Abstract
This study aimed to investigate the correlation between triglyceride glucose (TyG) index and coronary artery disease (CAD) with type 2 diabetes mellitus (T2DM) and identify the risk associated TyG index in different subgroups.
A total of 1665 eligible inpatients (CAD with T2DM group [n = 680], non-coronary artery disease without T2DM [n = 985]) were consecutively enrolled in this study. They were assigned into 4 subgroups: middle-aged, elderly, male, and female subgroups. Receiver operating characteristic curve diagnostic test and a logistic regression model was established to analyze the risk factors for CAD with T2DM.
TyG index is an independent risk factor for patients with CAD with T2DM. The risk of increased TyG index is greater in the middle-aged and male subgroups than in the elderly and female subgroups, respectively (all P < .05). The specificity and the positive predictive value of the TyG index is greater than the sensitivity and the negative predictive value, respectively (all P < .05).
Increased TyG index is a new independent risk factor for CAD with T2DM, and its risk is higher in the middle-aged and male subgroups than in the elderly and female subgroups, respectively. TyG index may be used as a clinical predictor of CAD with T2DM.
Keywords: coronary artery disease, risk factor, triglyceride glucose index, type 2 diabetes mellitus
1. Introduction
Coronary artery disease (CAD) is the major cause of death in middle-aged and elderly populations. A number of researches have been done to explore the risk factors of CAD to improve the diagnosis and prevention of CAD.[1,2] Recently, the influence of triglyceride glucose (TyG) index on type 2 diabetes mellitus (T2DM) and coronary artery disease (CAD) has attracted much attention from researchers. TyG index is a simple and inexpensive alternative indicator for insulin resistance, which not only is related to the occurrence and development of T2DM but also affects the development of coronary atherosclerosis plaque and promotes the occurrence of adverse cardiovascular events.[3–5] Previous studies have reported that TyG index predicts coronary artery calcification and CAD prognosis and is associated with the CAD severity.[6,7]
There are diversities in body hormones and metabolic levels among different ages and sexes populations, and the risk of some CAD risk factors are different among different ages and sexes.[8,9] However, no studies on the role of TyG index in CAD with T2DM in middle-aged and elderly populations and on the different risks of TyG index in CAD with T2DM in middle-aged and elderly populations and in both sexes have been conducted. Thus, this study aimed to investigate the relationship between TyG index and CAD with T2DM in middle-aged and elderly people and to determine the difference in the risk between middle-aged and elderly individuals and between sexes.
2. Methods
A total of 680 in patients with CAD with T2DM in the Department of Cardiology, Affiliated Hospital of Chengde Medical College, from November 2011 to May 2017, were consecutively enrolled in this retrospective study, and 985 non-coronary artery disease without T2DM inpatients were enrolled as the control group. All patients were assigned into the following subgroups according to age and sex: middle-aged (40≤ age <60 years, n = 821) and elderly (age ≥60 years, n = 844) subgroups and male (n = 671) and female (n = 994) subgroups. The inclusion criteria were as follows: age ≥40 years, CAD[10] (unstable angina; non-ST elevation myocardial infarction; ST-elevation myocardial infarction; and coronary angiography showing the stenosis at least or more than 50% in 1 or more of the left main, left anterior descending, left circumfex, right coronary, or their main branches), and T2DM[11] (diagnosed according to the American Diabetes Association of diabetes guidelines). The major exclusion criteria included recent acute coronary syndrome, type 1 diabetes or secondary diabetes, connective tissue disease, severe valvular heart disease, constrictive pericarditis, hypertrophic cardiomyopathy, clinical history of malignant cancer, and insufficient medical record for TyG index calculation. We conducted this study according to the standards of the Declaration of Helsinki on medical research, and the study protocol was approved by the Institutional Review Boards of The Affiliated Hospital of Chengde Medical University. All subjects provided written informed consent.
Demographic characteristics and clinical data of all patients, including age, sex, height, weight, hypertension, dyslipidemia, ischemic stroke, systolic blood pressure, diastolic blood pressure, and ejection fraction, were retrospectively obtained by master students. All blood samples were obtained after >8 hour of fasting and were analyzed for lipid profile, glucose levels, blood routine, and biochemistry routine. Subsequently, pulse pressure, body mass index (BMI), and TyG index were calculated. Pulse pressure was calculated as systolic blood pressure minus diastolic blood pressure; BMI, as weight (kg) divided by height (m2); and TyG index,[12] as in [fasting triglycerides (mg/dl)∗fasting glucose (mg/dl)/2].
All statistical analyses and data processes were performed using Statistical Software Package 19 (SPSS Inc., Chicago, IL) software. Kolmogorov-Smirnov test was performed to determine the distribution pattern of the continuous variables; all variables were skewed and expressed as quartile M (QR). Moreover, differences in patient characteristics between groups were tested with Mann–Whitney U test. Categorical variables were reported as percentages, and differences in patient characteristics between groups were tested with Chi-Squared test. Receiver operating characteristic curve diagnostic test was employed to determine the optimal cut-off point value for TyG index and diagnostic efficacy. The risk factors for CAD with T2DM were analyzed using a multivariate logistic regression model. Subsequently, a logistic regression model for the middle-aged and elderly subgroups and for the male and female subgroups was established, and the risk of TyG was compared. All statistical analyses were two-sided, and a P value <.05 was considered statistically significant.
3. Results
The proportion of male was higher, the median age and BMI were greater, and chest pain and abnormal wall motion were more common in the CAD with T2DM group than in the control group (all P < .001). The prevalence of smoking, hypertension, dyslipidemia, ischemic stroke, and TyG index ≥8.0 was significantly higher in the CAD with T2DM group (all P < .001). Similarly, the median of left ventricle end-diastolic diameters were higher in the CAD with T2DM group than in the control group; however, the median ejection fraction was lower in the CAD with T2DM group (all P < .001). Moreover, the median leukocyte and neutrophil percentages in the blood routine examination were higher whereas the median platelet counts were lower in the CAD with T2DM group than in the control group (all P < .001). Total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) were higher in the CAD with T2DM group, whereas high-density lipoprotein cholesterol (HDL-C) was higher in the control group (all P < .05) (Table 1).
Table 1.
Baseline characteristics of the CAD with T2DM and control groups.
| Variables | CAD with T2DM (n = 680) | T2DM without CAD (n = 156) | Control (n = 985) | χ2∗ | P∗ | χ2/Z† | P† |
| Male (%) | 311 (45.7) | 66 (42.3) | 360 (36.5) | 14.332 | .001 | 1.463 | <.001 |
| Age (years) | 63 (57,71) | 59 (53, 63) | 58 (53, 63) | 168.116 | <.001 | −12.697 | <.001 |
| BMI (kg/m2) | 26.0 (24.0, 28.5) | 26.2 (23.9, 28.7) | 25.0 (23.0, 27.3) | 39.816 | <.001 | −5.596 | <.001 |
| Chest pain (%) | 417 (63.9) | 66 (43.7) | 404 (41.6) | 78.86 | <.001 | 2.475 | <.001 |
| Smoking (%) | 222 (33.1) | 33 (21.2) | 244 (24.8) | 17.449 | <.001 | 1.505 | <.001 |
| Hypertension (%) | 483 (73.5) | 102 (67.1) | 445 (45.6) | 133.707 | <.001 | 3.306 | <.001 |
| Dyslipidemia (%) | 295 (45.8) | 63 (42.0) | 261 (27.0) | 63.705 | <.001 | 2.283 | <.001 |
| Ischemic stroke (%) | 100 (20.9) | 11 (7.7) | 63 (6.8) | 64.089 | <.001 | 3.598 | <.001 |
| Pulse pressure (mm Hg) | 56 (50,70) | 50 (44, 60) | 50 (40, 60) | 62.075 | <.001 | −7.68 | <.001 |
| Left atrium (cm) | 35 (32, 39) | 34 (31, 38) | 33 (30, 35) | 45.973 | <.001 | −6.723 | <.001 |
| Left ventricle end-diastolic diameter (cm) | 50 (46, 54) | 48 (46, 51) | 49 (46, 52) | 27.916 | <.001 | −5.16 | <.001 |
| Left ventricular | |||||||
| Ejection fraction (%) | 59 (54, 64) | 62 (57, 67) | 62 (58, 67) | 113.885 | <.001 | −10.213 | <.001 |
| Leukocyte (109/L) | 6.7 (5.5, 8.0) | 6.4 (5.4, 7.4) | 6.3 (5.3, 7.5) | 48.519 | <.001 | −4.598 | <.001 |
| Platelet (109/L) | 210 (170, 242) | 207 (167, 239) | 215 (181, 255) | 24.059 | <.001 | −5.295 | <.001 |
| Neutrophil (%) | 62.8 (55.7, 69.8) | 59.7 (54.5, 65.6) | 61.5 (54.2, 67.6) | 41.91 | <.001 | −2.371 | .018 |
| TC (mmol/L) | 4.31 (3.63, 5.20) | 4.22 (3.71, 5.06) | 4.24 (3.60, 4.90) | 5.468 | .065 | −8.378 | <.001 |
| HDL-C (mmol/L) | 1.0 (0.8, 1.2) | 1.07 (0.9, 1.37) | 1.1 (0.9, 1.4) | 70.846 | <.001 | −2.874 | .004 |
| LDL-C (mmol/L) | 2.34 (1.75, 3.06) | 2.13 (1.67, 2.61) | 2.33 (1.79, 2.88) | 16.803 | <.001 | −3.406 | .001 |
| Uric acid (mmol/L) | 304 (254, 369) | 291 (241, 346) | 291 (244, 343) | 11.877 | .003 | −9.898 | <.001 |
| Blood urea nitrogen (mmol/L) | 5.9 (4.8, 6.9) | 5.88 (4.83, 6.70) | 5.2 (4.3, 6.1) | 102.525 | <.001 | −4.29 | <.001 |
| Creatinine (μmol/L) | 67.2 (56.0, 80.6) | 63.2 (54.3, 75.3) | 63.5 (55.4, 72.9) | 19.489 | <.001 | 1.458 | .001 |
| Abnormal wall motion (%) | 302 (61.9) | 84 (64.1) | 432 (52.4) | 14.597 | .001 | 2.72 | <.001 |
| β-blockers (%) | 382 (57.7) | 62 (40.3) | 324 (33.4) | 95.324 | <.001 | 3.45 | <.001 |
| ACEI/ARB (%) | 328 (50.2) | 46 (30.1) | 219 (22.6) | 134.451 | <.001 | 7.314 | <.001 |
| Diuretics (%) | 152 (24.0) | 2 (1.3) | 40 (4.1) | 128.387 | <.001 | 9.007 | <.001 |
| Statin (%) | 627 (93.3) | 122 (78.2) | 594 (60.7) | 223.868 | <.001 | −19.187 | <.001 |
| TyG index | 9.42 (8.96, 9.83) | 9.46 (8.99, 10.06) | 8.72 (8.35, 9.13) | 417.848 | <.001 | −19.187 | <.001 |
In the middle-aged and elderly subgroups, the median TyG index in the CAD with T2DM group was significantly higher than that in the control group (both P < .05). In the male and female subgroups, the median TyG index in the CAD with T2DM group was also significantly higher than that in the control group (both P < .05) (Figs. 1 and 2)
Figure 1.
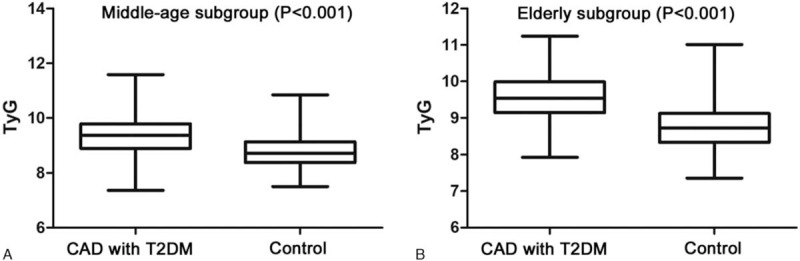
Comparison of TyG index between the CAD with T2DM and control groups in the middle-aged (A) and elderly subgroups (B).
Figure 2.
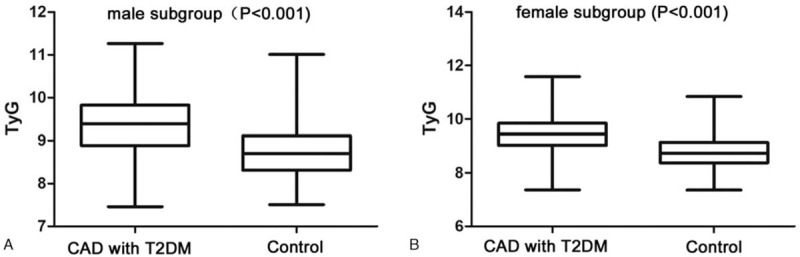
Comparison of TyG index between the CAD with T2DM and control groups in the male (A) and female subgroups (B).
Based on the TyG diagnostic test analysis, the area under the receiver operating characteristic curve (AUC) of the TyG index was 0.776, the optimal diagnostic cut-off point value was 8.0, and the sensitivity and specificity were 42.27% and 85.06%, respectively. The positive and negative predictive values were 98.09% and 7.51%, respectively. Moreover, the TyG index diagnostic test analysis revealed that in the middle-aged, elderly, male, and female subgroups, the AUC of the TyG index was 0.816, 0.755, 0.775, and 0.782, respectively; the optimal diagnostic cut-off point value was 8.2, 8.3, 8.3, and 8.2, respectively; the sensitivity was 33.43%, 55.24%, 51.77%, and 39.80%, respectively; the specificity was 95.28%, 75.00%, 82.86%, and 86.27%, respectively; the positive predictive value was 97.95%, 94.27%, 94.21%, and 96.21%, respectively; and the negative predictive value was 17.50%, 18.38%, 24.17%, and 14.08%, respectively. In all subgroups, the specificity and the positive predictive value of the TyG index was greater than the sensitivity and the negative predictive value, respectively, in the CAD with T2DM group. Furthermore, the middle-aged subgroup had the greatest AUC, diagnostic specificity, and positive predictive value (Table 2).
Table 2.
TyG index diagnostic test in different subgroups.
| Groups | AUC | 95% CI | P | Se (%) | Sp (%) | PPV (%) | NPV (%) | Cut-off point |
| All subjects | 0.776 | 0.753–0.799 | <.001 | 42.27 | 85.06 | 98.09 | 7.51 | 8.0 |
| Middle-aged subgroup | 0.816 | 0.784–0.847 | <.001 | 33.43 | 95.28 | 97.95 | 17.50 | 8.2 |
| Elderly subgroup | 0.755 | 0.722–0.787 | .017 | 55.24 | 75.00 | 94.27 | 18.38 | 8.3 |
| Male subgroup | 0.775 | 0.740–0.810 | <.001 | 51.77 | 82.86 | 94.21 | 24.17 | 8.3 |
| Female subgroup | 0.782 | 0.752–0.812 | <.001 | 39.80 | 86.27 | 96.21 | 14.08 | 8.2 |
TyG index was correlated with CAD risk factors; it was positively correlated with BMI, systolic blood pressure, pulse pressure, TC, LDL-C, and leukocyte and was negatively correlated with HDL-C (all P < .05). TyG index was also positively associated with the left atrium, uric acid, and heart rate (all P < .05) (Table 3).
Table 3.
Correlation array between TyG index and CAD risk factors.
| TyG index | BMI | Systolic blood pressure | Pulse pressure | Left atrium | TC | HDL-C | LDL-C | Uric acid | Leukocyte | Heart rate | |
| Age | .091∗∗ | −0.051 | .168∗∗ | .263∗∗ | .077∗∗ | −.028 | −.035 | 0.001 | 0.039 | 0.014 | .058∗ |
| TyG index | .195∗∗ | .148∗∗ | .117∗∗ | .108∗∗ | .278∗∗ | −.280∗∗ | .058∗ | .164∗∗ | .182∗∗ | .174∗∗ | |
| BMI | .152∗∗ | .055∗ | .276∗∗ | 0.031 | −.154∗∗ | 0.037 | .160∗∗ | 0.044 | .083∗∗ | ||
| Pulse pressure | .086∗∗ | 0.029 | 0.031 | 0.005 | −0.033 | 0.035 | −0.031 | ||||
| Left atrium | −.084∗∗ | −.132∗∗ | −.066∗ | .141∗∗ | 0.038 | −0.002 | |||||
| TC | .281∗∗ | .824∗∗ | 0.025 | 0.016 | .098∗∗ | ||||||
| HDL-C | .209∗∗ | −.167∗∗ | −.156∗∗ | −.068∗∗ | |||||||
| LDL-C | −0.017 | −0.009 | .069∗∗ | ||||||||
| Uric acid | .125∗∗ | .079∗∗ | |||||||||
| Leukocyte | .181∗∗ |
Univariate analysis was performed to determine the factors with P < .05; thereafter, multivariate logistic regression model analysis found that BMI ≥30 kg/m2, smoking, hypertension, dyslipidemia, ischemic stroke, pulse pressure ≥50 mm Hg, and TyG index ≥8.0 are all independent risk factors for CAD with T2DM, and the odds ratio (OR) value of these factors were was 1.749, 1.743, 2.960, 2.020, 2.449, 1.717, and 2.641, respectively (all P < .05). The risk of TyG index is higher than that of BMI ≥30 kg/m2, smoking, dyslipidemia, ischemic stroke, and pulse pressure ≥50 mm Hg (Table 4).
Table 4.
Multiple logistic regression model for CAD with T2DM.
| Unadjusted | Multivariate-adjusted | |||||
| Variables | OR | 95% CI | P | OR | 95% CI | P |
| BMI ≥30 kg/m2 | 2.214 | 1.580–3.103 | <.001 | 1.749 | 1.171–2.611 | .006 |
| Smoking | 1.502 | 1.211–1.863 | <.001 | 1.743 | 1.297–2.342 | <.001 |
| Hypertension | 3.353 | 2.706–4.154 | <.001 | 2.960 | 2.193–3.996 | <.001 |
| Dyslipidemia | 2.291 | 1.857–2.827 | <.001 | 2.020 | 1.528–2.671 | <.001 |
| Ischemic stroke | 3.598 | 2.567–5.043 | <.001 | 2.449 | 1.626–3.690 | <.001 |
| Pulse pressure ≥50 mm Hg | 1.952 | 1.581–2.409 | <.001 | 1.717 | 1.264–2.332 | .001 |
| TyG index≥8.0 | 4.168 | 2.292–7.577 | <.001 | 2.641 | 1.154–6.045 | .022 |
Moreover, based on the multivariate logistic regression models that were established for the middle-aged and elderly subgroups, BMI ≥30 kg/m2, smoking, hypertension, dyslipidemia, ischemic stroke, and TyG index ≥8.2 were all independent risk factors for middle-age patients with CAD with T2DM and BMI ≥30 kg/m2, hypertension, dyslipidemia, ischemic stroke, pulse pressure ≥50 mm Hg, and TyG index ≥8.3 were all independent risk factors for elderly patients with CAD with T2DM (all P < .05). The risk of increased TyG index is greater in the middle-aged group (OR 5.732) than in the elderly group (2.841) (Table 5).
Table 5.
Multivariate logistic regression model for the middle-aged and elderly subgroups.
| Unadjusted | Multivariate-adjusted | |||||
| Variables | OR | 95% CI | P | OR | 95% CI | P |
| Middle-aged subgroup | ||||||
| BMI ≥30 kg/m2 | 2.317 | 1.446–3.711 | <.001 | 1.884 | 1.049–3.384 | .034 |
| Smoking | 2.760 | 2.018–3.773 | <.001 | 3.327 | 2.160–5.123 | <.001 |
| Hypertension | 3.335 | 2.414–4.606 | <.001 | 3.302 | 2.117–5.150 | <.001 |
| Dyslipidemia | 2.691 | 1.968–3.679 | <.001 | 2.213 | 1.447–3.385 | <.001 |
| Ischemic stroke | 3.711 | 1.999–6.889 | <.001 | 2.341 | 1.059–5.172 | .036 |
| TyG index ≥8.2 | 10.142 | 4.077–25.230 | <.001 | 5.732 | 1.722–19.075 | .004 |
| Elderly subgroup | ||||||
| BMI ≥30 kg/m2 | 2.498 | 1.487–4.196 | <.001 | 1.969 | 1.091–3.552 | .024 |
| Hypertension | 2.893 | 2.151–3.893 | <.001 | 2.683 | 1.779–4.048 | <.001 |
| Dyslipidemia | 2.381 | 1.756–3.230 | <.001 | 1.679 | 1.128–2.499 | .011 |
| Ischemic stroke | 2.778 | 1.841–4.193 | <.001 | 2.090 | 1.278–3.419 | .003 |
| Pulse pressure ≥50 mm Hg | 1.786 | 1.317–2.423 | <.001 | 1.613 | 1.037–2.510 | .034 |
| TyG index ≥8.3 | 3.703 | 2.302–5.955 | <.001 | 2.841 | 1.495–5.399 | .001 |
Multivariate logistic regression models were also established for the male and female subgroups. BMI ≥30 kg/m2, hypertension, dyslipidemia, ischemic stroke, and TyG index ≥8.3 were independent risk factors for male patients with CAD with T2DM and hypertension, dyslipidemia, ischemic stroke, pulse pressure ≥50 mm Hg, and TyG index ≥8.2 were independent risk factors for female patients with CAD with T2DM (all P < .05). The risk of increased TyG index was greater in the male group (OR 3.935) than in the female group (OR 2.309) (Table 6).
Table 6.
Multiple logistic regression model for the male and female subgroups.
| Unadjusted | Multivariate-adjusted | |||||
| Variables | OR | 95% CI | P | OR | 95% CI | P |
| Male subgroup | ||||||
| BMI ≥30 kg/m2 | 3.528 | 1.993–6.244 | <.001 | 2.884 | 1.468–5.668 | .002 |
| Hypertension | 2.864 | 2.076–3.953 | <.001 | 3.473 | 2.255–5.349 | <.001 |
| Dyslipidemia | 2.548 | 1.819–3.569 | <.001 | 1.872 | 1.199–2.923 | .006 |
| Ischemic stroke | 3.008 | 1.734–5.218 | <.001 | 2.078 | 1.062–4.067 | .033 |
| TyG index ≥8.3 | 5.187 | 3.042–8.845 | <.001 | 3.935 | 1.893–8.178 | <.001 |
| Female subgroup | ||||||
| Hypertension | 3.918 | 2.923–5.253 | <.001 | 3.067 | 2.123–4.432 | <.001 |
| Dyslipidemia | 2.229 | 1.696–2.929 | <.001 | 1.949 | 1.392–2.728 | <.001 |
| Ischemic stroke | 4.199 | 2.731–6.454 | <.001 | 3.123 | 1.942–5.021 | <.001 |
| Pulse pressure ≥50 mm Hg | 2.402 | 1.791–3.222 | <.001 | 1.901 | 1.295–2.789 | .001 |
| TyG index ≥8.2 | 4.155 | 2.327–7.419 | <.001 | 2.309 | 1.127–4.729 | .022 |
4. Discussion
The main findings of this study were that TyG index is an independent risk factor for patients with CAD with T2DM and the median TyG index and the prevalence of increased TyG index are higher in the CAD with T2DM group than in control group. TyG index was first found by Guerrero-Romero to be associated with insulin resistance, and previous studies also showed that its predicted value for insulin resistance may be better than HOMA-IR.[13–15] In insulin resistance, the sensitivity and responsiveness to insulin metabolism weakens, which could in turn lead to vasoconstriction, promote inflammatory response and thrombosis, and accelerate coronary atherosclerosis.[16–18] A previous study also found that TyG index is a predictor of cardiovascular risk and is associated with cardiovascular outcomes in stable CAD with T2DM.[19] Moreover, TyG index is associated with coronary artery stiffness and calcification, influences the formation and progression of atherosclerotic plaque, and is associated with coronary stenosis, cardiovascular disease, and adverse cardiovascular events.[20–22]An observational cohort study found that TyG index is associated with an increased risk of coronary artery stenosis in patients with asymptomatic T2DM.[16] Cho et al[7] showed that TyG index is independently correlated with CAD and obstructive CAD in patients without T2DM and that it increases the risk of CAD. In addition, TyG index plays a potential role in CAD with T2DM. Elevated TyG index indicates insulin resistance, which in turn increases the risk of CAD, promotes the formation and development of coronary atherosclerotic plaque, and accelerates deterioration in CAD with T2DM.
In our study, the multivariate logistic regression models for middle-aged, elderly, male, and female subgroups found that TyG index is an independent risk factor for patients with CAD with T2DM. The risk of increased TyG index was different between the middle-aged and elderly subgroups and between the male and female subgroups. No studies on the risk of TyG index in CAD with T2DM according to different age and sex groups have been conducted; our study is the first to report that the risk of elevated TyG index is greater in the middle-aged and male groups than in the elderly and female groups, respectively.[23,24]
Previous studies have proven that the clinical features of CAD differ between middle-aged and older people as well as between sexes; moreover, some risk factors are different in each group.[25,26] Nakagomi et al[27] reported sex differences in the association between TyG index and arterial stiffness. This may be due to the age increased, the hormone secretion have changed, and body metabolism decreased.[28] There are differences of hormone secretion and composition in male and female, and the ability of lipid metabolism and glucose metabolism in the body are different, so, the tolerance to CAD risk factors and the development of cardiovascular disease may also different.[9,29] Therefore, TyG index can be used to predict CAD patients with T2DM, but we must pay attention to the differences among patients of different ages and genders, to promote making better prevention and treatment programs.
The specificity and the positive predictive value of the TyG index is greater than the sensitivity and the negative predictive value, respectively. The AUC of each group is >0.7, and the diagnostic efficiency of TyG index in the middle-aged subgroup is greater than that in the elderly subgroup; the diagnostic efficiency of TyG index is similar between the female and male subgroups. Thus, the TyG index could be used as a predictive indicator for CAD with T2DM, especially for middle-aged people regardless of sex.
Moreover, TyG index is associated with age, BMI, systolic blood pressure, pulse pressure, TC, LDL-C, leukocyte, and HDL-C. Previous studies found that TyG index could be used as a biomarker for atherosclerosis and is positively correlated with risk factors for CAD, such as BMI, systolic blood pressure, and LDL-C.[30–32] TyG index could be used as a biomarker for insulin resistance in patients with or without diabetes and is also associated with metabolic disorders and vasoconstriction.[33,34] These factors increase the risk of CAD and accelerate the development of CAD. Hence, an increased TyG index plays a potential role in CAD.
This study has some limitations. Firstly, there is a methodological error with biases between groups, with different populations and different vascular risks, which may limit the study. Secondly, as this was a single-center study, all patients were selected from one hospital within a specific period of time. Hence, a multi-regional and multi-ethnic study is needed in the future. Lastly, the sample size of the young population is extremely small, this study only explored the middle-aged and elderly population; thus, an investigation of a large sample including young individuals is warranted.
In conclusion, increased TyG index is an independent risk factor for patients with CAD with T2DM, and the risk differs between the middle-aged and elderly group and between males and females. TyG index could be used as a convenient and inexpensive clinical predictor of CAD with T2DM.
Acknowledgments
The authors are grateful to the assistance provided by the cardiology and radiology doctors and nurses at The Affiliated Hospital of Chengde Medical University.
Author contributions
Conceptualization: Yueqiao Si, Lixian Sun.
Formal analysis: Weichao Shan, Ying Zhang, Lixian Sun.
Funding acquisition: Yueqiao Si, Lixian Sun.
Investigation: Jingyi Liu, Chao Han.
Methodology: Yueqiao Si, Wenjun Fan.
Project administration: Yueqiao Si, Jingyi Liu.
Resources: Jingyi Liu.
Supervision: Yueqiao Si, Wenjun Fan, Chao Han.
Visualization: Yueqiao Si.
Writing – original draft: Yueqiao Si.
Writing – review & editing: Lixian Sun.
Footnotes
Abbreviations: AUC = area under the receiver operating characteristic curve, BMI = body mass index, CAD = coronary artery disease, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, OR = odds ratio, T2DM = type 2 diabetes mellitus, TC = total cholesterol, TyG = triglyceride glucose.
How to cite this article: Si Y, Fan W, Shan W, Zhang Y, Liu J, Han C, Sun L. Association between triglyceride glucose index and coronary artery disease with type 2 diabetes mellitus in middle-aged and elderly people. Medicine. 2021;100:9(e25025).
This study was supported by grants from Hebei Province Government Science and Technology Agency (Grant no. 17277769D) and 2020 Hebei Provincial Department of Education Graduate Innovation Funding Project (Grant no.CXZZSS2020126).
The authors declare that they have no competing interests.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The comparison among CAD with T2DM group, T2DM without CAD and Control group.
CAD with T2DM group compared with Control group. Because TyG index is not statistically significant between the CAD with DM and the DM without CAD group, the T2DM without CAD group were not performed statistical analysis.
BMI = body mass index, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, TC = total cholesterol, TyG = triglyceride glucose.
Data are presented as number (%) of patients, median (interquartile range).
AUC = area under the curve, CI = confidence interval, NPV = negative predictive value, PPV = positive predictive value, Se = sensitivity, Sp = specificity.
P < .01.
P < .001.
BMI = body mass index, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, TC = total cholesterol, TyG = triglyceride glucose.
BMI = body mass index, CI = confidence interval, OR = odds ratio, TyG = triglyceride glucose.
BMI = body mass index, CI = confidence interval, OR = odds ratio, TyG = triglyceride glucose index.
BMI = body mass index, CI = confidence interval, OR = odds ratio, TyG = triglyceride glucose index.
References
- [1].Malakar AK, Choudhury D, Halder B, et al. A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol 2019;234:16812–23. [DOI] [PubMed] [Google Scholar]
- [2].Lehmann N, Erbel R, Mahabadi AA, et al. Value of progression of coronary artery calcification for risk prediction of coronary and cardiovascular events: result of the HNR study (Heinz Nixdorf Recall). Circulation 2018;137:665–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jin JL, Cao YX, Wu LG, et al. Triglyceride glucose index for predicting cardiovascular outcomes in patients with coronary artery disease. J Thorac Dis 2018;10:6137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Navarro-Gonzalez D, Sanchez-Inigo L, Pastrana-Delgado J, et al. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: the Vascular-Metabolic CUN cohort. Prev Med 2016;86:99–105. [DOI] [PubMed] [Google Scholar]
- [5].Sanchez-Inigo L, Navarro-Gonzalez D, Fernandez-Montero A, et al. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest 2016;46:189–97. [DOI] [PubMed] [Google Scholar]
- [6].Park K, Ahn CW, Lee SB, et al. Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care 2019;42:1569–73. [DOI] [PubMed] [Google Scholar]
- [7].Cho YR, Ann SH, Won KB, et al. Association between insulin resistance, hyperglycemia, and coronary artery disease according to the presence of diabetes. Sci Rep 2019;9:6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yamamoto S, Hotta K, Ota E, et al. Effects of resistance training on muscle strength, exercise capacity, and mobility in middle-aged and elderly patients with coronary artery disease: a meta-analysis. J Cardiol 2016;68:125–34. [DOI] [PubMed] [Google Scholar]
- [9].Davies RE, Rier JD. Gender disparities in CAD: women and ischemic heart disease. Curr Atheroscler Rep 2018;20:51. [DOI] [PubMed] [Google Scholar]
- [10].Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: an Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation 2016;134:e123–55. [DOI] [PubMed] [Google Scholar]
- [11].Schoenborn NL, Crossnohere NL, Bridges JFP, et al. Patient perceptions of diabetes guideline frameworks for individualizing glycemic targets. JAMA Intern Med 2019;179:1642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Won KB, Kim YS, Lee BK, et al. The relationship of insulin resistance estimated by triglyceride glucose index and coronary plaque characteristics. Medicine 2018;97:e10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord 2008;6:299–304. [DOI] [PubMed] [Google Scholar]
- [14].Vasques AC, Novaes FS, de Oliveira Mda S, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract 2011;93:e98–100. [DOI] [PubMed] [Google Scholar]
- [15].Lee SH, Kwon HS, Park YM, et al. Predicting the development of diabetes using the product of triglycerides and glucose: the Chungju Metabolic Disease Cohort (CMC) study. PLoS One 2014;9:e90430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee EY, Yang HK, Lee J, et al. Triglyceride glucose index, a marker of insulin resistance, is associated with coronary artery stenosis in asymptomatic subjects with type 2 diabetes. Lipids Health Dis 2016;15:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bonora E, Kiechl S, Willeit J, et al. Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in Caucasian subjects from the general population: the Bruneck study. Diabetes Care 2007;30:318–24. [DOI] [PubMed] [Google Scholar]
- [18].Eddy D, Schlessinger L, Kahn R, et al. Relationship of insulin resistance and related metabolic variables to coronary artery disease: a mathematical analysis. Diabetes Care 2009;32:361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jin JL, Sun D, Cao YX, et al. Triglyceride glucose and haemoglobin glycation index for predicting outcomes in diabetes patients with new-onset, stable coronary artery disease: a nested case-control study. Ann Med 2018;50:576–86. [DOI] [PubMed] [Google Scholar]
- [20].Zhao S, Yu S, Chi C, et al. Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: the Northern Shanghai Study. Cardiovasc Diabetol 2019;18:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lee SB, Ahn CW, Lee BK, et al. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc Diabetol 2018;17:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shi W, Xing L, Jing L, et al. Value of triglyceride-glucose index for the estimation of ischemic stroke risk: insights from a general population. Nutr Metab Cardiovasc Dis 2019;21:30354–0. [DOI] [PubMed] [Google Scholar]
- [23].Lambrinoudaki I, Kazani MV, Armeni E, et al. The TyG index as a marker of subclinical atherosclerosis and arterial stiffness in lean and overweight postmenopausal women. Heart Lung Circ 2018;27:716–24. [DOI] [PubMed] [Google Scholar]
- [24].Arpon A, Milagro FI, Santos JL, et al. Interaction among sex, aging, and epigenetic processes concerning visceral fat, insulin resistance, and dyslipidaemia. Front Endocrinol 2019;10:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ermolao A, Gasperetti A, Rigon A, et al. Comparison of cardiovascular screening guidelines for middle-aged/older adults. Scand J Med Sci Sports 2019;29:1375–82. [DOI] [PubMed] [Google Scholar]
- [26].Wada H, Miyauchi K, Daida H. Gender differences in the clinical features and outcomes of patients with coronary artery disease. Expert Rev Cardiovasc Ther 2019;17:127–33. [DOI] [PubMed] [Google Scholar]
- [27].Nakagomi A, Sunami Y, Kawasaki Y, et al. Sex difference in the association between surrogate markers of insulin resistance and arterial stiffness. J Diabetes Complications 2020;34:107442. [DOI] [PubMed] [Google Scholar]
- [28].Sousa NFDS, Lima MG, Cesar CLG, et al. Active aging: prevalence and gender and age differences in a population-based study. Cad Saude Publica 2018;34:e00173317. [DOI] [PubMed] [Google Scholar]
- [29].Martinez C, Rikhi R, Haque T, et al. Gender identity, hormone therapy, and cardiovascular disease risk. Curr Probl Cardiol 2018;S0146-2806:30142–7. [DOI] [PubMed] [Google Scholar]
- [30].da Silva A, Caldas APS, Hermsdorff HHM, et al. Triglyceride-glucose index is associated with symptomatic coronary artery disease in patients in secondary care. Cardiovasc Diabetol 2019;18:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Salazar J, Bermudez V, Olivar LC, et al. Insulin resistance indices and coronary risk in adults from Maracaibo City, Venezuela: a cross sectional study. F1000Res 2018;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Locateli JC, Lopes WA, Simoes CF, et al. Triglyceride/glucose index is a reliable alternative marker for insulin resistance in South American overweight and obese children and adolescents. J Pediatr Endocrinol Metab 2019;32:1163–70. [DOI] [PubMed] [Google Scholar]
- [33].Angoorani P, Heshmat R, Ejtahed HS, et al. Validity of triglyceride-glucose index as an indicator for metabolic syndrome in children and adolescents: the CASPIAN-V study. Eat Weight Disord 2018;23:877–83. [DOI] [PubMed] [Google Scholar]
- [34].Irace C, Carallo C, Scavelli FB, et al. Markers of insulin resistance and carotid atherosclerosis. A comparison of the homeostasis model assessment and triglyceride glucose index. Int J Clin Pract 2013;67:665–72. [DOI] [PubMed] [Google Scholar]


