Abstract
Backgrounds:
Many studies have evaluated the effect of maternal fever on the development risk of congenital heart diseases (CHDs) in offspring, but the findings were inconsistent. Furthermore, a complete overview of the existing data was also missing. Therefore, we intend to provide updated epidemiologic evidence to estimate the association between maternal fever and the risk of overall CHDs and specific CHD phenotypes in offspring.
Methods:
Pubmed, Embase, and Web of Science were searched through March 2020 to identify eligible studies that assessed the association between maternal fever and CHDs risk in offspring. The summary risk estimates were calculated using random-effects models. Potential heterogeneity source was explored by subgroup analyses and potential publication bias was assessed by Begg funnel plots and Begg rank correlation test.
Results:
Sixteen studies involving 31,922 CHDs cases among 183,563 participants were included in this meta-analysis. Overall, mothers who had a fever experience during preconception and conception periods had a significantly higher risk of overall CHDs in offspring (odds ratio [OR] = 1.45, 95% confidence interval [CI]: 1.21–1.73) when compared with those who did not have a fever experience. For specific CHD phenotypes in offspring, a statistically significant association was found between maternal fever and risk of conotruncal defects (CTD) (OR = 1.38, 95%CI: 1.01–1.89), atrial septal defects (ASD) (OR = 1.48, 95% CI: 1.01–2.17), transposition of the great vessels (TGA) (OR = 1.81, 95% CI: 1.14–2.88), and right ventricular outflow tract obstruction (RVOTO) (OR = 1.66, 95% CI: 1.04–2.65). Relevant heterogeneity moderators have been identified by subgroup analyses, and sensitivity analyses yielded consistent results.
Conclusions:
Although the role of potential bias and evidence of heterogeneity should be carefully evaluated, our review indicates that maternal fever is significantly associated with the risk of CHDs in offspring, which highlights that preventing maternal fever during the preconception and conception periods play an important role in decreasing the risk of CHDs in offspring. However, given the limited number of current case-control studies, larger-sample prospective studies are required to further confirm our results. Besides, due to the underlying mechanisms between maternal fever and the risk of specific CHD phenotypes in offspring are still unreported, more research is needed to explore the possible mechanisms.
Keywords: congenital heart diseases, maternal fever, meta-analysis
1. Introduction
Congenital heart diseases (CHDs) are currently the most common birth defects in newborns, accounting for one-third of congenital anomalies.[1] Current epidemiological researches suggest that the worldwide prevalence of CHDs was 8.22 per 1000 live births, and approximately 1.35 million babies with CHDs were born each year globally, which have emerged as the largest cause of infant morbidity and mortality worldwide.[2,3] Although surgical interventional therapy has dramatically changed the natural history of CHDs,[4] some studies have found that the history of CHDs could increase the risk of cardiovascular disease[5] and neurodevelopmental disabilities[6,7] in later life. As of now, most studies have proved that the etiology of CHDs attributes to both the genetic and environmental factors,[8,9] with the details remaining unclear. Therefore, identification of risk factors for CHDs onset and subsequent efforts to prevent CHDs remain important priorities for research,[9] especially where it could be modified and avoided, which may be a key step in the implementation of primary prevention.
Over the past several decades, epidemiological researches on maternal exposures during pregnancy have provided some thread for the identification of potential risk factors for CHDs.[10–12] As maternal fever is not uncommon during pregnancy,[13] it is estimated that approximately 20.0 per 100 women reported experiencing fever on at least one occasion during pregnancy.[14] With the global attention of birth defect, fever is becoming increasingly recognized as an important public health problem, which has been confirmed as a likely teratogen in a variety of animal and human.[13,15] Meanwhile, previous studies have also reported that hyperthermia could interfere with protein synthesis through heat shock proteins, which can cause membrane and/or vascular rupture, cell death, and placental infarction.[13,16]
Furthermore, a previous review performed 6 years ago suggested that maternal fever was significantly associated with an increased risk of CHDs in offspring.[17] However, only seven original studies have been included in the prior review and only a limited number of confounding factors have been considered, which reduced the precision and robustness of the results and thereby weaken the persuasiveness of the evidence.[17] Meanwhile, the prior review did not pay attention to the association between different maternal exposure times and the risk of CHDs in offspring. Besides, several studies with inconsistent findings have been published after the publication of that review.
Therefore, considering the inconsistency of the existing studies and the insufficient statistical power of published review, we conducted an updated systematic review and meta-analysis to include the latest literature. This is expected to increase the statistical power, which will help to find a statistically significant difference. Our study has the following objectives:
-
1.
to review and summarize the epidemiologic relationship between maternal fever and risk of overall CHDs in offspring;
-
2.
to assess the association between maternal fever and risk of specific CHD phenotypes in offspring; and
-
3.
to identify the potential heterogeneity sources by subgroup and sensitivity analyses.
2. Methods
2.1. Literature search strategy
PubMed, Embase, and Web of Science Database were searched from its start data through March 01, 2020. We used and combined the following search terms:
-
1.
congenital heart disease, congenital heart defect, congenital heart malformation, congenital heart anomalies, congenital cardiac disease, congenital cardiac defect, congenital cardiac malformation, congenital cardiac anomalies, cardiovascular malformation, congenital cardiovascular disease, cardiovascular defect, and cardiovascular anomalies;
-
2.
fever, febrile, hyperthermia, and pyrexia.
The search strategy is shown in Supplemental Table 1. Additionally, the reference lists of retrieved articles and recent reviews were also manually reviewed. The present study was conducted and reported based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.[23] Ethical approval is not required because there is no patient recruitment or personal information collection, and the data included in our study were extracted from published literature.
2.2. Exposure and outcomes
In the present study, the exposure of interest was maternal fever which was defined as a temperature greater than 38 degrees or self-reported fever experience. The outcomes of interest were CHDs or specific CHD phenotypes including conotruncal defects (CTD), transposition of the great vessels (TGA), tetralogy of fallot, atrial septal defects (ASD), ventricular septal defects, left ventricular outflow tract obstruction, right ventricular outflow tract obstruction (RVOTO), hypoplastic left heart syndrome, which were diagnosed by experienced pediatric cardiologists using ultrasonography and confirmed by surgery. However, some of the included studies did not always define exposures and outcomes, and in such cases, we relied on the corresponding terminology in the original articles.
2.3. Study selection
Firstly, we performed an initial screening of titles or abstracts to broaden the inclusion criteria to obtain any relevant study. Secondly, we reviewed the full-text of all selected studies. Studies were considered for inclusion if they fulfilled the following criteria:
-
1.
were original epidemiology studies;
-
2.
had a cohort or case-control study design;
-
3.
the exposure of interest was maternal fever;
-
4.
the outcome of interest was overall CHDs or specific CHD phenotypes; and
-
5.
reported relative risks (RRs) or odds ratios (ORs), with corresponding 95% confidence intervals (CIs) (or provided sufficient information to calculate effect sizes).
Conversely, studies were excluded if they
-
1.
were review articles, or conference abstracts;
-
2.
did not provide any information to calculate effect sizes;
-
3.
had incomplete or unclear data, or
-
4.
were duplicate publications.
If more than 1 study involved the same population, the most recent and comprehensive study was selected.
2.4. Data extraction and quality assessment
Two independent authors extracted data and assessed study quality. Any disagreements were resolved through discussion among the authors until consensus was reached. Data extraction was performed by using a standardized data collection form. Information was recorded as follows: the first author name; publication year; geographic region; sample sources; study design; recruitment period; the number of cases/controls; reported time of maternal fever; assessment methods of fever; the outcome of interest; reported ORs and their 95% CIs for risk of CHDs; whether confounding factors were matched or adjusted; and quality score. The study quality of the included literature was assessed by using the Newcastle-Ottawa Scale.[24] When the study gains seven or more stars, it is considered of higher methodological quality.
2.5. Statistical analysis
ORs were used to estimate the association between maternal fever and risk of CHDs in offspring. In the presence of between-study heterogeneity, the combined ORs and their corresponding 95% CIs were calculated using random-effects models, otherwise, the fixed-effect model was used.[25] Homogeneity of effect size across studies was tested by using the Q statistics (significance level at P < .10) and I2 statistic (significance level at I2 > 50%), which is a quantitative measurement of inconsistency across studies.[26]
Subgroup analyses were conducted based on the following factors: continents; sample sources; study design; reported time of maternal fever; assessment methods of fever; whether confounding factors were matched or adjusted; and quality score of studies. We also performed sensitivity analyses to investigate the influence of a single study on the overall risk estimate by omitting one study at a time. Potential publication bias was assessed by Begg funnel plots and Begg rank correlation test (significance level at P < .10).[27] Considering the limited numbers of studies that examined specific CHD phenotypes, subgroup analyses, sensitivity analyses, and publication bias assessment were only conducted for the association between maternal fever and risk of overall CHDs in offspring.
All statistical analyses were performed using Stata version 12.0 and Review Manager Version 5.3. All reported P values were two-sided and P < .05 was considered statistically significant, except where otherwise specified.
3. Results
3.1. Literature search
The flowchart of the literature identification, screening, inclusion, and exclusion process is shown in Figure 1. We initially found 484 potentially eligible articles from PubMed, Embase, and Web of Science Database. Of these, 2 additional articles were found from reference lists. At first, 171 studies were removed because of duplicates. After 313 articles were screened based on titles or abstracts, 252 articles were excluded (15 articles were reviews and 237 articles were unrelated to our topics). Then, 61 potentially relevant articles were identified, which were carefully assessed by full-text, and 45 articles were further excluded for various reasons including
Figure 1.
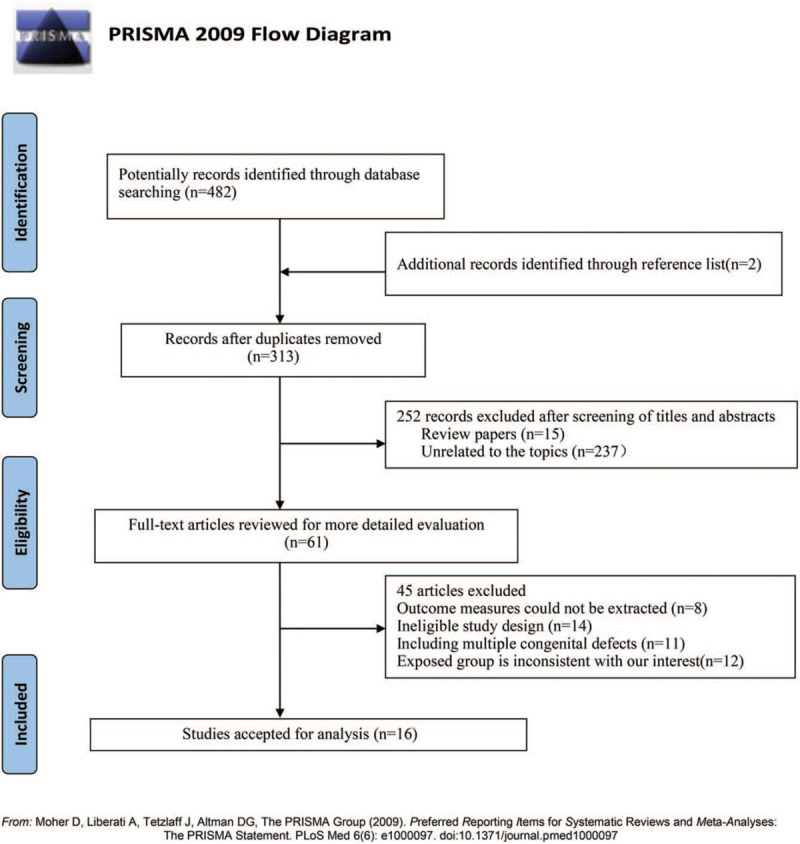
Flow chart of literature selection.
3.2. Study characteristics
The main characteristics of the included studies were provided in Table 1. These papers were published between 1989 and 2019, with a total of 31,922 CHDs cases among 183,563 participants. Among them, eight studies were conducted in North America, 4 studies in Europe, and 4 studies in Asia. Seven studies were based on hospitals, and the remaining 9 were based on population. Fifteen studies had a case-control design, while the other study had a cohort design.
Table 1.
Characteristic of studies of maternal fever and risk of congenital heart diseases in offspring.
| First author (reference) publication year | Geographic region | Study design | Sample source | Recruitment period | No. of Cases/Controls | Whether reported the exposure measurement standard | Period of exposure | reported CHDs | Whether the confounding factors were adjusted | Quality scores |
| Adams, MM et al[28] 1989 | America (North America) | case-control | Population | 1976-1980 | 83/1303 | No | pre-pregnancy and pregnancy | CTD | Crude | 6 |
| Stoll, C et al[29] 1989 | France (European) | case-control | Hospital | 1979–1986 | 801/801 | No | during pregnancy | CHDs/VSD/ASD | Crude | 6 |
| Tikkanen, J et al[30] 1991 | Finland (European) | case-control | Hospital | 1982–1984 | 573/1055 | Yes | the first trimester | CHDs/VSD/ASD | Crude | 5 |
| Zhang, J et al[31] 1993 | China (Asia) | case-control | Hospital | 1986–1987 | 10/94 | No | the first trimester | CHDs | Crude | 6 |
| Fixler, DE et al[32] 1998 | America (North America) | case-control | Population | NA | 89 /82 | Yes | pre-pregnancy and pregnancy | CHDs | Crude | 6 |
| Botto, LD et al[33] 2001 | America (North America) | case-control | Population | 1982–1983 | 905/3029 | No | pre-pregnancy and pregnancy | CHDs | Adjusted | 8 |
| Acs, N et al[34] 2005 | Hungary (European) | case-control | Hospital | 1980–1996 | 4480/38152 | No | the first trimester | CHDs | Crude | 7 |
| Cleves, MA et al[35] 2008 | America (North America) | case-control | Population | 1997–2003 | 3690/4760 | Yes | the first trimester | CHDs /CTD/VSD/ASD/TGA/TOF/HLHS/LVOTO/RVOTO | Adjusted | 8 |
| Liu, SW et al[36] 2009 | China (Asia) | case-control | Hospital | 2004–2005 | 164/328 | No | the first trimester | CHDs | Crude | 7 |
| Oster, ME et al[37] 2011 | America (North America) | case-control | Population | 1981–1989 | 2361/3435 | Yes | during pregnancy | CHDs /CTD/VSD/ASD/TGA/HLHS/LVOTO/RVOTO | Adjusted | 8 |
| Botto, LD et al[38] 2014 | America (North America) | case-control | Hospital | 1997–2005 | 7020/6746 | Yes | the first trimester | CTD/VSD/ASD/TGA/TOF/HLHS/LVOTO/RVOTO | Crude | 7 |
| Ou, YQ et al[18] 2016 | China (Asia) | case-control | Population | 2004–2013 | 4034/4034 | No | the first trimester | CHDs/VSD/ASD/TGA | Adjusted | 7 |
| Simeone, MS et al[19] 2016 | America (North America) | case-control | Population | 1997–2011 | 594/11829 | No | pre-pregnancy and pregnancy | CHDs/TOF/HLHS | Crude | 7 |
| Saaa, L et al[20] 2017 | Denmark (Europe) | cohort | Population | 1996–2002 | 656/69023 | No | the first trimester | CHDs | Adjusted | 7 |
| Lin, S et al[21] 2018 | America (North America) | case-control | Population | 1997–2007 | 5848/5742 | No | pre-pregnancy and pregnancy | CHDs | Crude | 7 |
| Guo, LQ et al[22] 2019 | China (Asia) | case-control | Hospital | 2014–2016 | 614/1228 | Yes | pre-pregnancy and pregnancy | CHDs | Adjusted | 8 |
Meanwhile, 6 studies reported maternal fever measurement standards, while the remaining 10 studies did not report measurement standards. The exposure time of fever in 8 studies was in the first trimester, 6 studies in prepregnancy and pregnancy, and 2 studies during pregnancy. Additionally, 6 studies adjusted or matched some potential confounding factors (such as maternal age, educational level, economic situation, and so on), and the remaining studies did control any factors when estimating the effect. Eleven studies were considered of higher methodological quality, achieving a quality score of more than 7.
3.3. Maternal fever and risk of CHDs in offspring
The association between maternal fever and risk of overall CHDs in offspring was shown in Figure 2. Overall, mothers who had a fever during preconception and conception periods compare with those who did not have a fever experience, were at a significantly higher risk of overall CHDs in offspring (OR = 1.45, 95%CI: 1.21–1.73; P < .001). However, substantial heterogeneity was found (P < .001; I2 = 80.0%).
Figure 2.
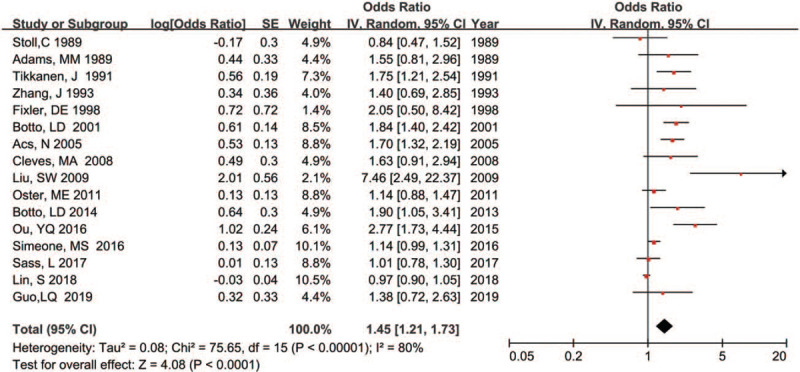
Forest plot of maternal fever and risk of overall CHDs in offspring.
Meanwhile, we also calculated the pooled estimate of the association between maternal fever and the risk of specific CHDs phenotypes in offspring, and the results were shown in Figures 3 and 4. Our results indicated that maternal fever experience was significantly associated with increased risk of most CHD phenotypes in offspring such as CTD (OR = 1.38, 95% CI: 1.01–1.89; P = .04), ASD (OR = 1.48, 95% CI: 1.01–2.17; P = .04), TGA (OR = 1.81, 95% CI: 1.14–2.88; P = .01), and RVOTO (OR = 1.66, 95% CI: 1.04–2.65; P = .03). Furthermore, substantial heterogeneity was not found except for RVOTO (P = .08; I2 = 61.0%). However, our results did not find a significantly association between maternal fever the risk of some specific CHD phenotypes in offspring including tetralogy of fallot (OR = 1.11, 95% CI: 0.94–1.32; P = .22), hypoplastic left heart syndrome (OR = 1.17, 95% CI: 0.94–1.47; P = .17), ventricular septal defects (OR = 1.21, 95% CI: 0.76–1.94; P = .43), and left ventricular outflow tract obstruction (OR = 1.08, 95% CI: 0.52–2.24; P = .84).
Figure 3.
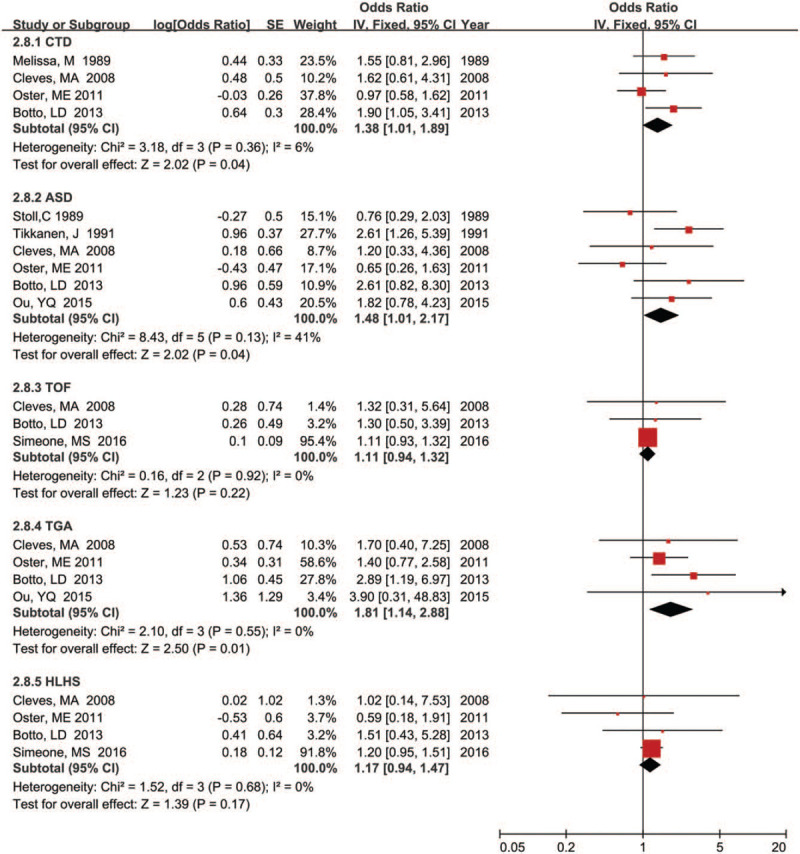
Forest plot of maternal fever and risk of CTD, ASD, TOF, TGA, and HLHS in offspring.
Figure 4.
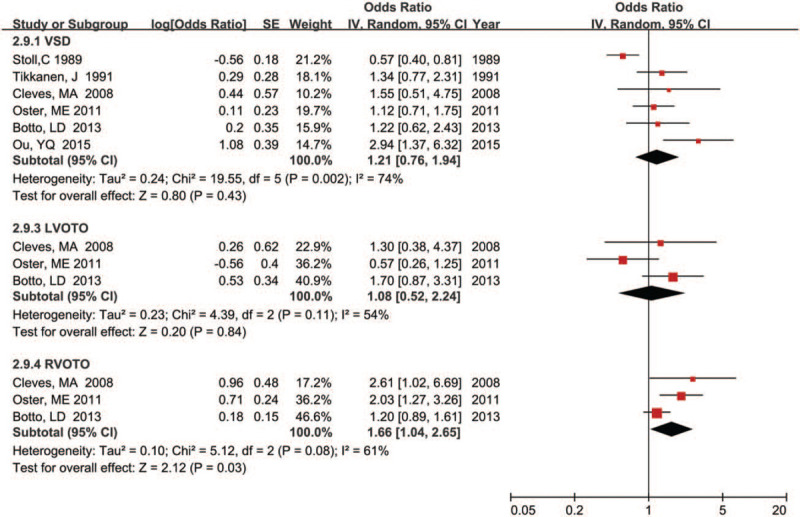
Forest plot of maternal fever and risk of VSD, LVOTO, and RVOTO in offspring.
3.4. Subgroup analyses
Subgroup analyses for the association between maternal fever and risk of total CHDs in offspring were summarized in Table 2. Overall, a significantly increased risk of developing total CHDs in offspring was found in most subgroups. After subgroup analyses, different study design (test for subgroup difference [TSD]: I2 = 83.4%), different exposure time of maternal fever (TSD: I2 = 70.1%), different geographic regions (TSD: I2 = 34.1%), and sample source (TSD: I2 = 22.6%) were identified as relevant heterogeneity moderators. However, statistically significant difference was found only in different study design (χ2 = 6.02; P = .01) and different exposure time of maternal fever (χ2 = 10.02; P = .02), while different geographic regions (χ2 = 3.03; P = .22) and different sample source (χ2 = 1.29; P = .26) were not statistically different. Subgroup analyses indicated that maternal fever in the first trimester of pregnancy was significantly associated with risk of total CHDs in offspring (OR = 1.79; 95% CI: 1.32–2.44), and also reported the association between maternal fever in 3 months before and during the pregnancy and risk of total CHDs in offspring (OR = 1.29; 95% CI: 1.01–1.64).
Table 2.
Subgroup analysis of association maternal fever and risk of congenital heart diseases in offspring.
| Measure of heterogeneity | |||||
| Subgroup variables | No. of studies | Pooled OR (95%CI) | χ2 | P | I2 |
| Geographic region | 3.03∗ | .22∗ | 34.1%∗ | ||
| North America | 8 | 1.31 (1.07–1.60) | 28.97 | <.001 | 76.0% |
| Europe | 4 | 1.30 (0.92–1.84) | 12.63 | .01 | 76.0% |
| Asia | 4 | 2.29 (1.25–4.20) | 9.32 | .03 | 68.0% |
| Study design | 6.02∗ | .01∗ | 83.4%∗ | ||
| Cohort | 1 | 1.01 (0.78–1.30) | – | – | – |
| Case-control study | 15 | 1.51 (1.24–1.83) | 74.90 | <.001 | 81.0% |
| Sample source | 1.29∗ | .26∗ | 22.6%∗ | ||
| Hospital | 7 | 1.64 (1.22–2.21) | 13.18 | .04 | 54.0% |
| Population | 9 | 1.33 (1.09–1.63) | 41.35 | <.001 | 81.0% |
| Exposure time | 10.02∗ | .02∗ | 70.1%∗ | ||
| First trimester | 8 | 1.79 (1.32–2.44) | 27.00 | <.001 | 74.0% |
| Pre-pregnancy and pregnancy | 6 | 1.29 (1.01–1.64) | 23.83 | <.001 | 79.0% |
| During pregnancy | 2 | 1.09 (0.87–1.37) | 0.88 | .35 | 0.00% |
| Whether reported the exposure measurement standard | 0.00∗ | .98∗ | 0.00%∗ | ||
| Yes | 6 | 1.42 (1.17–1.74) | 5.62 | .34 | 11.0% |
| No | 10 | 1.43 (1.14–1.80) | 62.01 | <.001 | 85.0% |
| Whether the confounding factors were adjusted | 0.08∗ | .78∗ | 0.00%∗ | ||
| Adjusted | 6 | 1.50 (1.11--2.03) | 21.78 | .001 | 77.0% |
| Unadjusted | 10 | 1.42 (1.13–1.79) | 42.68 | <.001 | 79.0% |
| Quality score | 0.02∗ | .90∗ | 0.00%∗ | ||
| < 7 | 5 | 1.43 (1.07–1.91) | 4.59 | .33 | 13.0% |
| ≥ 7 | 11 | 1.47 (1.19–1.81) | 64.76 | <.001 | 85.0% |
3.5. Sensitivity analyses
Sensitivity analyses were conducted to explore the potential sources of heterogeneity and to examine the robustness of the risk estimate when assessing the relationship between maternal fever and total CHDs in offspring. For the association between maternal fever and risk of overall CHDs in offspring, removing the poor-quality studies did not change the overall risk estimate (OR = 1.47; 95% CI: 119–1.81), with substantial evidence of heterogeneity (P < .001; I2 = 64.76%). Exclusion of 10 studies not adjusting for any confounding factors yielded similar results (OR = 1.50; 95% CI:1.11–2.03), but heterogeneity was still present (P < .001; I2 = 77.0%). Further exclusion of any single study at a time did not materially alter the overall risk estimate (Fig. 5).
Figure 5.
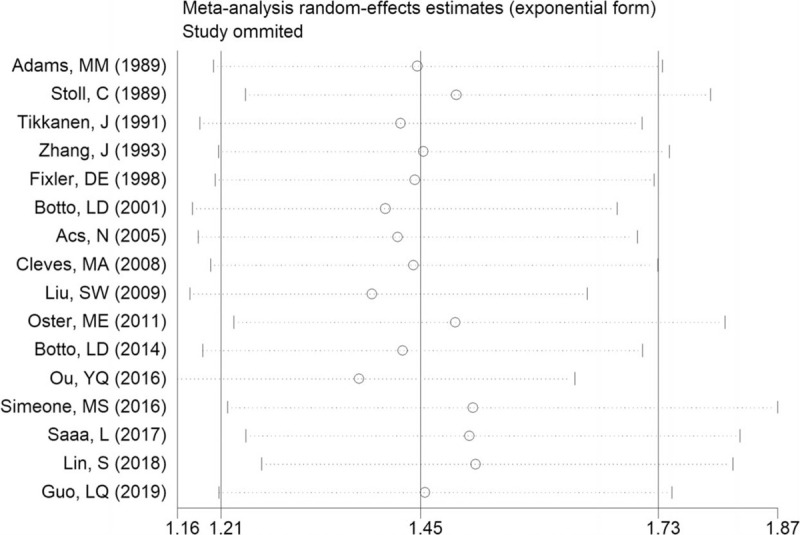
Sensitivity analysis for the association between maternal fever and risk of overall CHDs in offspring.
3.6. Publication bias
Begg rank correlation test along with the result of the funnel plot was applied to assess the asymmetry of the funnel plot and evaluate the significance of publication bias. Begg funnel plot showed a mild asymmetry in the visual examination (Fig. 6), but Begg rank correlation test (P = .47) indicated no evidence of publication bias among studies of maternal fever and the risk of overall CHDs in offspring.
Figure 6.
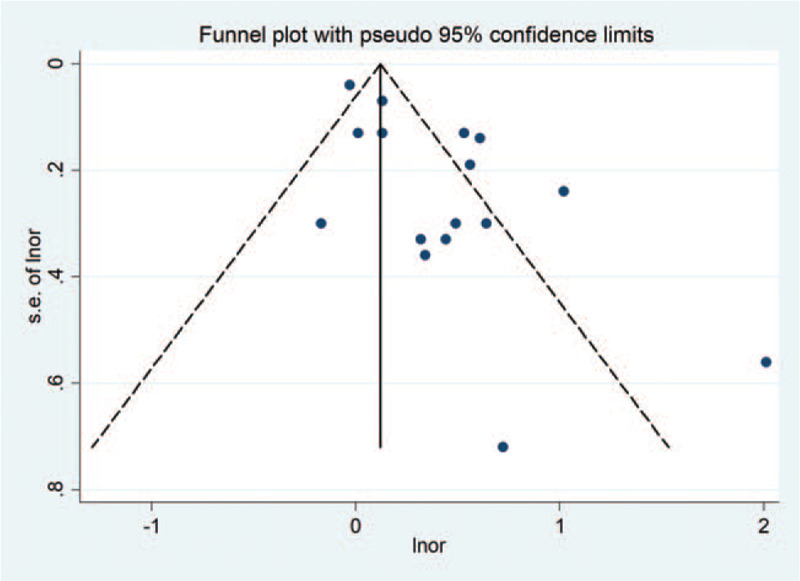
Begg funnel plot for the association between maternal fever and risk of overall CHDs in offspring.
4. Discussion
CHDs were considered to be the leading non-infectious cause of infant morbidity and mortality, accounting for 6.0% of neonatal death and 46% of congenital deaths respectively.[39,40] Almost all infants with CHDs need clinical or surgical intervention in the first year of life, which brings a serious economic burden to their families.[41]
Our study suggested that mothers who had a fever during preconception and conception periods compare with those who did not have a fever experience, were at a significantly higher risk of overall CHDs in offspring (OR = 1.45). Furthermore, our present study also indicated that maternal fever could significantly increase the risk of specific CHD phenotypes in offspring including CTD (OR = 1.38), ASD (OR = 1.48), TGA (OR = 1.81), and RVOTO (OR = 1.66). Besides, after subgroup and sensitivity analyses, the relationship between maternal fever and higher risks of developing overall CHDs in offspring still existed, which implied our results were robust and credible.
So far, 1 existing review[17] has evaluated the relation between maternal fever and CHDs in offspring. Our findings were generally consistent with the previous review, but our study has important strengths. On the one hand, compared with the previous review, our meta-analysis provides the most up-to-date evidence on this topic and expands the sample size, which enhanced statistical power to provide more precise and reliable risk estimates. On the other hand, our study fully considered the effect of geographic region, study design, sample source, exposure time, whether reported the exposure measurement standard, whether the confounding factors were adjusted, and quality score. We found that the association between maternal fever and risk of CHDs in offspring remains statistically significant after subgroup and sensitivity analyses. Furthermore, we also fully considered the relationship between maternal fever and specific CHD phenotypes in offspring. Our results indicated that maternal fever was significantly associated with some specific CHD phenotypes in offspring including CTD, ASD, TGA, and RVOTO.
To our knowledge, the underlying mechanisms involved in the relationship between maternal fever and the risk of CHDs in offspring are still unclear. The most probable explanation is the teratogenicity of hyperthermia. Some previous studies[15,42] have reported that hyperthermia has teratogenic effects not only on animals but also on humans. Meanwhile, these studies[15,42] also have found that hyperthermia was significantly associated with malformations including central nervous defects, craniofacial anomalies, heart defect, and so on. Nonetheless, the teratogenic mechanisms of maternal fever are unknown.[42] The potential hypothesis is that hyperthermia can activate temperature-sensitive TRPV1 and TRPV4 ion channels in neural crest cells, which can result in cardiac and craniofacial birth defects.[42]
Additionally, there are a few possible explanations for the association between maternal fever and risk of CHDs in offspring. On the one hand, the association between maternal fever and risk of CHDs in offspring may attribute to metabolic changes. Evidence indicates[13] that maternal fever can cause changes in the metabolic level of the mother. For example, hyperthermia could interfere with protein synthesis through heat shock proteins, which can cause membrane and/or vascular rupture, cell death, and placental infarction. On the other hand, maternal fever may lead to an abnormal gene. Previous studies[13,42] suggested that hyperthermia can cause interruption of normal sequence of gene, and even touch off the abnormal expression and mutation of the gene.
Substantial heterogeneity was observed among studies assessing the association between maternal fever and CHDs in offspring, which was not surprising given the differences in the study region and methodology. In the present study, we conducted subgroup and sensitivity analyses to explore the potential source of heterogeneity. Our subgroup analyses have identified the most relevant heterogeneity moderators including exposure time of maternal fever, study design, geographic region, and sample source. However, little significant alterations were found in risk estimates among most subgroups. Besides, sensitivity analyses also showed that there were no significant changes in the results by omitting 1 or more studies in turn. Both of these indicated that our results were little affected by heterogeneity, yet we also should interpret the results with caution because of heterogeneity.
Potential limitations should be taken into account. Firstly, considering limited numbers of studies for specific CHD phenotypes, subgroup analyses, sensitivity analyses, and publication bias assessment were only conducted for the association between maternal fever and risk of overall CHDs in offspring, so more studies should be included in future reviews, to provide further support for our results. Secondly, recall bias should be considered. Most of the studies included in our review were case-control studies and case-control studies are prone to recall bias, which may restrict the strength and quality of evidence. Although most case-control studies collected data information by structured questionnaires, standardized questionnaires, medical records, or interviews, which might to some extent help participants to accurately recall the information to some extent, we should still view the results with caution.
Thirdly, most of the studies included in our review did not give a strict definition and detailed information of maternal fever, which may increase the likelihood of misclassification bias, so the risk might be overestimated or underestimated. Fourth, residual confounding is of concern. Uncontrolled or unconsidered risk factors may produce potential biases, such as the duration of fever. Although the pooled risk estimate did not significantly change after restricting the analysis to studies that have adjusted for confounding factors, the possible effects of uncontrolled or unconsidered residual confounding cannot be ignored. Fifth, the Begg rank correlation test did not indicate any evidence of publication bias, but Begg funnel plot did show a little substantial asymmetry, which may influence the results. Sixth, since fever may be a clinical manifestation of a certain cause, we cannot rule out the possibility that the observed association between maternal fever and risk of CHDs in offspring is caused by a certain cause rather than the fever itself. Among the possible etiologies of fever, infection is the most common pathogenesis, which is often caused by viral, bacterial, and fungal organisms. However, it is difficult to distinguish the effects of fever on CHDs from the effects of other potential infections because reports of such exposures show a high level of concordance.[17] Therefore, we failed to control the influence of the cause of fever on the risk of CHDs in our study. Future studies should emphasize the precise collection of fever and its causes, which would contribute to providing more accurate and refined evidence for explaining the association between fever and risk of CHDs. Besides, the present review included only studies published in English, and additional research in other populations is warranted to generalize the findings.
5. Conclusion
In summary, based on our findings, the associations between maternal fever and risk of CHDs in offspring are significant. This association is present not only for overall CHDs in offspring but also for some specific CHD phenotypes in offspring. Our findings highlight that preventing maternal fever during the preconception and conception periods may contribute to reducing the risk of overall CHDs and some specific CHD phenotypes in offspring. However, given the limited number of current case-control studies, larger-sample prospective studies are required to further confirm our results. Besides, due to the underlying mechanisms between maternal fever and the risk of specific CHD phenotypes in offspring are still unreported, more research is needed to explore the possible mechanisms.
Acknowledgments
The authors would like to thank the editors and reviewers for their suggestions and all colleagues working in the Department of Cardiothoracic Surgery, Hunan Children's Hospital.
Author contributions
Conceptualization: Guihong Yang, Xicheng Deng, Peng Huang, Kai Zhang, Yunfei Li.
Data curation: Jianfeng Xiao, Peng Huang, Yunfei Li.
Formal analysis: Guihong Yang, Xicheng Deng, Jianfeng Xiao, Peng Huang, Kai Zhang, Yunfei Li.
Methodology: Guihong Yang, Jianfeng Xiao, Kai Zhang, Yunfei Li.
Software: Guihong Yang, Kai Zhang.
Writing – original draft: Guihong Yang, Xicheng Deng, Jianfeng Xiao, Peng Huang.
Writing – review & editing: Guihong Yang, Xicheng Deng.
Supplementary Material
Footnotes
Abbreviations: 95% CIs = 95% confidence intervals, ASD = atrial septal defects, CHDs = congenital heart diseases, CTD = conotruncal defects, ORs = odds ratios, RVOTO = right ventricular outflow tract obstruction, TGA = transposition of the great vessels, TSD = test for subgroup difference.
How to cite this article: Yang G, Deng X, Xiao J, Huang P, Zhang K, Li Y. Maternal fever during preconception and conception is associated with congenital heart diseases in offspring: an updated meta-analysis of observational studies. Medicine. 2021;100:9(e24899).
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors declare that they have no conflicts of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
ASD = atrial septal defect, CHDs = congenital heart diseases, CTD = conotruncal defects, HLHS = hypoplastic left heart syndrome, LVOTO = left ventricular outflow tract obstruction, NA = not available, RVOTO = right ventricular outflow tract obstruction, TGA = D-transposition of the great, TOF = tetralogy of fallout, VSD = ventricular septal defect.
Test for subgroup differences.
95% CI = confidence interval, OR = Odd Ratios.
References
- [1].Krasuski RA, Bashore TM. Congenital Heart Disease Epidemiology in the United States. Circulation 2016;134:110–3. [DOI] [PubMed] [Google Scholar]
- [2].Liu Y, Chen S, Zuhlke L, et al. Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol 2019;48:455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bernier PL, Stefanescu A, Samoukovic G, et al. The challenge of congenital heart disease worldwide: epidemiologic and demographic facts. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2010;13:26–34. [DOI] [PubMed] [Google Scholar]
- [4].van der Bom T, Zomer AC, Zwinderman AH, et al. The changing epidemiology of congenital heart disease. Nat Rev Cardiol 2011;8:50–60. [DOI] [PubMed] [Google Scholar]
- [5].Wang T, Chen L, Yang T, et al. Congenital heart disease and risk of cardiovascular disease: a meta-analysis of cohort studies. J Am Heart Assoc 2019;8:e12030.doi: 10.1161/JAHA.119.012030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gaynor JW, Stopp C, Wypij D, et al. Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics 2015;135:816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kim DS, Stanaway IB, Rajagopalan R, et al. Results of genome-wide analyses on neurodevelopmental phenotypes at four-year follow-up following cardiac surgery in infancy. Plos One 2012;7:e45936.doi: 10.1371/journal.pone.0045936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zaidi S, Brueckner M. Genetics and genomics of congenital heart disease. Circ Res 2017;120:923–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jenkins KJ, Correa A, Feinstein JA, et al. Noninherited risk factors and congenital cardiovascular defects: current knowledge: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation 2007;115:2995–3014. [DOI] [PubMed] [Google Scholar]
- [10].Zhang S, Wang L, Yang T, et al. Parental alcohol consumption and the risk of congenital heart diseases in offspring: an updated systematic review and meta-analysis. Eur J Prev Cardiol 2020;27:410–21. [DOI] [PubMed] [Google Scholar]
- [11].Zhao L, Chen L, Yang T, et al. Parental smoking and the risk of congenital heart defects in offspring: An updated meta-analysis of observational studies. Eur J Prev Cardiol 2019;27:1284–93. [DOI] [PubMed] [Google Scholar]
- [12].Hu CY, Huang K, Fang Y, et al. Maternal air pollution exposure and congenital heart defects in offspring: a systematic review and meta-analysis. Chemosphere 2020;253:126668.doi: 10.1016/j.chemosphere.2020.126668. [DOI] [PubMed] [Google Scholar]
- [13].Edwards MJ. Review: hyperthermia and fever during pregnancy. Birth Defects Res A Clin Mol Teratol 2006;76:507–16. [DOI] [PubMed] [Google Scholar]
- [14].Dreier JW, Andersen AM, Berg-Beckhoff G. Systematic review and meta-analyses: fever in pregnancy and health impacts in the offspring. Pediatrics 2014;133:e674–88. [DOI] [PubMed] [Google Scholar]
- [15].Graham JJ, Marshall J. Edwards: discoverer of maternal hyperthermia as a human teratogen. Birth Defects Res A Clin Mol Teratol 2005;73:857–64. [DOI] [PubMed] [Google Scholar]
- [16].Edwards MJ, Saunders RD, Shiota K. Effects of heat on embryos and foetuses. Int J Hyperthermia 2003;19:295–324. [DOI] [PubMed] [Google Scholar]
- [17].Shi QY, Zhang JB, Mi YQ, et al. Congenital heart defects and maternal fever: systematic review and meta-analysis. J Perinatol 2014;34:677–82. [DOI] [PubMed] [Google Scholar]
- [18].Ou Y, Mai J, Zhuang J, et al. Risk factors of different congenital heart defects in Guangdong, China. Pediatr Res 2016;79:549–58. [DOI] [PubMed] [Google Scholar]
- [19].Simeone RM, Tinker SC, Gilboa SM, et al. Proportion of selected congenital heart defects attributable to recognized risk factors. Ann Epidemiol 2016;26:838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sass L, Urhoj SK, Kjaergaard J, et al. Fever in pregnancy and the risk of congenital malformations: a cohort study. BMC Pregnancy Childbirth 2017;17:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lin S, Lin Z, Ou Y, et al. Maternal ambient heat exposure during early pregnancy in summer and spring and congenital heart defects - A large US population-based, case-control study. Environ Int 2018;118:211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Guo L, Zhao D, Zhang R, et al. A matched case-control study on the association between colds, depressive symptoms during pregnancy, and congenital heart disease in Northwestern China. Sci Rep 2019;9:589.doi: 10.1038/s41598-018-36968-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. W64. [DOI] [PubMed] [Google Scholar]
- [24].Wells GA, Shea BJ, O’Connell D, Peterson J, Welch V. Newcastle-Ottawa scale (NOS) for assessing the quality of non-randomized studies in the meta-analysis, Available www.ohri.ca/programs/clinical_epidemiology/oxford.htm (Accessed May 20, 2020). [Google Scholar]
- [25].DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials 2015;45(Pt A):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Adams MM, Mulinare J, Dooley K. Risk factors for conotruncal cardiac defects in Atlanta. J Am Coll Cardiol 1989;14:432–42. [DOI] [PubMed] [Google Scholar]
- [29].Stoll C, Alembik Y, Roth MP, et al. Risk factors in congenital heart disease. Eur J Epidemiol 1989;5:382–91. [DOI] [PubMed] [Google Scholar]
- [30].Tikkanen J, Heinonen OP. Maternal hyperthermia during pregnancy and cardiovascular malformations in the offspring. Eur J Epidemiol 1991;7:628–35. [DOI] [PubMed] [Google Scholar]
- [31].Zhang J, Cai WW. Association of the common cold in the first trimester of pregnancy with birth defects. Pediatrics 1993;92:559–63. [PubMed] [Google Scholar]
- [32].Fixler DE, Threlkeld N. Prenatal exposures and congenital heart defects in Down syndrome infants. Teratology 1998;58:6–12. [DOI] [PubMed] [Google Scholar]
- [33].Botto LD, Lynberg MC, Erickson JD. Congenital heart defects, maternal febrile illness, and multivitamin use: a population-based study. Epidemiology 2001;12:485–90. [DOI] [PubMed] [Google Scholar]
- [34].Acs N, Banhidy F, Puho E, et al. Maternal influenza during pregnancy and risk of congenital abnormalities in offspring. Birth Defects Res A Clin Mol Teratol 2005;73:989–96. [DOI] [PubMed] [Google Scholar]
- [35].Cleves MA, Malik S, Yang S, et al. Maternal urinary tract infections and selected cardiovascular malformations. Birth Defects Res A Clin Mol Teratol 2008;82:464–73. [DOI] [PubMed] [Google Scholar]
- [36].Liu S, Liu J, Tang J, et al. Environmental risk factors for congenital heart disease in the Shandong Peninsula, China: a hospital-based case-control study. J Epidemiol 2009;19:122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Oster ME, Riehle-Colarusso T, Alverson CJ, et al. Associations between maternal fever and influenza and congenital heart defects. J Pediatr 2011;158:990–5. [DOI] [PubMed] [Google Scholar]
- [38].Botto LD, Panichello JD, Browne ML, et al. Congenital heart defects after maternal fever. Am J Obstet Gynecol 2014;210:351–9. [DOI] [PubMed] [Google Scholar]
- [39].Ntiloudi D, Zegkos T, Bazmpani MA, et al. Pregnancy outcome in women with congenital heart disease: a single-center experience. Hellenic J Cardiol 2018;59:155–9. [DOI] [PubMed] [Google Scholar]
- [40].Ntiloudi D, Zegkos T, Koutsakis A, et al. Pregnancy in patients with congenital heart disease: a contemporary challenge. Cardiol Rev 2017;25:326–30. [DOI] [PubMed] [Google Scholar]
- [41].Hoffman J. The global burden of congenital heart disease. Cardiovasc J Afr 2013;24:141–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hutson MR, Keyte AL, Hernandez-Morales M, et al. Temperature-activated ion channels in neural crest cells confer maternal fever-associated birth defects. Sci Signal 2017;10: doi: 10.1126/scisignal.aal4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


