Abstract
Sleep disturbance and cognitive impairment now represent two of the most common and debilitating conditions facing seropositive (HIV+) individuals who are otherwise well controlled with antiretroviral therapy. Sleep-assessment-based biomarkers represent an important step towards improving understanding the unique mechanistic features that may link sleep disruption and cognition in HIV+ individuals and can ultimately advance early detection and treatment opportunities in this cohort. In this study, a risk score was computed via a generalized linear model (GLM), which optimally combines polysomnography (PSG) features extracted from EEG, EMG, and EOG signals, to distinguish 18 HIV+ Black male individuals with and without cognitive impairment. The optimal set of features was identified via the least absolute shrinkage and selection operator (LASSO) approach, and the risk separation between the two groups, i.e., cognitively normal and cognitive impaired, was significant (and has a P-value < .001). Interestingly, the optimal set of features were all EEG derived and sleep stage-specific. These preliminary findings suggest that sleep-based EEG markers may be used as a diagnostic and prognostic for cognition in HIV+ patients.
I. INTRODUCTION
With the advent of antiretroviral therapy (ART), the survival rate of seropositive HIV patients has increased dramatically. Several recent studies have shown improving life expectancies over time, among adults receiving ART [1]–[3]. As such, seropositive individuals are now living longer lives but face new challenges centered around more indolent, and debilitating chronic medical and psychiatric conditions, including dementia and sleep impairment [4]–[6]. Over the last two decades, there has been an increased focus on the presence and impact that sleep disturbance plays on the morbidity of HIV+ individuals [7]–[9], with mounting evidence suggesting that sleep disturbance is more common and debilitating in seropositive individuals compared to their seronegative peers [8], [10]. HIV+ individuals demonstrate symptoms of poor sleep at a rate of 30% to 73% [11] compared to 10% of the overall adult population in the community who suffers from poor sleep [12]. While individuals suffering from neurodegenerative diseases can disproportionately suffer from sleep disturbances [13], individuals with chronic sleep disturbance have also been found to be at greater risk of cognitive impairment [14]. The mechanism underlying the inter-relationship between poor sleep, cognitive decline and HIV seropositive is likely complex and multifactorial to include immunologically (vulnerability of blood-brain barrier once exposed to virus and hyperinflammatory state) [15], pharmacologically (side effects of ART therapy) [16], psychologically (higher risk of affective disorder), behaviorally (potential role of at-risk and healthy behaviors i.e. substance abuse, exercise, diet), socially (role of social support, resources, engagement), and even environmentally (sleep-conducive bedroom, neighborhood safety) based variables that influence the manifestation and severity of both sleep disorders and cognitive impairment [17], [18].
HIV-associated neurocognitive disorders have been reported in as high as 50% of all individuals with optimally controlled viral loads due to ART [10] and recent studies have also found a higher rate of insomnia in optimally managed ART patients compared to their seronegative peers. Many of these same studies, however, failed to find definitive features on PSG or subjective sleep questionnaires that predict cognitive function based their performance on neurocognitive assessments. Although Polysomnography (PSG) represents the gold standard for objectively characterizing sleep architecture, PSG does not accurately predict or discriminate between those individuals reporting poor sleep or in those individuals who clinically endorse insomnia diagnostic criteria [19].
Sleep staging which is primarily based on scoring criteria utilizing the macro EEG frequency patterns on the PSG (along with stage-dependent inclusion of eye movements and muscle tone) [20], is performed on every patient who completes the study. Yet, the scored data are not fully exploited to study biomarkers of cognition. The PSG also records several physiologic rhythms beyond brain activity, such as breathing patterns, heart rate variability, eye movements, and muscle tone. These recordings have not been collectively analyzed as a method to potentially develop biomarkers to identify and predict those with clinical complaints of poor sleep and the related symptoms that come with it including cognitive and affective disorders.
In this study, we set out to develop a sleep biomarker to predict those seropositive HIV individuals displaying cognitive impairment, a common functional impairment experienced by insomnia sufferers and individuals with HIV. The method for identifying a sleep biomarker utilizes the various physiologic tracings recorded in a sleep study to predict those individuals who also demonstrate cognitive impairment on a comprehensive neurocognitive assessment. To accomplish our goal, we used scored PSG recordings obtained from 18 HIV+ patients to construct a risk score using a generalized linear model (GLM). The risk score distributions for the cognitively normal and cognitively impaired were found to be separable (p<0.01), and EEG features were found to be most informative of risk.
II. Methods
A. Study Population
Our study population consists of 18 HIV+ patients. All participants were black males with an average age of 49.94 (±6.63) and an average BMI of 27.42 (±5.41). The data contains 13 “good sleepers” (PSQI ≤ 5) and 5 “bad sleepers” (PSQI > 5). All the seropositive HIV+ participants were recruited at Johns Hopkins Medical Institutions (JHMI) from an established HIV-research cohort at JHU [the Northeastern AIDS Dementia (NEAD)], Central Nervous System HIV Antiretroviral Therapy Effects Research (CHARTER) and other available HIV+ patients’ research cohorts. This study was approved by the JHMI IRB and all participants provided informed consent prior to enrollment. A full medical evaluation was conducted to ensure that the participant was medically, cognitively, and psychologically stable to participate. Participants were required to have a relatively low HIV viral load (3000 copies/ml), and those whose combination antiretroviral therapy (cART) regimen included efavirenz were excluded from the study due to its potential sleep-altering effects [4]. Participants were also dropped from the study if they screened positive for recreational drug use during the 2-week protocol.
B. Data Acquisition
The study data were collected between August 2008 and April 2011. The PSG was conducted in the Johns Hopkins Clinical Research Unit followed by 2-week-in-home actigraphy monitoring along with the completion of a full battery of cognitive, sleep, and functional questionnaires. The PSG was conducted by a trained staff of certified sleep technicians. The recording included six EEG channels collected in a contralateral ear reference montage (F3–A2, F4–A1, C3–A2, C4–A1, O1–A2, and O2–A1), two EOG channels, one for each eye (right EOG-A2 and left EOG-A2), three EMG channels (chin, right leg, and left leg) and one ECG channel. All signals were sampled at a sampling rate of 500 Hz. Fig. 1 shows a snapshot of PSG recording for approximately 2 minutes, including 6 EEG signals (i.e. F3–A2, F4–A1, C3–A2, C4–A1, O1–A2, and O2–A1), ECG, right and left EOG signals, EMG and left leg signals.
Fig. 1.
The Snapshot of PSG recording
C. Visual Sleep Stage Scoring
The recorded data were visually scored by an expert clinician in accordance with the American Academy of Sleep Medicine (AASM) Manual for Scoring Sleep [20]. The sleep cycle consists of five sleep stages, three non-rapid eye movement (NREM) stages; stage N1, stage N2, and stage N3, the rapid eye movement (REM) stage, and stage of wakefulness (W). Every 30-second window (epoch) of each patient’s recorded data was assigned a sleep stage by the same technician. All studies were reviewed and finalized by a certified sleep specialist.
D. Labeling
The American Academy of Neurology (AAN) describes two levels of cognitive impairment: minor cognitive motor disorder (MCMD) and the more severe HIV-associated dementia (HAD). The ‘Frascati’ criteria emphasizes that the essential feature of HIV-associated neurocognitive disorders (HAND) is cognitive disturbance; this revision eliminates the possibility of HIV neurocognitive disorders being diagnosed based on neuromotor and noncognitive psychiatric changes such as changes in personality or mood. Frascati describes three syndromes within the framework of HAND: asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND) and HAD. According to the AAN criteria, patients who have impairments in cognitive/behavioral function but no impairments in work or activities of daily living (ADLs) are classified as normal which can cause to miss the opportunity to detect early signs of neurocognitive impairment. The Frascati scale addresses limitations of AAN by identifying and more precisely classifying individuals with milder stages of HAND in terms of ANI and MND. On the other hand, a possible limitation of the Frascati rating scale, is that it relies on the individual’s subjective assessment of their functional status. Under-reporting of functional deficits can thereby occur when there is poor insight, and over-reporting can occur [21].
In this study, both Frascati and AAN cognitive impairment criteria have been adopted to cover each other’s limitations. According to this justification, each subject was categorized as cognitively impaired if both of his score on AAN and Frascati were ≥1. Based on this categorization,11 patients (out of 18) were classified as cognitively impaired and the rest (7 patients) were defined as normal [22].
E. Data Analysis
In this study, we aimed to construct a risk score: COG-Risk. The COG-risk score is a model that relates PSG features to the conditional probability of being cognitively impaired in the HIV+ population. The process of constructing the COG-Risk score involves three major steps: preprocessing to filter PSG signals, extracting features from EEG, EOG and EMG signals as biomarkers and finally, constructing a risk model using stepwise-GLM. Each step is explained in the following:
1). Preprocessing
The electrophysiological signals were filtered using third-order high- and low-pass Butterworth filters. The cutoff frequencies for each signal type were selected based on the AASM criteria [20]. Artifacts, such as those caused by movements, were not removed from the signals before feature extraction because they were considered important characteristics of the wake stage.
2). Feature Extraction
The continuous recordings were divided into non-overlapping 30-second epochs for feature extraction. A large list of features was computed from the PSG signals, including spectral-based features from EEG and EOG signals and energy-based features from EMG signals. Table I lists the complete set of features used in the model, as well as the physiological meaning of each feature. Spindles were detected using the Wendt algorithm [23]. The length of a single spindle was restricted to (0.5–2) seconds. The EOG features are combinations of the cross- and autocorrelations of the two EOG signals [24].
TABLE I.
Extracted Features from PSG Signals
| Feature # | Quantitative feature | Signal | AASM Justification |
|---|---|---|---|
| 1,2 | Delta Power FBI1: (0.5–2) | EEG: F3–A2, F4–A1 | Delta activity |
| 3,4 | Theta Power FBI: (4–7) | EEG: F3–A2, F4–A1 | Theta activity |
| 5,6 | Alpha Power FBI: (8–13) | EEG: F3–A2, F4–A1 | Alpha activity |
| 7,8 | Beta Power FBI: (13–30) | EEG: F3–A2, F4–A1 | Beta activity |
| 9 | Number of Spindles | EEG: C3–A2 | Spindles present |
| 10 | Maximum Spindle Duration | EEG: C3–A2 | Spindles present |
| 11 | EMG Energy | EMG Chin, Left Leg, Right Leg | EMG activity |
| 12:17 | EOG FBI: (0.3–35) | Right EOG and Left EOG | Eye movements present |
| 18:26 | EOG FBI: (0.1–0.3) | Right EOG and Left EOG | Eye movements present |
| 27:35 | EOG FBI: (0.3–0.45) | Right EOG and Left EOG | Eye movements present |
| 36:44 | EOG FBI: (0.1–0.45) | Right EOG and Left EOG | Eye movements present |
Frequency Band of Interest in (Hz).
3). GLM
Since our labels are zeros and ones, we assume them to be observations of Bernoulli random variables. Specifically, each patient i is either cognitively impaired (yi = 1) or normal (yi = 0); and the probability of observing yi = 1 is pi = pr(y = 1). We hypothesize that pi = F(PSG features) and seek to identify an accurate F. The GLM for Bernoulli observations is a prime candidate for finding F and is as follows [25]:
| (1) |
Here, for each epoch of 30 seconds, p is defined as the probability that the patient is cognitively impaired. The cognitive status of each patient was assigned to all epochs of his PSG recording. X is a matrix of the PSG derived features from the epochs of all participants. A constant (intercept) term was added to X as vector of ones. Our objective then was to design g such that the data likelihood function of our patients was maximized (maximum likelihood estimation). The GLM framework ensured a class of functions bounded between 0 and 1. The functions render a concave likelihood function (with a unique global maximum) which can be efficiently maximized over an unknown set of coefficients, β[26].
Applying GLM, we designed sleep stage-dependent models to decrease the influence of feature variety across each sleep stage among individuals and to boost the performance of the overall COG-risk. Five models each for one of the sleep stages including N1, N2, N3, REM, and W were built employing only features of the data from the same sleep stage. For each stage, first features were standardized to zero mean and unit variance to reduce the effects of individual differences; Next, GLM was built by 10-fold cross-validated least absolute shrinkage and selection operator (LASSO) approach. LASSO is a regression analysis method that performs both feature selection and regularization in order to enhance the prediction accuracy and interpretability of the statistical model it produces [27]. Regarding (1), only the features selected by LASSO- GLM from X influence p.
III. Results & Discussion
A. Distinguishability of Features
Through the cross-validated LASSO method, the optimized sleep stage-dependent models selected different features among the total of 44 features; respectively 13, 21, 21,16 and 22 features for N1, N2, N3, R, and W stages. Three features were commonly selected by all models. Table II lists these features, their AASM justifications, and their coefficients. The sign of the coefficient indicates whether there is a positive or negative correlation between each feature and the dependent variable (here the probability of being cognitively impaired). A positive coefficient indicates that as the value of the feature increases, the mean of the dependent variable also tends to increase. Conversely, a negative coefficient suggests that as the feature increases, the dependent variable tends to decrease.
TABLE II.
Features selected commonly among all sleep stages.
| Feature # | Quantitative feature | Signal | AASM Justification | β | ||||
|---|---|---|---|---|---|---|---|---|
| W | ||||||||
| 1 | Theta Power FBI: (4–7) | EEG (F4A1) | Theta activity | 0.56 | ||||
| 2 | Beta Power FBI: (13–30) | EEG (F3A2) | Beta activity | 0.1 | ||||
| 3 | Maximum Spindle Duration FBI: (13–30) | EEG (C3A2) | Spindles present | 0.44 | ||||
The distinguishability of these features among sleep stages can indicate the different patterns of sleep, such as different quality of sleep, among cognitively-impaired HIV+ individuals and those not impaired. Slow-wave and theta activity during sleep reflect important aspects of memory processing, as an evening-to-morning change in declarative memory correlated with delta and theta power during intervening sleep in both groups [28]. Our results suggest that sleep changes contribute to memory impairments by interfering with sleep-dependent memory establishment.
B. Optimized model
The boxplot in Fig. 2 shows the difference between the medians of the two groups, cognitively normal and cognitive impairment. As a result of the optimized model, acquiring the selected features, the separation between the two groups was significant (and has a P-value < .001).
Fig. 2.
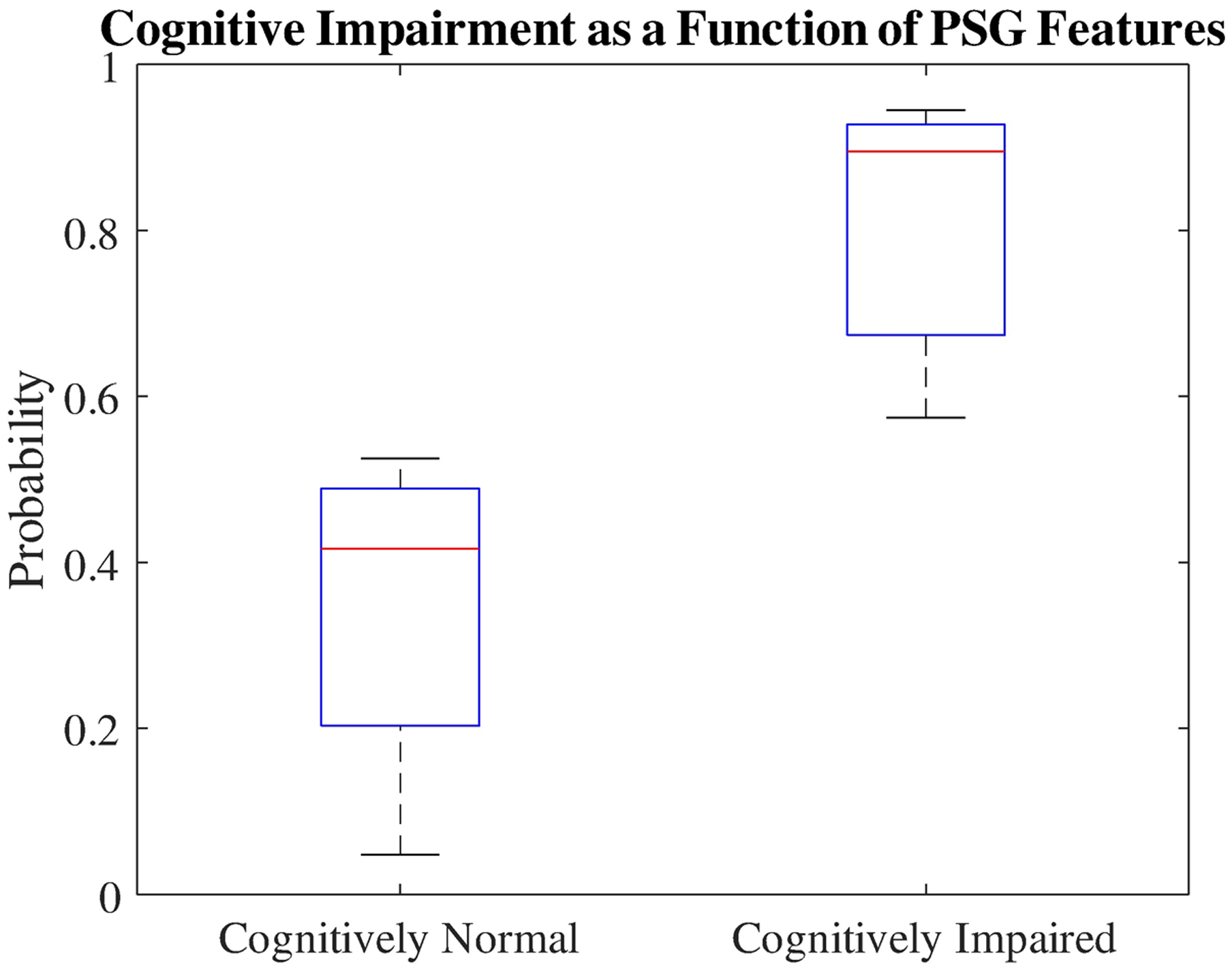
The probability of cognitively normal and impaired patients achieved by COG-risk score.
IV. Conclusion
In this study, we demonstrated an innovative usage of PSG recordings for the purpose of identifying cognitive impairment among individuals with the HIV in an effort to develop an at-risk cognitive biomarkers which can reduce human subjectivity, both on the part of the clinician and patients. We successfully constructed a risk score using GLM for Bernoulli observations, that significantly separates highly functioning HIV+ individuals with and without evidence of cognitive impairment. Cognitive impairment in HIV-infected individuals can be caused by HIV itself or by a combination of HIV in the blood and external factors. HIV+ individuals with cognitive impairment can have variety of symptons and changes in behavior, mood, movement, and/or thinking skills that challenge the early diagnosis of their disorder. Our approach may well have a future impact in the clinical setting by providing a method of early detection of cognitive decline in the HIV population, in a less invasive manner or less expensively than alternative methods particularly when the resource-intensive process of formal and comprehensive neurocognitive assessment is neither available nor feasible.
Strengths & Limitations
We developed and examined our risk score with more than 17000 epochs of PSG recordings. Although this amount of data was sufficient enough for our methodology, it was extracted from a small sample size of 18 participants. Moreover, our sample consisted of only HIV black males. Therefore, generalizing our results should be done with caution. Nonetheless, despite our small sample size, we believe our results demonstrate significant differences in sleep patterns of cognitively impaired HIV+ individuals compare to those are cognitively paired that warrant further investigation in a larger sample.
Acknowledgments
Research supported by The Johns Hopkins Catalyst Award; JHU CFAR NIH/NIAID fund 1P30AI094189-01A1; NSF 1609038.
Footnotes
The final publication is available at https://ieeexplore.ieee.org/abstract/document/9176592
Contributor Information
Hilda Azimi, University of Ottawa, Ottawa, ON, Canada..
Kristin M. Gunnarsdottir, Johns Hopkins University, Baltimore, MD, USA.
Sridevi V. Sarma, Johns Hopkins University, Baltimore, MD, USA..
Alyssa Gamaldo, Penn State - College of Health and Human Development, State College, PA, USA..
Rachel Marie E. Salas, Johns Hopkins School of Medicine, Baltimore, MD, USA..
Charlene Gamaldo, Johns Hopkins School of Medicine, Baltimore, MD, USA..
References
- [1].“Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. - PubMed - NCBI.” [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/24367482. [Accessed: 30-Oct-2019]. [DOI] [PMC free article] [PubMed]
- [2].“Life expectancy among HIV-positive patients in Rwanda: a retrospective observational cohort study. - PubMed - NCBI.” [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/25701995. [Accessed: 30-Oct-2019]. [DOI] [PubMed]
- [3].“Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. - PubMed - NCBI.” [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/18657708. [Accessed: 30-Oct-2019]. [DOI] [PMC free article] [PubMed]
- [4].Darko DF, Mccutchan JA, Kripke DF, Gillin JC, Goishan S, and D P, “Fatigue, sleep disturbance, disability, and indices of progression of HIV infection,” Am. J. Psychiatry, pp. 514–520, 1992. [DOI] [PubMed] [Google Scholar]
- [5].Allavena C et al. , “Prevalence and Risk Factors of Sleep Disturbance in a Large HIV-Infected Adult Population,” AIDS Behav., vol. 20, no. 2, pp. 339–344, February. 2016, doi: 10.1007/s10461-015-1160-5. [DOI] [PubMed] [Google Scholar]
- [6].Phillips KD, Sowell RL, Boyd M, Dudgeon WD, Hand GA, and Mind-Body Research Group, “Sleep quality and health-related quality of life in HIV-infected African-American women of childbearing age,” Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil, vol. 14, no. 4, pp. 959–970, May 2005. [DOI] [PubMed] [Google Scholar]
- [7].Gamaldo CE et al. , “Sleep and cognition in an HIV+ cohort: a multi-method approach,” J. Acquir. Immune Defic. Syndr. 1999, vol. 63, no. 5, August. 2013, doi: 10.1097/QAI.0b013e31829d63ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gamaldo CE et al. , “Sleep, Function and HIV: A Multi-Method Assessment,” AIDS Behav., vol. 17, no. 8, pp. 2808–2815, October. 2013, doi: 10.1007/s10461-012-0401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gamaldo CE and McArthur JC, “The Evaluation and Diagnosis of ‘Insomnia’ in Relation to Sleep Disturbance Prevalence and Impact in Early-Treated HIV-Infected Persons,” Clin. Infect. Dis, vol. 55, no. 10, pp. 1429–1430, November. 2012, doi: 10.1093/cid/cis698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gamaldo CE et al. , “Evaluating sleep and cognition in HIV,” J. Acquir. Immune Defic. Syndr. 1999, vol. 63, no. 5, pp. 609–616, August. 2013, doi: 10.1097/QAI.0b013e31829d63ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Reid S and Dwyer J, “Insomnia in HIV infection: a systematic review of prevalence, correlates, and management,” Psychosom. Med, vol. 67, no. 2, pp. 260–269, April. 2005, doi: 10.1097/01.psy.0000151771.46127.df. [DOI] [PubMed] [Google Scholar]
- [12].Ram S, Seirawan H, Kumar SKS, and Clark GT, “Prevalence and impact of sleep disorders and sleep habits in the United States,” Sleep Breath. Schlaf Atm, vol. 14, no. 1, pp. 63–70, February. 2010, doi: 10.1007/s11325-009-0281-3. [DOI] [PubMed] [Google Scholar]
- [13].“Chronic sleep disturbance and neural injury: links to neurodegenerative disease.” [Online]. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4734786/. [Accessed: 12-Jan-2020]. [DOI] [PMC free article] [PubMed]
- [14].“Assessment of sleep satisfaction in patients with dementia due to Alzheimer’s disease - Journal of Clinical Neuroscience.” [Online]. Available: https://www.jocn-journal.com/article/S0967-5868(14)00486-X/pdf. [Accessed: 12-Jan-2020]. [DOI] [PubMed]
- [15].Ivey NS, MacLean AG, and Lackner AA, “AIDS and the blood-brain barrier,” J. Neurovirol, vol. 15, no. 2, pp. 111–122, April. 2009, doi: 10.1080/13550280902769764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Montessori V, Press N, Harris M, Akagi L, and Montaner JSG, “Adverse effects of antiretroviral therapy for HIV infection,” CMAJ, vol. 170, no. 2, pp. 229–238, January. 2004. [PMC free article] [PubMed] [Google Scholar]
- [17].Nath A, Schiess N, Venkatesan A, Rumbaugh J, Sacktor N, and Mcarthur J, “Evolution of HIV dementia with HIV infection,” Int. Rev. Psychiatry, vol. 20, no. 1, pp. 25–31, January. 2008, doi: 10.1080/09540260701861930. [DOI] [PubMed] [Google Scholar]
- [18].Nokes KM and Kendrew J, “Correlates of Sleep Quality in Persons With HIV Disease,” J. Assoc. Nurses AIDS Care, vol. 12, no. 1, pp. 17–22, January. 2001, doi: 10.1016/S1055-3290(06)60167-2. [DOI] [PubMed] [Google Scholar]
- [19].Moyle G, Fletcher C, Brown H, Mandalia S, and Gazzard B, “Changes in sleep quality and brain wave patterns following initiation of an efavirenz-containing triple antiretroviral regimen,” HIV Med., vol. 7, pp. 243–7, June. 2006, doi: 10.1111/j.1468-1293.2006.00363.x. [DOI] [PubMed] [Google Scholar]
- [20].Berry RB et al. , “AASM Scoring Manual Updates for 2017 (Version 2.4),” J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med, vol. 13, no. 5, pp. 665–666, May 2017, doi: 10.5664/jcsm.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gandhi NS et al. , “Comparison of scales to evaluate the progression of HIV-associated neurocognitive disorder,” HIV Ther., vol. 4, no. 3, pp. 371–379, May 2010, doi: 10.2217/hiv.10.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].“Clinical-neuropathologic correlation in HIV-associated dementia | Neurology.” [Online]. Available: https://n.neurology.org/content/43/11/2230.short. [Accessed: 12-Jan-2020]. [DOI] [PubMed]
- [23].Wendt SL, Christensen JAE, Kempfner J, Leonthin HL, Jennum P, and Sorensen HBD, “Validation of a novel automatic sleep spindle detector with high performance during sleep in middle aged subjects,” Conf. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Conf, vol. 2012, pp. 4250–4253, 2012, doi: 10.1109/EMBC.2012.6346905. [DOI] [PubMed] [Google Scholar]
- [24].“A Novel Sleep Stage Scoring System: Combining Expert-Based Rules with a Decision Tree Classifier. - PubMed - NCBI.” [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/30441082. [Accessed: 15-Oct-2019]. [DOI] [PMC free article] [PubMed]
- [25].Dobson AJ, An introduction to generalized linear models, 2. ed. Boca Raton, Fla.: Chapman & Hall/CRC, 2002. [Google Scholar]
- [26].McCullagh P, Nelder JA: Generalized linear models. Chapman and Hall; London – New York: 1983, 261 S., £ 16,- - Enderlein - 1987 - Biometrical Journal - Wiley Online Library.” [Online]. Available: https://onlinelibrary.wiley.com/doi/abs/10.1002/bimj.4710290217. [Accessed: 12-Jan-2020]. [Google Scholar]
- [27].Tibshirani R, “Regression Shrinkage and Selection via the Lasso,” J. R. Stat. Soc. Ser. B Methodol, vol. 58, no. 1, pp. 267–288, 1996. [Google Scholar]
- [28].Westerberg CE et al. , “Concurrent impairments in sleep and memory in amnestic mild cognitive impairment,” J. Int. Neuropsychol. Soc. JINS, vol. 18, no. 3, pp. 490–500, May 2012, doi: 10.1017/S135561771200001X. [DOI] [PMC free article] [PubMed] [Google Scholar]


