity (rho = 0.48, P < .001). Small to moderate significant correlations were observed between PA intensity and duration of stance, swing and double support phases.
The data obtained suggest that the most relevant determinants associated with higher and more intense levels of PA in free-living conditions are gait speed and stride length. The simultaneous quantitative assessment of gait parameters and PA levels might represent a useful support for physical therapists in tailoring optimized rehabilitative and training interventions.
Keywords: accelerometer, gait, multiple sclerosis, physical activity, spatio-temporal parameter
1. Introduction
Muscular weakness, fatigue, spasticity, and poor balance negatively influence walking abilities in people with multiple sclerosis (pwMS) who exhibit a gait pattern characterized by reduced speed and step length and increased double support time.[1] The quality of gait typically tends to worsen with the progression of the disease,[2] to such a point that it represents a serious limitation in performing daily activities and severely reduce the quality of life.[3,4]
Physical activity (PA), especially when structured in the form of exercise, represents a very effective way to counteract several negative effects of the disease and, in particular, it is beneficial to walking abilities.[5,6] In this regard, 2 recent meta-analyses emphasized the strong link between PA and locomotion from different perspectives. Pearson et al,[7] who summarized the results of 13 studies on PA interventions specifically focused on walking, concluded that exercise is able to significantly increase walking speed (as measured using the 10 m Walking Test, 10MWT) and walking endurance, assessed using both the 6-m and 2-m walking test. The review by Streber et al,[8] which aimed at establishing correlates and determinants of PA in pwMS, stated that walking limitations are among the most important correlates of PA. However, despite the clear positive effects of PA mobility on pwMS, existing data suggest that such patients still appear reluctant not only to engage in structured PA programs, but are also characterized by a reduced amount of overall body movement, thus exhibiting a marked tendency to sedentary behavior.[9] This creates a vicious circle since physical inactivity promotes a reduction in cardiorespiratory fitness[10] and physical deconditioning[11] also acting as co-factors in the onset of comorbidities such as obesity, metabolic syndrome osteoporosis, etc.[12]
Given the importance of PA levels in MS, a key issue in correctly estimating the amount and intensity of PA performed by pwMS on a daily basis, is represented by the data collection methods. Although for this purpose self-reported data, in the form of diaries and recall questionnaires are still the most widespread technique owing to their low cost, easiness of use and versatility, it is noteworthy that such tools suffer from poor reliability and validity, participant recall bias and interpretation of questions[13] and usually overestimate amount and intensity of movement performed.[14] However, since a decade ago objective techniques (mostly based on the use of wearable accelerometers) have been successfully employed in acquiring more reliable, long-term PA data, which are not only limited to the number of daily steps, but also extend to PA intensity and energy expenditure.[15] The availability of continuous quantitative data on mobility of pwMS has made it possible to elucidate the relationship between PA levels and important features associated with MS, such as risk of falls,[16] cognitive performance,[17] self-efficacy[18] and quality of life.[19–20]
Although the mutual relationship between ambulation and PA in pwMS appeared quite clear in most previous studies, although to different degree of strength, there is still a lack of detailed information about the way in which specific features of the gait cycle are associated with amount and intensity of PA. Thus, to partly overcome such limit, this study aimed to verify the existence of possible relationships among PA parameters and the main spatio-temporal parameters of gait (i.e., speed, stride length, cadence, duration of stance, swing, and double support phase). To this purpose, data on PA performed by pwMS was acquired on the basis of 1 week of continuous monitoring with wearable accelerometers, while gait analysis was carried out in a clinical setting, using validated inertial sensors. Such information will not only allow further elucidation of the role of walking capabilities on propensity to mobility and movement, but will also provide suggestions on the modalities with which rehabilitative and training intervention should be articulated, with the final aim of reducing sedentary activity typical of most pwMS.
2. Methods
2.1. Participants
The study was performed in the period March-November 2018 and involved a convenience sample of 40 outpatients presenting with relapsing–remitting MS, followed at the Regional Center for Multiple Sclerosis of Sardinia (Cagliari, Italy) who consented to participate. They met the following criteria: diagnosis of MS according to the 2005 McDonald criteria[21–22]; age between 18 and 65 years; Expanded Disability Status Scale (EDSS,[23]) score ≤ 6; being clinically stable and on treatment with disease modifying agents at least for 6 months; free from other associated medical conditions able to severely influence gait and balance. The study was carried out in compliance with the ethical principles for research involving human subjects expressed in the Declaration of Helsinki and was approved by the local ethics committee. All participants signed an informed consent form after a detailed explanation of the purposes of the study and the methodology used in the experimental tests.
2.2. Data collection and processing: physical activity
Amount and intensity of PA were computed on the basis of data acquired by a tri-axial accelerometer (Actigraph GT3X, Acticorp Co., Pensacola, FL) previously validated for use in pwMS.[16,24] Participants were instructed to wear the device on the non-dominant wrist for 7 consecutive days 24 hours/day, allowing its removal only for showering, bathing and when performing water-based activities (i.e., swimming). The wrist was selected as the site of placement as in that location the device is generally better tolerated by pwMS, for both comfort and aesthetic reasons.[25] Moreover, this choice is likely to increases wear time compliance and allows acquiring data on sleep.[26,27] The devices were set to collect data using 60-seconds epochs at 30 Hz frequency. At the end of the week, data were downloaded and processed using the dedicated ActiLife software (v6.13.3 Acticorp Co. Pensacola, FL) to perform step counts, vector magnitude counts (VM, a composite of accelerometric counts from these 3 planes of motion) and PA intensity classification based on the procedure proposed by Sebastiao et al,[16] who derived the following intervals for the accelerometric counts per minute (cpm) to discriminate PA of different intensity in pwMS as follows (between parentheses the correspondent values of metabolic equivalent (MET) are reported):
sedentary behavior (SB) 0 to 99 cpm (<0.2 MET)
light intensity activity (LPA) 100 to 1721 cpm (0.2–3 MET)
moderate-to-vigorous (MVPA) intensity activity ≥ 1722 cpm (>3 MET)
Since such thresholds were obtained having the accelerometer positioned at the hip, we applied the correction for wrist placement automatically provided by the ActiLife software. The data acquired on a certain day were considered valid if wear time was at least 16 hours. Non-wear time was defined as a time interval of at least 60 consecutive minutes characterized by zero accelerometric counts.
2.3. Data collection and processing: quantitative gait analysis
A miniaturized lightweight inertial sensor, previously validated for use in pwMS (G-Sensor, BTS Bioengineering SpA, Italy) was employed to assess gait parameters.[28] Using a semi-elastic belt, the device was placed at the low back of participants, approximately at the L4-L5 vertebrae position. After a short familiarization phase, pwMS were asked to walk at a self-selected comfortable speed in the most natural manner possible along a straight 30-m trajectory ideally located at the center of a 2 m wide hallway. During the trial, the device acquired linear accelerations along its 3 axes at 100 Hz frequency which were transmitted in real-time via Bluetooth to a PC. Using dedicated software (G-Studio, BTS Bioengineering SpA, Italy), the following 6 spatio-temporal parameters were calculated:
Gait speed: the mean velocity of progression (m s−1);
Cadence: the rate at which a person walks (steps min−1);
Stride length: the longitudinal distance between successive ground contacts of the same foot (m);
Stance and swing phases duration (expressed as a percentage of the gait cycle): time during which the foot remains respectively in contact (stance) or not in contact (swing) with the ground;
Double support phase duration (expressed as a percentage of the gait cycle): time during which both feet are in contact with the ground.
2.4. Statistical analyses
The existence of possible relationships between PA and gait parameters was assessed using Spearman rank correlation coefficient rho by setting the level of significance at P < .05. Rho values of 0.1, 0.3, and 0.5 were assumed to be representative of small, moderate, and large correlations respectively, according to Cohen guidelines.[29] All analyses were performed using the IBM SPSS Statistics v.20 software (IBM, Armonk, NY).
3. Results
3.1. General comments
Of the 40 originally recruited participants, 3 withdrew from the study for personal reasons, and 6 were excluded because they did not meet the 16 hours/day wear-time criteria for all of the 7 consecutive days. Thus, the results presented here refer to 31 pwMS (17 female, 14 male), whose anthropometric and clinical data are reported in Table 1.
Table 1.
Anthropometric and demographic aspects of participants. Values are expressed as mean ± SD.
| Variable | Mean value | Range (min – max) |
| Age (years) | 52.5 ± 11.3 | 31–72 |
| Height (cm) | 164.9 ± 10.8 | 147–186 |
| Body mass (kg) | 63.8 ± 14.7 | 43–100 |
| EDSS score | 3.1 ± 1.7 | 1.0–6.0 |
3.2. Please insert Table 1 approximately here
In Tables 2 and 3 are reported the descriptive statistics for PA and gait parameters respectively, while in Figure 1 shows the average hourly trend for daily number of steps.
Table 2.
Physical activity parameters calculated for pwMS during one week of measurement. Values are expressed as mean ± SD.
| Physical Activity Parameters | |
| Step count (steps day−1) | 9233 ± 2567 |
| Vector magnitude (106 counts day−1) | 2.00 ± 0.58 |
| Physical Activity Intensity | |
| Sedentary behavior (%) | 53.03 ± 10.5 |
| Light intensity (%) | 31.68 ± 8.74 |
| MVPA (%) | 15.27 ± 5.69 |
Table 3.
Values of the spatio-temporal and kinematic parameters of gait. Values are expressed as mean ± SD.
| Spatio-temporal parameters of gait | |
| Gait speed (m s−1) | 0.98 ± 0.32 |
| Stride length (m) | 1.11 ± 0.33 |
| Cadence (steps min−1) | 108.6 ± 13.8 |
| Stance phase (% gait cycle) | 62.8 ± 4.9 |
| Swing phase (% gait cycle) | 37.2 ± 4.9 |
| Double support phase (% gait cycle) | 25.6 ± 8.6 |
Figure 1.
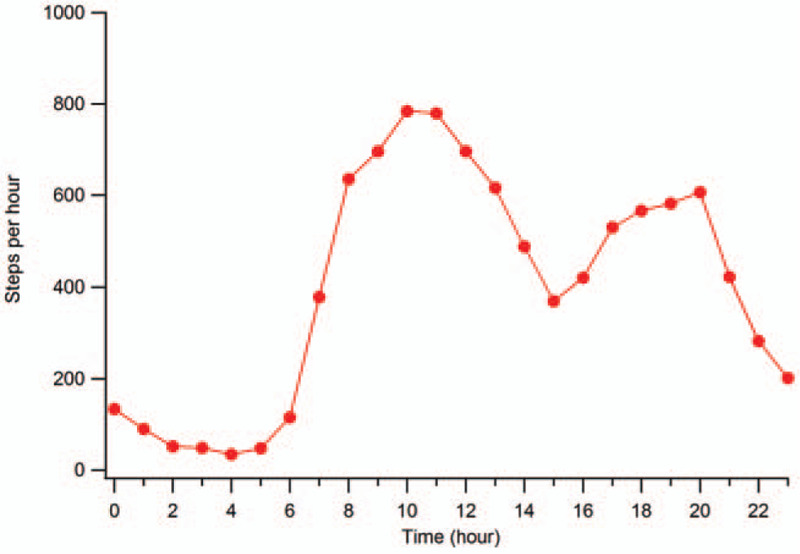
Hourly trend of step count for pwMS (average value calculated on 7 consecutive days).
The whole set of spatio-temporal parameters of gait of participants acquired from the instrumental analysis (see Table 3) depicts the typical pattern of individuals with MS characterized by reduced gait speed, stride length, and increased double support phase duration. The values obtained are in fairly good agreement, even from a quantitative point of view, with those typical of MS individuals with similar levels of disability.[27]
3.3. Correlation analysis
The results of the correlation analysis between PA and gait parameters are summarized in Table 4
Table 4.
Spearman correlation coefficients for correlation between physical activity intensity and spatio-temporal parameters of gait.
| Correlation between Physical Activity parameters and Gait parameters | ||||||
| Gait parameters | ||||||
| Gait speed | Stride length | Cadence | Stance | Swing | Double support | |
| PA parameters | ||||||
| Sedentary Behavior (%) | −0.442∗∗ | −0.493∗∗ | NS | 0.273∗ | −0.273∗ | 0.301∗ |
| Light intensity (%) | NS | 0.348∗∗ | −0.317∗ | NS | NS | −0.258∗∗ |
| MVPA (%) | 0.484∗∗ | 0.400∗∗ | NS | −0.268∗ | 0.268∗ | −0.286∗ |
| Step count | 0.623∗∗ | 0.413∗∗ | 0.403∗∗ | NS | NS | NS |
| Vector magnitude count | 0.562∗∗ | 0.452∗∗ | NS | −0.338∗∗ | 0.338∗∗ | −0.358∗∗ |
The correlation analysis detected moderate to large significant correlations between the percentage of time spent in SB and MVPA and all gait parameters with the exception of cadence. In particular, the highest values of rho were observed for gait speed, which was found negatively correlated with SB and positively correlated with MVPA (rho = –0.442 and 0.484 respectively, P < .001 in both cases) and for stride length (rho = –0.493 and 0.480, P < .001). Interestingly, the duration of the double support phase was the only gait parameter, together with the stride length, which was significantly correlated with all 3 levels of PA intensity. Finally, we found large positive correlations of both step count and VM counts with gait speed (rho = 0.623 and 0.562, P < .001) and moderate with stride length (rho = 0.413 and 0.452, P < .001). VM was also found moderately correlated with the duration of stance, swing, and double support phase.
4. Discussion
The aim of this study was to assess the existence of possible relationship between spatio-temporal parameters of gait and PA parameters, both quantitatively assessed, to understand what specific locomotion features might play an important role in promoting an active lifestyle in pwMS. In this context, the cohort of pwMS tested here exhibit the typical gait pattern observed in similar studies performed using wearable sensors (see the review by Vienne-Jumeau et al for details[30]), characterized by reduced speed and stride length and increased double support time. As regards the PA profile of participants, it is to be preliminarily observed that the use of an accelerometer to objectively assess amount and intensity of PA in MS is relatively recent (the earliest data on pwMS were presented by Ng et al in 1997,[31] but the systematic employment of such devices started less than 20 years ago[32,33]). The number of studies performed with this approach is still quite limited and they are characterized by much variability in terms of devices employed, acquisition protocols (e.g., wear time, device positioning, etc.) and data processing. There are other factors, such as M:F ratio of the tested cohorts, disability level and geographical location of the participants, that may affect the results.
When comparing the data obtained here with those of previous similar studies, we observe that the value of daily steps calculated for our cohort (9233) is similar to what reported by Shammas et al[34] and Romberg et al[35] for European pwMS, but well above those found in several investigations performed in the United States.[36–41] In the latter, the number of daily steps ranged from 5800 to 8860, depending on the average level of participants’ disability. Such bias, however, may also be attributed to the intrinsic propensity to walk which may differ significantly from country to country and has generally been recognized lowest in the United States with respect to EU countries, as found in several studies based on accelerometric, smartphone and fitness tracker data.[42–44] It should also be considered that in most of the studies cited, the inclusion criterion in terms of accelerometer wear time was 10 hours/day, while in our study we opted for a stricter limit of 16 hours/day (also considering the expected increased compliance owing to the wrist positioning of the device) and thus the number of missing steps associated with non-wear time probably resulted reduced.
Observing the PA intensity data of the pwMS tested, when raw accelerations were processed using the cut points proposed by Sebastiao et al,[16] the percentages of SB (53%) and LPA (32%) were found consistent with those reported in previous studies which indicate values from 60 to 70% for SB and 27 to 37% for LPA.[16,45–47] In contrast, our group exhibited significantly higher values for MVPA (15%), the typical values for pwMS being in the range 1% to 7%. It may be that such a discrepancy is due to the different positioning of the accelerometer, probably not fully compensated by the correction applied by the Actilife software. In this regard, it is noteworthy to be pointed out that previous studies observed that the largest absolute differences in accelerometric counts between hip and wrist occur for the most intense activities.[48] Thus, it is reasonable to hypothesize that the different site of placement generates a nonlinear error which is relatively low in magnitude for low-intensity activities and more severe for high-intensity activities.
The most innovative aspect of this study is represented by the search for possible correlations between PA and gait patterns, the latter investigated using quantitative gait analysis. Although previous studies reported the existence of significant correlations between accelerometric counts collected during real-life activities and spatio-temporal parameters of gait,[49] there is scarcity of data about the relationship between amount and intensity of daily PA and gait features. In this regard, our result suggest that gait speed and stride length appear to be the variables most strongly associated with the number of daily steps, SB and MVPA, but it was interesting to observe that the double support duration time was also found correlated with PA of any intensity, although with lower strength. As regards gait speed, our results are in line with previous studies which correlated the performance of timed tests (such as T25FWT and 10MWT) with PA levels. In particular, values of rho for correlation “speed vs MVPA” (0.48 in our group) in the range 0.33 to 0.40 were reported by Cederberg et al[46] and Baird et al.[47] In contrast, Shammas et al[34] found no significant correlation between PA intensity and gait speed, but it is to be recalled that they did not use a specific cut point for pwMS to classify it. However, the same authors (consistently with what reported in similar subsequent investigations[39,50]) reported a significant correlation between gait speed and daily steps count quite similar to what was found in the present study, as the coefficients of correlation ranged between 0.60 and 0.72 vs 0.62 here calculated.
Instead, the results for correlations between the percentage of light intensity PA and gait speed are quite contrasting, but there are few data in the literature to compare them with: Baird et al[47] detected no significant correlation (similarly to what was observed in the present study) while Cederberg et al[46] did (rho = 0.62).
Stride length was the only gait parameter that was found significantly correlated with all levels of PA intensity as well as step count and VM count. Reduced stride length, which is one of the typical features of the gait pattern in pwMS and is due to weakness, spasticity, reduced proprioception, and changes in motor integration, has been proven sensitive to physical rehabilitation and exercise[51,52] and it was also found associated with the risk of falling and fatigue[53,54]. Thus, it is reasonable to hypothesize that pwMS characterized by higher values of step length are in some way more inclined to be engaged in PA, even of moderate and vigorous intensity.
Finally, our results show that temporal parameters of gait, such as duration of stance, swing, and double support phases, are moderately correlated with PA intensity. In short, pwMS who exhibit increased duration of the stance phase (and correspondingly decreased duration of the swing phase) and increased double support are characterized by higher percentages of SB and reduced percentages of MVPA. The increase in stance and double support is a marker of cautious gait and reflect a protective strategy that favored stability and balance at the expense of speed.[55–57] Fear of falling, or even a general feeling of unsteadiness, may thus discourage individuals from performing PA and promote sedentary behavior.
Some limitations of the study are to be acknowledged. Firstly, the tested sample was predominantly composed of pwMS with low-mild disability (60% of them had an EDSS score of ≤3) living in an inner-city residential area and thus generalizations to different geographic and socio-economic contexts and to individuals more severely impaired are difficult. Secondly, to simplify the calculation procedure we employed a single set of cut-points to convert accelerations to PA intensity but actually, as demonstrated by Sandroff et al,[58] the thresholds suitable to define MVPA (and the other PA levels) depend on the disability, meaning that in presence of higher EDSS the threshold expressed in terms of cpm to discriminate MVPA decreases. At last, it is possible that the results might have been influenced by confounding factors like the simultaneous presence of men and women (which are known to have different gait features[59]) and the large range of disability level which characterized the participants. Such factors are certainly worthy of future specific analysis.
5. Conclusion
This study investigated the relationship between the amount and intensity of PA performed by individuals affected by MS with low-mild disability and their gait patterns, using objective techniques (i.e., accelerometers), with a set of cut-points for the accelerometric counts previously validated in pwMS. Our initial hypothesis, namely the existence of significant correlations between gait parameters and amount/intensity of performed PA, was substantially confirmed by the results. In particular, gait speed and stride length were the variables more strongly correlated with the number of daily steps and PA intensity of any level, but also stance, swing and double support duration were found to be associated with SB and MVPA. Taken together, such findings indicate that most gait parameters commonly assessed in pwMS may also provide a rough idea about the propensity of the individual to engage in PA. Although further studies on larger cohorts are needed to better elucidate the role of clinical and demographic factors, such as disability level, gender and socio-economic status, it appears important to perform continuous monitoring of PA in pwMS, as well as acquiring quantitative measures of their gait patterns, to plan adequate training or physical therapy programs tailored to individuals’ needs to maximize their effectiveness.
Acknowledgments
The authors wish to thank the participants of the study and their families for their valuable support.
The authors wish to thank all the participants and their caregivers for the valuable support.
Author contributions
Conceptualization: Massimiliano Pau.
Formal analysis: Massimiliano Pau, Micaela Porta.
Funding acquisition: Massimiliano Pau.
Investigation: Giancarlo Coghe, Eleonora Cocco.
Methodology: Massimiliano Pau.
Resources: Eleonora Cocco.
Writing – original draft: Massimiliano Pau.
Writing – review & editing: Massimiliano Pau, Eleonora Cocco.
Footnotes
Abbreviations: 10MWT = 10 m Walking Test, cpm = counts per minute, EDSS = Expanded Disability Status Scale, LPA = light intensity physical activity, MET = metabolic equivalent, MS = multiple sclerosis, MVPA = moderate-to-vigorous physical activity, PA = physical activity, pwMS = people with multiple sclerosis, SB = sedentary behaviour, VM = vector magnitude.
How to cite this article: Pau M, Porta M, Coghe G, Cocco E. What gait features influence the amount and intensity of physical activity in people with multiple sclerosis? Medicine. 2021;100:9(e24931).
This work was partly supported by FISM – Fondazione Italiana Sclerosi Multipla (Italian Foundation for Multiple Sclerosis) grant code 2017/R/19.
The datasets generated and analyzed during the current study are publicly available at Mendeley Data (https://data.mendeley.com/datasets/kj57yp96s7/1).
This research was approved by the Ethics Committee of ATS Sardegna (Prot. 102/2018/CE). Then, written informed consent was obtained from all participants.
The authors have no conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
MVPA = moderate-to-vigorous intensity activity.
P < .05.
P < .01.
MVPA = moderate-to-vigorous intensity activity.
References
- [1].Cameron MH, Wagner JM. Gait abnormalities in multiple sclerosis: pathogenesis, evaluation, and advances in treatment. Curr Neurol Neurosci Rep 2011;11:507–15. [DOI] [PubMed] [Google Scholar]
- [2].Kister I, Chamot E, Salter AR, et al. Disability in multiple sclerosis: a reference for patients and clinicians. Neurology 2013;80:1018–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ertekin Ö, Özakbaş S, İdiman E. Caregiver burden, quality of life and walking ability in different disability levels of multiple sclerosis. Neuro Rehabil 2014;34:313–21. [DOI] [PubMed] [Google Scholar]
- [4].Kohn CG, Baker WL, Sidovar MF, et al. Walking speed and health-related quality of life in multiple sclerosis. Patient 2014;7:55–61. [DOI] [PubMed] [Google Scholar]
- [5].Snook EM, Motl RW. Effect of exercise training on walking mobility in multiple sclerosis: a meta-analysis. Neurorehabil Neural Repair 2009;23:108–16. [DOI] [PubMed] [Google Scholar]
- [6].Motl RW, Goldman MD, Benedict RH. Walking impairment in patients with multiple sclerosis: exercise training as a treatment option. Neuropsychiatr Dis Treat 2010;16:767–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pearson M, Dieberg G, Smart N. Exercise as a therapy for improvement of walking ability in adults with multiple sclerosis: a meta-analysis. Arch Phys Med Rehabil 2015;96:1339–48e7. [DOI] [PubMed] [Google Scholar]
- [8].Streber R, Peters S, Pfeifer K. Systematic review of correlates and determinants of physical activity in persons with multiple sclerosis. Arch Phys Med Rehabil 2016;97:633–45. [DOI] [PubMed] [Google Scholar]
- [9].Motl RW, Learmonth YC, Pilutti LA, et al. Top 10 research questions related to physical activity and multiple sclerosis. Res Q Exerc Sport 2015;86:117–29. [DOI] [PubMed] [Google Scholar]
- [10].Motl RW, Goldman M. Physical inactivity, neurological disability, and cardiorespiratory fitness in multiple sclerosis. Acta Neurol Scand 2011;123:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sandroff BM, Klaren RE, Motl RW. Relationships among physical inactivity, deconditioning, and walking impairment in persons with multiple sclerosis. J Neurol Phys Ther 2015;39:103–10. [DOI] [PubMed] [Google Scholar]
- [12].Giesser BS. Exercise in the management of persons with multiple sclerosis. Ther Adv Neurol Disord 2015;8:123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Silfee VJ, Haughton CF, Jake-Schoffman DE, et al. Objective measurement of physical activity outcomes in lifestyle interventions among adults: a systematic review. Prev Med Rep 2018;11:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Boon RM, Hamlin MJ, Steel GD, et al. Validation of the New Zealand Physical Activity Questionnaire (NZPAQ-LF) and the International Physical Activity Questionnaire (IPAQ-LF) with accelerometry. Br J Sports Med 2010;44:741–6. [DOI] [PubMed] [Google Scholar]
- [15].Casey B, Coote S, Galvin R, et al. Objective physical activity levels in people with multiple sclerosis: meta-analysis. Scand J Med Sci Sports 2018;28:1960–9. [DOI] [PubMed] [Google Scholar]
- [16].Sebastião E, Learmonth YC, Motl RW. Lower physical activity in persons with multiple sclerosis at increased fall risk: a cross-sectional study. Am J Phys Med Rehabil 2017;96:357–61. [DOI] [PubMed] [Google Scholar]
- [17].Sandroff BM, Dlugonski D, Pilutti LA, et al. Physical activity is associated with cognitive processing speed in persons with multiple sclerosis. Mult Scler Relat Disord 2014;3:123–8. [DOI] [PubMed] [Google Scholar]
- [18].Motl RW, Snook EM, McAuley E, et al. Symptoms, self-efficacy, and physical activity among individuals with multiple sclerosis. Res Nurs Health 2006;29:597–606. [DOI] [PubMed] [Google Scholar]
- [19].Motl RW, McAuley E. Pathways between physical activity and quality of life in adults with multiple sclerosis. Health Psychol 2009;28:682–9. [DOI] [PubMed] [Google Scholar]
- [20].Motl RW, McAuley E, Snook EM, et al. Physical activity and quality of life in multiple sclerosis: intermediary roles of disability, fatigue, mood, pain, self-efficacy and social support. Psychol Health Med 2009;14:111–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the McDonald criteria. Ann Neurol 2005;58:840–6. [DOI] [PubMed] [Google Scholar]
- [23].Kurtze JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–52. [DOI] [PubMed] [Google Scholar]
- [24].Sandroff BM, Motl RW, Suh Y. Accelerometer output and its association with energy expenditure in persons with multiple sclerosis. J Rehabil Res Dev 2012;49:467–75. [DOI] [PubMed] [Google Scholar]
- [25].Sandroff BM, Motl RW, Pilutti LA, et al. Accuracy of stepwatch (and Actigraph accelerometers for measuring steps taken among persons with multiple sclerosis. PLoS One 2014;9:e93511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kos D, Nagels G, D’Hooghe MB, et al. Measuring activity patterns using actigraphy in multiple sclerosis. Chronobiol Int 2007;24:345–56. [DOI] [PubMed] [Google Scholar]
- [27].Buchan DS, McSeveney F, McLellan G. A comparison of physical activity from Actigraph GT3X+ accelerometers worn on the dominant and non-dominant wrist. Clin Physiol Funct Imaging 2019;39:51–6. [DOI] [PubMed] [Google Scholar]
- [28].Pau M, Caggiari S, Mura A, et al. Clinical assessment of gait in individuals with multiple sclerosis using wearable inertial sensors: comparison with patient-based measure. Mult Scler Relat Disord 2016;10:187–91. [DOI] [PubMed] [Google Scholar]
- [29].Cohen J. Statistical power analysis. Curr Dir Psychol Sci 1992;1:98–101. [Google Scholar]
- [30].Vienne-Jumeau A, Quijoux F, Vidal PP, et al. Wearable inertial sensors provide reliable biomarkers of disease severity in multiple sclerosis: a systematic review and meta-analysis. Ann Phys Rehabil Med 2020;63:138–47. [DOI] [PubMed] [Google Scholar]
- [31].Ng AV, Kent-Braun JA. Quantitation of lower physical activity in persons with multiple sclerosis. Med Sci Sports Exerc 1997;29:517–23. [DOI] [PubMed] [Google Scholar]
- [32].Pearson OR, Busse ME, van Deursen RW, et al. Quantification of walking mobility in neurological disorders. QJM 2004;97:463–75. [DOI] [PubMed] [Google Scholar]
- [33].Snook EM, Mojtahedi MC, Evans EM, et al. Physical activity and body composition among ambulatory individuals with multiple sclerosis. Int J MS Care 2005;7:137–42. [Google Scholar]
- [34].Shammas L, Zentek T, von Haaren B, et al. Home-based system for physical activity monitoring in patients with multiple sclerosis (pilot study). Biomed Eng Online 2014;13:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Romberg A, Ruutianen J, Daumer M. Physical activity in finnish persons with multiple sclerosis. J Nov Physiother 2013;3:3. [Google Scholar]
- [36].Motl RW, McAuley E, Snook EM, et al. Validity of physical activity measures in ambulatory individuals with multiple sclerosis. Disabil Rehabil 2006;28:1151–6. [DOI] [PubMed] [Google Scholar]
- [37].Sandroff BM, Dlugonski D, Weikert M, et al. Physical activity and multiple sclerosis: new insights regarding inactivity. Acta Neurol Scand 2012;126:256–62. [DOI] [PubMed] [Google Scholar]
- [38].Dlugonski D, Pilutti LA, Sandroff BM, et al. Steps per day among persons with multiple sclerosis: variation by demographic, clinical, and device characteristics. Arch Phys Med Rehabil 2013;94:1534–9. [DOI] [PubMed] [Google Scholar]
- [39].Cavanaugh JT, Gappmaier VO, Dibble LE, et al. Ambulatory activity in individuals with multiple sclerosis. J Neurol Phys Ther 2011;35:26–33. [DOI] [PubMed] [Google Scholar]
- [40].Fjeldstad C, Fjeldstad AS, Pardo G. Use of accelerometers to measure real-life physical activity in ambulatory individuals with multiple sclerosis: a pilot study. Int J MS Care 2015;17:215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Engelhard MM, Patek SD, Lach JC, et al. Real-world walking in multiple sclerosis: separating capacity from behavior. Gait Posture 2018;59:211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bassett DR, Wyatt HR, Thompson H, et al. Pedometer-measured physical activity and health behaviors in U.S. Adults Med Sci Sports Exerc 2010;42:1819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Althoff T, Sosič R, Hicks JL, et al. Large-scale physical activity data reveal worldwide activity inequality. Nature 2017;547:336–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kosecki D. Fitbit's Healthiest Cities, countries, and days for 2017. 2017 [accessed on December 20, 2020]. Available at: https://blog.fitbit.com/fitbit-year-in-review/ [Google Scholar]
- [45].Ezeugwu V, Klaren RE, A. Hubbard E, et al. Mobility disability and the pattern of accelerometer-derived sedentary and physical activity behaviors in people with multiple sclerosis. Prev Med Rep 2015;2:241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cederberg KL, Motl RW, McAuley E. Physical Activity, sedentary behavior, and physical function in older adults with multiple sclerosis. J Aging Phys Act 2018;26:177–82. [DOI] [PubMed] [Google Scholar]
- [47].Baird JF, Cederberg KLJ, Sikes EM, et al. Physical activity and walking performance across the lifespan among adults with multiple sclerosis. Mult Scler Relat Disord 2019;35:36–41. [DOI] [PubMed] [Google Scholar]
- [48].Hildebrand M, Van Hees VT, Hansen BH, et al. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med Sci Sports Exerc 2014;46:1816–24. [DOI] [PubMed] [Google Scholar]
- [49].Motl RW, Pilutti L, Sandroff BM, et al. Accelerometry as a measure of walking behavior in multiple sclerosis. Acta Neurol Scand 2013;127:384–90. [DOI] [PubMed] [Google Scholar]
- [50].Shah VV, McNames J, Mancini M, et al. Quantity and quality of gait and turning in people with multiple sclerosis, Parkinson's disease and matched controls during daily living. J Neurol 2020;267:1188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pau M, Corona F, Coghe G, et al. Quantitative assessment of the effects of 6 months of adapted physical activity on gait in people with multiple sclerosis: a randomized controlled trial. Disabil Rehabil 2018;40:144–51. [DOI] [PubMed] [Google Scholar]
- [52].Leone C, Kalron A, Smedal T, et al. Effects of rehabilitation on gait pattern at usual and fast speeds depend on walking impairment level in multiple sclerosis. Int J MS Care 2018 Sep; 20:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kalron A, Frid L, Gurevich M. Concern about falling is associated with step length in persons with multiple sclerosis. Eur J Phys Rehabil Med 2015;51:197–205. [PubMed] [Google Scholar]
- [54].Kalron A. Association between perceived fatigue and gait parameters measured by an instrumented treadmill in people with multiple sclerosis: a cross-sectional study. J Neuroeng Rehabil 2015;12:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Benedetti MG, Piperno R, Simoncini L, et al. Gait abnormalities in minimally impaired multiple sclerosis patients. Mult Scler 1999;5:363–8. [DOI] [PubMed] [Google Scholar]
- [56].Martin CL, Phillips BA, Kilpatrick TJ, et al. Gait and balance impairment in early multiple sclerosis in the absence of clinical disability. Mult Scler 2006;12:620–8. [DOI] [PubMed] [Google Scholar]
- [57].Kalron A, Dvir Z, Givon U, et al. Gait and jogging parameters in people with minimally impaired multiple sclerosis. Gait Posture 2014;39:297–302. [DOI] [PubMed] [Google Scholar]
- [58].Sandroff BM, Riskin BJ, Agiovlasitis S, et al. Accelerometer cut-points derived during over-ground walking in persons with mild, moderate, and severe multiple sclerosis. J Neurol Sci 2014;340:50–7. [DOI] [PubMed] [Google Scholar]
- [59].Pau M, Corona F, Pilloni G, et al. Do gait patterns differ in men and women with multiple sclerosis? Mult Scler Relat Disord 2017;18:202–8. [DOI] [PubMed] [Google Scholar]


