Supplemental Digital Content is available in the text.
Background:
Anemia is highly prevalent in India, especially in children. Exposure to ambient fine particulate matter (PM2.5) is a potential risk factor for anemia via. systemic inflammation. Using health data from the National Family and Health Survey 2015–2016, we examined the association between ambient PM2.5 exposure and anemia in children under five across India through district-level ecological and individual-level analyses.
Methods:
The ecological analysis assessed average hemoglobin levels and anemia prevalence (hemoglobin < 11 g/dL considered anemic) by district using multiple linear regression models. The individual-level analysis assessed average individual hemoglobin level and anemia status (yes/no) using generalized linear mixed models to account for clustering by district. Ambient PM2.5 exposure data were derived from the Multiangle Imaging SpectroRadiometer (MISR) level 2 aerosol optical depth (AOD) data and averaged from birth date to date of interview.
Results:
The district-level ecological analysis found that, for every 10 μg m–3 increase in ambient PM2.5 exposure, average anemia prevalence increased by 1.90% (95% CI = 1.43, 2.36) and average hemoglobin decreased by 0.07 g/dL (95% CI = 0.09, 0.05). At the individual level, for every 10 μg m–3 increase in ambient PM2.5 exposure, average hemoglobin decreased by 0.14 g/dL (95% CI = 0.12, 0.16). The odds ratio associated with a 10-μg m–3 increase in ambient PM2.5 exposure was 1.09 (95% CI = 1.06, 1.11). There was evidence of effect modification by wealth index, maternal anemia status, and child BMI.
Conclusion:
Our results suggest that ambient PM2.5 exposure could be linked to anemia in Indian children, although additional research on the underlying biologic mechanisms is needed. Future studies on this association should specifically consider interactions with dietary iron deficiency, maternal anemia status, and child BMI.
Keywords: Anemia; Children; Ambient PM2.5 exposure; India; Association
What this study adds
To our knowledge, there have been no previous studies done to assess the association between ambient PM2.5 exposure and anemia in children in India. Across all main models, we found that children exposed to higher levels of ambient PM2.5 were at higher risk of being anemic after adjusting for potential confounders such as diet, sex, wealth index, maternal anemia status, and accounting for clustering by district. This study adds to the body of global evidence highlighting the adverse effects of ambient PM2.5 exposure on human health. There is no conflict of interest.
Introduction
As of 2016, exposure to ambient fine particulate matter (PM2.5) was the fifth leading cause of death globally, according to the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD).1 In India, exposure to ambient PM2.5 is the third leading cause of death.2 India has some of the highest levels of ambient PM2.5 in the world, exceeding the World Health Organization (WHO)-recommended annual mean air quality guideline of 10 μg m–3 by orders of magnitude. Exposure to ambient PM2.5 has been associated with adverse cardiovascular, respiratory, and mortality outcomes, and with child health outcomes that include low birth weight and stunted growth.3–7 The body of epidemiologic studies conducted in India about the impact of ambient PM2.5 exposure on health continues to expand.2,8,9 However, a general lack of information on the association between ambient PM2.5 exposure and many health outcomes still remain, especially from exposure-response analyses.
One such outcome is anemia. Globally, India carries the largest burden of anemia, especially among women and children.10–12 Anemia, measured via. low-blood hemoglobin concentration, is characterized by a decreased oxygen-carrying capacity of the blood. There are several types of anemia, broadly falling into two etiological categories: deficiency in the production of red blood cells (erythrocytes) by the bone marrow and increased rate of erythrocyte destruction (hemolysis). The former could be due to the deficiency of hematopoietic nutrients such as iron and vitamin B12. The latter occurs in specific hemolytic anemias, and the prevalence of these, including hemoglobinopathies, has increased from about 308 million in 1990 to about 506 million in 2017.1,13 Chronic inflammation from any cause operates in both etiologic ways to cause a mild anemia. Inflammation can reduce erythrocyte production and hemoglobin by reducing dietary iron absorption and erythropoiesis and by reducing erythrocyte lifespan.14
Given that the Indian diet has a low iron density, along with evidence of low iron stores based on scattered surveys, it might be logical to infer that most of the burden of anemia is due to iron deficiency.15,16 According to the India National Family and Health Survey 2015–2016 (NFHS-4), 53.1% of women in India with 15–49 years of age and 58.5% of children under five were anemic.17 Despite programs such as the National Iron Plus Initiative aiming to reduce iron deficiency anemia via. iron supplementation and food fortification, anemia prevalence is still high.18 Chronic systemic inflammation is the second leading cause of anemia after dietary iron deficiency, though the two causes are not mutually exclusive.14,19 Exposure to air pollution, especially PM2.5, has been shown to induce systemic inflammation.6,20,21
Most existing studies that have examined exposure to ambient particulate matter and anemia outcomes have been conducted in the United States, Europe, and China and have observed associations between increased exposure to PM and increased anemia prevalence/decreased hemoglobin concentration among elderly individuals.22,23 Additionally, a few studies have observed a strong association between exposure to biomass fuel burning and anemia in pregnant women and infants.24–27 A recent study conducted in Lima, Peru, established an association between exposure to ambient PM2.5 and moderate/severe anemia in children under five.28 The association between ambient PM2.5 exposure and anemia in children under five in India has not yet been examined.
The lack of available spatiotemporal ambient PM2.5 exposure data across India until recent years had limited the number and type of studies that could be performed about associations between PM2.5 exposure and potential health effects. While the network of in situ ground-monitoring sites has improved over time in India, sites are mostly located over urban locations and do not provide exposure data continuous over space and time. Advent of satellite remote sensing techniques over the last two decades allows us to obtain long-term ambient PM2.5 exposure from satellite retrievals of aerosol optical depth (AOD) and study its association with childhood anemia in India.
Long-term ambient PM2.5 exposure could lead to chronic systemic inflammation, which could reduce iron absorption and thus exacerbate the effects of dietary iron deficiency, leading to the onset of anemia.14,29 We hypothesized that long-term ambient PM2.5 exposure would be associated with decreased hemoglobin levels and increased odds of anemia in children after adjusting for relevant covariates. We conducted an ecological (district-level) analysis and an individual-level analysis using satellite retrievals of PM2.5 exposure data and outcome/covariate data from the NFHS-4. To our knowledge, this is the first study of its scale to evaluate this association among children in India.
Methods
Study population
Our study population was obtained from the National Family and Health Survey (NFHS-IV), a nationally representative household survey administered across all 640 districts of India. Data for this survey were collected between January 2015 and November 2016.17 The objective of the NHFS-IV was to collect information about women’s reproductive and sexual health, demographics, lifestyle factors, family life, and birth outcomes, with the aim of informing policy and gauging progress in India’s health sector. The survey used a stratified two-stage sample based on India’s 2011 census as a sampling frame. Primary Sampling Units (PSUs) were villages in rural areas and census enumeration blocks (CEBs) in urban areas. Final PSUs were separated into clusters, and 22 households were selected using systematic sampling from each urban and rural cluster. Questionnaires were administered to collect information about all members in the selected households.
The NHFS-IV administered multiple questionnaires: a household questionnaire, a women’s questionnaire, a men’s questionnaire, and a biomarker questionnaire. Information about children was collected using the women’s, household, and biomarker questionnaires. The women’s questionnaire was administered to women between the ages of 15 and 49 years. Household questionnaires had a 98% response rate, although women’s questionnaires had a 97% response rate. There is little evidence of selection bias due to nonresponse, as indicated by exceptionally high response rates. For further information about sampling design, see the National Family and Health Survey IV Report by the International Institute for Population Sciences.17
Ambient PM2.5 exposure assessment
In the ecological analysis, the primary exposure was five-year average ambient PM2.5 exposure per district, modeled as a continuous variable. We averaged exposure between 2010 and 2015 because the earliest year of birth for children in the study was 2010, and the survey was primarily administered in 2015. For the individual-level analysis, individual exposure to ambient PM2.5 was calculated by averaging district-level exposure from month of birth to month of interview administration for each child. Absence of systematic ground-based PM2.5 measurements at a desirable spatial resolution prompted the use of satellite-derived ambient PM2.5 for this study. We used the Multiangle Imaging SpectroRadiometer (MISR) retrieved level 2 AOD data to estimate the PM2.5, with the help of a spatially and temporally varying conversion factor (η) at 50 × 50 km horizontal resolution. η is derived from the GEOS-Chem chemical transport model simulations and depends on aerosol vertical distribution, emission, and meteorological factors such as temperature, relative humidity, and precipitation. Details about the conversion factor η and the calibration and validation of the data are discussed elsewhere.30–32 We applied this at district level using the district shape files in a GIS platform. We generated monthly ambient PM2.5 exposure for 6 years (2010–2015) for use in this study.30 The monthly ambient PM2.5 data we obtained was bias-corrected against available in situ measurement data and was observed to perform satisfactorily when compared with in situ measurement sites with ±8% uncertainty.30,34 We note that lack of reference grade in situ monitoring sites over the rural locations limits extensive validation of the satellite-derived ambient PM2.5 data over these locations. Satellite-retrieved ambient PM2.5 exposure was averaged within a district boundary to obtain the district-level estimates used in this study.
Hemoglobin concentrations and anemia prevalence estimates
For the ecological analysis, the primary outcomes were district-level anemia prevalence and average hemoglobin concentration by district, measured in g/dL. Anemia prevalence information by district for the ecological analysis was obtained from the India NFHS-4 survey. The primary outcomes of the individual-level analysis were the presence of anemia (“yes” if hemoglobin was <11 g/dL and “no” if it was above 11 g/dL) and hemoglobin level.
Blood specimens were collected from all children aged between 6 and 59 months who resided in the households selected for the survey. Consent was obtained from parent(s) or guardian(s). Blood samples were drawn using a finger or heel prick. Hemoglobin concentrations were measured on-site using the HemoCue Hb 201+ analyzer by trained medical personnel in each district.17,34,35 Blood samples were dried overnight and packaged using a systematic protocol in air-tight plastic bags the following day. After all biomarker testing in each PSU was completed, samples were sent via. Speed Post to the designated laboratory for testing.
Confounders and effect modifiers
All information about covariates was obtained from the NFHS-IV questionnaires administered to mothers of the participating children. We calculated the Dietary Diversity Score (DDS) as a proxy for iron deficiency using the method created by Krebs-Smith et al. 1987 and covered it briefly here.36 Food groups included in DDS calculations were cereals/roots, vegetables/fruits, legumes/pulses, meat/fish, eggs, milk, and Vitamin A-rich foods (e.g., leafy greens, fortified foods). Information about oils/fats was not available for this study population and so was not included in the DDS calculation. Participants were assigned a 1 for every food group consumed and 0 if otherwise. Food groups were summed for each participant to derive a DDS, with possible values between 0 and 6. For the ecological analysis, models were adjusted for average DDS by district, although individual DDS was used for the individual-level models.
Exposure to biomass burning has been linked to anemia in children, so we adjusted for (household-level) cooking fuel type in the individual-level analysis.25,26 Subjects were assigned to clean fuel (biogas, LPG, electricity, no fuel used), kerosene, or biomass (wood, straw/shrubs/grass, agricultural crops, animal dung, coal/lignite, charcoal, other). Ecological models were not adjusted for household cooking fuel type.
Body mass index (BMI) in the NFHS-4 questionnaire was presented as z scores. Obesity has been linked to low-level inflammation and subsequent disruptions in iron trafficking.37 Interpretation of levels of BMI is based on number of standard deviations (SD) from median z-score.17,38,39 Children with BMI z scores +2 SD or more from the median were considered overweight/obese, and those that were –2 SD or less from the median were considered thin/wasted. Average BMI z-score by district was adjusted for in the ecological models, although individual BMI z-scores were used for the individual analysis models.
Socioeconomic status (SES) was quantified using individual wealth index, with levels “poorest,” “poorer,” “middle,” “richer,” and “richest.” Principal component analysis was used to derive scores assigned at the household level, factoring in consumer goods owned (quality and quantity), transportation method, toilet facilities, and flooring materials. Individuals were then ranked based on household wealth score and were subsequently divided into the five equal categories mentioned above, each containing 20% of the population.17
Other covariates used in the analysis were secondhand smoke exposure (yes/no), type of residence (urban/rural), sex (female/male), age in years (continuous), and maternal anemia status (yes/no). For the ecological analysis, covariates were averaged by district, although individual data was used in the individual-level analysis.
Statistical model
R Version 3.3.3 (R Core Team, Vienna, Austria) was used to conduct all statistical analyses.40 Figures were created using R and QGIS Version 2.14.15-Essen (QGIS Development Team, Chicago, IL).41 We first performed an ecological analysis in which we estimated the impact of mean PM2.5 exposure on average anemia prevalence and average hemoglobin levels by district using standard multiple linear regression models. We then evaluated the association of interest at the individual level using generalized linear mixed-effects models to account for clustering by district. We assessed nonlinearity using penalized splines and determined if there were deviations when compared with the generalized linear mixed-effects models using Akaike Information Criteria (AIC). The confounders that were adjusted for were determined a priori and included in the final models. We also assessed effect modification of PM2.5 exposure by wealth index (SES), sex, maternal anemia status, residence type, and DDS using multiplicative interaction terms. A new model was run for each interaction of interest.
Results
Study population and characteristics
The original NFHS-4 dataset contained 259,627 observations; of these, 161,070 observations had missing exposure, outcome, and covariate data (Figure 1). The majority of these observations were omitted due to missing data on variables used to derive dietary diversity score (DDS). Between 31% and 66% of observations in each district were missing DDS variables. See eFigure 1 (http://links.lww.com/EE/A114) for visualization of missing DDS data. Additionally, phlebotomy samples were not available for 50,132 children. We finally included 98,557 children in the individual analysis across 636 districts. In the ecological analysis, 638 of 640 districts were included, as two districts (Mahesana and Kabirdham) had missing anemia prevalence data. Characteristics of the children included in the analysis can be found in Table 1.
Figure 1.
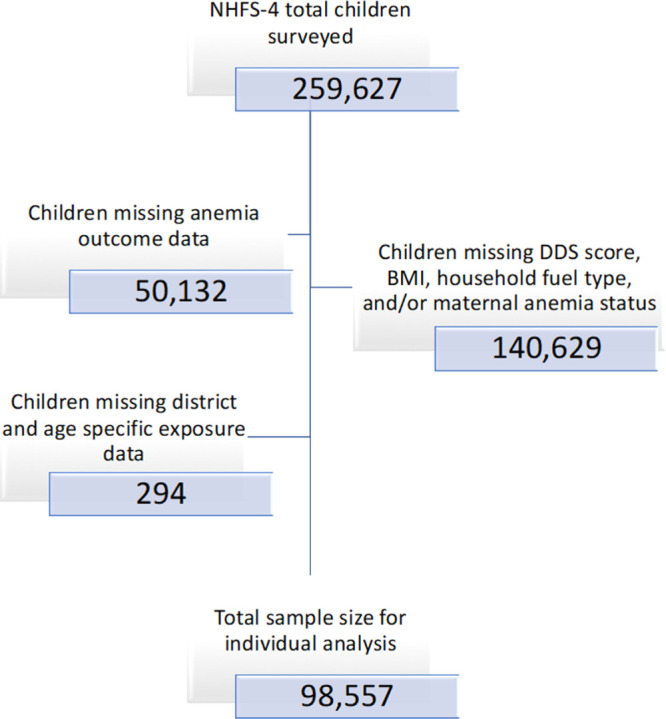
Missing observations from NFHS-4 sorted by variables of interest.
Table 1.
Characteristics of 98,557 children under five included in individual analysis from the Demographic and Health Survey 2014–2015 by anemia category.
| Variable | Total(n = 98,557) | Anemica,b (n = 62,100) | Not anemic(n = 36,457) |
|---|---|---|---|
| Hemoglobin (g/dL) (mean (SD)) | 10.4 (1.6) | 9.5 (1.2) | 11.9 (0.8) |
| PM2.5 exposure (μg m–3) (mean (SD)) | 53.3 (23.3) | 55.7 (22.5) | 49.1 (23.9) |
| Sex (%) | |||
| Female | 51.1 | 50.5 | 52.1 |
| Age (yrs) (mean (SD)) | 2.1 (1.3) | 1.9 (1.2) | 2.4 (1.4) |
| DDSb (%) | |||
| 0 | 31.1 | 31.4 | 30.7 |
| 1 | 23.2 | 24.0 | 22.0 |
| 3 | 30.8 | 31.1 | 30.2 |
| 6 | 14.8 | 12.4 | 17.1 |
| Secondhand smoke (%) | |||
| Yes | 57.6 | 57.4 | 58.0 |
| Wealth Indexc (%) | |||
| Poorest | 28.3 | 30.3 | 25.0 |
| Poorer | 24.3 | 24.0 | 24.9 |
| Middle | 20.1 | 19.7 | 20.8 |
| Richer | 15.6 | 15.0 | 16.8 |
| Richest | 11.6 | 11.0 | 12.4 |
| Residence type (%) | |||
| Urban | 21.6 | 21.3 | 22.3 |
| Cooking fuel (%) | |||
| Clean fuel | 26.3 | 25.1 | 28.2 |
| Kerosene | 0.7 | 0.7 | 0.9 |
| Biomass | 73.0 | 74.2 | 71.0 |
| Maternal anemia (%) | |||
| Yes | 58.1 | 64.0 | 47.9 |
| BMId (%) | |||
| –2 SD | 3.5 | 3.3 | 3.6 |
| Median | 94.2 | 94.3 | 94.1 |
| +2 SD | 2.4 | 2.4 | 2.3 |
aChild considered anemic if blood hemoglobin <11.0 g/dL.
bDDS is a composite measure of diet quality used as a proxy for iron deficiency. It is calculated by considering consumption of various food groups (see Methods). Cutoffs determined by quartiles of DDS (DDS range is 0–6).
cWealth index was determined using household-level cores based on quality/quantity of consumer goods, transportation method, toilet facilities, and flooring materials. Scores were divided into quintiles from lowest to highest.
dChildren with BMI z-scores that are -2 SD or more from the median are considered underweight; children with BMI z scores that are +2 SD or more from the median are considered overweight.
BMI, body mass index; DDS, diet diversity score.
About 63% of the included children were anemic. Children with anemia were on average slightly younger compared with children without anemia, tended to be from lower wealth index levels, and had higher percentages of maternal anemia. See Figure 2 for district-level prevalence of anemia and district-level ambient PM2.5 exposure levels and eFigure 2 (http://links.lww.com/EE/A114) for visualization of variability in ambient PM2.5 exposure between 2010 and 2015. Although districts with larger areas seemed to have higher variability in ambient PM2.5 exposure, standard deviations rarely exceeded 10 μg m–3. Summary statistics for India’s 36 states/UTs are presented in eTable 1 (http://links.lww.com/EE/A114). There was more variability in ambient PM2.5 exposure in the states with the highest anemia prevalence. Summary statistics for the variables of both the included and omitted observations were similar (see eTable 2; http://links.lww.com/EE/A114).
Figure 2.
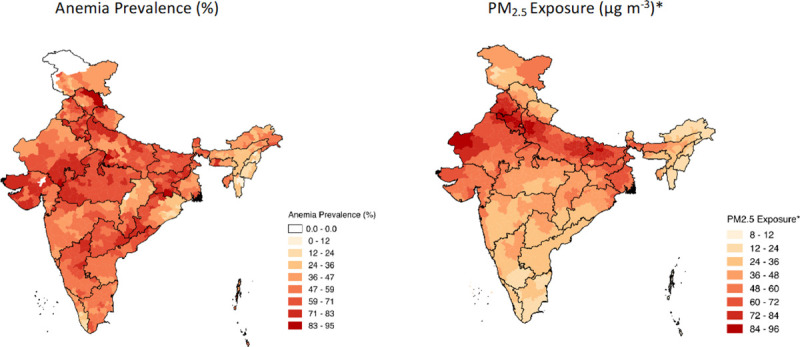
(L) Prevalence of anemia by district within each state in India; children considered anemic if Hb < 11 g/dL. (R) PM2.5 exposure by district in each state, measured in μg m–3.
Ecological analysis
In multivariable models adjusting for relevant confounders, anemia prevalence increased with increasing ambient PM2.5 exposures (Table 2). For every 10 μg m–3 increase in ambient PM2.5 exposure, we observed a 0.07 g/dL (0.09, 0.05) decrease in average hemoglobin level. Additionally, for every 10 μg m–3 increase in ambient PM2.5, we observed a 1.90% (1.43, 2.36) increase in average anemia prevalence.
Table 2.
District- and individual-level effects of every 10 μg m–3 increase in ambient PM2.5 exposure on hemoglobin and anemia in India (number of districts: 638 and number of children: 98,557).
| Ecologicala,c | Sample size (n) | Change in Hb (g/dL) (95% CI) | Change in anemia prevalence (%) (95% CI) |
| 638 | –0.07 (–0.09, –0.05) | 1.90 (1.43, 2.36) | |
| Individualb,d | Sample size (n) | Change in Hb (g/dL) (95% CI) | Adjusted odds ratio of anemia (95% CI) |
| 98,557 | –0.14 (–0.16, –0.12) | 1.09 (1.06, 1.11) |
aAnalysis unit for ecological analysis was district, PM2.5 exposure modeled as long-term district average (2010–2015).
bAnalysis unit for individual analysis was individual child, PM2.5 exposure modeled as average individual exposure from birth month to month of interview.
cFinal models were adjusted by average DDS, percent female, proportion of children exposed to secondhand smoke, percent urban (vs. rural), average BMI z score, percent biomass, and average wealth index.
dFinal models were adjusted by sex, DDS, age in years, residence type (urban vs. rural), maternal anemia status, (household) biomass exposure, individual wealth index, individual BMI z score, and secondhand smoke exposure; district-level random effects.
BMI, body mass index; DDS, diet diversity score.
Individual-level analysis
Four districts (North East Delhi, Central Delhi, Diu, and Yanam) are excluded because the exposure data could not be generated separately for these districts as they are smaller than MISR level 2 pixel size. As presented in Table 2, across India, for every 10 μg m–3 increase in ambient PM2.5 exposure, adjusted average hemoglobin level decreased by 0.14 g/dL (0.16, 0.12). When modeling anemia as a binary outcome (yes/no), for a 10 μg m–3 increase in ambient PM2.5 exposure, the adjusted odds ratio of anemia was about 1.09 (1.06, 1.11). Other significant variables in the analysis included DDS, wealth index, BMI, exposure to secondhand smoke, type of cooking fuel, maternal anemia status, and age.
Effect modification
We did not find evidence of effect modification by sex, DDS, residence type, and secondhand smoke exposure. There was evidence of effect modification by wealth index, maternal anemia status, and BMI (Table 3). Higher levels of wealth index appeared to be protective compared with lower levels when exposed to the same amount of ambient PM2.5. Children whose mothers were anemic had higher odds of anemia compared with children whose mothers were not anemic. Finally, effect estimates were larger for higher BMI z-scores compared with lower z-scores. We observed that children with higher BMI z-scores had higher odds of anemia compared with children with lower z-scores when exposed to the same amount of ambient PM2.5.
Table 3.
Assessing individual-level effect modification of the association between each 10 μg/m–3 increase in ambient PM2.5 exposures, hemoglobin, and anemia by relevant covariates for 98,557 children under five or younger participating in the NFHS-4.
| Variabled | Hb (g/dL) (95% CI) | P value for interaction | Odds ratio of anemia (95% CI) | P value for interaction |
|---|---|---|---|---|
| Sex | ||||
| Male (reference) | –0.05 (–0.06, –0.03) | 1.00 | ||
| Female | 0.02 (–0.05, 0.09) | 0.47 | 1.03 (0.93, 1.15) | 0.57 |
| DDS | ||||
| 0 (reference) | –0.04 (–0.06, –0.02) | 1.00 | ||
| 1 | –0.04 (–0.13, 0.05) | 0.13 | 1.11 (0.96, 1.28) | 0.995 |
| 2 | –0.07 (–0.18, 0.03) | 0.15 | 1.12 (0.95, 1.32) | 0.44 |
| 3 | –0.05 (–0.15, 0.05) | 0.07 | 1.10 (0.95, 1.29) | 0.14 |
| 4 | –0.07 (–0.19, 0.05) | 0.38 | 1.22 (1.01, 1.46) | 0.71 |
| 5 | –0.04 (–0.19, 0.11) | 0.60 | 1.07 (0.85, 1.35) | 0.63 |
| 6 | –0.05 (–0.20, 0.10) | 0.67 | 1.08 (0.86, 1.36) | 0.74 |
| Secondhand smoke | ||||
| No (reference) | –0.04 (–0.06, –0.02) | 1.00 | ||
| Yes | –0.01 (–0.08, 0.07) | 0.002 | 1.04 (0.93, 1.17) | 0.02 |
| Wealth Index | ||||
| Poorest (reference) | –0.04 (–0.05, –0.02) | 1.00 | ||
| Poorer | 0.08 (–0.02, 0.18) | 0.29 | 0.89 (0.76, 1.04) | 0.34 |
| Middle | 0.16 (0.05, 0.26) | 0.35 | 0.86 (0.73, 1.02) | 0.56 |
| Richer | 0.38 (0.27, 0.50) | <0.001 | 0.64 (0.53, 0.76) | 0.01 |
| Richest | 0.47 (0.34, 0.60) | 0.02 | 0.58 (0.47, 0.70) | 0.35 |
| Residence type | ||||
| Rural (Reference) | –0.04 (–0.06, –0.03) | 1.00 | ||
| Urban | –0.02 (–0.10, 0.07) | < 0.001 | 1.01 (0.89, 1.15) | 0.001 |
| Maternal anemia | ||||
| No (reference) | –0.05 (–0.06, –0.03) | 1.00 | ||
| Yes | –0.41 (–0.48, –0.34) | 0.39 | 1.77 (1.59, 1.97) | 0.70 |
| BMIc | ||||
| –2 SD | 0.02 (0.07, –0.03) | 1.02 (0.96, 1.07) | ||
| Median | –0.034 (–0.04, –0.031) | 0.44 | 1.066 (1.060, 1.073) | 0.73 |
| +2 SD | –0.08 (–0.14, –0.03) | 1.12 (1.05, 1.20) |
aModels were adjusted for sex, DDS, residence type (urban vs. rural), wealth index, maternal anemia status, (household) cooking fuel type, BMI z-score, and secondhand smoke exposure; district-level random effects.
bResults presented for a 10 μg m–3 increase in ambient PM2.5 exposure and holding all other covariates constant.
cBMI modeled as a continuous variable; cutoffs presented are for underweight/thin, median z-score, and overweight.
dStratified effect estimates presented holding all other model covariates constant.
BMI, body mass index; DDS, diet diversity score.
Assessing nonlinearity
We assessed potential nonlinearities in the associations of interest using penalized smoothing splines, presented in Figure 3. However, the generalized linear mixed models were found to be more robust than the penalized splines fit, based on AIC.
Figure 3.
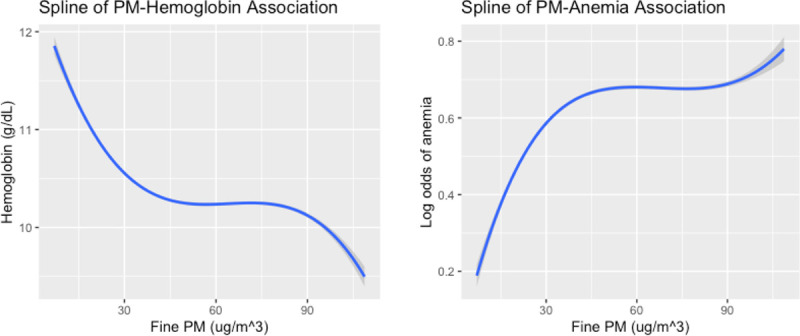
Penalized splines of the association between ambient PM2.5 exposure and anemia outcomes (L) Spline used to assess potential nonlinearity in association between ambient PM2.5 exposure (μg m–3) and hemoglobin level (g/dL) at the individual level. (R) Spline was used to assess nonlinearity between ambient PM2.5 exposure (μg m–3) and anemia status. Shaded regions denote 95% Confidence bands.
Discussion
This study aimed to assess the association between ambient PM2.5 exposure and anemia in children under five across India. Through the results of this study, we observed that children exposed to higher levels of ambient PM2.5 on average had lower hemoglobin levels and higher odds of being anemic. Although the study design does not allow us to comment on causality, the results of this preliminary study do highlight an association between our exposure and outcome of interest. Childhood anemia in India is an endemic problem; as of 2016, nearly 60% of children in India were anemic.17 The introduction of the National Iron Plus Initiative in 2011 sought to expand the beneficiaries of the National Nutritional Anemia Prophylaxis Program to children with 6–59 months of age.42 Although anemia decreased by about 11% between 2006 and 2016, it remains a major issue despite an increase in available food fortified with iron.43 It is clear that other potential risk factors for childhood anemia must be identified and understood.
The association between air pollution and anemia has been studied in a limited capacity. This is the first study, to our knowledge, that examines the association between ambient PM2.5 exposure and anemia across India in children under five. Morales-Ancajima et al. presented similar findings in children under five in a study conducted in Lima, Peru.28 This study modeled ambient PM2.5 exposure in quintiles and observed statistically significant associations between increased ambient PM2.5 exposure quintile and increased hemoglobin levels (4Q: –0.026; 5Q: –0.048), as well as increased odds of moderate/severe anemia (2Q, 3Q: 1.09, 4Q: 1.19, 5Q: 1.24), among children between the ages of 6 and 59 months. Our results are similar to these, even though childhood anemia prevalence in India (about 58.5% as of 2016) is higher than in Peru (39.6%).28 Another existing study is that of Nikolić et al, which looked to examine the effects of air pollution on erythrocytes in children between the ages of 11 and 14 years.44 The study found that anemia prevalence was significantly higher in children exposed to higher levels of air pollution compared with lower levels (RR = 3.76 [2.06-6.88]). However, this study failed to adjust for dietary information, which likely biased the results away from the null. A larger body of research exists examining the association between air pollution and anemia in the elderly population.22
The biologic plausibility of this study is based on existing literature that establishes a clear link between exposure to high levels of air pollution to inflammation, and subsequently to anemia of inflammation, via. elevated levels of C-reactive proteins (CRP).45–48 The majority of studies to establish this link have focused on elderly populations. There are several proposed mechanisms through which chronic inflammation could have an impact on erythrocytes and hemoglobin concentration.14 First, by immune activation, inflammation leads to changes in iron trafficking in the body through the secretion of cytokines, most notably interleukin-6 (IL-6). These stimulate the liver to produce hepcidin, which binds to the cellular transmembrane iron exporter, ferroportin, and inhibits both dietary iron absorption in the duodenum and the recycling of iron from senescent erythrocytes through macrophages. In effect, there is less iron for hemoglobin formation, and the inflammatory cytokines may also independently suppress erythropoiesis. Although inflammation can occur in several clinical conditions such as cancer and multiple organ dysfunctions, another reason of public health importance is obesity, which can induce a low-grade activation of the innate immune system.49 The inflammatory response also induces an increase in cytokines, which reduces iron trafficking. In India, inappropriate fat accumulation has been observed even at normal body size, and adiposity has been shown to reduce iron absorption in women.37,50,51
There is a growing body of evidence suggesting that air pollution is associated with child obesity and child BMI.52–54 Our results suggest that children with higher BMI z-scores may be more likely to have anemia compared with those with lower BMI z-scores at the same level of PM2.5 exposure. High child BMI could have a role to play in how PM2.5 exposure affects anemia outcomes in children in India. However, child BMI can also serve as a marker of other health and lifestyle conditions; lower BMI could be indicative of stunting, premature birth, and malnutrition, which may be associated with risk of anemia.55,56 The results regarding effect modification of the association between PM2.5 exposure and anemia by BMI encourage further research specifically about this interaction and its biologic mechanisms.
In this study, dietary diversity score (DDS) was used primarily as a proxy for iron deficiency; iron deficiency is the leading cause of anemia worldwide.16 DDS has been declared an appropriate metric to adjust for the presence of basic micronutrients; many studies have used DDS as a proxy for adequate nutrition.57–59 Although we did not specifically adjust for vegetarianism, DDS was also able to capture whether children consumed meat or not. We observed that as average DDS increased, average hemoglobin level also increased, indicating that as children received better nutrition, their hemoglobin levels improved. There was no evidence of effect modification of the association between PM2.5 exposure and anemia by DDS.
We observed evidence that both wealth index and maternal anemia status were effect modifiers for the association of interest. Compared with children of lower SES, children of higher SES status had higher levels of hemoglobin and lower odds of having anemia when exposed to the same amount of PM2.5. Children of higher SES are more likely to have adequate access to nutrition, less overall exposure to harmful air pollutants, and overall slightly better health outcomes compared with children of lower SES, which could potentially explain why the same level of PM2.5 exposure brings about significantly different anemia outcomes between levels of SES.60,61
Maternal anemia status could also be a modifier of the association between PM2.5 exposure and anemia. Children whose mothers were anemic had, on average, lower hemoglobin levels and higher odds of having anemia when compared with children whose mothers were not anemic when ambient PM2.5 exposure was held constant. This suggests that family history of anemia could play a role in how the body reacts to ambient PM2.5 exposure.62 Our findings here indicate that further research about family history as a risk factor for anemia and gene-environment interaction in this context could be valuable to better understand the observed results.
Indoor air pollution, most often caused by burning of biomass as cooking fuel, has also been recognized as a threat to human health.2,63,64 In 2016, India launched the Pradhan Mantri Ujjwala Yojana (PMUY), aimed at provided women and children safe access to Liquefied Petroleum Gas (LPG) and electricity. Since the launch of this intervention, household PM2.5 exposure levels have been significantly reduced.65 The methodology used in this study should be replicated using NHFS-V (2020) data to observe whether this reduction in household air pollution has in any way reduced ambient PM2.5 levels and whether this is reflected in the association with anemia outcomes in children. Such results would provide strong evidence for the transition to cleaner household energy.
Using splines, we observed potential nonlinearities in the associations between ambient PM2.5 exposure and both anemia outcomes, although models with the linear terms had an improved fit relative to those with the splines (Figure 3). It is possible that these nonlinearities can be attributed to some threshold effect of ambient PM2.5 exposure on anemia outcomes, although the biologic mechanisms at play are not clear. What these results do indicate is that exposure to ambient PM2.5 at both lower levels (i.e., below about 30 10 μg/m–3) and extremely high levels could be associated with increased odds of anemia and decreased hemoglobin levels. A 2020 study conducted by Elbarbary et al, which assessed the association between ambient air pollution exposure and anemia prevalence/hemoglobin levels in older Chinese adults, detected nonlinearities in dose-response curves for PM and NO2 exposure with respect to hemoglobin levels and anemia prevalence in the study population.48 There is inconsistency in the results across the limited studies that have been conducted that examine air pollution and its association with anemia. This is likely because these studies have all focused on different geographic regions and demographic groups. Although this has implications for generalizability, most of the existing studies have detected some level of association, indicating that this association warrants further research focusing on vulnerable groups such as children, elderly populations, and women of reproductive age. Future studies should specifically examine the potential mechanisms underlying the observed nonlinearities between ambient PM2.5 exposure and anemia outcomes.
There are assumptions and limitations to consider when assessing the results of this study. For the purposes of exposure assignment, we have assumed that children did not leave their district of birth. Linear regression assumptions were verified using model diagnostics, residual plots, and subject matter knowledge. We have assumed that DDS is an adequate proxy for iron deficiency; data about iron intake was not available and should be more specifically factored in for future studies on this association. Additionally, the NFHS-4 dataset was missing information about consumption of oils/fats, which is a category included in calculation of DDS. These data were missing for all children in the dataset. Oils/fats are not as important of an indicator for iron consumption as are vegetables, fruits, and meat, so we have assumed that this missingness has not biased the results. Although most of the omitted observations were missing information about DDS, the missingness appeared nondifferential and would have biased results toward the null, if at all. Another limitation of this study is the cross-sectional study design, as it limits ability to assess temporality of exposure and outcome. However, the aim of this study was not to establish causality but was rather to assess whether an association was present. We were also unable to adjust for in-utero PM2.5 exposure; prenatal exposure to PM2.5 has been linked with outcomes such as reduced fetal growth and could have an impact on the development of multiple systems involved in hematology.
This study is the first of its kind conducted in children under five across India. Using publicly available data allowed us to perform this analysis on a large, representative sample and allowed us to adjust for relevant confounders. The main aim of the study was to conduct an exploratory analysis to determine the existence and magnitude of the association between ambient PM2.5 exposure and anemia in children. Our results indicate evidence of an association, thus reinforcing already existing findings. The results of this study indicate that further epidemiologic and toxicologic studies should be conducted to further understand this relationship and the biologic mechanisms that drive it. Additionally, our results add to the growing body of evidence suggesting that meeting targets for the National Clean Air Program would improve child health and that air pollution control should be a top priority in India.
Conflicts of interest statement
The authors declare that they have no conflicts of interest with regard to the content of this report.
Acknowledgments
U.M. acknowledges funding from Fulbright-Nehru Student Research Scholarship to work on project at IIT-Delhi. S.D. acknowledges financial support for the Institute Chair Professor position. DST-FIST program (SR/FST/ESII-016/2014) is acknowledged for providing computing support at IIT-Delhi. J.E.H. was supported by US National Institutes of Health grant P30 ES000002. Authors acknowledge the comments by the reviewers that helped improve the manuscript.
UNNATI MEHTA initiated the work during a Fulbright-Nehru Student Research grant at the Indian Institute of Technology-Delhi, India, and is now at the Harvard University T. H. Chan School of Public Health, Boston, USA, where the work was completed.
SOURANGSU CHOWDHURY is now at Max Planck Institute for Chemistry, Mainz, Germany.
Data sources
MISR data are archived in NASA Langley Research Atmospheric Data Science Center. NFHS-4 India health data can be accessed via. the Demographic and Health Survey webpage. Email U.M. for computing code.
S.D. and U.M. conceived the study and wrote the initial manuscript. U.M. carried out the analysis. S.C. generated the exposure data. S.G. helped in calculating DDS and provided input for analysis. J.H. and A.K. provided input for the analysis. All authors provided comments and contributed to the final version of the manuscript.
Supplementary Material
Footnotes
Published online 7 January 2021
Sponsorships or competing interests that may be relevant to content are disclosed at the end of the article.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.environepidem.com).
References
- 1.The Lancet. GBD 2017: a fragile world. Lancet. 2018; 392:1683. [DOI] [PubMed] [Google Scholar]
- 2.India State-Level Disease Burden Initiative Air Pollution Collaborators. The impact of air pollution on deaths, disease burden, and life expectancy across the states of India: the Global Burden of Disease Study 2017. Lancet Planet Health. 2019; 3:e26–e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pun V, Mehta S, Dowling R. Air pollution and child stunting—a systematic review and meta-analysis. Environ Epidemiol. 2019; 3:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun X, Luo X, Zhao C, et al. The associations between birth weight and exposure to fine particulate matter (PM2.5) and its chemical constituents during pregnancy: a meta-analysis. Environ Pollut. 2016; 211:38–47. [DOI] [PubMed] [Google Scholar]
- 5.Thurston GD, Kipen H, Annesi-Maesano I, et al. A joint ERS/ATS policy statement: what constitutes an adverse health effect of air pollution? An analytical framework. Eur Respir J. 2017; 49:1600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brook RD, Rajagopalan S, Pope CA, III, et al. ; American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010; 121:2331–2378. [DOI] [PubMed] [Google Scholar]
- 7.Franklin BA, Brook R, Arden Pope C, III. Air pollution and cardiovascular disease. Curr Probl Cardiol. 2015; 40:207–238. [DOI] [PubMed] [Google Scholar]
- 8.Spears D, Dey S, Chowdhury S, Scovronick N, Vyas S, Apte J. The association of early-life exposure to ambient PM2.5 and later-childhood height-for-age in India: an observational study. Environ Health. 2019; 18:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balakrishnan K, Ghosh S, Thangavel G, et al. Exposures to fine particulate matter (PM2.5) and birthweight in a rural-urban, mother-child cohort in Tamil Nadu, India. Environ Res. 2018; 161:524–531. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen PH, Scott S, Avula R, Tran LM, Menon P. Trends and drivers of change in the prevalence of anaemia among 1 million women and children in India, 2006 to 2016. BMJ Glob Health. 2018; 3:e001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Institute for Health and Metrics Evaluation. GBD Compare. Global Burden of Disease. 2015. Available at: https://vizhub.healthdata.org/gbd-compare/#. Accessed 3 January 2020. [Google Scholar]
- 12.GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016; 388:1659–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.India State-Level Disease Burden Initiative Collaborators. Nations within a nation: variations in epidemiological transition across the states of India, 1990–2016 in the Global Burden of Disease Study. Lancet. 2017; 390:2437–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood. 2019; 133:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Indian Council of Medical Research (ICMR). Nutrient Requirements and Recommended Dietary Allowances for Indians: A Report of the Expert Group of the Indian Council of Medical Research. National Institute of Nutrition; 2010. [Google Scholar]
- 16.De Benoist B, McLean E, Egli I, Cogswell M. Worldwide Prevalence on Anaemia 1993-2005. World Health Organization; 2008. [Google Scholar]
- 17.International Institute for Population Sciences (IIPS). National family health survey (NFHS-4) 2015-2016. IIPS ICF. 2017; 4:297–299. [Google Scholar]
- 18.Ministry of Health and Family Welfare. Anaemia Mukt Bharat: Intensified National Iron Plus Initiative (I-NIPI) - Operational Guidelines for Programme Managers. Government of India; 2018. [Google Scholar]
- 19.World Health Organization. The global prevalence of anaemia in 2011. WHO. 2015; 43. Available at: http://www.who.int/nutrition/publications/micronutrients/global_prevalence_anaemia_2011/en/. Accessed 2 January 2020. [Google Scholar]
- 20.Wu W, Jin Y, Carlsten C. Inflammatory health effects of indoor and outdoor particulate matter. J Allergy Clin Immunol. 2018; 141:833–844. [DOI] [PubMed] [Google Scholar]
- 21.Delfino RJ, Staimer N, Tjoa T, et al. Associations of primary and secondary organic aerosols with airway and systemic inflammation in an elderly panel cohort. Epidemiology. 2010; 21:892–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honda T, Pun VC, Manjourides J, Suh H. Anemia prevalence and hemoglobin levels are associated with long-term exposure to air pollution in an older population. Environ Int. 2017; 101:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seaton A, Soutar A, Crawford V, et al. Particulate air pollution and the blood. Thorax. 1999; 54:1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page CM, Patel A, Hibberd PL. Does smoke from biomass fuel contribute to anemia in pregnant women in Nagpur, India? A cross-sectional study. PLoS One. 2015; 10:e0127890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishra V, Retherford RD. Does biofuel smoke contribute to anaemia and stunting in early childhood? Int J Epidemiol. 2007; 36:117–129. [DOI] [PubMed] [Google Scholar]
- 26.Kyu HH, Georgiades K, Boyle MH. Biofuel smoke and child anemia in 29 developing countries: a multilevel analysis. Ann Epidemiol. 2010; 20:811–817. [DOI] [PubMed] [Google Scholar]
- 27.Machisa M, Wichmann J, Nyasulu PS. Biomass fuel use for household cooking in Swaziland: is there an association with anaemia and stunting in children aged 6-36 months? Trans R Soc Trop Med Hyg. 2013; 107:535–544. [DOI] [PubMed] [Google Scholar]
- 28.Morales-Ancajima VC, Tapia V, Vu BN, Liu Y, Alarcón-Yaquetto DE, Gonzales GF. Increased outdoor PM2.5 concentration is associated with moderate/severe anemia in children aged 6-59 months in Lima, Peru. J Environ Public Health. 2019; 2019:6127845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005; 352:1011–1023. [DOI] [PubMed] [Google Scholar]
- 30.Dey S, Di Girolamo L, van Donkelaar A, Tripathi SN, Gupta T, Mohan M. Variability of outdoor fine particulate (PM 2.5) concentration in the Indian subcontinent: a remote sensing approach. Remote Sens Environ. 2012; 127:153–161. [Google Scholar]
- 31.van Donkelaar A, Martin RV, Brauer M, et al. Global estimates of ambient fine particulate matter concentrations from satellite-based aerosol optical depth: development and application. Environ Health Perspect. 2010; 118:847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Donkelaar A, Martin RV, Brauer M, Boys BL. Use of satellite observations for long-term exposure assessment of global concentrations of fine particulate matter. Environ Health Perspect. 2015; 123:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chowdhury S, Dey S, Smith KR. Ambient PM2.5 exposure and expected premature mortality to 2100 in India under climate change scenarios. Nat Commun. 2018; 9:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.HemoCue. Anemia. 2020. Available at: https://www.hemocue.com/en/health-areas/anemia. Accessed 21 August 2020.
- 35.Sanchis-Gomar F, Cortell-Ballester J, Pareja-Galeano H, Banfi G, Lippi G. Hemoglobin point-of-care testing: the HemoCue system. J Lab Autom. 2013; 18:198–205. [DOI] [PubMed] [Google Scholar]
- 36.Krebs-Smith SM, Smiciklas-Wright H, Guthrie HA, Krebs-Smith J. The effects of variety in food choices on dietary quality. J Am Diet Assoc. 1987; 87:897–903. [PubMed] [Google Scholar]
- 37.Kurpad AV, Varadharajan KS, Aeberli I. The thin-fat phenotype and global metabolic disease risk. Curr Opin Clin Nutr Metab Care. 2011; 14:542–547. [DOI] [PubMed] [Google Scholar]
- 38.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007; 85:660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson LN, Carsley S, Lebovic G, et al. Misclassification of child body mass index from cut-points defined by rounded percentiles instead of Z-scores. BMC Res Notes. 2017; 10:639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.R Core Team. R: A Language and Environment for Statistical Computing. 2019. Available at: https://www.r-project.org/.
- 41.QGIS Development Team. QGIS Information System. 2017. Available at: https://qgis.org/en/site/.
- 42.Kapil U, Kapil R, Gupta A. National iron plus initiative: current status & future strategy. Indian J Med Res. 2019; 150:239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.International Institute for Population Science. National Family Health Survey (NFHS-3), 2005-06: India. International Institute for Population Sciences (IIPS) and Macro International; 2007. [Google Scholar]
- 44.Nikolić M, Nikić D, Stanković A. Effects of air pollution on red blood cells in children. Polish J Environ Stud. 2008; 17:267–271. [Google Scholar]
- 45.Viehmann A, Hertel S, Fuks K, et al. ; Heinz Nixdorf Recall Investigator Group. Long-term residential exposure to urban air pollution, and repeated measures of systemic blood markers of inflammation and coagulation. Occup Environ Med. 2015; 72:656–663. [DOI] [PubMed] [Google Scholar]
- 46.Bárány P. Inflammation, serum C-reactive protein, and erythropoietin resistance. Nephrol Dial Transplant. 2001; 16:224–227. [DOI] [PubMed] [Google Scholar]
- 47.Hsu CY, Bates DW, Kuperman GJ, Curhan GC. Relationship between hematocrit and renal function in men and women. Kidney Int. 2001; 59:725–731. [DOI] [PubMed] [Google Scholar]
- 48.Elbarbary M, Honda T, Morgan G, et al. Ambient air pollution exposure association with anaemia prevalence and haemoglobin levels in Chinese older adults. Int J Environ Res Public Health. 2020; 17:3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011; 121:2111–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aigner E, Feldman A, Datz C. Obesity as an emerging risk factor for iron deficiency. Nutrients. 2014; 6:3587–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herter-Aeberli I, Thankachan P, Bose B, Kurpad AV. Increased risk of iron deficiency and reduced iron absorption but no difference in zinc, vitamin A or B-vitamin status in obese women in India. Eur J Nutr. 2016; 55:2411–2421. [DOI] [PubMed] [Google Scholar]
- 52.Adair H, Arroyo V. WHO | Air Pollution and Child Health: Prescribing Clean Air. World Health Organization; 2018. [Google Scholar]
- 53.Limaye S, Salvi S. Obesity and asthma: the role of environmental pollutants. Immunol Allergy Clin North Am. 2014; 34:839–855. [DOI] [PubMed] [Google Scholar]
- 54.Jerrett M, McConnell R, Wolch J, et al. Traffic-related air pollution and obesity formation in children: a longitudinal, multilevel analysis. Environ Health. 2014; 13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramachandran P, Gopalan HS. Assessment of nutritional status in Indian preschool children using WHO 2006 growth standards. Indian J Med Res. 2011; 134:47–53. [PMC free article] [PubMed] [Google Scholar]
- 56.Sahu SK, Ganesh Kumar S, Vishnu Bhat B, et al. Malnutrition among under-five children in India and strategies for control. J Nat Sci Biol Med. 2015; 6:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steyn NP, Nel JH, Nantel G, Kennedy G, Labadarios D. Food variety and dietary diversity scores in children: are they good indicators of dietary adequacy? Public Health Nutr. 2006; 9:644–650. [DOI] [PubMed] [Google Scholar]
- 58.Habte TY, Krawinkel M. Dietary diversity score: a measure of nutritional adequacy or an indicator of healthy diet? J Nutr Heal Sci. 2016; 3. [Google Scholar]
- 59.Belachew A, Tewabe T. Under-five anemia and its associated factors with dietary diversity, food security, stunted, and deworming in Ethiopia: systematic review and meta-analysis. Syst Rev. 2020; 9:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pappachan B, Choonara I. Inequalities in child health in India. BMJ Paediatr Open. 2017; 1:e000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Varadharajan KS, Thomas T, Kurpad AV. Poverty and the state of nutrition in India. Asia Pac J Clin Nutr. 2013; 22:326–339. [DOI] [PubMed] [Google Scholar]
- 62.Kotecha PV. Nutritional anemia in young children with focus on Asia and India. Indian J Community Med. 2011; 36:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith KR. National burden of disease in India from indoor air pollution. Proc Natl Acad Sci U S A. 2000; 97:13286–13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dutta K, Shields KN, Edwards R, Smith KR. Impact of improved biomass cookstoves on indoor air quality near Pune, India. Energy Sustain Dev. 2007; 11:19–32. [Google Scholar]
- 65.Sharma D, Jain S. Impact of intervention of biomass cookstove technologies and kitchen characteristics on indoor air quality and human exposure in rural settings of India. Environ Int. 2019; 123:240–255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


