Abstract
Introduction:
Due to the diversity of reports and on the rates of medications errors (MEs) in Saudi Arabia, we performed the first meta-analysis to determine the rate of medications errors in Saudi Arabia using meta-analysis in the hospital settings.
Methods:
We conducted a systematic literature search through August 2019 using PubMed, EMBASE, CINAHL, PsycINFO, and Google Scholar to identify all observational studies conducted in hospital settings in Saudi Arabia that reported the rate of MEs. A random-effects models were used to calculate overall MEs, as well as prescribing, dispensing, and administration error rates. The I2 statistics were used to analyze heterogeneity.
Results:
Sixteen articles were included in this search. The total incidence of MEs in Saudi Arabia hospitals was estimated at 44.4%. Prescribing errors, dispensing errors, and adminstration errors incidents represent 40.2%, 28.2%, and 34.5% out of the total number of reported MEs, respectively. However, between-study heterogeneity was also generally found to be >90% (I-squared statistic).
Conclusions:
This study demonstrates the MEs common in health facilities. Additional efforts in the field are needed to improve medication management systems in order to prevent patient harm incidents.
Keywords: administration error, dispensing error, medication errors, prescribing error, Saudi Arabia
1. Introduction
Medication errors (MEs) are major issue in every healthcare system. They are ranked as one of the most common medical errors in the practice, according to the reports of the Joint Commission.[1] Medication errors have an enormous impact on the health care system, including patients and payers.[2] They remain the eighth leading cause of amenable and preventable death in the United States of America (USA), causing about 225,000 deaths each year.[3] According to various studies on drug related hospital admissions, 5% to 6% of all hospitalizations are due to medication-related problems.[4] It was estimated that MEs causing hospitalization in the UK occur in approximately 10% of inpatients; and nearly 50% of them are preventable.[5] From the economic standpoint, these may have significant economic repercussions. It has been estimated that the annual cost of MEs in the world is approximately US$42 billion.[6]
Medication errors in hospital settings have become a topic of top priority in all nations and represent a significant challenge. There are evidence shows that the transition of care on admission to the hospital and between different units are risk points for MEs.[7,8] There are many potential reasons for MEs to happen at hospital. Patients commonly receive new drugs or have alternate drugs due to drug formularies limitations which could limit to certain medications during the hospitalization.[9] In addition, the lack of communication, understanding, and collaboration among providers is a significant factor in preventable MEs after hospital.[10]
Disseminating information about the rate of MEs in hospitals is the first step toward tackling this issue. Direct observation has been found to be the most accurate method for defining the real rates of ME.[11] To date, a considerable number of studies have evaluated the rates of ME in different regions of Saudi Arabia. However, their results significantly vary (41.6%–70%).[12–15] The reason for the observed variation in ME rates between studies can be explained, at least in part, by the definitions of MEs and the methods used in studies to determine the frequency of MEs. The diversity of methods for determination of MEs and extensive statistics on the rates of ME pushed healthcare planners and policymakers to deal with restrictions on using the results.
Given the above diversity on the rates of ME in Saudi Arabia, it is important to use stringent to combine the previous results of ME rates together to quantify the real prevalence of MEs on national level. Thus, to fill a significant gap in the literature, this research was designed to determine the pooled estimate of ME rates in Saudi Arabia and those occurring at different stages in the hospital setting (i.e., prescribing, dispensing, and drug administration phases) using meta-analysis.
2. Methods
2.1. Information sources and search
In this research, the systematic review was done in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA) guidelines.[16] Databases including PubMed, EMBASE, CINAHL, PsycINFO, and Google Scholar, which have electronically published studies from January 1966 to August 2019, were searched to find relevant Arabic and English language articles that examined the rate of MEs. We used the following key terms to search a database: incident, frequency, rate, or percentage, prevalence rates, medication error, drug error, medication mistake, dispensing error, administration error, transcribing error, prescribing error, drug mistake, administration mistake, prescribing mistake, dispensing mistake, transcribing mistake, preparation mistake, Saudi, and their Arabic equivalents, where above search terms were used in Arabic to search for articles published in Arabic language. We also added the names of cities with and and or operators in the abstracts and titles of articles and synonyms and other search terms to ensure that we did not overlook relevant studies. The reference lists of previous studies were accessed to find more previous studies if they were impossible to find in databases. The evaluation was done by 2 researchers. Disagreements between the 2 reviewers were brought to a third investigator for resolution.
2.2. Selection of studies
First, we conducted a preliminary examination of the titles on all studies selected from the search to obtain information relevant to our research. Second, we screened for the abstracts and skimmed full text when needed to assess the inclusion of the articles.
2.3. Inclusion/exclusion criteria of studies
The inclusion criteria were peer-reviewed observational and interventional studies conducted in hospital settings in Saudi Arabia that reported the rate of MEs in any age groups and in any phase of the pharmaceutical process. We excluded opinion pieces and letters to the editor, qualitative studies, brief reports, case reports, editorial comments, and review articles. Additionally, we did not make use of studies that reported MEs using voluntary reports. Only studies using direct observation methods by the investigators were included as this method is recognized as effective in identifying MEs that actually occur.[11] Studies with no data or insufficient data (i.e., total number medications and numbers of ME were not reported) were also excluded. We had no language restrictions to include studies that are not published in English language.
2.4. Definition of medication error
To identify and describe actual or potential MEs, we adopted a universally accepted definition of “medication error” that was approved by the National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP)[17]: “a medication error is any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the health care professional, patient or consumer.” Several methods are used in classifying MEs. In this study, we used an approach that based the classification on the stages of the medication-use process in hospitals, specifically, prescribing, dispensing, and administration. Definitions of each of the ME categories are well documented in the existing literature.[18,19]
2.5. Extraction of data
We developed a standardized data extraction instrument for the study that contains a checklist of items that should be included in the studies, that is, the title, year of research, the first author's name, the sample size of the study, a study place, the frequency of overall occurrence of MEs and distribution across phases of the medication process, the type of methodology and study sampling, and the quality score of the study.
2.6. Quality assessment
To assess the quality of each study included, we used a thirteen-question scoring system (Table 1) utilized by previous studies.[20–23] A score of 1 was assigned to each question if the study met the requirements of the quality assessment question, and a score of 0 was assigned if the study did not satisfy such a requirement. Criteria were graded no score if the requirement was unclear or not reported. The total score for all studies obtained from 13 questions was calculated by collecting the obtained scores.
Table 1.
A list of quality assessment questions.
| No. | Assessment question | ScoreYes = 1No = 0 |
| 1 | Are the research questions clearly stated? | |
| 2 | Is the definition of what constitutes a medication error clearly stated? | |
| 3 | Are the error categories clearly specified? | |
| 4 | Are the error categories clearly defined? | |
| 5 | Is the sampling method and calculation of sample size described clearly? | |
| 6 | Is the denominator clearly defined? | |
| 7 | Is the data collection method described clearly? | |
| 8 | Are the measures used reliable? | |
| 9 | Is the setting in which study conducted described? | |
| 10 | Are the measures in place to ensure that results valid? | |
| 11 | Is the limitations of study listed? | |
| 12 | Are any assumptions made mentioned? | |
| 13 | Ethical approval was obtained |
2.7. Data synthesis and statistical analysis
A random-effects meta-analysis approach was employed to estimate the overall ME rates as well as the error rates of prescribing, dispensing, and administration. The entire analyses involved in this study were conducted using Comprehensive Meta-Analysis software (Biostat, Englewood, NJ). Event rates were noted from articles, and 95% confidence intervals (95% CI) were calculated. For the purpose of this study, we performed 4 group analyses according to the type of errors. Most studies were analyzed in more than 1 group. By using this measure (numerator/denominator) for each study, we were able to calculate the integrated indicators for every one of these groups. The first group specifically included errors regarding medication orders. Errors in MEs were defined as numerators and MEs as denominators. The second group included errors in the prescribing (numerator) of total MEs (denominator). The third group included errors in dispensing (numerators) and total MEs (denominator). Finally, the fourth group included administration errors as numerators and total MEs as denominators. In cases where the numerator equals the denominator, the study was excluded from the specific group analysis to avoid any imprecise estimate.
We also assessed the statistical heterogeneity scores using the I2 statistic test (in which a value of >75% was regarded as “large heterogeneity” and a value of <40% was regarded as “heterogeneity force is not important”). In this paper, we assessed the possibility of publication bias in meta-analyses across the selected studies using the funnel plot and Kendall's tau and Egger bias test. As another way to address publication bias, a fail-safe N was calculated to determine the number of non-significant studies (i.e., with an error rate of zero) that would be necessary to reduce the overall error rate to zero. If we needed a large number of studies, there would be less reason for concern about publication bias.[24] All significance testing was 2-sided, and the results were considered statistically significant at P < .05. In this study, we assessed the rates of the ME in Saudi Arabia based on the completed and published data from previous studies; thus, this study did not need to obtain Institutional Review Board approval.
3. Results
Figure 1 shows a flow diagram of the study selection, based on the inclusion and exclusion criteria. As of date, 16 studies were eligible to be included in this meta-analysis (Table 2). Two reviewers independently conducted the quality assessment on all studies using a 13-question scoring system. The quality assessment showed that only 1 study had a cumulative quality score of 5; 1 study had a cumulative quality score of 6; 2 studies (12.5.%) scored 7; 2 studies (12.5.%) scored 8; 2 studies (12.5.%) scored 9; 3 studies (18.7.%) scored 10; and the remaining studies (31.2%) scored >10.
Figure 1.
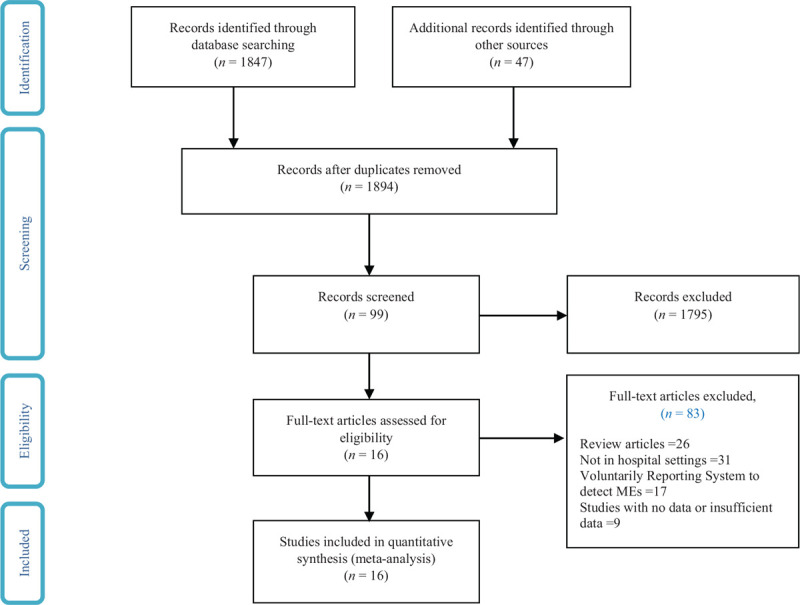
Search flow diagram.
Table 2.
Selected articles about medication errors.
| Study's names | Publication year | Study design | Type of hospital | Setting | Method of quantification | Sample size | Total no. of prescriptions during the study period | Prevalence of total medication error | Type of error | Duration | Error rate | Quality |
| Irshaid et al[25] | 2005 | Retrospective cross-sectional | Asir Central Hospital | Outpatient Departments | Direct observational of medication orders | NA | 3796 | 3567 | Overall medication errors | 12 mo | Overall medication errors = 0.94 | 8 |
| Dibbi et al[26] | 2006 | Retrospective cross-sectional | Hera General Hospital | NA | Direct observational of medical records | 2627 | 2627 | 2627 | Prescribing errors, dispensing errors, administration errors | 24 mo | Prescribing errors = 0.47, dispensing errors = 0.34, administration errors = 0.19 | 9 |
| Khoja et al[27] | 2011 | Retrospective Cross-Sectional | Primary Health Care Clinics | Public and Private Clinics | Direct observational of Prescribed medication | 81 | 5299 | 990 | Overall medication errors | 1 Working Day | Overall medication errors = 0.18 | 7 |
| Al-Dhawailie et al[28] | 2011 | Prospective observational study | Tertiary university teaching hospital | Inpatient pharmacy | Direct observational of inpatient medication orders for medical ward | 113 | 1582 | 113 | Overall medication errors | 1 mo | Overall medication errors = 0.07 | 10 |
| Al-Jeraisy et al[13] | 2011 | Retrospective cohort | King Abdulaziz Medical City | General pediatric wards and the pediatric intensive care unit | Direct observational of physician medication orders and patients’ files | 44.5 | 2380 | 1333 | Overall medication errors | 5 wk | Overall medication errors = 0.56 | 10 |
| Abuyassin et al[29] | 2011 | Prospective observational study | Tertiary Hospital | Hospital Departments | Direct observational of prescribed medication | 60 | 564 | 209 | Overall medication errors | Over 2 mo | Overall medication errors = 0.37 | 12 |
| Qureshi et al[30] | 2011 | Prospective cohort | Three government-funded primary health care (PHC) centres | Outpatient setting | Direct observational of Prescribed medication | 100 | 1182 | 753 | Overall medication errors | 12 mo | Overall medication errors = 0.61 | 9 |
| Alakhali et al[31] | 2014 | Retrospective cross-sectional | Hospital in Abha | Outpatient | Direct observational of prescribed medication histories | NA | 1850 | 201 | Overall medication errors, prescribing errors, dispensing errors, administration errors | 2 mo | Overall medication errors = 0.92, prescribing errors = 0.93, dispensing errors = 0.03, administration errors = 0.04 | 7 |
| Alanazi et al[32] | 2015 | Retrospective cross-sectional | King Abdulaziz Medical City | Emergency Department | Direct observational of medical charts | 5752 | 5752 | 2657 | Overall medication errors | 3 mo | Overall medication errors = 0.46 | 8 |
| Al-Jadhey et al[33] | 2015 | Prospective cohort | Four tertiary hospitals | Surgical and Intensive Care Units (ICUs) | Direct observational of prescribed medication | 3985 | 4041 | 1676 | Overall medication errors | Over 4 mo | Overall medication errors = 0.39 | 12 |
| Abdallah et al[34] | 2016 | Retrospective cross-sectional | King Saud Medical City | ICU sections | Direct observational of medical records | 114 | 130 | 116 | Overall medication errors | 1 mo | Overall medication errors = 0.89 | 11 |
| Alomi et al[35] | 2017 | Prospective observational study | King Salman Hospital, | Inpatient | Direct observational of documented medication errors forms | 805 | NA | 3089 | Prescribing errors, dispensing errors, administration errors | 9 mo | Prescribing errors = 0.44, dispensing errors = 0.02, administration errors = 0.34 | 5 |
| Albahouth et al[36] | 2018 | Prospective observational study | Ten primary care | Internal Medicine Department | Direct observational of prescribed medication | 70 | 994 | 174 | Overall medication errors | 2 mo | Overall medication errors = 0.17 | 10 |
| Abdulghani et al[37] | 2018 | Prospective cross-sectional study | Tertiary care hospital | Hospital ward | Direct observational of prescribed medication | 286 | 3085 | 537 | Prescribing errors | 3 mo | Prescribing errors = 0.17 | 11 |
| Mazhar et al[38] | 2018 | Prospective observational study | Tertiary care teaching hospital | Internal medicine ward. | Direct observational of prescribed medication | 375 | 409 | 226 | Overall medication errors | 3 mo | Overall medication errors = 0.76 | 11 |
| Albaraki et al[39] | 2018 | Retrospective Pilot | Seven military hospitals | Stock Control Departments’ | Direct observational of prescribed medication histories | NA | 759 | 3 | Overall medication errors | 6 mo | Overall medication errors = 0.004 | 6 |
As shown in Table 2, a total of 16 studies involve the cross-sectional, cohort, and pilot study, and the study hospital setting included a hospital ward; internal medicine ward; outpatient and inpatient setting; tertiary care and primary care; public and private clinics; Intensive Care Unit (ICU) and Neonatal Intensive Care Unit (NICU) sections; surgical, medical and cardiac intensive care units; department of children and obstetrics; gynecology; emergency department; and the stock control departments. Of the 16 selected studies, 7 articles were published between 2005 and 2011, and the remaining 9 articles were published between 2014 and 2018. All articles were published in English.
3.1. Statistical results
3.1.1. Medication error rate
Figure 2 shows the forest plot of the overall ME rate. In this analysis, out of 16 studies, 13 studies met the criteria and had enough information to be included in the meta-analysis (i.e., denominator data were reported, and the numerator does not equal the denominator). There was broad variation among studies in the rate of overall MEs. In a total of 26,808 medication orders from these studies, ME rates ranged from 0.04% to 94%. The lowest reported rate of MEs was found in the Albaraki et al study.[39] According to the result of the random-effect model, the overall hospital ME rate per medication was calculated as 0.444, with 95% CI: 0.435 to 0.452, which was statistically significant at 5% level. The number of missing studies that would be needed to nullify the significance of the estimate (classic fail-safe N) of the meta-analysis was 185. There is no indication of publication bias according to visual inspection of the funnel plot (Fig. 3) or Kendall's tau statistic (P = .714) or Egger's test (intercept a = 4.1; P = .702). The main analysis indicated a significant amount of heterogeneity (I2 statistic [I2 = 99.973% and P < .001]).
Figure 2.
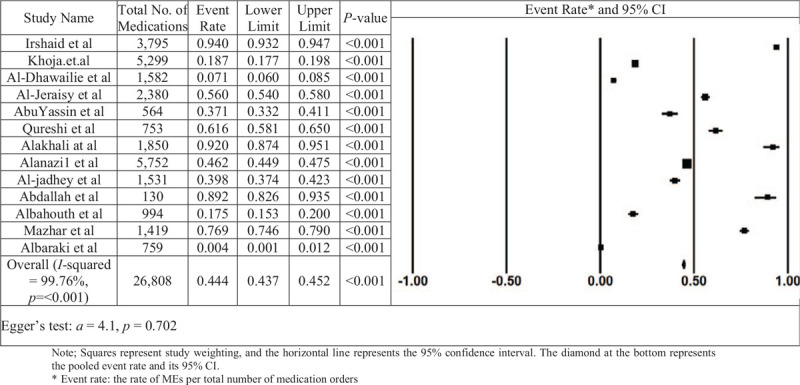
The rate of medication errors in Saudi Arabia in study and overall estimation.
Figure 3.
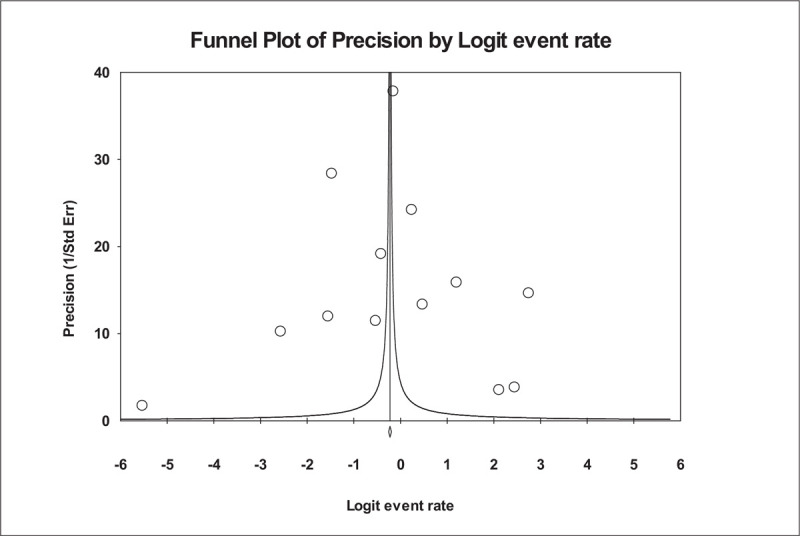
Funnel plot of precision by Logit Event Rate. This is a display of the study's effect size on a logit scale against its respective precision for each study included in the meta-analysis. Dots represent point estimates plotted over precision measures (1/standard error). The vertical line represents the summary estimate rate using random-effects meta-analysis.
3.1.2. Prescribing-error rate
Four studies were used to estimate the prescribing-error rate. The prescribing-error rates reported across these studies varied from 5.26% to 36.26%. The results, reported in Figure 4, showed that the prescribing-error rate is 0.402, with a 95% CI of 0.392 to 0.412 and a value of P < .001. Thus, the random-effect rate was 40.2%. The number of missing studies that would be needed to nullify the significance of the estimate of the meta-analysis was 248. There was no significant publication bias found according to a visual inspection of the funnel plot (Fig. 5) or Kendall's tau statistic (P = .496) or Egger's test (intercept a = 2.9; P = .908). According to the results of the heterogeneous test, there was a high degree of heterogeneity between the studies included in the analysis, depending on the results obtained from the I2 statistic (I2 = 99.973% and P < .001).
Figure 4.
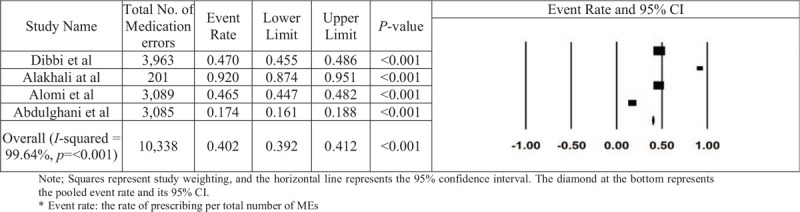
The rate of prescribing errors in Saudi Arabia in each paper and the overall estimation.
Figure 5.
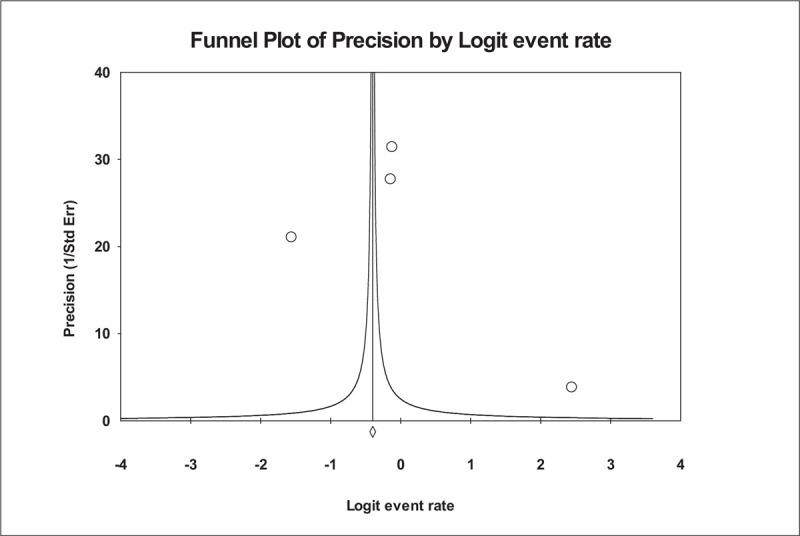
Funnel plot of precision by Logit Event Rate.
3.1.3. Dispensing-error rate
The dispensing-error rates for each of the 3 studies and overall estimate are reported in Figure 6. Only 3 studies were used to estimate the dispensing-error rate. The integrated prescribing-error rate was calculated as 0.282, with a 95% CI of 0.269 to 0.295 and a value of P < .001. The number of non-significant studies needed to nullify the significance of the estimate was 975. An analysis of publication bias was conducted. There is some asymmetry indicating the possibility of missing studies according to visual inspection of the funnel plot (Fig. 7). However, the asymmetry is not confirmed by either Kendall's tau statistic (P = .601) or Egger's test (intercept a = −10.08; P = .444). Heterogeneity between included studies did show significance (I2 = 99.973% and P < .001).
Figure 6.
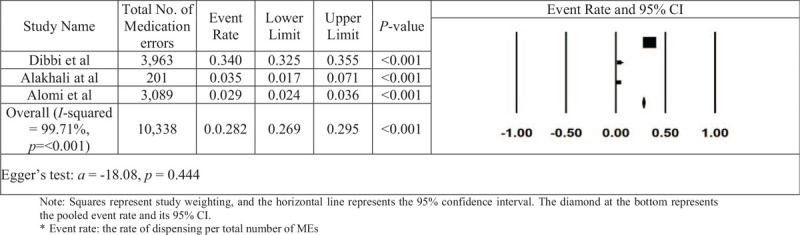
The rate of dispensing errors in Saudi Arabia in each paper and the overall estimation.
Figure 7.
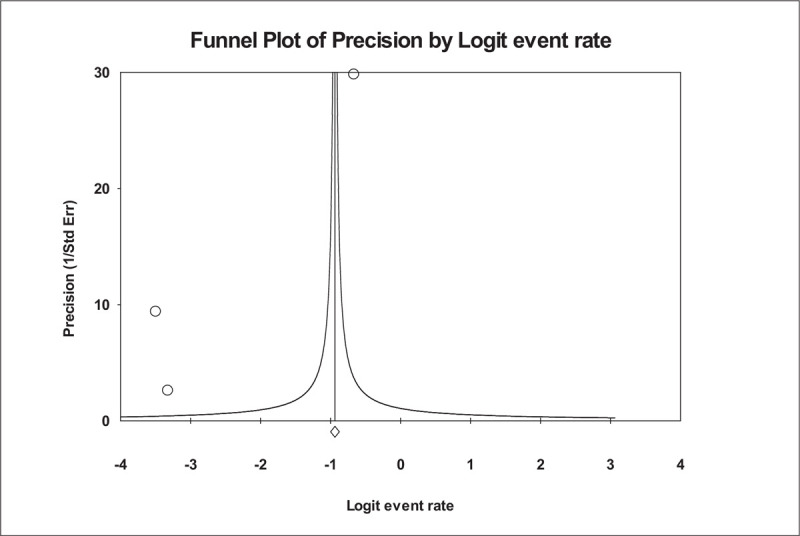
Funnel plot of precision by Logit Event Rate.
3.1.4. Administration-error rate
Three studies were used for this subgroup analysis to estimate the administration-error rate (Fig. 8). The integrated prescribing-error rate was calculated as 0.282, with a 95% CI of 0.269 to 0.295 and a value of P < .001. The number of missing studies that would be needed to change the results of the meta-analysis was 975. A visual inspection of the plot (Fig. 9) indicated some bias; however, the existence of this publication bias could not be confirmed with Kendall's tau statistic (P = .601) or Egger's test (intercept a = −10.18; P = .777). There was a high degree of heterogeneity between the studies included in the analysis, depending on the results obtained from the I2 statistic (I2 = 99.74% and P < .001).
Figure 8.
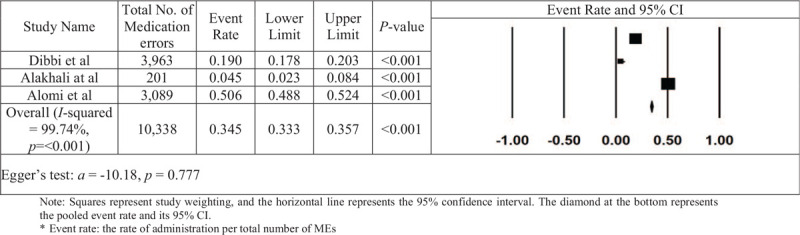
The rate of administration errors in Saudi Arabia in each paper and the overall estimation.
Figure 9.
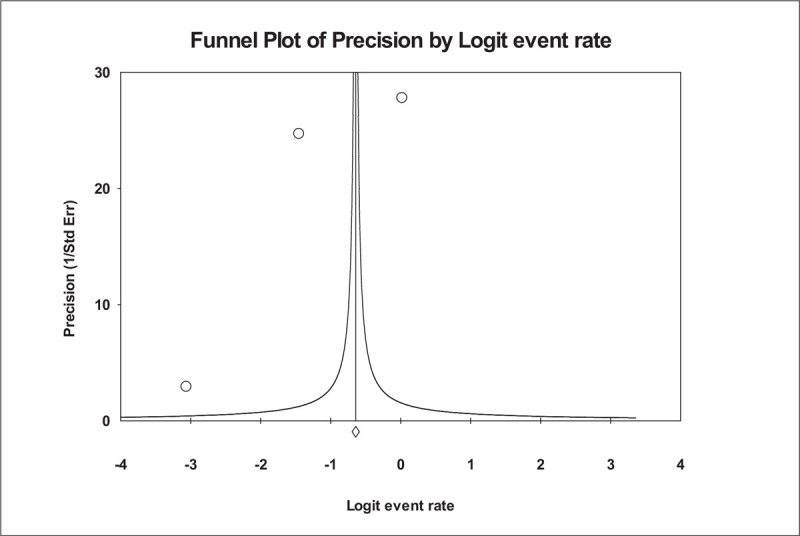
Funnel plot of precision by Logit Event Rate.
4. Discussion
Medication errors cause serious problems for patients and increase the patients’ mortality and hospital cost. In this meta-analysis, we found a significant variation in the reported rates of MEs in different hospitals in Saudi Arabia. The authors were able to use 16 studies in Saudi Arabia to evaluate the rate of MEs in hospitals during the stages of prescribing, dispensing, and administration. The integrated ME rate in Saudi hospitals was estimated to be 44.4%, higher than those reported by the only meta-analyses published in relation to MEs in hospital.[40] In the review of Taghizadeh et al,[40] they included 33 out of 323 articles in hospital settings. The pooled rate of MEs in Iranian hospitals was 31.8%. These findings called to attention the high rates of MEs, which warrants serious concern about patient safety in the healthcare system in Saudi Arabia.
This study highlighted the most frequently reported category of MEs according to medication-use process stages. We observed a high rate of MEs in the medication-prescribing stage. This estimate is higher than those reported by previous studies.[41–45] The high rate of prescribing errors among physicians in Saudi Arabia is a serious threat to patients and Saudi's health care system. Unfortunately, prescribing errors that occur in hospital settings have been a major issue for decades.
The future prevention of prescribing errors should focus on their root causes. Previous studies have recognized several important causes of this type of ME. The leading causes for prescribing errors were inadequate drug knowledge and experience, insufficient staff, increased patient load, time pressures and distractions while prescribing, incomplete supervision, miscommunication of prescribing decisions, increased reliance on pharmacists and nurses to identify and correct prescribing errors, and finally the lack of protocol/policy in how certain drugs are prescribed.[44–49]
To reduce prescribing error in Saudi Arabia, several recommendations can be proposed. First, there is a need to raise awareness about prescribing errors and promote safety culture among prescribers. Secondly, improvement must be made to the system of reporting ME by providing specific on-the-job training in the prescribing error reporting system and encourage prescribers to promptly report such errors. Thirdly, health care organizations should offer guidelines for clear and effective communication among health care providers. Finally, more appropriate policies and procedures should be put in place to reduce prescribing errors in Saudi Arabia.
Errors in administration, the final step in the medication pathway, remain a serious safety problem. In our study, administration errors were the second most frequently reported types of errors at 34.5% of drug MEs. This observation was found to be higher than those reported by a previous study.[50] According to a study done by Lan et al, insufficient knowledge of pharmacology was found to be the most significant difficulty nurses encountered when administering medications.[51] Thus, in the clinical environment, it is an institution's role to improve nurses’ working procedures and knowledge by implementing an educational training program about drugs and potential MEs.[52]
Although there has been a real increase in the adoption of new technologies such as automated dispensing systems,[53] dispensing errors accounted for 28.2% of the total MEs in Saudi hospitals; the results are significantly higher than studies performed previously in health institutions in different countries.[44,45,54] High workload, distractions, and working environment were both subjectively and objectively reported as contributing to dispensing errors.[55,56] It is crucial that the medication dispensing system that involves preventing and detecting MEs offers the possibility of connecting all the steps of care of the patient, in addition to preventing dispensing errors.
Currently, MEs in hospital settings are of a greater degree in comparison to those in other healthcare settings.[57] Our results demonstrate that Saudi healthcare settings share much of the challenges in medication safety observed in most countries around the world. While we did not evaluate it in the current research, we feel it is important to call for additional studies to elucidate the nature and define the causal factors of these MEs that, when eliminated, would prevent recurrence of these errors. Clearly, additional focus is needed on prescribing-error prevention strategies in the hospital settings in Saudi Arabia. Probably, computerizing the medication process system in hospital settings and pharmacological education of prescribers and nurses could help to reduce MEs.
To explore the potential source of heterogeneity across studies, sensitivity analyzes were performed by excluding studies one at a time to determine whether heterogeneity was substantially modified. When these studies were excluded from the analysis, the heterogeneity among studies was essentially unchanged. Thus, we cannot rule out that the bias and imprecision of I2 when only a low number of studies in some analyses were used.[58]
This analysis has its own limitations, and these should be noted before the implications of the current findings are considered. First, as noted previously, a significant heterogeneity across the selected studies points to caution in the interpretation of our findings. However, the heterogeneity may emanate from the variety of the studies’ characteristics, initially, the different study durations and settings that complicated the studies’ grouping. For example, studies with longer duration of data collection and conducted at outpatient and emergency departments represented higher medication error rates compared to other settings. Second, although the funnel plots assume a bias among the studies being examined in some group analyses, the bias was not evident by the Kendall's and Egger's tests. In addition, fail-safe numbers showed that publication bias is unlikely to be a problem. Finally, the meta-analysis included a few studies that make it difficult to generalize the results.
5. Conclusions
Understanding the MEs that occur in hospitals is an essential step toward improving the safety of patients. The results of this study provide data on the rates and types of MEs in hospitals in Saudi Arabia. Through this meta-analysis, we learned that the most common errors were prescribing errors, followed by administration errors. Because of this concern, there is an urgent need for additional work in the field to improve medication management systems in order to prevent patient harm due to MEs. This information can be used to prioritize the future studies to identify the causes of errors occurring at different stages in the hospital setting as well as to develop intervention directed at reducing the risk to patients from MEs.
Acknowledgments
The authors would like to thank the Saudi Association for Scientific Research (SASR) for providing their intellectual, technical, and logistical support throughout the project.
Author contributions
Conceptualization: Ziyad S Almalki, Nasser Alqahtani, Abdulhadi Alqahtani, Nawaf Alotaibi.
Data curation: Ziyad S Almalki, Najwa Tayeb Salway, Mona Marzoq Alharbi, Abdulhadi Alqahtani, Tahani Alshammari.
Formal analysis: Ziyad S Almalki, Nasser Alqahtani, Najwa Tayeb Salway, Mona Marzoq Alharbi.
Funding acquisition: Ziyad S Almalki.
Investigation: Najwa Tayeb Salway, Abdulhadi Alqahtani.
Methodology: Ziyad S Almalki, Nasser Alqahtani, Najwa Tayeb Salway, Mona Marzoq Alharbi, Abdulhadi Alqahtani, Tahani M Alotaibi.
Project administration: Ziyad S Almalki, Tahani M Alotaibi.
Resources: Abdulhadi Alqahtani.
Software: Ziyad S Almalki, Tahani M Alotaibi, Tahani Alshammari.
Supervision: Najwa Tayeb Salway.
Validation: Mona Marzoq Alharbi.
Writing – original draft: Ziyad S Almalki, Nasser Alqahtani, Najwa Tayeb Salway, Mona Marzoq Alharbi, Nawaf Alotaibi, Tahani Alshammari.
Writing – review & editing: Ziyad S Almalki, Nasser Alqahtani, Najwa Tayeb Salway, Abdulhadi Alqahtani, Nawaf Alotaibi, Tahani Alshammari.
Footnotes
Abbreviations: CI = confidence interval, ICU = Intensive Care Unit, MEs = medication errors, NCC MERP = National Coordinating Council for Medication Error Reporting and Prevention, NICU = Neonatal Intensive Care Unit, PRISMA = Preferred Reporting Items for Systematic Review and Meta-Analysis.
How to cite this article: Almalki ZS, Alqahtani N, Salway NT, Alharbi MM, Alqahtani A, Alotaibi N, Alotaibi TM, Alshammari T. Evaluation of medication error rates in Saudi Arabia: A protocol for systematic review and meta-analysis. Medicine. 2021;100:9(e24956).
This project was supported by the Deanship of Scientific Research at Prince Sattam bin Abdulaziz University.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
ICU = Intensive Care Unit.
References
- [1].The Joint Commission. Sentinel Event Alert: Preventing Pediatric Medication Errors. Issue 39, April 11, 2008. Available from: http://www.jointcommission.org/SentinelEvents/SentinelEventAlert/sea 39.htm. Accessed May 30, 2019. [Google Scholar]
- [2].Cousins DD, Heath WM. The National Coordinating Council for medication error reporting and prevention: promoting patient safety and quality through innovation and leadership. Jt Comm J Qual Patient Saf 2008;34:700–2. [DOI] [PubMed] [Google Scholar]
- [3].Miller G. The best health care system in the world? Soc Work 2013;58:181–3. [DOI] [PubMed] [Google Scholar]
- [4].Einarson TR. Drug-related hospital admissions. Ann Pharmacother 1993;27:832–40. [DOI] [PubMed] [Google Scholar]
- [5].Sekhar MS, Mary CA, Anju P, et al. Study on drug related hospital admissions in a tertiary care hospital in South India. Saudi Pharm J 2011;19:273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Aitken M, Gorokhovich L. Advancing the responsible use of medicines: applying levers for change. SSRN Electronic J 2012. [Google Scholar]
- [7].Weant KA, Bailey AM, Baker SN. Strategies for reducing medication errors in the emergency department. Open Access Emerg Med 2014;6:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wong JD, Bajcar JM, Wong GG, et al. Medication reconciliation at hospital discharge: evaluating discrepancies. Ann Pharmacother 2008;42:1373–9. [DOI] [PubMed] [Google Scholar]
- [9].Nickerson A, MacKinnon NJ, Roberts N, et al. Drug-therapy problems, inconsistencies and omissions identified during a medication reconciliation and seamless care service. Healthc Q 2005;8:65–72. [DOI] [PubMed] [Google Scholar]
- [10].Munday A, Kelly B, Forrester J, et al. Do general practitioners and community pharmacists want information on the reasons for drug therapy changes implemented by secondary care? Brit J Gen Pract 1997;47:563–6. [PMC free article] [PubMed] [Google Scholar]
- [11].Flynn EA, Barker KN, Pepper GA, et al. Comparison of methods for detecting medication errors in 36 hospitals and skilled-nursing facilities. Am J Health Syst Pharm 2002;59:436–46. [DOI] [PubMed] [Google Scholar]
- [12].Sadat-Ali M, Al-Shafei BA, Al-Turki RA, et al. Medication administration errors in Eastern Saudi Arabia. Saudi Med J 2010;31:1257–9. [PubMed] [Google Scholar]
- [13].Al-Jeraisy MI, Alanazi MQ, Abolfotouh MA. Medication prescribing errors in a pediatric inpatient tertiary care setting in Saudi Arabia. BMC Res Notes 2011;4:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mazhar F, Akram S, Al-Osaimi YA, et al. Medication reconciliation errors in a tertiary care hospital in Saudi Arabia: admission discrepancies and risk factors. Pharm Pract (Granada) 2017;15:864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Al-Rashoud I, Al-Ammari M, Al-Jadhey H, et al. Medication discrepancies identified during medication reconciliation among medical patients at a tertiary care hospital. Saudi Pharm J 2017;25:1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. [DOI] [PubMed] [Google Scholar]
- [17].National Coordinating Council for Medication Error Reporting and Prevention (NCCMERP). Definition and Types of Medication Errors. http://www.nccmerp.org/about-medication-errors. Accessed May 30, 2019. [Google Scholar]
- [18].Potts AL, Barr FE, Gregory DF, et al. Computerized physician order entry and medication errors in a pediatric critical care unit. Pediatrics 2004;113:59–63. [DOI] [PubMed] [Google Scholar]
- [19].Williams DJ. Medication errors. RCPE 2007;37:343–6. [Google Scholar]
- [20].Salmasi S, Khan TM, Hong YH, et al. Medication errors in the Southeast Asian countries: a systematic review. PLoS One 2015;10:e0136545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Alsulami Z, Conroy S, Choonara I. Medication errors in the Middle East countries: a systematic review of the literature. Eur J Clin Pharmacol 2013;69:995–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ghaleb MA, Barber N, Franklin BD, et al. A systematic review of medication errors in pediatric patients. Ann Pharmacother 2006;40:1766–76. [DOI] [PubMed] [Google Scholar]
- [23].Thomas B, Paudyal V, MacLure K, et al. Medication errors in hospitals in the Middle East: a systematic review of prevalence, nature, severity and contributory factors. Eur J Clin Pharmacol 2019;75:1269–82. [DOI] [PubMed] [Google Scholar]
- [24].Fragkos KC, Tsagris M, Frangos CC. Publication bias in meta-analysis: confidence intervals for Rosenthal's fail-safe number. Int Sch Res Notices 2014;2014:825383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Irshaid YM, Al Homrany M, Hamdi AA, et al. Compliance with good practice in prescription writing at outpatient clinic in Saudi Arabia. E Mediterr Health J 2005;11:922–8. [PubMed] [Google Scholar]
- [26].Dibbi HM, Al-Abrashy HF, Hussain WA, et al. Causes and outcome of medication errors in hospitalized patients. Saudi Med J 2006;27:1489–92. [PubMed] [Google Scholar]
- [27].Khoja T, Neyaz Y, Quresh N, et al. Medication errors in primary care in Riyadh city, Saudi Arabia. E Mediterr Health J 2011;17:156–9. [PubMed] [Google Scholar]
- [28].Al-Dhawailie AA. Inpatient prescribing errors and pharmacist intervention at a teaching hospital in Saudi Arabia. Saudi Pharm J 2011;19:193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].AbuYassin B, Aljadhey H, Al-Sultan M, et al. Accuracy of the medication history at admission to hospital in Saudi Arabia. Saudi Pharm J 2011;19:263–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Qureshi N, Neyaz Y, Khoja T, et al. Effectiveness of three interventions on primary care physicians medication prescribing in Riyadh city, Saudi Arabia. E Mediterr Health J 2011;17:172–9. [PubMed] [Google Scholar]
- [31].Alakhali K, Ansari S, Alavudeen S, et al. Medication errors at the outpatient pharmacy in Aseer Region Kingdom of Saudi Arabia. Eur J Clin Pharm 2014;16:144–6. [Google Scholar]
- [32].Alanazi M, Al-Jeraisy M, Salam M. Prevalence and predictors of antibiotic prescription errors in an emergency department, Central Saudi Arabia. Drug Healthc Patient Saf 2015;7:103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Aljadhey H, Mahmoud MA, Ahmed Y, et al. Incidence of adverse drug events in public and private hospitals in Riyadh, Saudi Arabia: the (ADESA) prospective cohort study. BMJ Open 2016;6:e010831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Abdallah MS, Sheikh MA, Alaqqad A, et al. PRN medications ordering practice at a large intensive care unit in Saudi Arabia. J Intensive Crit Care 2016;2:1–5. [Google Scholar]
- [35].Alomi YA, Alshabaar N, Lubad N, et al. Inpatient medication errors and pharmacist intervention at Ministry of Health Public Hospital, Riyadh, Saudi Arabia. PTB Rep 2019;5:44–8. [Google Scholar]
- [36].Albahouth Z, Almurshadi S, Almudaiheem H, et al. Prevalence and patterns of medication errors across the primary health care centers in the capital city of Saudi Arabia, Riyadh. Eur J Pharm Med Res 2018;5:363–9. [Google Scholar]
- [37].Abdulghani KH, Aseeri MA, Mahmoud A, et al. The impact of pharmacist-led medication reconciliation during admission at tertiary care hospital. Int J Clin Pharm Net 2018;40:196–201. [DOI] [PubMed] [Google Scholar]
- [38].Mazhar F, Haider N, Al-Osaimi YA, et al. Prevention of medication errors at hospital admission: a single-centre experience in elderly admitted to internal medicine. Int J Clin Pharm Net 2018;40:1601–13. [DOI] [PubMed] [Google Scholar]
- [39].Albaraki SM, Aldakheel SI, Alnasser AA, et al. Healthcare practitioners malpractices and medication errors of narcotics dispensing and handling in multiregional hospitals in Saudi Arabia. J Pharm Sci 2018;3: [Google Scholar]
- [40].Taghizadeh A, Moosazadeh M, Nesami MB, et al. Determination of the prevalence of medication errors in Iranian Hospital: a systematic review and meta-analysis. Acta Med Mediterr 2016;32:1525–33. [Google Scholar]
- [41].Koumpagioti D, Varounis C, Kletsiou E, et al. Evaluation of the medication process in pediatric patients: a meta-analysis. J Pediatr 2014;90:344–55. [DOI] [PubMed] [Google Scholar]
- [42].Dean B, Schachter M, Vincent C, et al. Prescribing errors in hospital inpatients: their incidence and clinical significance. Qual Saf Health Care 2002;11:340–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Saghafi F, Zargarzadeh AH. Medication error detection in two major teaching hospitals: what are the types of errors? J Res Med Sci: official journal of Isfahan University of Medical Sciences 2014;19:617. [PMC free article] [PubMed] [Google Scholar]
- [44].Leape LL, Bates DW, Cullen DJ, et al. Systems analysis of adverse drug events. ADE Prevention Study Group. JAMA 1995;274:35–43. [PubMed] [Google Scholar]
- [45].Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA 1995;274:29–34. [PubMed] [Google Scholar]
- [46].Aljadhey H, Mahmoud MA, Hassali MA, et al. Challenges to and the future of medication safety in Saudi Arabia: a qualitative study. Saudi Pharm J 2014;22:326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Franklin BD, Reynolds M, Shebl NA, et al. Prescribing errors in hospital inpatients: a three-centre study of their prevalence, types and causes. Postgrad Med J 2011;87:739–45. [DOI] [PubMed] [Google Scholar]
- [48].Dean B, Schachter M, Vincent C, et al. Causes of prescribing errors in hospital inpatients: a prospective study. Lancet 2002;359:1373–8. [DOI] [PubMed] [Google Scholar]
- [49].Tully MP, Ashcroft DM, Dornan T, et al. The causes of and factors associated with prescribing errors in hospital inpatients. Drug Safety 2009;32:819–36. [DOI] [PubMed] [Google Scholar]
- [50].Berdot S, Gillaizeau F, Caruba T, et al. Drug administration errors in hospital inpatients: a systematic review. PLoS One 2013;8:e68856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lan Y, Wang K, Yu S, et al. Medication errors in pediatric nursing: assessment of nurses’ knowledge and analysis of the consequences of errors. Nurs Educ Today 2013;34:821–8. [DOI] [PubMed] [Google Scholar]
- [52].Al-Otaibi H, Moawed SA, Al-Harbi MF. Nurses’ medication errors in the Pediatric Emergency Departments in Saudi Arabia. Middle East J Nurs 2018;12:3–13. [Google Scholar]
- [53].Alsultan MS, Khurshid F, Mayet AY, et al. Hospital pharmacy practice in Saudi Arabia: dispensing and administration in the Riyadh region. Saudi Pharm J 2012;20:307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].James KL, Barlow D, McArtney R, et al. Incidence, type and causes of dispensing errors: a review of the literature. Int J Pharm Pract 2009;17:9–30. [PubMed] [Google Scholar]
- [55].Anacleto TA, Perini E, Rosa MB, et al. Medication errors and drug dispensing systems in a hospital pharmacy. Clinics 2005;60:325–32. [DOI] [PubMed] [Google Scholar]
- [56].Cohen MR. Medication Errors. 2nd ed.2007;Washington: American Pharmacists Association, 680. [Google Scholar]
- [57].Valizadeh F, Ghasemi S, Najaf SS, et al. Errors in medication orders and the nursing staff 's reports in medical notes of children. Iran J Pediatr 2008;18:33–40. [Google Scholar]
- [58].Von Hippel PT. The heterogeneity statistic I2 can be biased in small meta-analyses. BMC Med Res Methodol 2015;15:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


