Supplemental Digital Content is available in the text.
Abstract
Background:
A number of studies have linked long-term exposure to particulate matter with aerodynamic diameter <2.5 µm (PM2.5) with mortality, but most of these studies were conducted in Europe and North America. Studies in Asian countries had been conducted at relatively high exposures. We evaluated the association of long-term exposure to PM2.5 and natural-cause and cause-specific mortality in Japan, where PM2.5 levels are relatively low compared with levels in other Asian countries.
Methods:
A cohort of 75,531 participants underwent basic health checkups in Okayama City in 2006 or 2007. We followed the participants until the end of 2016. Average PM2.5 levels from 2006 to 2010 were obtained and assigned to the participants by geographical location. We used the Cox proportional hazard models to estimate hazard ratios for a 5-μg/m3 increase in PM2.5 levels for natural-cause or cause-specific mortality, adjusting for potential confounders.
Results:
PM2.5 exposure was associated with increased risk of mortality; the hazard ratios were 1.29 (95% confidence interval = 1.18, 1.41) for mortality from natural causes, 1.16 (1.02, 1.32) for cardiorespiratory mortality, and 1.63 (1.13, 2.34) for lung cancer mortality. PM2.5 exposure was more strongly associated with cardiorespiratory mortality from hypertension, pneumonia and influenza, and chronic obstructive pulmonary disease than with ischemic heart disease or cerebrovascular disease. Elderly participants and smokers tended to have higher effect estimates.
Conclusion:
Long-term exposure to PM2.5 can increase the risk of natural-cause, cardiorespiratory, and lung cancer mortality in Japan.
What this study adds
This study shows that long-term exposure to PM2.5 can increase the risk of natural-cause, cardiorespiratory, and lung cancer mortality in Japan, where PM2.5 levels are relatively low compared with levels in other Asian countries.
Introduction
A number of studies have linked long-term exposure to air pollution, especially particulate matter with aerodynamic diameter <2.5 µm (PM2.5), with all-cause, cardiorespiratory, and lung cancer mortality.1 Most of these studies, however, were conducted in Europe and North America.2–5 Asian countries differ from Western countries in lifestyle, disease incidence, and pollution levels, and evidence on the health effects of long-term exposure to PM2.5 in Asian countries is still limited.
Several epidemiological studies in Asian countries have evaluated the effect of long-term exposure to air pollution on mortality.6–11 For example, a cohort study from Hong Kong utilized PM2.5 data from satellite surveillance (median = 35.3 µg/m3) or a land-use regression model (median = 42.2 µg/m3) to demonstrate that PM2.5 exposure increased the risk of mortality from all natural and cardiovascular causes.8,9 A Chinese cohort study reported positive associations between PM2.5 exposure and nonaccidental, cardiorespiratory, and lung cancer mortality using PM2.5 data (mean = 43.7 µg/m3) from the Global Burden of Disease project.10 These studies, however, were conducted in areas with relatively higher PM2.5 levels, and studies in areas of lower PM2.5 levels are needed to assess exposure-response functions between PM2.5 and mortality in Asian countries.
In the present study, we evaluated the association of long-term exposure to PM2.5 and natural-cause and cause-specific mortality in Japan, where PM2.5 levels are relatively lower than in other Asian countries.
Methods
Participants
We followed up a cohort of 76,591 individuals who were enrolled in basic health checkups in Okayama City, Japan, in the fiscal year of 2006 or 2007 (i.e., between April 2006 and March 2008). Okayama City is an urbanized area in the western part of Japan, with a population exceeding 0.7 million. These basic health checkups were conducted under the Health and Medical Service Act for the Aged and were provided to residents who were >40 years of age to screen for medical conditions such as cardiovascular disease. The residents eligible for the basic health checkups were those covered by National Health Insurance, which is 1 of 2 types of health insurance system available in Japan, whereas the other is Employee’s Health Insurance (employment-based health insurance). For example, about 50.4% of the residents in Okayama City were eligible for the basic health checkups in the fiscal year of 2006 and about 34.2% of the eligible participants participated in the health checkups.12 Medical history, self-rated health, lifestyle habits, and activity were self-reported from participants. Physical assessment (height, weight, and blood pressure at rest), blood analysis (complete blood count and biochemical blood tests), urinalysis (urine protein, blood, and sugar), and electrocardiography were conducted for each participant in medical facilities. We followed the participants for survival until the end of 2016. Twenty-two participants were excluded because we could not ascertain their survival status or cause of death, leaving 76,569 participants in the analysis.
Air pollution measurements
We focused on PM2.5 as the main air pollutant in the present study. We used the information on annual modeled PM2.5 data provided by the Atmospheric Composition Analysis Group at Dalhousie University.13 The group provided ground-level PM2.5 concentration data estimated by combining aerosol optical depth with the GEOS-Chem chemical transport model and calibrated to global ground-based observations of PM2.5 using geographically weighted regression. After obtaining the dust- and sea salt- removed PM2.5 global estimates (approximately 1 × 1 km), we calculated annual average PM2.5 concentrations for each census area in Okayama City between 2006 and 2010. For privacy reasons, we could not obtain the exact address of each participant, and used census-level information instead. The census area, approximately corresponding to the area code, is the smallest area used for the National Census, and its median size in Okayama City is 0.19 km2. After obtaining participants’ locations at the census level, we assigned modeled PM2.5 concentrations in each census to the participants and used the 5-year average concentrations (2006–2010) as the main exposure indicator. We excluded 1,038 participants who were assigned negative PM2.5 concentrations in any of these years, leaving 75,531 participants living in the 765 census areas in the analysis. The median number of the participants living in the 765 census areas was 63 and the number ranged from 1 to 1,541.
Mortality
After identifying the survival status of the participants, we evaluated the causes of death for deceased participants by linking records to the vital statistics database of the Ministry of Health, Labor, and Welfare in Japan. We focused on natural-cause and cause-specific mortality. The underlying causes of death were coded according to the 10th International Classification of Diseases (ICD-10). The numbers of deaths from natural causes (ICD-10 code: A00-R99), cardiorespiratory disease (I10-69/J00-J99), and lung cancer (C33-C34) were determined. We also focused on specific causes of death for cardiorespiratory disease mortality as follows: Circulatory disease (I10-69), ischemic heart disease (IHD) (I20-I25), other cardiac diseases (such as dysrhythmias, heart failure, or cardiac arrest) (I26-51), cerebrovascular disease (I60-69), respiratory disease (J00-J99), pneumonia and influenza (J10-22), and chronic obstructive pulmonary disease (COPD) and allied conditions (J40-47).
Statistical analysis
We used Cox proportional hazard models to estimate hazard ratios (HRs) for a 5-μg/m3 increase in PM2.5 levels for natural-cause or cause-specific mortality, adjusting for individual-level potential confounders and area-level socioeconomic status in the model. For privacy reasons, we only could obtain the month of death or censorship. For each study participant, therefore, person-years were counted from April 2006 to the month of death or censorship (i.e., move to other municipalities or the end of the study in December 2016), whichever occurred first. Although we do not know the exact dates when participants underwent the basic health checkup, we assumed that they were already living in the corresponding census area in April 2006.
We first adjusted for the age group (<50, 50 to <60, 60 to <70, 70 to <80, or ≥80 years) and sex to evaluate the association between PM2.5 exposure and natural-cause or cause-specific mortality (cardiorespiratory disease and lung cancer). We then adjusted for other individual-level potential confounders such as examination year (dichotomous; 2006 or 2007), current disease status (i.e., taking treatment) for hypertension (dichotomous) and diabetes mellitus (dichotomous), effort to reduce dietary salt intake (dichotomous), effort to consume more vegetables (dichotomous), alcohol consumption (dichotomous; drinker including regular and occasional drinker or never drinker), smoking status (dichotomous; current smoker or not), smoking quantity of smokers (number of smoking per day) (continuous), squared quantity of smokers (continuous), smoking years of smokers (continuous), squared smoking years of smokers (continuous), regular exercise (dichotomous; having regular exercise of more than 30 minutes per day for more than 1 year or not), height (continuous), body mass index (categorical; quartile), and total cholesterol (continuous). Body mass index was defined as body weight (kg) divided by height squared (m2). We included the height variable to reflect individual socioeconomic status14–16 and total cholesterol because it has been shown to be associated with a lower risk of mortality, possibly owing to inflammatory or nutritional processes.17 Efforts to reduce dietary salt intake and consume more vegetables were obtained from the self-reported questions related to dietary awareness of the participants. All individual-level variables were obtained at the baseline health checkup and the variables other than examination year, height, body mass index, and total cholesterol were self-reported. We then adjusted for the proportion of white-collar workers over 15 years of age in the census as an indicator of area-level socioeconomic status in the model. We defined managerial, professional or technical, and clerical workers as white-collar workers, while sales, service, security, agriculture, fishery, manufacturing process, transport, construction, carrying, and others as nonwhite-collar workers. The data were obtained from the 2015 National Census.
In subsequent analyses, we entered the quartile PM2.5 exposure into the model instead of the linear PM2.5 format. Moreover, we estimated HRs for specific causes of death for cardiorespiratory disease mortality (defined above) in the fully-adjusted model. We also examined the presence of effect modification by stratifying the participants by age (<70 or ≥70 years), sex (male or female), current smoking status (smoker or not), body mass index (above or below the median value of 22.6), hypertension, and diabetes mellitus; P-values for interaction were calculated.
In the sensitivity analysis, we used the concentration in 2006 and the 2-year average of the concentrations from 2006 and 2007 as alternative exposure indicators. Moreover, most of the heart failure (ICD-10 code: I50) mortality are considered to be reclassified as IHD mortality,18 so we combined the heart failure mortality with the category of IHD mortality and repeated the analysis.
All confidence intervals (CIs) were calculated at the 95% level, and P-values for interaction smaller than 0.05 were considered significant. Stata SE version 15 (StataCorp, College Station, Texas) was used for all analyses. The study was approved by the Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences Institutional Review Board (No. 1801-034).
Results
The average values of PM2.5 concentration in the census areas (with SD) were 14.0 (1.0) μg/m3, and PM2.5 levels ranged from 12.2 to 15.3 μg/m3 (Figure 1). Mean age of the participants was 69.9 years, and more women (67.9%) were enrolled (Table 1). Although the participants from the lowest PM2.5 quartile were the oldest, those from the highest PM2.5 quartile tended to smoke, drink alcohol, attempt to consume more vegetables, exercise regularly, have higher total cholesterol levels, and live in the areas with higher proportions of white-collar workers. Age-standardized mortality rates for natural-cause and cause-specific mortality stratified by sex and the PM2.5 quartile are shown in eTable 1; http://links.lww.com/EE/A45 and women had lower mortality in any cause.
Figure 1.

A map of study area and fine particulate matter (PM2.5) exposure distribution.
Table 1.
Demographic characteristics of study participants by quartile fine particulate matter exposure (n = 75,531)
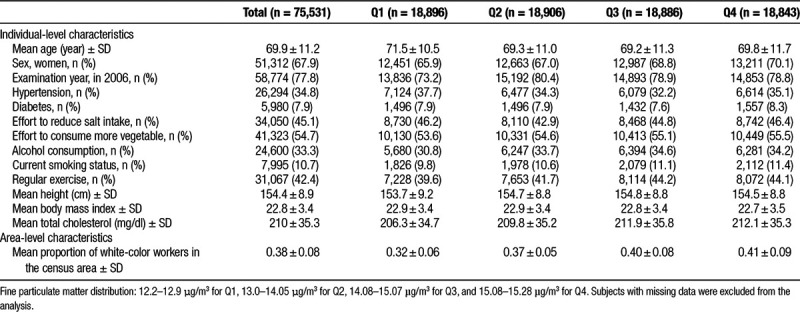
Table 2 shows the HRs for natural-cause and cause-specific mortality. In the crude models, PM2.5 was not associated with increased risk of mortality. After adjusting for potential confounders, however, the elevated risks became apparent, and PM2.5 was associated with increased risk of natural-cause and cause-specific mortality; the HRs for a 5-μg/m3 increase in PM2.5 were 1.29 (95% CI = 1.18, 1.41) for all-cause mortality, 1.16 (1.02, 1.32) for cardiorespiratory mortality, and 1.63 (1.13, 2.34) for lung cancer mortality. When we used the quartile category for PM2.5, the risk increased almost linearly, especially for mortality from natural causes (Figure 2).
Table 2.
Adjusted HRs for a 5-μg/m3 increase in fine particulate matter and 95% CIs for natural-cause and cause-specific mortality (n = 75,531)
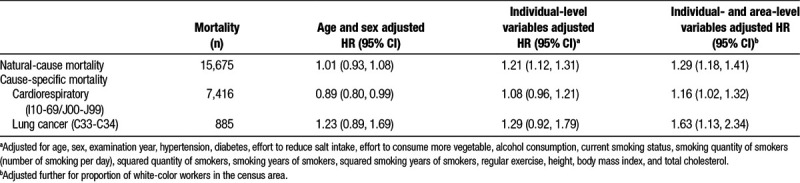
Figure 2.
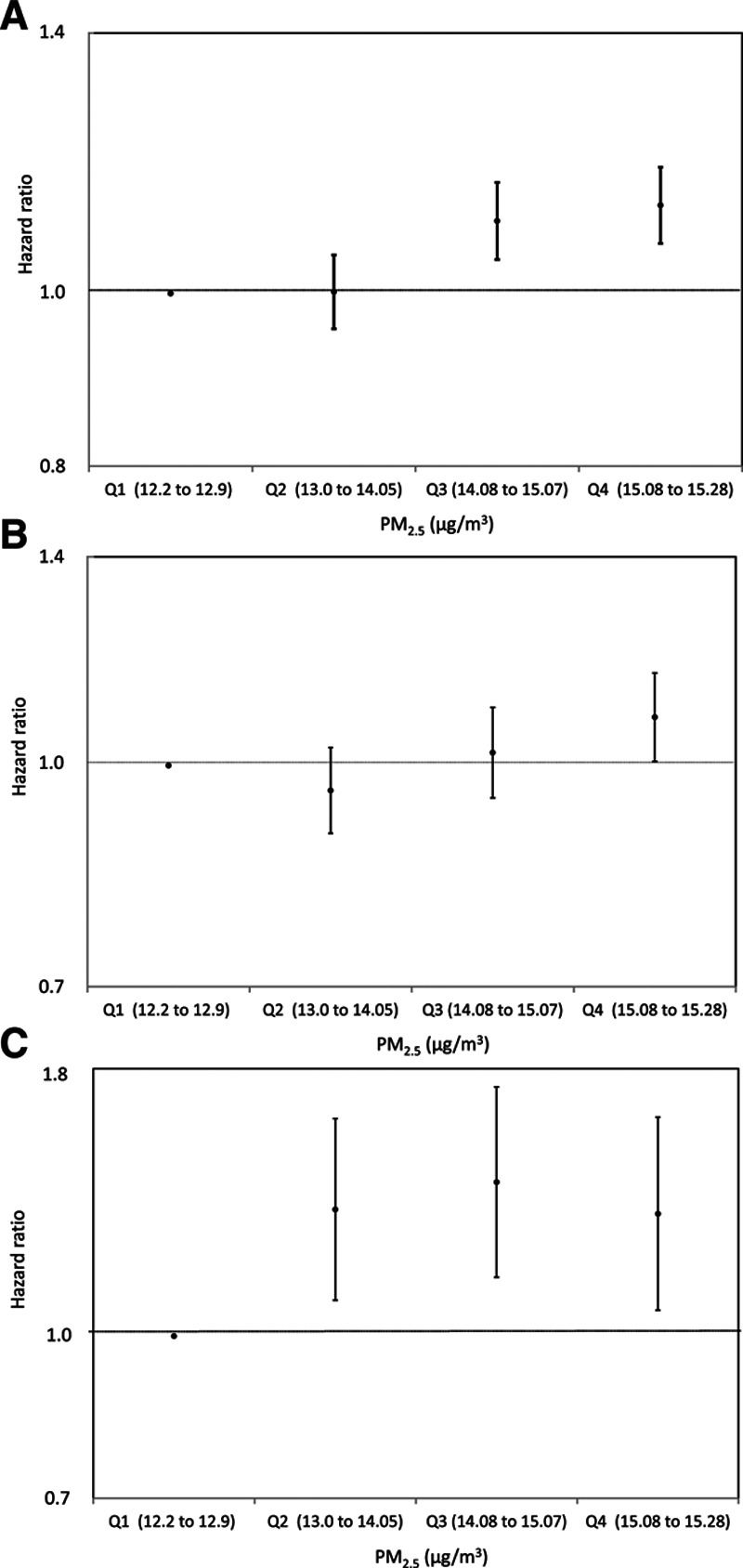
HRs for the association of quartile fine particulate matter (PM2.5) exposure and (A) natural-cause, (B) cardiorespiratory disease, and (C) lung cancer mortality. Error bars denote the 95% CIs.
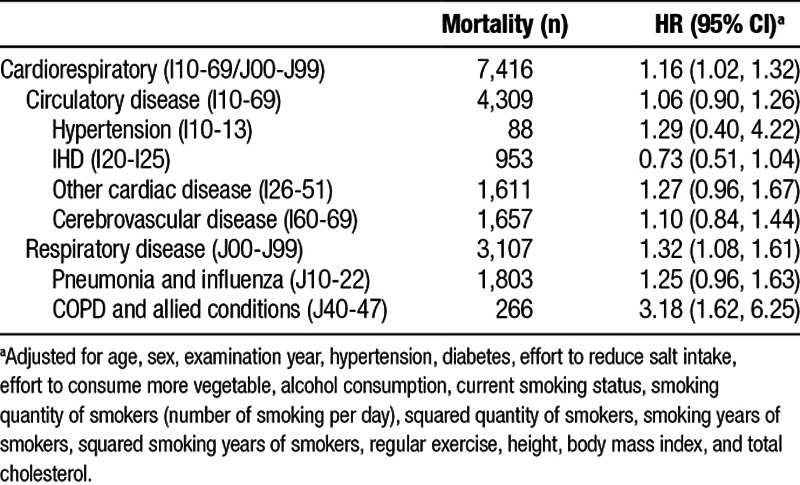
Table 3 shows the HRs for the specific causes of death. PM2.5 exposure was more strongly associated with hypertension, pneumonia and influenza, and COPD; the HR for COPD and allied conditions was 3.18 (1.62, 6.25).
Table 3.
Adjusted HRs for a 5-μg/m3 increase in fine particulate matter and 95% CIs for cardiorespiratory mortality (n = 75,531)
When we stratified the participants by individual factors, the elderly and smokers tended to have higher effect estimates, and P-values for interaction were statistically significant (Figure 3).
Figure 3.

Association of fine particulate matter (PM2.5) exposure and natural-cause mortality, stratified by participant characteristics. P-values for interaction are shown. BMI, body mass index; DM, diabetes mellitus; HTN, hypertension. Error bars denote the 95% CIs.
The PM2.5 concentration in the census areas declined gradually. The concentrations of 2006, the 2-year average of 2006 and 2007, and the 5-year average (2012–2016) were 15.3, 14.8, and 13.5 μg/m3, respectively, and all were strongly correlated with the main exposure indicator in the present study, i.e., correlation coefficients with 5-year average concentrations from 2006 to 2010 were 0.99 for all. When we used the concentration in 2006 and the 2-year average of the concentrations from 2006 and 2007 as alternative exposure indicators in the sensitivity analysis, the results did not change substantially (eTable 2; http://links.lww.com/EE/A45). Finally, when we combined the heart failure mortality with the IHD mortality, the HRs for a 5-μg/m3 increase in PM2.5 was 1.08 (95% CI = 0.84, 1.37).
Discussion
In the present study, we evaluated the association of long-term exposure to PM2.5 and natural-cause and cause-specific mortality in Okayama, Japan. We found that long-term exposure was associated with increased risk of natural-cause, cardiorespiratory, and lung cancer mortality. For cardiorespiratory mortality, PM2.5 exposure was more strongly associated with mortality from respiratory disease, hypertension, pneumonia and influenza, and COPD than with other causes. Elderly and smoking participants tended to have higher effect estimates.
The positive associations of PM2.5 and natural-cause and cardiorespiratory mortality are consistent with those reported by previous studies in both Western and Asian countries,1,8–10 but the effect estimates obtained in the present study were higher: The percent excess risk estimated for natural-cause mortality was 29% per 5 μg/m3 (or 66% per 10 μg/m3), much higher than the estimates from an American cancer cohort study (4% per 10 μg/m3)5 and Medicare cohort (7.3% per 10 μg/m3),3 the ESCAPE study in Europe (7% per 5 μg/m3),2 and studies conducted in Hong Kong (14% or 6% per 10 μg/m3)8,9 and China (9% per 10 μg/m3)10 in Asian individuals. The differences in exposure assessment, concentrations in PM2.5 (e.g., the lower PM2.5 concentrations in Japan than in other Asian countries), particle composition, disease pattern between Western and Asian countries, the associations between PM2.5 and specific causes of diseases (e.g., the stronger association with mortality from respiratory than circulatory disease in the present study), or the demographic characteristics may act together to explain the differences in the effect estimates. For example, our study participants had larger fraction of elderly or female participants compared to the ESCAPE study2 or the study in China10 or lower BMI compared to the ESCAPE study,2 the Medicare cohort,3 or the study in Hong Kong.8,9 As we observed below, although some of the interactions were not significant, elderly or female participants and the participants with lower body mass index tended to have higher effect estimates compared to the others in the present study. But, these explanations would not fully explain the higher effect estimates in the present study and further studies in Japan are needed to examine this. The elevated risk of lung cancer is also consistent with the conclusions of the International Agency for Research on Cancer on the carcinogenicity of PM2.5,19 a meta-analysis,20 and other studies conducted in China10 and Japan.21
In relation to specific cardiorespiratory causes of death, PM2.5 was more strongly associated with respiratory disease (i.e., pneumonia and COPD) than with cardiac and cerebrovascular disease. Although a study based in North America also suggested a positive association between PM2.5 exposure and respiratory disease mortality,22 most studies in Western countries found a stronger effect estimate for IHD, followed by cerebrovascular disease and respiratory disease.22,23 Although a study from Hong Kong also observed a high HR for IHD and did not find a positive association with respiratory disease,8 a Chinese study in men observed stronger effects for COPD or stroke than for IHD.10 Moreover, a Japanese cohort study that converted larger particles to PM2.5 using a conversion factor reported positive associations of particles with lung cancer and respiratory disease mortality.21 These contrasting findings between Western and Asian countries may also be driven by differences in exposure assessment, particle composition, disease prevalence, diagnosis of the diseases, or study populations (e.g., lower body mass index among Asians). But, in the sensitivity analysis that combined the heart failure mortality with the IHD mortality, the effect estimates were elevated and consistent with studies in Western countries.23 Also, our previous cohort study in Japan that evaluated long-term exposure to nitrogen dioxide using a land-use regression model demonstrated elevated HRs for IHD11; more refined exposure modeling may therefore provide other insights in the Asian setting.
Moreover, in the present study, elderly and smoking participants tended to have higher effect estimates for mortality from natural causes, which may highlight the vulnerability of these populations to air pollution. Although a previous review did not note similar effect modifications,23 some studies demonstrated that smoking and air pollution had greater than additive effects,24–26 consistent with our study. Future studies are warranted to investigate this.
The present study has several strengths. First, selection bias would be small, because we could identify the survival status of most of the participants. Second, background information obtained by baseline questionnaire enabled us to adjust for potential confounders extensively.
In contrast, several limitations should be noted. First, there is a possibility of exposure misclassification because we could only assign PM2.5 exposure to the census level for privacy reasons. This misclassification would be predominantly a Berkson error, making the effect estimates less precise.27 Second, we used the 5-year average concentrations from 2006 to 2010 as a main exposure indicator and did not account for yearly changes in PM2.5, assuming that the spatial pattern in PM2.5 was preserved in the study area. Although the concentrations in PM2.5 declined gradually, the observed correlation coefficients between concentrations in different years were high, and Okayama City was geographically stable and well-developed. Also, the sensitivity analyses which used the alternative exposure indicators were robust (eTable 2; http://links.lww.com/EE/A45). Thus, our assumption would be reasonable. Third, follow-up information was available on a monthly basis only, but this nondifferential outcome misclassification would only move the effect estimates toward the null. Finally, residual confounding is possible because individual-level potential confounders were mostly collected by self-report and we could not obtain individual-level socioeconomic variables. We adjusted, however, for the proportion of white-collar workers and for height as an indicator of area-level and individual socioeconomic status in the main analyses, respectively.15,16
Conclusion
The present study shows that long-term exposure to PM2.5 can increase the risk of natural-cause, cardiorespiratory, and lung cancer mortality in Japan, where PM2.5 levels are relatively lower than in other Asian countries. PM2.5 was more strongly associated with respiratory outcomes, which is inconsistent with findings from studies in Europe and North America. Future work leveraging more precise exposure measurements may provide further insights, but this study offers compelling evidence on the harmful effects of PM2.5.
Conflict of interest statement
The authors declare that they have no conflicts of interest with regard to the content of this report.
ACKNOWLEDGMENTS
We appreciate the valuable support of Hiroaki Matsuoka and Saori Irie in collecting the data. We thank Dean Meyer, PhD, ELS from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Supplementary Material
Footnotes
Published online 20 May 2019
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.environepidem.com).
The results reported herein correspond to specific aims of grant No. 17K09085 to investigator T.Y. from JSPS KAKENHI. The sponsor has had no involvement in the study design, the collection, analysis, and interpretation of data, the writing of the report, or the decision to submit the article for publication.
Data: The data are not available to other researchers because we do not have any right to disclose the data.
References
- 1.World Health Organization. Denmark: WHO Regional Office for Europe; 2013. Review of evidence on health aspects of air pollution—REVIHAAP Project: final technical report. [PubMed] [Google Scholar]
- 2.Beelen R, Raaschou-Nielsen O, Stafoggia M, et al. Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet 2014383785–795 [DOI] [PubMed] [Google Scholar]
- 3.Di Q, Wang Y, Zanobetti A, et al. Air pollution and mortality in the Medicare population. N Engl J Med 20173762513–2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dockery DW, Pope CA, III, Xu X, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med 19933291753–1759 [DOI] [PubMed] [Google Scholar]
- 5.Pope CA, III, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 20022871132–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao J, Yang C, Li J, et al. Association between long-term exposure to outdoor air pollution and mortality in China: a cohort study. J Hazard Mater 20111861594–1600 [DOI] [PubMed] [Google Scholar]
- 7.Tseng E, Ho WC, Lin MH, Cheng TJ, Chen PC, Lin HH. Chronic exposure to particulate matter and risk of cardiovascular mortality: cohort study from Taiwan. BMC Public Health 201515936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong CM, Lai HK, Tsang H, et al. Satellite-based estimates of long-term exposure to fine particles and association with mortality in elderly Hong Kong residents. Environ Health Perspect 20151231167–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y, Tang R, Qiu H, et al. Long term exposure to air pollution and mortality in an elderly cohort in Hong Kong. Environ Int 201811799–106 [DOI] [PubMed] [Google Scholar]
- 10.Yin P, Brauer M, Cohen A, et al. Long-term fine particulate matter exposure and nonaccidental and cause-specific mortality in a large national cohort of Chinese men. Environ Health Perspect 2017125117002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yorifuji T, Kashima S, Tsuda T, et al. Long-term exposure to traffic-related air pollution and the risk of death from hemorrhagic stroke and lung cancer in Shizuoka, Japan. Sci Total Environ 2013443397–402 [DOI] [PubMed] [Google Scholar]
- 12.Okayama Prefecture. Basic health checkups [in Japanese]. Available at: http://www.pref.okayama.jp/page/357398.html. Accessed February 2, 2019.
- 13.van Donkelaar A, Martin RV, Brauer M, et al. Global estimates of fine particulate matter using a combined geophysical-statistical method with information from satellites, models, and monitors. Environ Sci Technol 2016503762–3772 [DOI] [PubMed] [Google Scholar]
- 14.Honjo K, Iso H, Inoue M, Tsugane S. Adult height and the risk of cardiovascular disease among middle aged men and women in Japan. Eur J Epidemiol 20112613–21 [DOI] [PubMed] [Google Scholar]
- 15.Tyrrell J, Jones SE, Beaumont R, et al. Height, body mass index, and socioeconomic status: mendelian randomisation study in UK Biobank. BMJ 2016352i582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magnusson PK, Rasmussen F, Gyllensten UB. Height at age 18 years is a strong predictor of attained education later in life: cohort study of over 950,000 Swedish men. Int J Epidemiol 200635658–663 [DOI] [PubMed] [Google Scholar]
- 17.Liang Y, Vetrano DL, Qiu C. Serum total cholesterol and risk of cardiovascular and non-cardiovascular mortality in old age: a population-based study. BMC Geriatr 201717294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens GA, King G, Shibuya K. Deaths from heart failure: using coarsened exact matching to correct cause-of-death statistics. Popul Health Metr 201086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loomis D, Grosse Y, Lauby-Secretan B, et al. ; International Agency for Research on Cancer Monograph Working Group IARC The carcinogenicity of outdoor air pollution. Lancet Oncol 2013141262–1263 [DOI] [PubMed] [Google Scholar]
- 20.Hamra GB, Guha N, Cohen A, et al. Outdoor particulate matter exposure and lung cancer: a systematic review and meta-analysis. Environ Health Perspect 2014122906–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katanoda K, Sobue T, Satoh H, et al. An association between long-term exposure to ambient air pollution and mortality from lung cancer and respiratory diseases in Japan. J Epidemiol 201121132–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pun VC, Kazemiparkouhi F, Manjourides J, Suh HH. Long-term PM2.5 exposure and respiratory, cancer, and cardiovascular mortality in older US adults. Am J Epidemiol 2017186961–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoek G, Krishnan RM, Beelen R, et al. Long-term air pollution exposure and cardio- respiratory mortality: a review. Environ Health 20131243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu K, Qiu G, Chan KH, et al. Association of solid fuel use with risk of cardiovascular and all-cause mortality in rural China. JAMA 20183191351–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner MC, Cohen A, Jerrett M, et al. Interactions between cigarette smoking and fine particulate matter in the Risk of Lung Cancer Mortality in Cancer Prevention Study II. Am J Epidemiol 20141801145–1149 [DOI] [PubMed] [Google Scholar]
- 26.Turner MC, Cohen A, Burnett RT, et al. Interactions between cigarette smoking and ambient PM2.5 for cardiovascular mortality. Environ Res 2017154304–310 [DOI] [PubMed] [Google Scholar]
- 27.Armstrong BG. Effect of measurement error on epidemiological studies of environmental and occupational exposures. Occup Environ Med 199855651–656 [DOI] [PMC free article] [PubMed] [Google Scholar]


