Abstract
Surgical site infection (SSI) is a costly postoperative complication with a decrease in the quality of life. We aimed to probe the predictive role of peripheral blood inflammation markers for SSI following mesh repair of groin hernia (GH).
This retrospective study assessed the data of 1177 patients undergoing elective mesh repair of GH (open/laparoscopy) in the absence of antibiotic prophylaxis. The relation between demographics, surgical factors, pre-surgical laboratory results and the occurrence of SSI were investigated by univariate and multivariate analyses. Receiver operating characteristic analysis was performed to determine the optimal threshold of parameters and compare their veracity.
The overall SSI rate was 3.2% with 1-year follow-up (38 superficial and 1 deep SSI). Patients with SSI had significant higher pre-surgical neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) than those without (P = .029 and P = .045, respectively); their NLR and PLR correlated positively with postoperative total days of antibiotic treatment for SSI (r = .689, P = .000; r = .493, P = .001; respectively). NLR and PLR had larger areas under the receiver operating characteristics curves than neutrophil (.875 vs. .601; P = .000; .726 vs. .601; P = .017). The combination of PLR and neutrophil/NLR raised the predictive sensitivity of PLR for SSI (sensitivity: PLR: 74.36%; PLR + neutrophil: 82.05%; PLR + NLR: 83.57%). On multivariate analyses, higher preoperative NLR (cut-off 2.44) and PLR (cut-off 125.42) were independent predictors for SSI.
Higher pre-surgical NLR and PLR may be valuable predictors for SSI following elective mesh repair of GH.
Keywords: inflammation, mesh repair of groin hernia, neutrophil-to-lymphoc-yte ratio, platelet-to-lymphocyte ratio, predictor, surgical site infection
1. Introduction
The use of prosthetic meshes in groin hernia (GH) repair has become the rule nowadays. However, the implantation of mesh may increase the risk for surgical site infections (SSIs). Although laparoscopic technique may help reduce the incidence of SSI, the reported overall SSI rate following mesh repair of GH (open and laparoscopy) was 2.59% in the latest Cochrane meta-analysis published in 2018,[1] which was higher than the traditional SSI rate of a clean operation (< 2%). SSI often results in an increase in hospitalization time, associated increase in expenses, and a decrease in quality of life. Mesh infections are the most serious cases, which usually call for excision of the mesh.[2] Some assessment tools are needed to predict SSI preoperatively in the hope of alleviating the occurrence and severity of SSI.
The reported risk factors for SSI including obesity, diabetes, age > 65 years, steroid use, operative factors, nonsterilized instruments, days of drainage, and concurrent ipsilateral hydrocele repair, range widely and remain controversial. Inflammation was considered as one of the important phases in an acute wound heal.[3] On the other hand, patients’ enhanced presurgical activation of pro-inflammatory cascades may increase host susceptibility to infection.[4] However, to our knowledge, there has been no report about inflammatory factors’ predictive effect on the development of SSI following mesh repair for GH.
White blood cell count and neutrophil counts are traditional inflammation markers with the question of their sensitive and specific. Recently, there is increasing evidence indicating that some other peripheral blood markers including: monocyte, eosnophils, basophil granulocyte, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio are effective laboratory markers of systemic inflammation.[5,6] Studies have demonstrated their predictive role not only on the progress and prognosis of many internal diseases, but also infectious complications following various surgical procedures such as cesarean section,[7] rectal cancer surgery,[8] curative gastrectomy for gastric cancer[9] and hepatectomy.[10] Recent studies also generalize the application of NLR to the field of hernia surgery and it was be found to be significantly associated with persistent postsurgical pain bowel,[11] the presence of a strangulated inguinal hernia,[12] and bowel resection in incarcerated groin hernia patients.[13]
On account of the relationship between patients’ preoperative systemic inflammatory status and infection, as well as the potential predictive role of routine laboratory test for inflammatory activity, we reviewed SSI occurring after elective mesh repair for GH (open and laparoscopy) in our institution, aiming to explore whether there is any correlation between readily available blood factors and SSI and therefore help prevent SSI.
2. Methods
2.1. Patients and study design
All the elective open and laparoscopic mesh repair for GH performed in the absence of antibiotic prophylaxis between January 2010 and September 2018 in our hospital were identified from the electronic medical records. The repairs were performed by at least one chief surgeon. Regularly the skin was shaved with a razor the day before surgery and prepared using iodophor. Lichtenstein repair was applied for open approach while trans-abdominal preperitoneal repair and totally extraperitoneal repair were used for laparoscopic approach. Both operative procedures were performed under the standard techniques as published previously. Patients were routinely required to return for a postoperative clinical examination 30 days after discharge. Details of hospitalization, subsequent visit and any additional hospital visits within 1 year after surgery were obtained through electronic patient records and available paper document.
Notably, patients undergoing open mesh repair procedure from 2010 to 2015 were excluded, as these data had been analysed in our former report.[14] Patients with antibiotic prophylaxis, those with inadequate information and those undergoing another concomitant surgery were also excluded. The institutional review board of the First Affiliated Hospital of Shantou University Medical College approved this study (No. 2017145). The informed consent was waived because of the anonymous retrospective collection of patients’ information.
2.2. Data collection
Electronic medical records were reviewed for SSI at 1 month and 1 year after elective mesh repair for GH. This review was performed by querying the word SSI in an individual's chart. If no SSI was documented in the physicians note, then SSI was not noted.
Moreover, variables were collected as follows with the aim to explore the risk factors for SSI: demographic factors, accompanied diseases, chronic drug therapy that may have an effect on the development of SSI, American Society of Anesthesiologists score, hernia type, hernia characteristics, prior history of GH repairs, lengths of preoperative hospital stay, surgical technique, operative time, intraoperative blood loss, surgical drains and pre-operative blood examination (within 24 hours prior to the surgical operations routinely). Serum NLR and PLR were calculated as the absolute neutrophil or platelet count divided by the absolute lymphocyte, respectively. lymphocyte-to-monocyte ratio was calculated as the absolute lymphocyte count divided by the absolute monocyte count. We did not consider about type of anesthesia as an additional risk factor for SSI.
SSI was diagnosed by the surgeons adapted from guidelines by the U.S. Centers for Disease Control and Prevention.[15] Superficial SSI was defined as an infection that arose within 30 days postoperatively and involved only skin or subcutaneous tissue. Deep SSI was defined as an infection arose within 1 year postoperatively and involved the fascial and muscle layers.
2.3. Statistical analyses
Statistical analyses were performed using SPSS 23.0 (IBM Corp., Armonk, NY). Continuous data are presented as median with inter-quartile range and analysed by the Mann-Whitney U test. Categorical variables are presented in counts and percentages, and compared by Fisher's exact test. The Pearson correlation coefficient was evaluated to assess correlations between variables. Variables with a P value < .05 in the univariate analysis were then entered into the multivariate analysis, which was performed by a forward stepwise logistic regression model. In the forward selection process, each model was built using pairwise deletion, without the examination of interaction terms.[16] We evaluate the goodness of fit of the model by the Hosmer-Lemeshow test. Receiver operating characteristics (ROC) curves were used to determine a cut-offs for continuous variables.
3. Results
A total of 1177 patients without prophylactic antibiotic were finally enrolled in the analysis (647 open and 530 laparoscopy). We excluded 258 patients: 222 had been analysed in our former report, 16 with missing data, and 20 were transferred for further treatment postoperatively resulting in lost follow-up. There were 39 SSIs (38 superficial SSIs and 1 deep SSI) developing among the 1177 patients within 1 year after elective mesh repair for GH, revealing a 3.2% (39/1342) overall SSI rate in the absence of antibiotic prophylaxis. SSIs were observed within a mean interval of 4.6 (4.0) days postoperatively (range, 1–18 days) during the original admission, with most (82.1%, 32/39) found within the first post-operative week. The causative agent was isolated in the secretions from surgical site in 21 infected patients. The most common microorganism isolated was staphylococcus aureus. All these patients were resolved by intravenous antibiotic treatment and/or wound drainage (12.8%, 5/39), without removal of the mesh.
Table 1 presented demography and clinical features on the basis of SSI status and results of univariate analysis. Body mass index, current smoker, diabetes mellitus, repair of recurrence, neutrophil (NEU), lymphocyte (LYM), platelet (PLT), serum albumin, NLR, and PLR were found to be associated with SSI by univariate analysis.
Table 1.
Univariate analysis of risk factors predicting surgical site infection following mesh repair of groin hernia.
| Variables | All | SSI | No SSI | OR | 95%CI | P-value |
| Total | 1177 | 39 (3.2)∗ | 1138 (96.8)∗ | |||
| Demographic factors | ||||||
| Age (yr) | 59 (42–70) | 61 (41–70) | 63 (51–71) | 0.988 | 0.969–1.006 | .192 |
| Gender (male) | 1124 (95.5) | 39 (100) | 1085 (92.2) | 1.856 | 0.250–13.779 | .545 |
| BMI (kg/m2) | 22.8 (20.6–24.6) | 23.8 (21.3–25.3) | 22.7 (20.5–24.5) | 1.114 | 1.008–1.231 | .034 |
| Current smoker, yes | 139 (11.8) | 10 (25.6) | 129 (11.3) | 2.697 | 1.285–5.663 | .009 |
| Comorbidity | ||||||
| Diabetes mellitus, yes | 57 (4.8) | 5 (12.8) | 52 (4.6) | 3.071 | 1.154–8.176 | .025 |
| Hypertension, yes | 199 (16.9) | 7 (17.9) | 192 (16.9) | 1.078 | 0.469–2.478 | .860 |
| Pulmonary disease, yes | 56 (4.8) | 2 (5.1) | 54 (4.7) | 1.085 | 0.255–4.621 | .912 |
| Malignancy, yes | 44 (3.7) | 3 (7.7) | 41 (3.6) | 2.230 | 0.659–7.540 | .197 |
| Chronic drug treatment | ||||||
| Steroid, yes | 55 (4.7) | 2 (5.1) | 53 (4.7) | 1.107 | 0.260–4.714 | .891 |
| Statin, yes | 53 (4.5) | 1 (2.6) | 52 (4.6) | 0.550 | 0.074–4.081 | .558 |
| Procedure factors | ||||||
| ASA score (III–IV), yes | 65 (5.5) | 3 (7.7) | 62 (5.5) | 1.446 | 0.433–4.827 | .549 |
| Type of hernia | 0.610 | 0.263–1.419 | .251 | |||
| Direct | 173 (14.7) | 5 (12.8) | 168 (14.8) | |||
| Indirect | 962 (81.7) | 34 (87.2) | 928 (81.5) | |||
| Combined | 42 (3.6) | 0 | 42 (3.7) | |||
| Hernia characteristics | 0.844 | 0.519–1.372 | .493 | |||
| Left | 412 (35.0) | 15 (38.5) | 397 (34.9) | |||
| Right | 600 (51.0) | 20 (51.3) | 580 (51.0) | |||
| Bilateral | 165 (14.0) | 4 (10.3) | 161 (14.1) | |||
| Duration of inguinal hernia presentation (years) | 1.0 (0.2–3.1) | 2.0 (0.5–3.6) | 1.0 (0.2–3.1) | 1.006 | 0.998–1.014 | .139 |
| Repair of recurrence, yes | 29 (2.5) | 3 (7.7) | 26 (2.3) | 0.281 | 0.081–0.970 | .045 |
| Preoperative stay (days) | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | 0.984 | 0.820–1.182 | .865 |
| Pre-operative blood results† | ||||||
| WBC (×109/L) | 6.8 (5.7–8.1) | 6.8 (5.9–8.5) | 6.8 (5.7–8.1) | 1.014 | 0.953–1.078 | .667 |
| NEU (×109/L) | 3.9 (3.1–2.0) | 6.6 (6.0–7.8) | 3.8 (3.1–4.8) | 1.505 | 1.350–1.678 | .000 |
| LYM (×109/L) | 2.0 (1.6–2.5) | 1.7 (1.2–2.2) | 2.0 (1.6–2.5) | 0.477 | 0.295–0.771 | .003 |
| MON (×109/L) | 0.5 (0.4–0.6) | 0.6 (0.5–0.7) | 0.5 (0.4–0.6) | 1.305 | 0.554–3.072 | .542 |
| EOS (×109/L) | 0.2 (0.1–0.3) | 0.1 (0.1–0.3) | 0.2 (0.1–0.3) | 1.887 | 0.761–4.679 | .170 |
| BASO (×109/L) | 0.03 (0.02–0.04) | 0.03 (0.02–0.04) | 0.03 (0.02–0.04) | 3.472 | 0.108–111.265 | .482 |
| HGB (g/L) | 141.0 (130.0–150.0) | 138.0 (127.0–144.0) | 141.0 (130.0–150.0) | 0.988 | 0.972–1.003 | .988 |
| PLT (×109/L) | 217 (184–253) | 249 (212–299) | 215 (183–253) | 1.008 | 1.004–1.013 | .000 |
| ALB (g/L) | 40.5 (37.6–42.8) | 38.8 (36.4–42.5) | 40.6 (37.7–42.9) | 0.924 | 0.866–0.986 | .018 |
| NLR | 2.0 (1.4–2.7) | 4.0 (3.0–6.7) | 1.9 (1.4–2.6) | 1.037 | 1.004–1.071 | .029 |
| PLR | 107.0 (85.2–143.5) | 142.6 (115.8–212.8) | 105.9 (83.5–142.1) | 1.002 | 1.000–1.003 | .045 |
| LMR | 3.8 (2.9–5.0) | 3.2 (1.9–4.2) | 3.9 (3.0–5.0) | 1.011 | 0.998–1.024 | .108 |
| Intraoperative factors | ||||||
| Surgical technique | 5.824 | 2.261–14.998 | .001 | |||
| Laparoscopic | 530 (45.0) | 5 (12.8) | 525 (46.1) | |||
| Open | 647 (55.0) | 34 (87.2) | 613 (53.9) | |||
| Duration of surgery (min) | 73 (53–93) | 76 (61–96) | 72 (53–93) | 1.006 | 0.998–1.014 | .139 |
| Intraoperative blood loss (ml) | 5 (5–10) | 5 (5–10) | 5 (5–10) | 1.033 | 0.991–1.077 | .123 |
| Use of postsurgical drains, yes | 278 (23.6) | 7 (17.9) | 271 (23.8) | 0.700 | 0.305–1.604 | .399 |
Among patients with SSI, pre-surgical NLR levels were significantly negatively related with postoperative period of SSI (r = -.473, P = .002), while it was positively correlated with the total days of antibiotic treatment for SSI (r = .689, P = .000). Among patients with SSI, pre-surgical PLR levels were significantly positively correlated with the total days of antibiotic treatment for SSI (r = .493, P = .001), whereas there was no significant correlation between PLR and postoperative period of SSI (r = -.251, P = .123).
ROC curve analysis showed that preoperative NEU, NLR and PLR levels could remarkly predict SSI (Fig. 1A). The optimal cut-offs of NLR and PLR to predict SSI were identified to be 2.44 and 125.42, respectively [NLR: area under the ROC curve (AUC) = .875, sensitivity: 97.44%, specificity: 70.3%; PLR: AUC = .723, sensitivity: 74.36%, specificity: 64.24%]. Pairwise comparison of ROC curves (Table 2) showed that both the NLR and PLR have better predictive ability than that of NEU (P = .000 and P = .0165, respectively). Fig. 1B presented the predictive veracity of different hematological indices combinations: the combination of NLR and PLR got the best AUC of .608.
Figure 1.

ROC curves of presurgical neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphoc-yte ratio (PLR) for predicting surgical site infection (SSI) (A); ROC curves of the combinations of NLR, PLR and neutrophil (NEU) for predicting SSI (B).
Table 2.
Pairwise comparison of receiver operating characteristics curves.
| Difference between areas | 95%CI | P-value | |
| NLR vs. NEU | 0.274 | 0.203–0.344 | .000 |
| PLR vs. NEU | 0.121 | 0.0222–0.221 | .0165 |
| NLR vs. PLR | 0.152 | 0.0927–0.212 | .000 |
In the multivariable analysis, we excluded 3 variables: neutrophil, lymphocyte, and platelet, with the purpose of avoiding multi collinearity. Three variables including current smoker, diabetes mellitus, and surgical technique, failed to be involved in the model equation by the forward stepwise procedure, thus, they were excluded in final results of the multivariate analysis. The ultimate statistical results of the forward stepwise process (Fig. 2) identified as the independent risk factors for SSI: NLR > 2.44 [odds ratio [OR], 2.203; 95% confidence interval, 1.711 - 2.836], and PLR > 125.42 (OR, 1.162; 95% confidence interval, 1.018 - 1.326). The analysis displayed a well Hosmer-Lemeshow test value indicative of goodness of fit (χ2 = 9.096, P = .334).
Figure 2.

Forward stepwise multivariable logistic regression analysis of risk factors association with SSI following mesh repair of GH. Three variables (with a P < 0.05 in the univariate analysis) including current smoker, diabetes mellitus, and surgical technique failed to be in the model equation. ALB = serum albumin, BMI = body mass index, NLR = neutrophil-to-lymphocyte ratio, PLR = platelet-to-lymphocyte ratio.
A subgroup analysis investigating the predictive ability of preoperative NLR and PLR levels on SSI in patients with certain demographics and clinical factors was then performed to further confirm the association between SSI and NLR, PLR levels in these groups. NLR could significantly distinguish the SSI and non-SSI patients in all subgroups (Fig. 3A), whereas PLR was an effective predictor for SSI in most subgroups, with the exception of age < 65 years, not current smoker and not diabetes mellitus (Fig. 3B).
Figure 3.
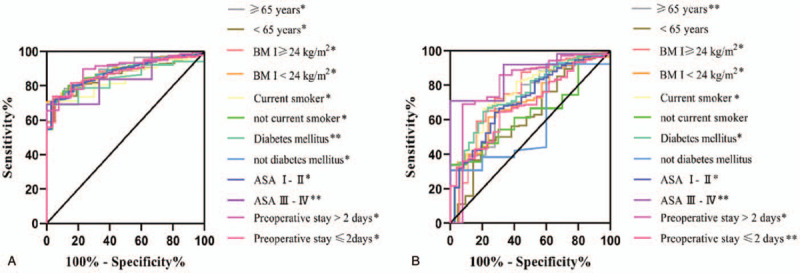
ROC curves of presurgical neutrophil-to-lymphocyte ratio (NLR) (A) and platelet-to-lymp-hocyte ratio (PLR) (B) for predicting surgical site infection (SSI) in subgroups. ASA = American Society of Anesthesiologists. BMI = body mass index. ∗∗P < .05, ∗P < .000.
4. Discussion
Even under scrupulous aseptic conditions and with a skillful technique, SSI is still regarded as a common but noticeable problem. Antibiotic prophylaxis may be a valid intervention to prevent SSI. Accurate and comprehensive pre-surgical risk assessment can help to select the best candidates for antibiotic prophylaxis. The result of our study showed that preoperative NLR and PLR were valuable predictors of SSI following mesh repair for GH. Their predictive ability exceeded that of preoperative NEU. To the best of our knowledge, this is the first study to reveal that preoperative NLR and PLR can help in identifying patients more prone to develop SSI following mesh repair for GH.
Despite the accepted key roles of NEU in host defence, observations from human studies revealed that NEUs might become inappropriately activated, and cause tissue damage in inflammatory diseases via their inap-propriate activation to release reactive oxygen species (ROS) and other tissue-damaging molecules.[17] Additionally, lymphocytopaenia may result from cytokines, and the instructions from neutrophilia. Therefore, we can assume that higher NLR level identifies patients with enhanced presurgical systemic inflammation, and this activation of pro-inflammatory cascades have been proved to destroy surgical site healing.[3] On the other hand, synergy between high circulating NEU and low LYM may be the explanation for the NLR's better predictive ability of SSI than that of NEU. The cause for higher presurgical NLR level observed in patients with SSI may be the higher incidence of current smoker and diabetes mellitus found in these patients; yet both current smoker and diabetes mellitus were not independent risk factors for SSI in this study. Cigarette smoke can activate inflammation via the activation of some transcription factors such as nuclear factor-κB (NF-κB) and interleukin-6 (IL-6) even in normal conditions.[18] It has been reported that NLR increases in correlation with pack/years of cigarettes.[19] Inflammatory disorders play an important role in the development and progress of diabetes mellitus and lead to tissue damage including nerve and artery.[20] Thus, these patients’ intensive pre-surgical systemic inflammatory status resulted in a higher pre-surgical NLR level.
Our findings revealed that using a cut point of > 2.44, preoperative NLR level predicted postoperative SSI with a high sensitivity (97.44%) and specificity (70.3%). The negative correlation between NLR and the postoperative period of SSI, further confirming higher NLR as a valuable predictor for SSI. The cut-off value here was within the recognized normal reference intervals for NLR (0.88–4.0).[21] NLR has displayed important predictive value for postoperative complications after many surgery procedures,[7–10] whereas there were only a fraction of studies focusing on SSI specifically. Preoperative NLR's cut-off value of this study was lower than those reported in literature (NLR > 3.85 - 6.2).[21–26] GH is mainly a type of focal disease and the mean degrees of basal systematic inflammation of our patients may be lower than that of cancer patients or patients with spinal disease.[11] However, a lower threshold may help to find out the surgical patients with intermediate risk of postsurgical SSI, who could not be usually identified early. It has also been reported[22] that a lower level of pre-surgical NLR was significant related to SSI, because local insufficient aggregation of NEU may delay wound healing after head and neck reconstruction. The opposite conclusion of the two studies may due to the different types of surgical interventions and different disease states. In view of the possibility that mesh implantation may initiate an acute inflammatory cellular response postoperatively,[27] a prospective study with the analysis of postoperative NLR's levels was required to ascertain the exact cut-off value of NLR.
Platelets (PLT) contain a mass of proinflammatory agents are possible to release highly active microparticles. The activated PLT are able to increase IL-6 production. PLR has been accepted as indicator revealing shifts in PLT and LYM counts due to acute inflammatory. Increasing evidence has suggested that PLR can afford valuable information to clinicians when coming across some infection related diseases, such as chronic obstructive pulmonary disease,[5] rheumatic diseases[28] and diabetic foot infection.[20] Our study discovered that pre-surgical PLR level was more closely associated with SSI than with NEU, maybe due to the interaction between circulating platelets and lymphocytes in the vessel lumen at sites of vascular damage.[28] However, pre-surgical PLR level was not significantly related to the postoperative period of SSI and its AUC was significantly lower than that of NLR. The combination of PLR and NEU, NLR raised the predictive sensitivity for SSI, thus, we recommended that it was better to combine the results of PLR and NLR/NEU to offer a better predictive effect of SSI.
Similar to NLR, higher incidences of current smoker and diabetes mellitus were found in the patients with SSI than those without. Tobacco smoke has been proved to be associated with heightened PLT activation and inflammation.[29] Abnormal platelet function lead to a higher morbidity and mortality diabetes mellitus.[30] Therefore, these patients’ enhanced pre-surgical systemic inflammatory status may be reflected by higher pre-surgical PLR levels. Unlike NLR, there have been very few studies focusing on the PLR's predictive role of SSI after various types of surgeries.[22] This study found that, at a cut-off value of 125.42, the sensitivity and specificity of the pre-surgical PLR in predicting SSI were 74.36% and 64.24%, respectively, with an AUC of 0.723. As we can not obtain reference range of PLR from the literature, we are not able to validate this result. This cut-off value may demand further investigation.
Furthermore, we found that there were positive and statistically significant correlations between pre-surgical NLR level, as well as PLR level, and the total days of the antibiotic treatment for SSI. This result indicated that higher pre-surgical NLR and PLR level predicted longer time of antibiotic treatment when encountering SSI, hence assisting in SSIs’ efficacious treatment. To date, only one retrospective study[31] has reported significant correlations between pre-surgical NLR level and antibiotic doses after dental treatment. As 6 of the patients with SSI received combination antibiotic therapy and the rest of these patients received monotherapy, it was difficult to calculate each patient's total antibiotic doses.
Subgroup analysis of the predictive ability of preoperative NLR and PLR levels on SSI showed a useful predictive value of NLR in every subgroup of patients; as well as the effective predictive function of PLR in most subgroups, with the exception of age < 65 years, not current smoker and not diabetes mellitus. While our subgroup analysis highlighted the predictive value of pre-surgical NLR and PLR level for SSI in several patient populations, the small number of patients in each subgroup restrict the possibility of these results to apply to clinical practice and should be viewed with caution.
The debate remains open on the effectiveness of antibiotic prophylaxis for high-risk patients who undergoing mesh repair for GH because of the limited data on this issue. One data analysis of 1254 patients from 3 prospective, randomized, multicenter studies demonstrated that antibiotic prophylaxis significantly reduced the rates of SSI after inguinal hernia repair in high-risk patients by 72%.[32] Our findings may assist in recognizing the partial patients for antibiotic prophylaxis who did call for it.
The primary limitations of our study were the retrospective property, small sample size, and the single racial group. Although we focused on mesh repairs, the retrospective property resulted in only one mesh infection identified in our study. What's more, we lacked data of several potential risk factors related to SSI due to our observational study design, such as the dosage of smoking, intraoperative body temperature and perioperative blood glucose. Attention had to be particularly paid to postoperative NLR and PLR levels due to the possibility that mesh implantation may induce an acute inflammatory cellular response as mentioned above. On the other hand, preoperative hair removal was actually carried out the day prior to surgery in our hospital because of our traditional care policy. The Centers for Disease Control and Prevention guidelines recommended shaving immediately before operation,[33] as bacteria may not have as long to propagate in nicks and damaged surfaces during the pre-surgical period. The timing of hair removal here might increase the risk of SSI. Additionally, we could not entirely rule out the impact of surgical technique (open and laparoscopy) on SSI. Laparoscopic approach seemed to be preferable in terms of risk for both superficial SSI and mesh infection,[34] mainly because of the short incision and the way for mesh introduced to the preperitoneal space different with that of open approach. Our results showed a significantly lower SSI rate of laparoscopic approach compared with open surgery in the univariate analysis, but surgical technique was not found to be an independent factor by forward stepwise multivariable logistic regression analysis, possibly due to the limited sample size. Prospective studies with larger patient series are required to confirm the predictive effect of pre-surgical NLR and PLR.
5. Conclusions
In conclusion, our analysis suggested that presurgical higher NLR and PLR levels were significantly associated with the occurrence of SSI in patients without preoperative antibiotic prophylaxis after elective mesh repair for GH. The role of preoperative NLR and PLR is a novel finding and warrants further exploration - they appeared to be objective, cheap and convenience biomarkers for the early prediction of SSI following elective mesh repair for GH.
Author contributions
Conceptualization: Yeye Zhuo, De Cai, Xinxin Li.
Data curation: Yeye Zhuo, De Cai.
Investigation: Yeye Zhuo.
Project administration: Juntian Chen.
Validation: Qian Zhang.
Writing – original draft: Yeye Zhuo, De Cai, Xinxin Li.
Writing – review & editing: Yeye Zhuo, De Cai, Juntian Chen, Qian Zhang, Xinxin Li.
Footnotes
Abbreviations: AUCs = area under the ROC curves, GH = groin hernia, LYM = lymphocyte, NEU = neutrophil, NLR = neutrophil-to-lymphocyte ratio, PLR = platelet-to-lymphocyte ratio, PLT = platelet, ROC = receiver operating characteristics, SSI = surgical site infection.
How to cite this article: Zhuo Y, Cai D, Chen J, Zhang Q, Li X. Pre-surgical peripheral blood inflammation markers predict surgical site infection following mesh repair of groin hernia. Medicine. 2021;100:9(e25007).
YZ and DC contributed equally to this work.
This work was supported by the Medical Research Fund of Guangdong Province in China (grant number A2018447) and Science and technology plan medical and health category project of Shantou (grant number 180404094011014. The fundings were mainly used for the data collection in this study, and the study designation, analysis, interpretation of data and writing were also almost carried out without the supporting of the funding.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Continuous data are presented as median (inter-quartile range).
Categorical data are presented as the number (percentage).
There were 39 infected cases among 1342 hernias which were surgically treated (1012 unilateral hernia and 165 bilateral hernia repairs).
ALB = serum albumin, ASA = American Society of Anesthesiologists, BASO = basophil granulocyte, BMI = body mass index, EOS = eosnophils, GH = groin hernia, HGB = hemoglobin, LMR = lymphocyte-to-monocyte ratio, LYM = lymphocyte, MON = monocyte, NEU = neutrophil, NLR = neutrophil-to-lymphocyte ratio, PLR = platelet-to-lymphocyte ratio, PLT = platelet, SSI = surgical site infection, WBC = white blood cell.
Reference ranges: WBC: 3.5–9.5 × 109/L; NEU: 1.8–6.3 × 109/L; LYM: 1.1–3.2 × 109/L; MON: 0.1–0.6 × 109/L; EOS: 0.02–0.52 × 109/L; BASO: 0.0–0.06 × 109/L; HGB: 115–150 g/L; PLT: 125–350 × 109/L; ALB: 40–55.0 g/L.
NEU = neutrophil, NLR = neutrophil-to-lymphocyte ratio, PLR = platelet-to-lymphocyte ratio.
References
- [1].Lockhart K, Dunn D, Teo S, et al. Mesh versus non-mesh for inguinal and femoral hernia repair. Cochrane Database Syst Rev 2018;9:D11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Orelio CC, van Hessen C, Sanchez-Manuel FJ, et al. Antibiotic prophylaxis for prevention of postoperative wound infection in adults undergoing open elective inguinal or femoral hernia repair. Cochrane Database Syst Rev 2020;21;4:CD003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Oishi Y, Manabe I. Macrophages in inflammation, repair and regeneration. Int Immunol 2018;11:511–28. [DOI] [PubMed] [Google Scholar]
- [4].Liew PX, Kubes P. The Neutrophil's Role During Health and Disease. Physiol Rev 2019;99:1223–48. [DOI] [PubMed] [Google Scholar]
- [5].CaoYuan Yao, XiaoLi Liu, Ze Tang. Prognostic Role of Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio for Hospital Mortality in Patients With AECOPD. Int J Chron Obstruct Pulmon Dis 2017;12:2285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tunyaporn Kamonvarapitak, Akihisa Matsuda, Satoshi Matsumoto, et al. Preoperative Lymphocyte-To-Monocyte Ratio Predicts Postoperative Infectious Complications After Laparosc-opic Colorectal Cancer Surgery. Int J Clin Oncol 2020;25:633–40. [DOI] [PubMed] [Google Scholar]
- [7].Reut Rotem, Miriam Erenberg, Misgav Rottenstreich, et al. Early prediction of post cesarean section infection using simple hematological biomarkers: a case control study. Eur J Obstet Gynecol Reprod Biol 2020;245:8–8. [DOI] [PubMed] [Google Scholar]
- [8].Jones HG, Qasem E, Dilaver N, et al. Inflammatory cell ratios predict major septic complications following rectal cancer surgery. Int J Colorectal Dis 2018;33:857–62. [DOI] [PubMed] [Google Scholar]
- [9].Mohri Y, Tanaka K, Toiyama Y, et al. Impact of Preoperative Neutrophil to Lymphocyte Ratio and Postoperative Infectious Complications on Survival After Curative Gastrectomy for Gastric Cancer: A Single Institutional Cohort Study. Medicine (Baltimore) 2016;95:e3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dan-Yun Ruan, Ze-Xiao Lin, Yang Li, et al. Poor oncologic outcomes of hepatocellular carcinoma patients with intra-abdominal infection after hepatectomy. World J Gastroenterol 2015;14, 21:5598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dario Bugada, Patricia Lavand’homme, Andrea Luigi Ambrosoli, et al. Effect of Preoperative Inflammatory Status and Comorbidities on Pain Resolution and Persistent Postsurgical Pain After Inguinal Hernia Repair. Mediators Inflamm 2016;5830347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhou H, Ruan X, Shao X, et al. Clinical value of the neutrophil/lymphocyte ratio in diagnosing adult strangulated inguinal hernia. Int J Sur 2016;36(Pt A):76–80. [DOI] [PubMed] [Google Scholar]
- [13].Xingming Xie, Shu Feng, Zhongling Tang, et al. Neutrophil-to-Lymphocyte Ratio Predicts the Severity of Incarcerated Groin Hernia. Med Sci Monit 2017;23:5558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhuo Y, Zhang Q, Tang D, et al. The effectiveness of i.v. cefuroxime prophylaxis of surgical site infection after elective inguinal hernia repair with mesh: A retrospective observational study. Eur J Clin Pharmacol 2016;9:1033–9. [DOI] [PubMed] [Google Scholar]
- [15].Mangram AJ, Horan TC, Pearson ML, et al. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. AM J INFECT CONTROL 1999;27:97–132. [PubMed] [Google Scholar]
- [16].de Mestre AM, Rose BV, Chang YM, et al. Multivariable analysis to determine risk factors associated with early pregnancy loss in thoroughbred broodmares. Theriogenology 2019;124:18–23. [DOI] [PubMed] [Google Scholar]
- [17].Nathalie Thieblemont, Helen L Wright, Steven W Edwards, et al. Human Neutrophils in Auto-Immunity. Semin Immunol 2016;28:159–73. [DOI] [PubMed] [Google Scholar]
- [18].Kunnumakkara AB, Shabnam B, Girisa S, et al. Inflammation, NF-(B, and Chronic Diseases: How are They Linked? Crit Rev Immunol 2020;40:1–39. [DOI] [PubMed] [Google Scholar]
- [19].Tulgar YK, Cakar S, Tulgar S, et al. The effect of smoking on neutrophil/lymphocyte and platelet/lymphocyte ratio and platelet indices: a retrospective study. Eur Rev Med Pharmacol Sci 2016;20:3112–8. [PubMed] [Google Scholar]
- [20].Tuna Demirdal, Pinar Sen. The significance of neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and lymphocyte-monocyte ratio in predicting peripheral arterial disease, peripheral neuropathy, osteomyelitis and amputation in diabetic foot infection. Diabetes Res Clin Pract 2018;44:118–25. [DOI] [PubMed] [Google Scholar]
- [21].Chao-Jun Shen, Tao Miao, Zhang-Fu Wang, et al. Predictive Value of Post-Operative Neutrophil/Lymphocyte Count Ratio for Surgical Site Infection in Patients Following Posterior Lumbar Spinal Surgery. Int Immunopharmacol 2019;74:105705. [DOI] [PubMed] [Google Scholar]
- [22].Yoko Maruyama, Keita Inoue, Keita Mori, et al. Neutrophil-lymphocyte Ratio and Platelet-Lymphocyte Ratio as Predictors of Wound Healing Failure in Head and Neck Reconstruction. Acta Otolaryngol 2016;137:106–10. [DOI] [PubMed] [Google Scholar]
- [23].Deniz Bolat, Yusuf Kadir Topcu, Ozgu Aydogdu, et al. Neutrophil to Lymphocyte Ratio as a Predictor of Early Penile Prosthesis Implant Infection. Int Urol Nephrol 2017;49:947–53. [DOI] [PubMed] [Google Scholar]
- [24].Ho-Jin Son, Jong-Lyel Roh, Seung-Ho Choi, et al. Nutritional and hematologic markers as predictors of risk of surgical site infection in patients with head and neck cancer undergoing major oncologic surgery. Head Neck 2018;40:596–604. [DOI] [PubMed] [Google Scholar]
- [25].Masano Sagawa, Kazuhiko Yoshimatsu, Hajime Yokomizo, et al. Worse Preoperative Status Based on Inflammation and Host Immunity Is a Risk Factor for Surgical Site Infections in Colorectal Cancer Surgery. J Nippon Med Sch 2017;84:224–30. [DOI] [PubMed] [Google Scholar]
- [26].Inose H, Kobayashi Y, Yuasa M, et al. Procalcitonin and neutrophil lymphocyte ratio after spinal instrumentation surgery. Spine (Phila Pa 1976) 2019;44:E1356–61. [DOI] [PubMed] [Google Scholar]
- [27].Heymann F, von Trotha KT, Preisinger C, et al. Polypropylene mesh implantation for hernia repair causes myeloid cell-driven persistent inflammation. JCI Insight 2019;4:e123862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gasparyan AY, Ayvazyan L, Mukanova U, et al. The Platelet-to-Lymphocyte Ratio as an Inflammatory Marker in Rheumatic Diseases. Ann Lab Med 2019;39:345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sarah Hom, Li Chen, Tony Wang, et al. Platelet Activation, Adhesion, Inflammation, and Aggregation Potential Are Altered in the Presence of Electronic Cigarette Extracts of Variable Nicotine Concentrations. Platelets 2016;27:694–702. [DOI] [PubMed] [Google Scholar]
- [30].Gabriele Giacomo Schiattarella, Albino Carrizzo, Federica Ilardi, et al. Rac1 Modulates Endothelial Function and Platelet Aggregation in Diabetes Mellitus. J Am Heart Assoc 2018;7:e007322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dogruel F, Gonen Z-B, Gunay-Canpolat D, et al. The Neutrophil-to-Lymphocyte Ratio as a Marker of Recovery Status in Patients With Severe Dental Infection. Med Oral Patol Oral Cir Bucal 2017;22:e440–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pessaux P, Lermite E, Blezel E, et al. Predictive risk score for infection after inguinal hernia repair. Am J Surg 2006;192:165–71. [DOI] [PubMed] [Google Scholar]
- [33].Mangram AJ, Horan TC, Pearson ML, et al. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 1999;27:97–132. [PubMed] [Google Scholar]
- [34].Kockerling F, Bittner R, Jacob D, et al. Do we need antibiotic prophylaxis in endoscopic inguinal hernia repair? Results of the Herniamed Registry. SURG ENDOSC 2015;29:3741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


